Introduction
Thyroid carcinoma, which is a common malignant tumor
of the endocrine system (1), has
one of the highest incidence rates for malignant tumors in a number
of regions (1,2), such as the United States (3), Korea (4) and southern European countries
(5). According to the latest data
from the American Cancer Society, there were 52,070 new cases and
2,170 mortalities of thyroid carcinoma in 2019 (3). According to its pathological
characteristics, thyroid cancer can be divided into papillary
thyroid cancer, follicular thyroid cancer, medullary thyroid
carcinoma and undifferentiated thyroid carcinoma (6). At present, the treatments for
differentiated thyroid cancer are mainly surgical resection,
thyroid-stimulating hormone inhibition treatment, radioactive
iodine treatment and molecular-targeted therapy (7). However, due to the strong invasive and
migratory nature of thyroid cancer and its high degree of
malignancy, recurrence or distant metastasis often occurs in a
number of patients after treatment (8). Thus, investigation of the molecular
mechanisms involved in the metastasis of thyroid carcinoma, and
development of molecular markers and targets of thyroid carcinoma
are important for the treatment of the cancer.
Long non-coding (lnc)RNAs are RNAs with >200
nucleotides in length and without protein-coding functions
(9). lncRNAs have been reported to
regulate numerous biological processes (9); for example, lncRNAs participate in the
growth and development of the human body and occurrence of numerous
diseases (9), such as cancer
(10) and glomerular and
tubulointerstitial kidney disease (11). A previous study indicated that the
genome is widely transcribed and regulated by lncRNAs, and various
lncRNAs serve important roles in different biological processes,
such as in chromatin remodeling, transcription, cleavage and
translation (12). Gene expression
profiling of tumors is indicative of abnormal expressions of
lncRNAs in tumors, and functional studies have reported that
lncRNAs are involved in general mechanisms underlying tumorigenesis
(13,14).
ZFAS1 serves a role in atherosclerosis (15) and a variety of cancer types
(16–19), such as ovarian cancer, breast
cancer, prostate cancer and hepatocellular carcinoma. ZFAS1 is a
candidate biomarker predictive of the prognosis of thyroid
carcinoma (20). Han et al
(20) reported that Homo
sapiens (hsa)-microRNA (miRNA/miR)-150-5p and hsa-miR-590-3p
are competitive endogenous RNAs related to ZFAS1 in thyroid cancer
cells. ZFAS1 promotes progression of papillary thyroid carcinoma by
sponging miR-590-3p and increasing high-mobility group AT-hook 2
expression (21). lncRNAs sponge
different miRNAs to regulate the cellular functions (22). Additionally, it has been reported
that miR-302a-3p serves a role in a variety of diseases, such as
hepatocellular carcinoma (23) and
pancreatic ductal adenocarcinoma (24). Long intergenic non-protein coding
RNA (LINC)01016 promotes the malignant phenotype of endometrial
cancer cells by regulating the miR-302a-3p/miR-3130-3p/nuclear
transcription factor Y subunit α/SATB homeobox 1 axis (25). In addition, miR-302a-3p suppresses
the progression of hepatocellular carcinoma by inhibiting
proliferation and invasion of the tumor cells (23). However, the role of miR-302a-3p in
thyroid carcinoma has not been reported.
The present study investigated the role of ZFAS1 in
the proliferation, migration, invasion and epithelial-mesenchymal
transition (EMT) of thyroid carcinoma cells, and explored the
downstream miRNA and target gene via which ZFAS1 exerted its
regulatory effects on thyroid carcinoma cells.
Materials and methods
Patients
This study was approved by the Ethics Board of The
First Hospital of Qiqihar (approval no. QR20180503112). Samples
(n=30) from carcinoma as well as the adjacent tissue (≥5 cm away
from the cancer tissue) were extracted from patients (age range,
28–65 years; mean age, 41.2±8.24 years; males, 11; females, 19)
diagnosed with thyroid carcinoma in the First Hospital of Qiqihar
between June 2018 and April 2019. The inclusion criteria of
patients in this study were as follows: Patients who were
identified as thyroid carcinoma via pathological examination; and
patients who did not receive radiotherapy or chemotherapy before
the surgery. Written informed consent was obtained from patients in
this study. Based on the median expression value of ZFAS1, the
patients were separated into low and high expression groups.
Cell culture
Nthy-ori3-1 (Shanghai YaJi Biological Technology
Co., Ltd.; http://www.yajimall.com/) (26), MDA-T68 [American Type Culture
Collection (ATCC)] (27), SW579
(ATCC) (28), B-CPAP (The Cell Bank
of Type Culture Collection of the Chinese Academy of Sciences)
(29) and TPC-1 (The Cell Bank of
Type Culture Collection of the Chinese Academy of Sciences)
(30) cell lines were cultured in
RPMI 1640 (cat. no. 21875091; Thermo Fisher Scientific, Inc.). TT
cells (ATCC) (28) were cultured in
DMEM (cat no. D0819; Sigma-Aldrich; Merck KGaA). The media all
contained 10% FBS (cat. no. F8192; Sigma-Aldrich; Merck KGaA) and
incubated with the cells at 37°C with 5% CO2.
Experimental design
To investigate the effects of using small
interfering (si)RNA to downregulate ZFAS1 expression, TT and SW579
cells were divided into the following groups: i) Control (without
transfection); ii) si-Control (transfected with 50 nM si-Control);
and iii) si-ZFAS1 (transfected with 50 nM si-ZFAS1). The si-Control
(5′-UUCUCCGAACGUGUCACGUTT-3′) and si-ZFAS1
(5′-CUAACUGCCUACCUGCAUATT-3′) were obtained from Shanghai
GenePharma Co., Ltd., and the cells were transfected using
Lipofectamine® 3000 (cat. no. L3000015; Thermo Fisher
Scientific, Inc.). To specify the linkage between miR-302a-3p and
ZFAS1 on the hallmarks of thyroid carcinoma, TT and SW579 cells
were divided into the following groups: i) Control (without
transfection); ii) inhibitor-negative control (NC; transfected with
50 nM miR-302a-3p inhibitor-NC; iii) inhibitor (transfected with 50
nM miR-302a-3p inhibitor); iv) inhibitor + si-ZFAS1 (transfected
with 50 nM miR-302a-3p inhibitor and 50 nM si-ZFAS1); and v)
si-ZFAS1 (transfected with 50 nM si-ZFAS1). The miR-302a-3p
inhibitor-NC (5′-CAGUACUUUUGUGUAGUACAA-3′) and miR-302a-3p
inhibitor (5′-UCACCAAAACAUGGAAGCACUUA-3′) were obtained from
Shanghai GenePharma Co., Ltd, and the cells (2×104
cells/well; 96-well plate) were transfected using
Lipofectamine® 3000 (cat. no. L3000015; Thermo Fisher
Scientific, Inc.). The cells were cultured for 24 h prior to
subsequent experimentation.
Reverse transcription-quantitative
(RT-q)PCR
The total RNAs were extracted from the tissue
samples and cells (1×106 cells) using TRIzol®
reagent (cat. no. 15596018; Thermo Fisher Scientific, Inc.). For
miRNA analysis, cDNA synthesis was performed on 200 ng of total RNA
using a TaqMan™ MicroRNA Reverse Transcription Kit (cat. no.
4366597; Thermo Fisher Scientific, Inc.) according the
manufacturer's protocol. Reverse transcription conditions included:
42°C for 30 min and at 85°C for 5 min. The qPCR reactions was
performed using 2 µl cDNA solution, 5 µl TaqMan 2X Perfect Master
Mix (Takara Biotechnology Co., Ltd.), 0.25 µl gene-specific primers
and 2.75 µl of nuclease-free water in a final volume of 10 µl with
a Bio-Rad IQ5 thermocycler (Bio-Rad Laboratories, Inc.) under the
following conditions: Initial denaturation at 95°C for 3 min,
followed by 40 cycles at 95°C for 30 sec, 62°C for 30 sec and 72°C
for 25 sec. The U6 gene was used as an internal control. For mRNA
analysis, the mRNA templates were reverse transcribed into cDNAs
using PrimeScript RT reagent kit (Takara Biotechnology Co., Ltd.).
Reverse transcription conditions: At 37°C for 30 min and at 85°C
for 5 min. According to the protocol of FastStart™ Universal
SYBR-Green Master (Rox; cat. no. 4913850001; Roche Diagnostics), 14
µl 2X SYBR-Green master mix, 1 µl forward primer (10 µM), 1 µl
reverse primer (10 µM), 3 µl cDNA template and 6 µl double
distilled H2O were mixed and reacted in a Bio-Rad IQ5
thermocycler (Bio-Rad Laboratories, Inc.) under the following
conditions: 95°C for 90 sec, 95°C for 25 sec, 65°C for 20 sec, 72°C
for 30 sec for 40 cycles. GAPDH was used as an internal control.
mRNA expression levels were calculated by the 2−ΔΔCq
method (31). The primers used for
RT-qPCR were shown in Table I.
 | Table I.Primers used in the study. |
Table I.
Primers used in the study.
| Gene | Primer sequence
(5′→3′) |
|---|
| LncRNA ZFAS1 | F:
CTATTGTCCTGCCCGTTAGAGCTATTGTCCTGCCCGTTAGAG |
|
| R:
GTCAGGAGATCGAAGGTTGTAG |
| miR-302a-3p |
AATAAGTGCTTCCATGTTTTGGTGA |
| Cyclin D1 | F:
GTCTTCCCGCTGGCCATGAACTAC |
|
| R:
GGAAGCGTGTGAGGCGGTAGTAGG |
| MMP2 | F:
GGAGGCACGATTGGTCTG |
|
| R:
TTGGTTTCCGCATGGTCT |
| MMP9 | F:
TGTACCGCTATGGTTACACT |
|
| R:
CCTCAAAGGTTTGGAAT |
| E-cadherin | F:
TAACCGATCAGAATGAC |
|
| R:
TTTGTCAGGGAGCTCAGGAT |
| N-cadherin | F:
AGTGAGCCTGCAGATTTTAAGGTGGATG |
|
| R:
CACTTGCCACTTTTCCTGGGTCTCTT |
| GAPDH | F:
CGCTTCACGAATTTGCGTGTCAT |
|
| R:
GAAGATGGTGATGGGATTTC |
| U6 | F:
TGCGGGTGCTCGCTTCGGCAGC |
|
| R:
CCAGTGCAGGGTCCGAGGT |
Transwell assay
The TT and SW579 cells (1×106) were
collected at the logarithmic growth phase, and pipetted into the
upper chamber (containing serum-free medium) of a Transwell insert
(8-µm) pre-coated with Matrigel (BD Bioscience; at 37°C for 4 h).
The lower chamber was supplemented with 10% FBS mixed in 400 µl
medium. Transwell was incubated at 37°C with 5% CO2 for
24 h. Next, cells remaining on the surface of the upper chamber
were removed with a cotton swab, the invading cells were fixed with
4% paraformaldehyde for 15 min at room temperature and then stained
with 0.2% crystal violet for 10 min at room temperature. The cells
in the lower chamber were observed under a light microscope
(magnification, ×200), and the cells were counted using Image J
software (version 1.8.0; National Institutes of Health).
Bioinformatics and dual-luciferase
reporter assay
The interactions between ZFAS1 and miR-302a-3p, and
cyclin D1 (CCND1) and miR-302a-3p were predicted by Starbase
(version 2.0; http://starbase.sysu.edu.cn). The mutants of ZFAS1 and
CCND1 were built using a Quick-Change Site-Directed Mutagenesis kit
(Agilent Technologies, Inc.). pGL3 plasmid encoding a luciferase
reporter gene was purchased from Promega Corporation. Recombinant
plasmids containing the wild-type (WT) ZFAS1-3′-untranslataed
region (UTR), WT CCND1-3′-UTR or corresponding mutant sequences
were constructed. The TT and SW579 cells (1×105
cells/well) were seeded in a 24-well plate, and the cells were
co-transfected with miR-302a-3p mimic (40 nM;
5′-UAAGUGCUUCCAUGUUUUGGUGA-3′; Shanghai GenePharma Co., Ltd.) or
miRNA control (40 nM; 5′-UUCUCCGAACGUGUCACGUTT-3′; Shanghai
GenePharma Co., Ltd.), recombinant plasmid (20 ng) or corresponding
mutants (20 ng) using Lipofectamine 3000. Plasmid pRL-Thymine
kinase (TK; Promega Corporation) was used as an internal reference
luciferase. The cells were cultured for 48 h prior to the detection
of luciferase activity using a Dual-Glo luciferase assay kit
(Promega Corporation). The firefly luciferase activity was
normalized to Renilla luciferase activity.
Wound healing assay
TT and SW579 cells (1×106) were collected
at the logarithmic growth phase. A gap in the middle of the cell
layer was created using a sterile 200-µl pipette tip by scratching
the monolayer of cells. After washing, the cells were treated with
serum-free medium for 48 h and incubated with 5% CO2 at
37°C. The images were captured using a light microscope
(magnification, ×100) and analyzed via ImageJ software 1.8.0
(National Institutes of Health). The mean distance between the
upper, middle and bottom edges of the gap were measured and
recorded.
Cell Counting Kit-8 (CCK-8) assay
The TT and SW579 cells (1×106) were
transfected with si-ZFAS1 or miR-302a-3p inhibitor, divided into
groups (control, si-control, si-ZFAS1, inhibitor-NC, inhibitor,
inhibitor + si-ZFAS1) and cultured for 24, 48 and 72 h. The cell
viability was detected by a CCK-8 assay (cat. no. 96992-100TESTS-F;
Sigma-Aldrich; Merck KGaA) according to the manufacturer's
protocol. The absorbance was determined at 450 nm using a Multiskan
microplate reader (Thermo Fisher Scientific, Inc.).
Western blotting
Following cell transfection for 24 h,
1×106 cells were obtained and lysed using RIPA lysate
(cat. no. R0278; Sigma-Aldrich; Merck KGaA) with protease inhibitor
(cat. no. S8830; Sigma-Aldrich; Merck KGaA) to extract the total
protein. The bicinchoninic acid method (cat. no. BCA1;
Sigma-Aldrich; Merck KGaA) was used to determine the concentration
of total protein. The proteins (25 µg/lane) were separated by 12%
SDS-PAGE, and then transferred to a PVDF membrane. The membrane was
blocked with 5% non-fat milk for 1 h at room temperature and
incubated with anti-cyclin D1 (1:10,000; cat. no. ab134175; 34
kDa), matrix metallopeptidase (MMP)-9 (1 µg/ml; cat. no. ab73734;
78 kDa), MMP2 (1:1,000; cat. no. ab37150; 72 kDa), E-cadherin
(1:10,000; cat. no. ab40772; 97 kDa), N-cadherin (1 µg/ml; cat. no.
ab18203; 130 kDa); and GAPDH (1:10,000; cat. no. ab181602; all
purchased from Abcam) primary antibodies overnight at 4°C. The
membrane was then washed with TBS-Tween 20 (0.1% Tween-20) and
incubated with horseradish peroxide-conjugated goat anti-rabbit
secondary antibody (1:2,000; cat. no. ab205718; Abcam) for 1 h at
room temperature. The proteins blots were developed using
SignalFire™ ECL Reagent (cat. no. 6883; Cell Signaling Technology,
Inc.) and quantified using ImageJ Software (version 1.46; National
Institutes of Health).
Statistical analysis
Data were expressed as the mean ± SD of three
independent experiments. The statistical differences between two
groups were analyzed by paired and unpaired Student's t-test,
whereas differences among multiple groups were analyzed by one- or
two-way analysis (for the CCK-8 data) of variance followed by
Tukey's post hoc test. Pearson's correlation coefficient test was
used for the analysis of correlation, Fisher's exact test and
χ2 test used to analyze associations between categorical
variables. All results were analyzed using GraphPad Prism 8.0
(GraphPad Software, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
ZFAS1 expression levels in tissues and
cell lines of thyroid carcinoma are upregulated and positively
associated with the proliferation of thyroid carcinoma cells
In order to determine the expression and effects of
ZFAS1 in thyroid carcinoma, the expression levels of ZFAS1 in tumor
tissues and cell lines were detected. In addition, the cell
viabilities of TT and SW579 cells with silencing ZFAS1 were
determined. The results demonstrated that ZFAS1 expression levels
were upregulated in thyroid carcinoma tissues compared with in
adjacent tissues, and were upregulated in MDA-T68, TT, SW579,
B-CPAP and TPC-1 cells compared with in Nthy-ori3-1 cells
(P<0.01; Fig. 1A and B). In
addition, the associations between ZFAS1 expression and clinical
characteristics were analyzed, and the results demonstrated that
ZFAS1 expression levels were significantly associated with tumor
size, lymph node status and tumor stage (Table II). In addition, ZFAS1 expression
levels were significantly decreased in the si-ZFAS1 group compared
with those of the si-control group in TT and SW579 cells
(P<0.01; Fig. 1C and D).
Notably, the cell viabilities of TT and SW579 cells were
significantly lower in the si-ZFAS1 group compared with those of
the si-Control group after 24, 48 and 72 h (P<0.05 or P<0.01;
Fig. 1E and F). These results
suggested that the expression levels of ZFAS1 were elevated in
thyroid carcinoma and associated with the proliferation of thyroid
carcinoma.
 | Table II.Association between ZFAS1 expression
and clinical characteristics. |
Table II.
Association between ZFAS1 expression
and clinical characteristics.
|
Characteristics | ZFAS1-low
cases | ZFAS1-high
cases | P-value |
|---|
| Age at diagnosis,
years |
|
| 0.269 |
|
≤45 | 10 | 7 |
|
|
>45 | 5 | 8 |
|
| Sex |
|
| 0.256 |
|
Male | 7 | 4 |
|
|
Female | 8 | 11 |
|
| Location |
|
| 0.514 |
| Left
lobe | 7 | 4 |
|
| Right
lobe | 5 | 9 |
|
|
Bilateral | 2 | 1 |
|
|
Isthmus | 1 | 1 |
|
| Focus type |
|
| 0.121 |
|
Unifocal | 8 | 12 |
|
|
Multifocal | 7 | 3 |
|
| Tumor size |
|
| 0.003a |
|
T1-T2 | 12 | 4 |
|
|
T3-T4 | 3 | 11 |
|
| Lymph node
status |
|
| 0.028a |
|
N0 | 10 | 4 |
|
|
N1 | 5 | 11 |
|
| Tumor stage |
|
| 0.010a |
|
I–II | 12 | 5 |
|
|
III–IV | 3 | 10 |
|
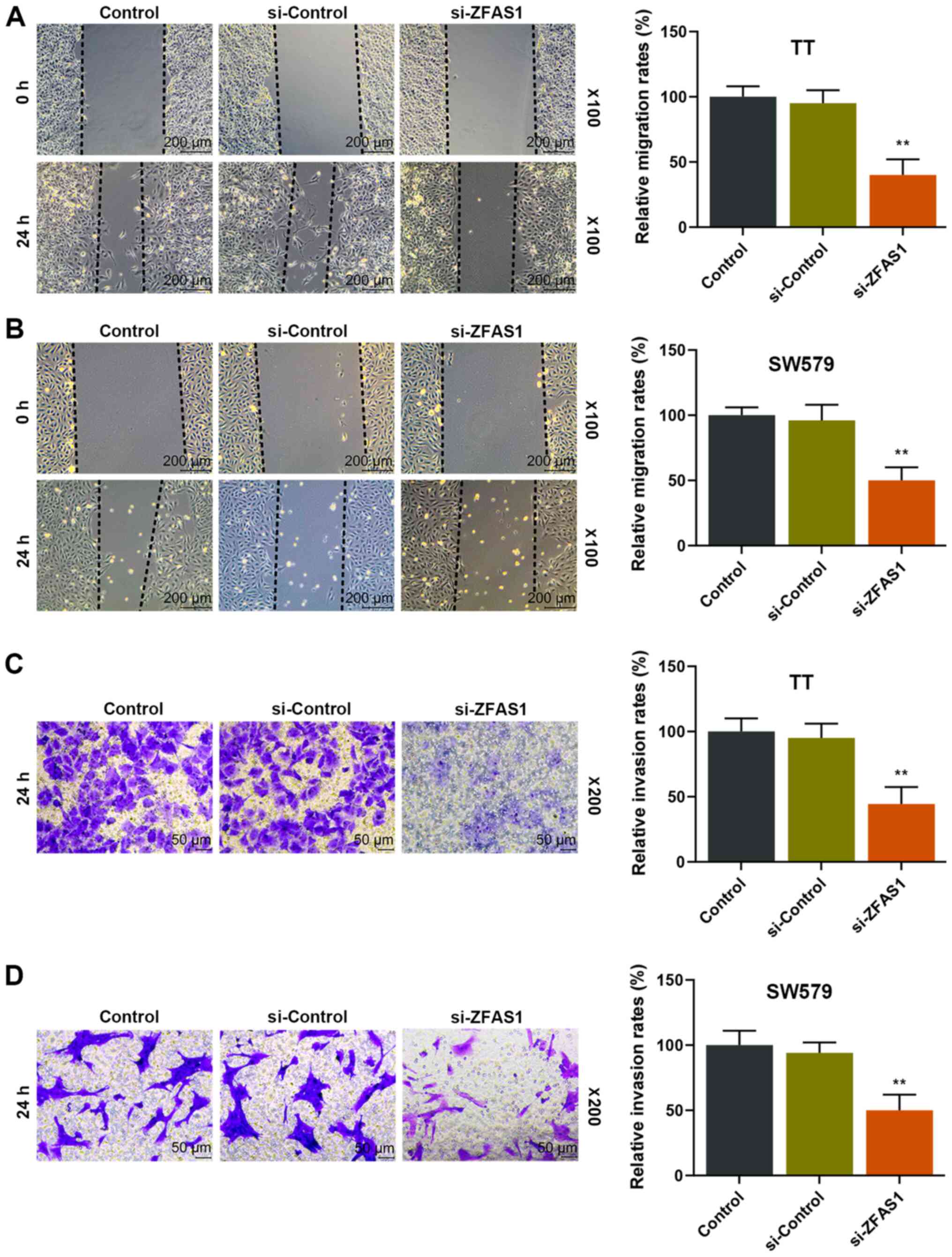
Reduction of ZFAS1 expression levels
reduces the migratory and invasive ability of thyroid carcinoma
cells
TT and SW579 cells were transfected with si-ZFAS1 to
further observe the effects of ZFAS1 on the migratory and invasive
ability of thyroid carcinoma cells. The results demonstrated that
the migration and invasion rates of TT and SW579 cells were
significantly decreased in the si-ZFAS1 group compared with the
si-Control group after 24 h (P<0.01; Fig. 2), suggesting that ZFAS1 expression
was positively associated with the migratory and invasive ability
of thyroid carcinoma cells.
miR-302a-3p is targeted by ZFAS0
Bioinformatics predicted that miR-302a-3p was the
target gene of ZFAS1 (Fig. 3A);
thus, the relationship between miR-302a-3p and ZFAS1 was further
examined. The results demonstrated that the relative luciferase
activity was significantly decreased in the ZFAS1-WT + mimic groups
in TT and SW579 cells compared with those of the ZFAS1-WT + blank
groups (P<0.01; Fig. 3B and C),
which indicated that miR-302a-3p was a target of ZFAS1. In
addition, the results demonstrated that miR-302a-3p expression
levels were significantly decreased in thyroid carcinoma tissues
compared with those of adjacent tissues (P<0.01; Fig. 3D), and that there was a negative
relationship between the expression levels of ZFAS1 and miR-302a-3p
(Fig. 3E). In addition, the
expression levels of miR-302a-3p in the inhibitor group were
significantly decreased compared with those of the inhibitor-NC
group, but were significantly higher in the inhibitor + si-ZFAS1
group compared with those of the inhibitor group in TT and SW579
cells (P<0.01; Fig. 3F and G).
In addition, the miR-302a-3p expression levels in the si-ZFAS1
group were significantly increased compared with those in the
inhibitor + si-ZFAS1 group (P<0.01; Fig. 3F and G). Taken together, these
results suggested that ZFAS1 may target miR-302a-3p to regulate the
activity of thyroid carcinoma cells.
Downregulation of ZFAS1 expression
levels eliminates the positive effects of miR-302a-3p inhibition on
the proliferation, migration and invasion of thyroid carcinoma
cells
To investigate the roles of ZFAS1 and miR-302a-3p in
the progression of thyroid carcinoma, the changes of the cell
viability, migration and invasion in thyroid carcinoma cells
treated with or without si-ZFAS1 and miR-302a-3p inhibitor were
examined. The results demonstrated that the cell viability, and
migration and invasion rates in the inhibitor group of TT and SW579
cells were significantly increased compared with those of the
inhibitor-NC group, but they were significantly decreased in the
inhibitor + si-ZFAS1 group compared with those of the inhibitor
group (P<0.01; Fig. 4). In
addition, the cell viability, and migration and invasion rates were
significantly decreased in the si-ZFAS1 group compared with the
inhibitor + si-ZFAS1 group (P<0.01; Fig. 4). These results suggested that
downregulation of ZFAS1 may attenuate the reduced expression of
miR-302a-3p in thyroid carcinoma.
 | Figure 4.Effects of downregulating ZFAS1 on
the cell viability, migration and invasion of TT and SW579 cells
treated with miR-302a-3p inhibitor. OD values in Control,
inhibitor-NC, inhibitor, inhibitor + si-ZFAS1 and si-ZFAS1 groups
in (A) TT and (B) SW579 cells. Relative migration rates of (C) TT
and (D) SW579 cells in each group. Scale, 200 µm; magnification,
×100. Relative invasion rates of (E) TT and (F) SW579 cells in each
group. Scale, 50 µm; magnification, ×200. **P<0.01 vs.
inhibitor-NC; ##P<0.01 vs. inhibitor;
^^P<0.01 vs. inhibitor + si-ZFAS1. OD, optical
density; NC, negative control; si, small interfering; miR,
microRNA. |
miR-302a-3p targets CCND1
The gene via which ZFAS1 and miR-302a-3p exerted
their regulatory roles in thyroid carcinoma was determined via
bioinformatic analysis. Bioinformatic analysis predicted that CCND1
is targeted by miR-302a-3p in the development of thyroid carcinoma
(Fig. 5A). In addition,
dual-luciferase reporter assay results demonstrated that the
relative luciferase activities of TT and SW579 cell lines were
significantly lower in the CCND1-WT + mimic groups compared with
those of CCND1-WT + blank groups (P<0.01; Fig. 5B and C).
Downregulation of ZFAS1 expression
levels reverses the promotive effects of miR-302a-3p inhibitor on
CCND1 expression in thyroid carcinoma cells
Whether CCND1 expression was regulated by ZFAS1 and
miR-302a-3p in TT and SW579 cells was explored. The results
demonstrated that CCND1 expression levels were significantly
increased in TT and SW579 cells in the inhibitor group compared
with those of the inhibitor-NC group (P<0.01; Fig. 6). In addition, CCND1 expression
levels in the inhibitor + si-ZFAS1 group were significantly reduced
compared with those of the inhibitor group, but were significantly
increased compared with those in the si-ZFAS1 group (P<0.01;
Fig. 6). These results suggested
that the expression levels of miR-302a-3p and CCND1 were negatively
associated, but that the expression levels of ZFAS1 and CCND1 were
positively associated.
Downregulation of ZFAS1 expression
levels eliminates the positive effects of miR-302a-3p inhibition on
EMT of thyroid carcinoma cells
The expression levels of MMP2, MMP9, E-cadherin and
N-cadherin were measured to detect the EMT of TT and SW579 cells
with or without silencing the expression levels of miR-302a-3p and
ZFAS1. The results demonstrated that the expression levels of MMP2,
MMP9 and N-cadherin were significantly increased in the inhibitor
group compared with those of the inhibitor-NC group (P<0.01;
Fig. 7). In addition, the
expression levels of MMP2, MMP9 and N-cadherin were significantly
decreased in the inhibitor + si-ZFAS1 group compared with the
inhibitor group, but were significantly increased compared with in
the si-ZFAS1 group (P<0.01; Fig.
7). However, the changes in the expression levels of E-cadherin
were the opposite to those observed MMP2, MMP9 and N-cadherin
(P<0.01; Fig. 7). Thus, the
results indicated that EMT was affected by miR-302a-3p and was
regulated by ZFAS1.
 | Figure 7.Effects of downregulating ZFAS1
expression on the epithelial-mesenchymal transition capability of
TT and SW579 cells treated with miR-302a-3p inhibitor. Protein
blots of MMP2, MMP9, E-cadherin and N-cadherin in Control,
inhibitor-NC, inhibitor, inhibitor + si-ZFAS1 and si-ZFAS1 groups
of (A) TT and (B) SW579 cells. Relative protein expressions of
MMP2, MMP9, E-cadherin and N-cadherin in each group of (C) TT and
(D) SW579 cells. Relative mRNA expressions of MMP2, MMP9,
E-cadherin and N-cadherin in each group of (E) TT and (F) SW579
cells. **P<0.01 vs. inhibitor-NC; ##P<0.01 vs.
inhibitor; ^^P<0.01 vs. inhibitor + si-ZFAS1. miR,
microRNA; NC, negative control; si, small interfering; MMP, matrix
metallopeptidase. |
Discussion
The present study revealed that ZFAS1 expression was
increased in thyroid carcinoma tissues and cell lines, and that
downregulation of ZFAS1 expression decreased the proliferation,
migration, invasion and EMT of the tumor cells. These properties,
however, were promoted by the inhibition of miR-302a-3p expression,
potentially by effects on CCND1 expression. These novel findings of
a possible regulatory pathway may contribute to the development of
interventions for thyroid carcinoma.
The results of the present study demonstrated that
ZFAS1 expression levels were increased in thyroid carcinoma tissues
and cell lines, and the proliferation, migration and invasion of TT
and SW579 cells were reduced after silencing ZFAS1 expression
levels. Dong et al (32)
reported that ZFAS1 overexpression facilitates the development of
clear cell renal cell carcinoma. Additionally, Xie et al
(33) demonstrated that ZFAS1
promotes the metastasis of colorectal cancer by sponging miR-484.
Thus, ZFAS1 is potentially a regulator in the progression of
thyroid carcinoma.
The MMPs are a family of endogenous proteolytic
enzymes with cofactors as metal ions (34). Hydrolyzed proteins require
Zn2+ and Ca2+ to fulfill their functions
(34). During the hydrolysis
process, the MMP family serves an important role in hydrolyzing
most of the extracellular matrix (ECM) components (35). The degradation of ECMs by MMPs
affects a number of pathologically related physiological processes,
such as the development of cancer, arthritis, genetic diseases,
chronic renal failure and cardiovascular diseases (36,37).
In cancer-related studies, abnormally expressed MMPs mainly affect
tumor cell invasion and migration (37–39).
MMP2 and MMP9 are two major members in MMPs and are widely used as
the biomarkers of EMT in cancer research (40). EMT refers to the process during
which epithelial cells change their protein expression levels and
transform into mesenchymal cells under the effects of external
factors (41). The cadherin family,
a class of Ca2+-dependent transmembrane glycoproteins,
serves an important role in tissue morphogenesis and coordination
of cell movement (42). E-cadherin
and N-cadherin are two representatives of the cadherin family and
are associated with EMT (43,44).
Thus, increases in MMP2, MMP9, N-cadherin expression levels, and
decreased E-cadherin expression are indicative of EMT process.
The results of the present study indicated that
miR-302a-3p expression was downregulated in thyroid carcinoma
tissue and targeted by ZFAS1. In addition, inhibition of
miR-302a-3p expression increased the proliferation, migration,
invasion and EMT of thyroid carcinoma cells, and such effects were
partially reversed by silencing ZFAS1. Zhang et al (45) demonstrated that inhibiting
miR-302a-3p expression targets the suppressor of the cytokine
signalling 5/STAT3 signaling axis and further promotes the
metastasis of pancreatic cancer. Additionally, Pan et al
(25) reported that miR-302a-3p
overexpression is involved in the regulation of endometrial cancer,
and it inhibits growth of endometrial cancer cells and is sponged
by LINC01016. In addition, Ye et al (23) observed that the upregulation of
miR-302a-3p suppresses the metastatic potential of hepatocellular
carcinoma. Thus, miR-302a-3p expression serves a protective role in
thyroid carcinoma. Taken together, it is hypothesized that the loss
of miR-302a-3p expression contributes to the promotion of thyroid
carcinoma, and this may be partially reversed by the downregulation
of ZFAS1 expression.
The cyclin family are a class of proteins widely
existing in eukaryotic cells (46);
they function periodically in the cell cycle and act on
cyclin-dependent kinases (CDKs) to regulate cell cycle progression
(46). Among them, CCND1 is a
highly conserved cell cycle family protein (47). CCND1 binds to CDKs, such as CDK4 or
CDK6, to form complexes and act as their regulatory subunit,
promoting cell cycle progression from G1 to S phase and completing
the regulation of cell cycle (47).
The overexpression of CCND1 occurs in different tumors, such as in
breast cancer and gastric cancer, and promotes cell invasion and
migration, leading to poor prognosis (48–50).
Guo et al (51) observed
that the lncRNA NR2F1-AS1 sponged miRNA-338-3p to upregulate CCND1
expression and promote thyroid carcinoma progression. In addition,
Jeon et al (52) proposed
that the CCND1 splice variant may serve as a biomarker for the
diagnostic and prediction of thyroid carcinoma. These findings
confirmed that the overexpression of CCND1 serves a key role in the
promotion of thyroid carcinoma. The results of the present study
demonstrated that CCND1 was a target of miR-302a-3p, and that the
inhibition of miR-302a-3p expression increased CCND1 expression
levels, which were reversed by the downregulation of ZFAS1
expression. Thus, it is hypothesized that CCND1 may be the target
gene through which ZFAS1 and miR-302a-3p exert their regulatory
functions in thyroid carcinoma. However, the present study also has
some limitations. For example, downregulated lncRNA ZFAS1 was only
demonstrated to inhibit the proliferation, migration and invasion
of thyroid cancer cells in vitro by regulating
miR-302a-3p/CCND1. These result needs to be further confirmed in
in vivo experiments. In addition, the effect of ZFAS1
expression on thyroid cancer also needs to be further studied.
The results of the present study demonstrated that
the downregulation of ZFAS1 targeted and increased the expression
of miR-302a-3p, which further suppressed the expression of CCND1,
resulting in the inhibition of the proliferation, migration,
invasion and EMT of thyroid carcinoma. These findings contribute to
the development of drug for the treatment of thyroid carcinoma,
however, the specific regulatory network should be further
specified.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WC designed and conceived the study, and wrote the
manuscript. LZ, HL, YL, QZ, DX and WF acquired, analyzed and
interpreted the data. All authors agree to be accountable for all
aspects of the work in ensuring that questions related to the
accuracy or integrity of the work are appropriately investigated
and resolved. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was approved by the ethics board of The
First Hospital of Qiqihar (approval no. QR20180503112). Written
informed consent was obtained from patients in the present
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liebner DA and Shah MH: Thyroid cancer:
Pathogenesis and targeted therapy. Ther Adv Endocrinol Metab.
2:173–195. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pellegriti G, Frasca F, Regalbuto C,
Squatrito S and Vigneri R: Worldwide increasing incidence of
thyroid cancer: Update on epidemiology and risk factors. J Cancer
Epidemiol. 2013:9652122013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ahn HS, Kim HJ and Welch HG: Korea's
thyroid-cancer ‘Epidemic’-screening and overdiagnosis. N Engl J
Med. 371:1765–1767. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Colonna M, Uhry Z, Guizard AV, Delafosse
P, Schvartz C, Belot A and Grosclaude P; FRANCIM network, : Recent
trends in incidence, geographical distribution, and survival of
papillary thyroid cancer in France. Cancer Epidemiol. 39:511–518.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rhee YH, Moon JH, Choi SH and Ahn JC:
Low-level laser therapy promoted aggressive proliferation and
angiogenesis through decreasing of transforming growth factor-β1
and increasing of Akt/Hypoxia inducible factor-1α in anaplastic
thyroid cancer. Photomed Laser Surg. 34:229–235. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Callender GG, Carling T, Christison-Lagay
E and Udelsman R: Surgery for thyroid cancer. Curr Opin Endocrinol
Diabetes Obess. 43:443–458. 2014.
|
|
8
|
Sipos J and Mazzaferri E: Thyroid cancer
epidemiology and prognostic variables. Clin Oncol. 22:395–404.
2010. View Article : Google Scholar
|
|
9
|
Ponting CP, Oliver PL and Reik W:
Evolution and functions of long noncoding RNAs. Cell. 136:629–641.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Y and Tang L: The application of
lncRNAs in cancer treatment and diagnosis. Recent Pat Anticancer
Drug Discov. 13:292–301. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ignarski M, Islam R and Müller RU: Long
non-coding RNAs in kidney disease. Int J Mol Sci. 20:32762019.
View Article : Google Scholar
|
|
12
|
Xu YZ, Chen FF, Zhang Y, Zhao QF, Guan XL,
Wang HY, Li A, Lv X, Song SS, Zhou Y and Li XJ: The long noncoding
RNA FOXCUT promotes proliferation and migration by targeting FOXC1
in nasopharyngeal carcinoma. Tumour Biol. 39:10104283177060542017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Iyer MK, Niknafs YS, Malik R, Singhal U,
Sahu A, Hosono Y, Barrette TR, Prensner JR, Evans JR, Zhao S, et
al: The landscape of long noncoding RNAs in the human
transcriptome. Nat Genet. 47:199–208. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li HJ, Li X, Pang H, Pan JJ, Xie XJ and
Chen W: Long non-coding RNA UCA1 promotes glutamine metabolism by
targeting miR-16 in human bladder cancer. Jpn J Clin Oncol.
45:1055–1063. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tang X, Yin R, Shi H, Wang X, Shen D, Wang
X and Pan C: lncRNA ZFAS1 confers inflammatory responses and
reduces cholesterol efflux in atherosclerosis through regulating
miR-654-3p-ADAM10/RAB22A axis. Int J Cardiol. 315:72–80. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou HL, Zhou YF and Feng ZT: Long
noncoding RNA ZFAS1 promotes hepatocellular carcinoma proliferation
by epigenetically repressing miR-193a-3p. Eur Rev Med Pharmacol
Scis. 23:9840–9847. 2019.
|
|
17
|
Zhang J, Quan LN, Meng Q, Wang HY, Wang J,
Yu P, Fu JT, Li YJ, Chen J, Cheng H, et al: miR-548e sponged by
ZFAS1 regulates metastasis and cisplatin resistance of OC by
targeting CXCR4 and let-7a/BCL-XL/S signaling axis. Mol Ther
Nucleic Acids. 20:621–638. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang S, Wang J, Yao T and Tao M: lncRNA
ZFAS1/miR-589 regulates the PTEN/PI3K/AKT signal pathway in the
proliferation, invasion and migration of breast cancer cells.
Cytotechnology. 72:415–425. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pan J, Xu X and Wang G: lncRNA ZFAS1 is
involved in the proliferation, invasion and metastasis of prostate
cancer cells through competitively binding to miR-135a-5p. Cancer
Manage Res. 12:1135–1149. 2020. View Article : Google Scholar
|
|
20
|
Han CG, Huang Y and Qin L: Long non-coding
RNA ZFAS1 as a novel potential biomarker for predicting the
prognosis of thyroid Cancer. Med Sci Monit. 25:2984–2992. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tong H, Zhuang X, Cai J, Ding Y, Si Y,
Zhang H and Shen M: Long noncoding RNA ZFAS1 promotes progression
of papillary thyroid carcinoma by sponging miR-590-3p and
upregulating HMGA2 expression. Onco Targets Ther. 12:7501–7512.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tam C, Wong JH, Tsui SKW, Zuo T, Chan TF
and Ng TB: lncRNAs with miRNAs in regulation of gastric, liver, and
colorectal cancers: Updates in recent years. App Microbiol
Biotechnol. 103:4649–4677. 2019. View Article : Google Scholar
|
|
23
|
Ye Y, Song Y, Zhuang J, Wang G, Ni J,
Zhang S and Xia W: MicroRNA-302a-3p suppresses hepatocellular
carcinoma progression by inhibiting proliferation and invasion.
Onco Targets Ther. 11:8175–8184. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Luo Z, Yi ZJ, Ou ZL, Han T, Wan T, Tang
YC, Wang ZC and Huang FZ: RELA/NEAT1/miR-302a-3p/RELA feedback loop
modulates pancreatic ductal adenocarcinoma cell proliferation and
migration. J Cell Physiol. 234:3583–3597. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pan X, Li D, Huo J, Kong F, Yang H and Ma
X: LINC01016 promotes the malignant phenotype of endometrial cancer
cells by regulating the miR-302a-3p/miR-3130-3p/NFYA/SATB1 axis.
Cell Death Dis. 9:3032018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao M, Sano D, Pickering CR, Jasser SA,
Henderson YC, Clayman GL, Sturgis EM, Ow TJ, Lotan R, Carey TE, et
al: Assembly and initial characterization of a panel of 85
genomically validated cell lines from diverse head and neck tumor
sites. Clin Cancer Res. 17:7248–7264. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Henderson YC, Ahn SH, Ryu J, Chen Y,
Williams MD, El-Naggar AK, Gagea M, Schweppe RE, Haugen BR, Lai SY
and Clayman GL: Development and characterization of six new human
papillary thyroid carcinoma cell lines. J Clin Endocrinol Metab.
100:E243–E252. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dutil J, Chen Z, Monteiro AN, Teer JK and
Eschrich SA: An interactive resource to probe genetic diversity and
estimated ancestry in cancer cell lines. Cancer Res. 79:1263–1273.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fabien N, Fusco A, Santoro M, Barbier Y,
Dubois PM and Paulin C: Description of a human papillary thyroid
carcinoma cell line. Morphologic study and expression of tumoral
markers. Cancer. 73:2206–2212. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schweppe RE, Klopper JP, Korch C,
Pugazhenthi U, Benezra M, Knauf JA, Fagin JA, Marlow LA, Copland
JA, Smallridge RC and Haugen BR: Deoxyribonucleic acid profiling
analysis of 40 human thyroid cancer cell lines reveals
cross-contamination resulting in cell line redundancy and
misidentification. J Clin Endocrinol Metab. 93:4331–4341. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dong D, Mu Z, Wei N, Sun M, Wang W, Xin N,
Shao Y and Zhao C: Long non-coding RNA ZFAS1 promotes proliferation
and metastasis of clear cell renal cell carcinoma via targeting
miR-10a/SKA1 pathway. Biomed Pharmacother. 111:917–925. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xie S, Ge Q, Wang X, Sun X and Kang Y:
Long non-coding RNA ZFAS1 sponges miR-484 to promote cell
proliferation and invasion in colorectal cancer. Cell Cycle.
17:154–161. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kapoor C, Vaidya S, Wadhwan V, Kaur G and
Pathak A: Seesaw of matrix metalloproteinases (MMPs). J Cancer Res
Ther. 12:28–35. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Itoh Y: Metalloproteinases in rheumatoid
arthritis: Potential therapeutic targets to improve current
therapies. Progress in Molecular Biology and Translational Science.
Elsevier; pp. 327–338. 2017, View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gheissari A, Meamar R, Abedini A,
Roomizadeh P, Shafiei M, Samaninobandegani Z, Tabrizi Z, Mahmoudi
F, Merrikhi A and Najafi Tavana E: Association of matrix
metalloproteinase-2 and matrix metalloproteinase-9 with endothelial
dysfunction, cardiovascular disease risk factors and thrombotic
events in children with end-stage renal disease. Iran J kidney Dis.
12:169–177. 2018.PubMed/NCBI
|
|
37
|
Balistreri CR, Allegra A, Crapanzano F,
Pisano C and Ruvolo G: Matrix metalloproteinases (MMPs), their
genetic variants and miRNA in mitral valve diseases: Potential
biomarker tools and targets for personalized treatments. J Heart
Valve Dis. 25:463–474. 2016.PubMed/NCBI
|
|
38
|
Li F, Jin D, Guan L, Zhang CC, Wu T, Wang
YJ and Gao DS: CEP55 promoted the migration, invasion and
neuroshpere formation of the glioma cell line U251. Neurosci Lett.
705:80–86. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cheng D, Jiang S, Chen J, Li J, Ao L and
Zhang Y: The increased lncRNA MIR503HG in preeclampsia modulated
trophoblast cell proliferation, invasion, and migration via
regulating matrix metalloproteinases and NF-κB signaling. Dis
Markers. 2019:49768452019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang X, Yang B, She Y and Ye Y: The lncRNA
TP73-AS1 promotes ovarian cancer cell proliferation and metastasis
via modulation of MMP2 and MMP9. J Cell Biochem. 119:7790–7799.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yang J and Weinberg RA:
Epithelial-mesenchymal transition: At the crossroads of development
and tumor metastasis. Dev Cell. 14:818–829. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Frismantiene A, Philippova M, Erne P and
Resink TJ: Cadherins in vascular smooth muscle cell (patho)biology:
Quid nos scimus? Cell Sign. 45:23–42. 2018. View Article : Google Scholar
|
|
43
|
Abdallah RA, Abdou AG, Abdelwahed M and
Ali H: Immunohistochemical expression of E- and N-Cadherin in
nodular prostatic hyperplasia and prostatic carcinoma. J Microsc
Ultrastruct. 7:19–27. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Oystese KAB, Berg JP, Normann KR, Zucknick
M, Casar-Borota O and Bollerslev J: The role of E and N-cadherin in
the postoperative course of gonadotroph pituitary tumours.
Endocrine. 62:351–360. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang Z, Li J, Guo H, Wang F, Ma L, Du C,
Wang Y, Wang Q, Kornmann M, Tian X and Yang Y: BRM
transcriptionally regulates miR-302a-3p to target SOCS5/STAT3
signaling axis to potentiate pancreatic cancer metastasis. Cancer
Lett. 449:215–225. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Malumbres M and Barbacid M: Cell cycle,
CDKs and cancer: A changing paradigm. Nat Rev Cancer. 9:153–166.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li X, Huo X, Li W, Yang Q, Wang Y and Kang
X: Genetic association between cyclin D1 polymorphism and breast
cancer susceptibility. Tumour Biol. 35:11959–11965. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Neumeister P, Pixley FJ, Xiong Y, Xie H,
Wu K, Ashton A, Cammer M, Chan A, Symons M, Stanley ER and Pestell
RG: Cyclin D1 governs adhesion and motility of macrophages. Mol
Biol Cell. 14:2005–2015. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhong Z, Yeow WS, Zou C, Wassell R, Wang
C, Pestell RG, Quong JN and Quong AA: Cyclin D1/cyclin-dependent
kinase 4 interacts with filamin A and affects the migration and
invasion potential of breast cancer cells. Cancer Res.
70:2105–2114. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ullah Shah A, Mahjabeen I and Kayani MA:
Genetic polymorphisms in cell cycle regulatory genes CCND1 and CDK4
are associated with susceptibility to breast cancer. J BUON.
20:985–993. 2015.PubMed/NCBI
|
|
51
|
Guo F, Fu Q, Wang Y and Sui G: Long
non-coding RNA NR2F1-AS1 promoted proliferation and migration yet
suppressed apoptosis of thyroid cancer cells through regulating
miRNA-338-3p/CCND1 axis. J Cell Mol Med. 23:5907–5919. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Jeon S, Kim Y, Jeong YM, Bae JS and Jung
CK: CCND1 splice variant as a novel diagnostic and predictive
biomarker for thyroid cancer. Cancers (Basel). 10:4372018.
View Article : Google Scholar
|