Introduction
Lung cancer is the leading cause of death,
accounting for one third of all cancer-related deaths and seriously
threatens the lives and health of people worldwide (1). Xuanwei, a county-level city in Yunnan
province, China, is one of the areas with the highest lung cancer
occurrence and mortality rates in the world (2,3).
Previous etiological and epidemiological studies have confirmed
that lung cancer in Xuanwei County has its own unique
epidemiological characteristics mainly due to polycyclic aromatic
hydrocarbons (PAHs) and indoor coal-fired particles. First, lung
cancer rates in Xuanwei tended to be higher in rural areas compared
with that in urban areas according to the data of Chinese national
retrospective surveys (4). Second,
the incidence of lung cancer in Xuanwei women (120 per 100,000) was
much higher compared with the national average (22.9 per 100,000
women) (4). Third, the age of onset
of lung cancer in Xuanwei has been reported to be 15–25 years
younger than that of places with high incidences of lung cancer,
such as Shanghai (4). Lung cancer
epidemiology demonstrated family aggregation and indicated that
genetic variation might serve an important role in Xuanwei lung
cancer tumorigenesis and progression (5–7).
However, there were few studies on genes unique in Xuanwei lung
cancer tumorigenesis and progression. The Xuanwei lung cancer cell
line XWLC-05 was established by F.C. Yan et al in 2007
(8). It was derived from a female
patient who was a 68-year-old Xuanwei permanent resident and
diagnosed with moderately differentiated lung adenocarcinoma.
XWLC-05 has since been used in various research studies including
antitumor drug screening and cancer molecular-targeted therapy
(9,10). However, the molecular mechanisms of
lung cancer progression in Xuanwei County remain to be elucidated.
Despite the efforts made in treatment in recent years, the 5-year
overall survival rate of patients with non-small cell lung cancer
(NSCLC) is still ~18% although ~60–70% of them have been diagnosed
in the early stages of lung cancer (11,12).
Therefore, elucidating the molecular mechanisms of occurrence and
metastasis of lung cancers is of great significance for the
clinical treatment of lung cancer in Xuanwei populations.
MicroRNAs (miRNAs) are highly conserved endogenous
non-coding single-stranded RNAs with a length of 18–25 bp that
regulate gene expression by binding to the 3′ untranslated region
(3′-UTR) of target transcripts leading to mRNA degradation or
suppressing translation into protein (13–15).
Previous studies suggest that miRNAs serve critical regulatory
roles in essential biological and pathological processes via
complicated but precise regulation networks (16–18).
Therefore, the deletion, mutation or abnormal expression of miRNAs
are closely linked to tumor progression. Numerous studies have
suggested the roles of miRNAs in carcinogenesis and progression of
lung cancer (19–22).
Through high-throughput sequencing, the present
study identified that miR-218 expression levels in NSCLC patients
were significantly lower than those in paired distal control
tissues. Previous studies confirm that miR-218 acts as a
tumor-suppressor miRNA in a number of types of cancer, including
lung cancer, breast cancer, glioma, hepatocellular carcinoma,
gastric cancer, colorectal cancer and prostate cancer (23–29).
As a tumor-suppressor miRNA, miRNA (miR)-218 is downregulated in
tumor progression and is associated with prognosis in NSCLC
(30–32). Furthermore, miR-218 can participate
in tumor progression by regulating target genes including PXN,
IL6R, JAK3, SLUG, ZEB2, EGFR, MEF2D, CDCP1, RUNX2, HMGB1, ETK,
HOXA1, CDK6 and ROBO1 (31–42).
The present study revealed for the first time, to the best of the
authors' knowledge, the expression levels and therapeutic potential
of miR-218 in XWLC-05. Furthermore, it was demonstrated that
overexpression of miR-218 could suppress cell proliferation, cell
migration and invasion and induce cell apoptosis by regulating
B-cell lymphoma 2 (BCL-2), BMI1 proto-oncogene, polycomb
ring finger (BMI-1), phosphatase and tensin homolog
(PTEN) and YY1 transcription factor (YY1) in NSCLC.
BCL-2 is an oncogene and is also central in the apoptosis
pathway. Overexpression of BCL-2 has been reported in a
number of types of human cancer, including breast, gastric and lung
cancer (43–45). Oncogene BMI-1 is
overexpressed in various types of human cancer and serves a
critical role in malignant transformation, proliferation, cell
cycle, apoptosis and distant metastasis (46,47). A
previous study revealed that BMI-1 is involved in the
self-renewal, differentiation and tumor initiation of cancer stem
cells (48). As a powerful tumor
suppressor, PTEN is frequently mutated in lung cancer and is
the negative regulator of the PI3K/mTOR/AKT oncogenic signaling
pathway. A number of studies have confirmed that PTEN is
crucial for cell proliferation, invasion and survival, and loss of
function of PTEN is frequently observed in a number of types
of cancer (49–51). Yin Yang 1 (YY1) (also known as
NF-E1, UCRBP and CF1) is a ubiquitous and multifunctional zinc
finger transcription factor and can regulate multiple genes
associated with multiple cellular processes including cellular
differentiation, DNA repair, autophagy and cell survival (52–54).
However, the role of YY1 in NSCLC progression remains
controversial. Notably, a previous study confirmed that YY1
can act as both oncogene and tumor-suppressor gene in breast cancer
and lung cancer (52–54).
The current study evaluated the underlying roles and
mechanisms of miR-218 in NSCLC progression. It was identified that
the expression of miR-218 in XWLC-05 was significantly lower
compared with that in BEAS-2B. Overexpression of miR-218 could
suppress cell proliferation, invasion and migration, and induce
cell apoptosis in XWLC-05 and NCI-H157 cells. The regulatory
mechanisms between miR-218 and its downstream BCL-2, BMI,
PTEN and YY1 gene axis were further explored in XWLC-05
and NCI-H157 cells to investigate the specificity and similarity of
Xuanwei NSCLC as compared with other NSCLC. The findings revealed
that miR-218 could be a potential therapeutic target for NSCLC.
Furthermore, the present study will provide a theoretical basis for
lung cancer treatment in high-risk areas worldwide.
Materials and methods
Cell strains and cell lines
Normal human lung epithelial cell line BEAS-2B,
Xuanwei lung adenocarcinoma cell line XWLC-05 and human lung
squamous cell carcinoma cell line NCI-H157 were all provided by
Yunnan Cancer Hospital (The Third Affiliated Hospital of Kunming
Medical University) and confirmed via short tandem repeat
profiling. Lung adenocarcinoma cell line NCI-H1975 was provided by
the Stem Cell Bank, Chinese Academy of Sciences. Large cell lung
cancer cell line NCI-H460SM was kindly provided by Dr Ming-Sound
Tsao (University of Toronto, Canada) (55).
Major reagents
RPMI-1640 medium, phosphate buffer and 0.25%
trypsin-EDTA were purchased from HyClone (Cytiva). Fetal bovine
serum (FBS), DMEM and Opti-MEM were purchased from Gibco (Thermo
Fisher Scientific, Inc.). miRcute miRNA Isolation kit, miRcute
miRNA First-strand cDNA Synthesis kit and miRcute miRNA qPCR kit
were purchased from Tiangen Biotech Co., Ltd. iTaq™ Universal
SYBR® Green Supermix and iScript™ cDNA Synthesis kit
were purchased from Bio-Rad Laboratories, Inc. miR-218 (cat. no.
HmiRQP0327) and U6 (cat. no. HmiRQP9001) primers were bought from
GeneCopoeia, Inc. YY1 and BMI-1 monoclonal antibodies were
purchased from Abcam. BCL-2 and PTEN monoclonal antibodies were
purchased from ProteinTech Group, Inc. Lipofectamine®
2000 transfection reagent was purchased from Invitrogen (Thermo
Fisher Scientific, Inc.). Matrigel was purchased from BD
biosciences. pGpU6/EGFP/Neo-miR-218 and pGpU6/EGFP/Neo-miR-NC were
purchased from Shanghai GenePharma Co., Ltd. The insert sequence of
miR-218 was
5′-TGCTGTTGTGCTTGATCTAACCATGTGTTTTGGCCACTGACTGACACATGGTTACAAGCACAA-3′.
The insert sequence of miR-NC was
5′-AATTCGTTCTCCGAACGTGTCACGTGTTTTGGCCACTGACTGACACGTGACATTCGGAGAAA-3′.
Cell culture
Cell culture and maintenance were conducted by the
following established procedures: The cell lines were expanded at
low passages and stored in liquid nitrogen until use. Cell lines
were cultured in RPMI-1640 or DMEM accordingly and supplemented
with 10% FBS at 37°C in a 5% CO2 incubator till they
reached 70–80% confluence. Cells were washed once with 1X PBS and
digested with 0.25% trypsin-EDTA, then harvested for the subsequent
analysis.
Bioinformatics analysis
To predict possible targets of miR-218, a
bioinformatical analysis was performed using TargetScan 7.2
(http://www.targetscan.org/vert_72/).
In the meantime, MiRTarBase (http://mirtarbase.mbc.nctu.edu.tw/php/index.php) and
StarBase V3.0 databases (http://starbase.sysu.edu.cn/) were used to predict the
relationship between mir-218 and its possible target genes.
Cell transfection
Transfection was performed using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). XWLC-05 and NCI-H157 cells were cultured and
grown to 70–80% confluence at 37°C in a 5% CO2
incubator. Then 1 ml Trypsin-EDTA (0.25% trypsin, 1 mM EDTA) was
added for digestion at 37°C for 3 min following washing with 1X
PBS. Then 10 ml 10% FBS complete medium was added to terminate
digestion. The cells were counted using the hemocytometer and then
diluted to the desired density. The density of 2×105
cells/ml was used for reverse transcription-quantitative (RT-q) PCR
and 2×106 cells/ml for western blot analysis. Then
appropriate cells were added into a 6-well cell culture plate. When
the cells were cultured at 37°C in a 5% CO2 incubator
overnight and reached 70–80% confluence, the RPMI-1640 culture
medium containing 10% FBS was removed and cells were washed with 1X
PBS twice. The transfection of pGpU6/EGFP/Neo-miR-218 or
pGpU6/EGFP/Neo-miR-NC involved 0.5 ml of Opti-MEM at a final
concentration of 8 µg/ml and 16 µl of Lipofectamine®
2000 per well. Then, 6 h following transfection, the culture medium
was replaced with RPMI-1640 containing 10% FBS. At 48 h following
transfection, the cell status and enhanced green fluorescent
protein (EGFP) expression were observed under a light and
fluorescence microscope at ×40 and ×100 magnification, and the
cells of each group were collected for subsequent RT-qPCR
detection. Each cell line was divided into three groups: (i) A
blank control group (containing only culture medium); (ii) a
negative control (NC) group (transfected with
pGpU6/EGFP/Neo-miR-NC); and (iii) a transfection group (transfected
with pGpU6/EGFP/Neo-miR-218).
RT-qPCR detection for miR-218, BCL-2,
BMI-1, PTEN and YY1
The total RNA of 1×106 cells was
extracted by using miRcute miRNA Isolation kit. RNA concentration
(>300 ng/µl) and purity (OD 260/OD 280 ~2.0) were measured using
a NanoDrop ND-1000 (Thermo Fisher Scientific, Inc.), and then the
integrity of the RNA was detected by 1.0% agarose gel
electrophoresis with ethidium bromide staining. cDNA was
synthesized according to the protocols of the cDNA synthesis kit,
with a reaction volume at 20 µl, including a 4 µl 5X iScript
reaction mix, 1 µl iScript reverse transcriptase, 1 µg RNA
template, 0.5 µl 10 µmol/l miR-218 or U6 primer and
RNase-free water. The reverse transcription conditions were 25°C
for 5 min, 42°C for 30 min and 85°C for 5 min. Then, qPCR was
performed according to the manufacturer's protocols. The reaction
system was 20 µl, including 10 µl 2X Supermix, 1 µl 10 µmol/l each
upstream and downstream primers, 2 µl cDNA template and 6
µl RNase-free water. Reaction conditions were: 95°C for 30
sec, 95°C for 15 sec and 60°C for 1 min with 40 cycles. The levels
of miR-218 (with U6 as reference gene) and the mRNA levels
of PTEN, BCL-2, BMI-1 and YY1 (with RPS13 as
the reference) were then detected in the cells 48 h following
transfection. The primers used for the amplification of each gene
are given in Table I. In the above
experiment, two wells were set for each condition and all reactions
were repeated three times. The experimental results were calculated
with the formula 2−ΔΔCq to obtain the amount of the
relative gene expression (56).
 | Table I.Primer sequences for qPCR. |
Table I.
Primer sequences for qPCR.
| Gene | Primer sequence
(5′→3′) |
|---|
| BCL-2 | F:
TCCAGGATAACGGAGGCT |
|
| R:
GCCAAACTGAGCAGAGTCTTC |
| BMI-1 | F:
GTCCTATTTGTGATGTCCAAGTTC |
|
| R:
GCAACCTCTCCTCTATCTTCATTA |
| PTEN | F:
CCGAAAGGTTTTGCTACCATTCT |
|
| R:
AAAATTATTTCCTTTCTGAGCATTCC |
| YY-1 | F:
GCACAAAGATGTTCAGGGATAA |
|
| R:
AAGGGCTTCTCTCCAGTATGA |
| RPS13 | F:
GTTGCTGTTCGAAAGCATCTTG |
|
| R:
AATATCGAGCCAAACGGTGAA |
Cell viability detected by MTT
assay
At 48 h following transfection, the cells of each
group were digested with 0.25% trypsin-EDTA for ~2–3 min until the
adherent cells were detached. The trypsinization process was
terminated with medium containing 10% FBS. Then the cells were
transferred into a centrifuge tube and centrifuged at 300 × g for 5
min at room temperature following cell counting using a
hemocytometer. Each well of the 96-well cell culture plate was
seeded with 200 μl cell suspension (1×104
cells/ml) and each group was repeated with 6 replicates. The medium
was removed and 20 μl MTT label reagent was added to each
well and the liquid in the well was carefully removed after 4 h
incubation at 37°C. DMSO (150 μl) was added to each well and
the 96-well cell culture plate was placed on an oscillator for 10
min to completely dissolve. The absorbance was determined by the
microplate reader at 490 nm wavelength and the plate was assayed
each day for 7 days.
Cell migration detected by scratch
assay
The cells of each group were digested 48 h after
transfection and seeded into the 6-well cell culture plate for 24 h
at 37°C. After inoculation, RPMI-1640 culture medium containing 10%
FBS was removed and monolayer cells were scratched with 10
µl pipette tips using the same amount of force, making sure
each scratch was as wide as possible. Loose cells were then gently
washed away with 1X PBS buffer. RPMI-1640 culture medium
supplemented with 3% FBS was added and images were captured under a
light microscope according to previous studies (22,57,58).
The cells were returned to the cell incubator for 72 h at 37°C and
images were captured every 24 h at the same position as the
previous image. Finally, the migration ability was then calculated
by the following formula according to the change in the scratch
distance that was measured by Image J software. Migration rate (%)
= (the scratch distance at time 0-the scratch distance at indicated
time point)/the scratch distance at time 0)x100%.
Cell invasion ability detected by
Transwell assay
The cells were collected by trypsinization 48 h
after transfection and the cell density was adjusted to
5×105/ml using serum-free medium. A total of 60 µl
Matrigel (diluted 7 times with serum-free medium) was added into
the upper chambers and incubated at 37°C in 5% CO2
atmosphere for 1 h. Then, 200 µl of cell suspension
(5×105 cells/ml) was inoculated into the Matrigel-coated
upper chamber (8 µm pore size; Costar; Corning, Inc.).
Subsequently, 600 µl culture medium containing 20% FBS was added to
the lower chamber. After incubation at 37°C in 5% CO2
atmosphere for 24 h, cells were fixed and stained with 0.2% crystal
violet for 1 h at room temperature. The excess stain was then
washed away slowly with water and the images were obtained using a
light microscope at ×40 and ×100 magnification. The cell number was
counted, and the result was compared among all groups.
Cell apoptosis detected by flow
cytometry and transmission electron microscopy
DUTP-FITC/propidium iodide (PI) staining was used
for detection of apoptotic cells and the Coulter DNA Prep reagents
kit for DNA cell cycle analysis. Cells were collected 48 h after
transfection and adjusted at 5×106/ml before being
centrifuged at 300 × g for 5 min at room temperature and the
supernatant discarded. The precipitated cells were washed once with
3 ml ice-cold PBS and centrifuged at 300 × g for 5 min at 4°C.
Precooled 70% ethanol was added and the cells maintained at 4°C for
2 h. Then cells were pelleted by centrifugation at 300 × g for 5
min at 4°C and 3 ml ice-cold PBS was added to re-suspend cells for
5 min. The cell suspension was filtered through a 400-mesh screen
and then centrifuged at 300 × g for 5 min at 4°C. The PBS was then
discarded. In a dark room, 1 ml PI stain was used at 4°C for 30
min. Cell cycle and apoptosis were measured using a Beckman-Coulter
flow cytometer (Beckman Coulter, Inc.) and WINCYCLE software
version 3.0 (Beckman Coulter, Inc.).
Another batch of cells was centrifuged at 300 × g
for 5 min at room temperature and collected at
~5×106/ml. Following washing with PBS twice, the
supernatant was discarded, and 1 ml 2.5% glutaraldehyde was added
to suspend cells and then transferred into a 1.5 ml Eppendorf tube.
The cells were centrifuged at room temperature at 1,200 × g for 15
min and fixed for 1 h with 2.5% glutaraldehyde, then washed for 2 h
in phosphate buffer to remove the glutaraldehyde as much as
possible and fixed for 1 h at room temperature with 1% osmium
solution. Following fixation, 50% ethanol was added to dehydrate
for 10 min, 70% ethanol for 10 min, 90% ethanol for 10 min, 90%
acetone for 10 min and 100% acetone for three times (10 min each).
Acetone and epoxy resin at a 1:1 mixture was used to embed the
cells for 2 h and then pure embedding agent (fully impregnated with
epoxy resin) added for several hours or overnight. Epoxy resin was
used for embedding at 62°C for 2 days. Following sectioning,
ultrathin sections (70 nm) were double stained with 2% lead acetate
uranium for 20 min at room temperature. Changes in cell morphology
were observed and images were captured by transmission electron
microscopy (TEM) at ×8,000 and ×10,000 magnification.
Western blotting
Cells were digested 48 h after transfection and
washed with ice-cold 1X PBS twice and then lysed with 200 µl
RIPA buffer (Beyotime Institute of Biotechnology) containing
proteinase inhibitors (Roche Diagnostics) and the protein
concentration was determined using the BCA protein assay kit
(Beyotime Institute of Biotechnology). Samples with equal amounts
of protein (80 µg) per lane were taken and mixed with 4X loading
buffer and then denatured. Proteins were separated using 10%
SDS-polyacrylamide gels and transferred onto a PVDF membrane (EMD
Millipore). The membranes were blocked for 1 h at room temperature
with 5% BSA (Beijing Solarbio Science & Technology Co., Ltd.)
and then incubated at 4°C overnight with the primary antibodies:
Anti-phosphatase and tensin homolog (PTEN) (ProteinTech Group,
Inc., cat. no. 60300-1-Ig, dilution 1:1,00), anti-BCL-2
(ProteinTech Group, Inc., cat. no. 60178-1-Ig, dilution 1:1,000),
anti-polycomb complex protein BMI-1 (BMI-1) (Abcam, cat. no.
ab126783, dilution 1:1,000), anti-transcriptional repressor protein
YY1 (YY1) (Abcam, cat. no. ab109237, dilution 1:2,000). After
washing the membranes with TBST, the membranes were incubated for 2
h at room temperature with 1:4,000 dilution of the horseradish
peroxidase-conjugated secondary antibody (Cell Signaling
Technology, Inc., cat. no. 7076) or with 1:2,000 dilution of the
horseradish peroxidase-conjugated secondary antibody (Cell
Signaling Technology, Inc., cat. no. 7074). GAPDH (Abmart
Pharmaceutical Technology Co., Ltd. cat. no. M20028; 1:5,000) was
used as a loading control. Protein expression was detected using
the enhanced chemiluminescence (ECL-Plus) reagents (EMD Millipore).
The relative protein levels were analyzed using Image J software
(version 1.52a; National Institutes of Health).
Statistical methods
All experimental data were imaged with GraphPad
Prism 7 (GraphPad Software, Inc.) and Image J (National Institutes
of Health). All data were analyzed with GraphPad Prism 7. All
results represent the average of triplicate experiments expressed
as the mean ± standard deviation. Statistical analysis was
performed using one-way ANOVA with Dunnett's post-hoc test where
all comparisons were against a single control, with Tukey's
post-hoc test where all groups were compared with one another.
P<0.05 was considered to indicate a statistically significant
difference.
Results
miR-218 expression levels are markedly
decreased in NSCLC cell lines
The total RNA of lung cancer cell lines (NCI-H1975,
NCI-H157, NCI-H460SM, XWLC-05) and the normal lung cell line
BEAS-2B were extracted and their OD260/OD280 values were all
>1.9. The results of electrophoresis demonstrated that the
integrity of RNA was reliable and could be used as a template for
cDNA synthesis. In order to determine the expression of miR-218 in
various types of lung cancer cells, BEAS-2B, a normal lung cell
line, was selected as the calibrated strain in the present study.
qPCR was performed to detect miR-218 expression in the 4 lung
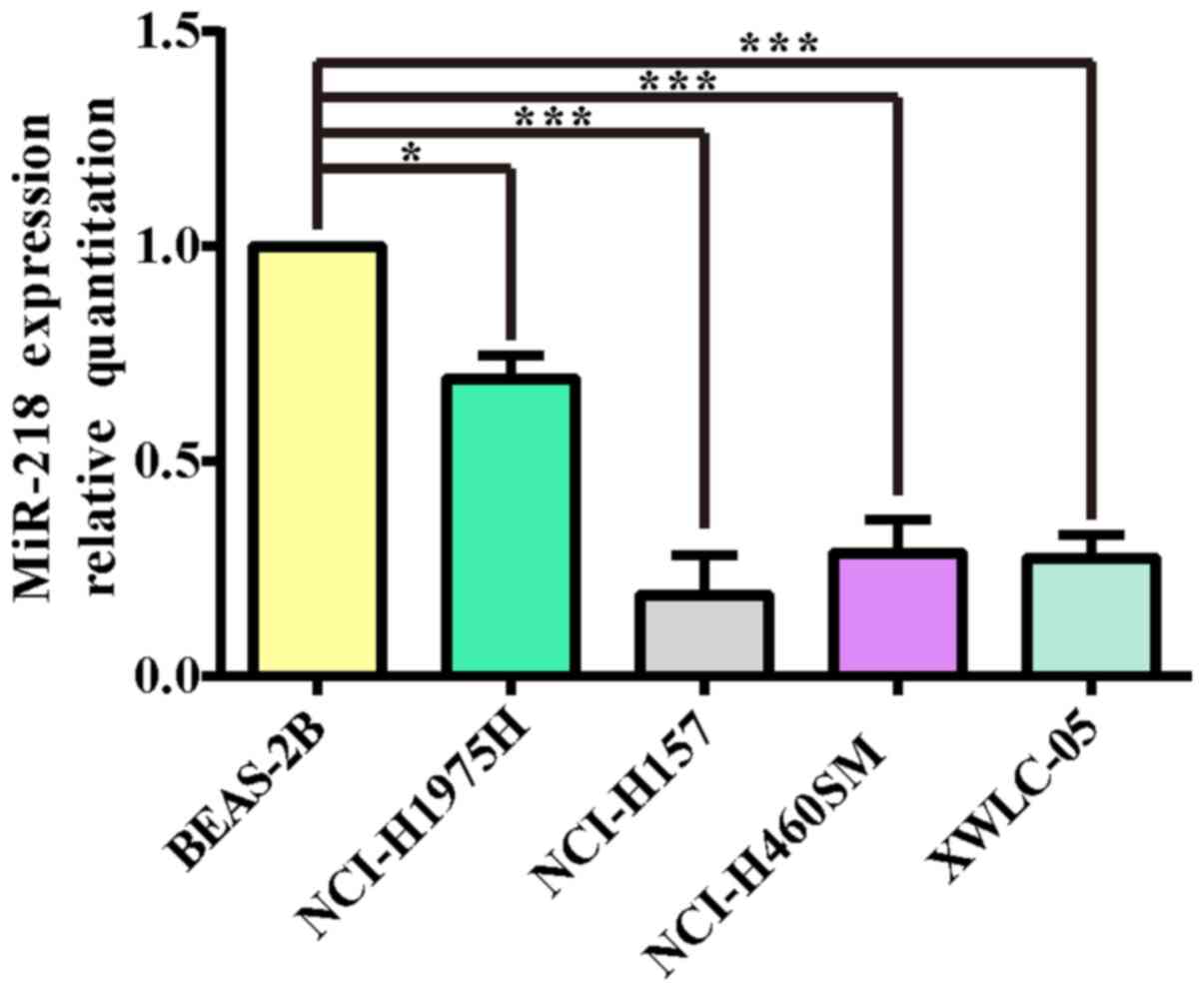
cancer cell lines. As shown in Fig.
1, the expression levels of miR-218 in non-small lung cancer
cell lines were lower compared with normal human lung epithelial
cell line BEAS-2B (P<0.05 and P<0.001 respectively). These
results also indicated that XWLC-05 and NCI-H157 cells could be
used for the subsequent miR-218 overexpression experiments.
Transfection optimization
TargetScan algorithm revealed that BMI-1,
BCL-2 and YY-1 mRNA were potential targets for miR-218
(Fig. 2A). It was therefore
essential to optimize transfection conditions in vitro and
evaluate the efficacy of miR-218 overexpression. XWLC-05 and
NCI-H157 cells were transfected for 24, 48 and 72 h respectively,
then EGFP levels were detected by fluorescence microscope and
miR-218 expression levels were measured by qPCR. Fluorescence
microscope observation revealed that pGpU6/EGFP/Neo-miR-218 or
pGpU6/EGFP/Neo-miR-NC plasmid group showed higher EGFP-positive
cells in both cell lines at 48 h after transfection, and
demonstrated high gene transfection efficiency (Fig. 2B). qPCR results demonstrated that
miR-218 expression level was significantly increased by the
pGpU6/EGFP/Neo-miR-218 plasmid in both cell lines at 48 h after
transfection (P<0.001; Fig.
2C).
 | Figure 2.miR-218 target genes and cell
transfection efficiency. (A) The 3′-UTR of BMI-1, BCL-2 and YY1
mRNA are potential targets for miR-218 and the seed matching
sequences are marked in red, as predicted by TargetScan. (B) The
expression of green fluorescent protein transfected with
pGpU6/EGFP/Neo-miR-218 or pGpU6/EGFP/Neo-miR-NC for 48 h was
observed using a fluorescence inverted microscope (magnification
×40; scale bar, 200 µm for NCI-H157; magnification, ×100; scale
bar, 50 µm for XWLC-05). (C) The expression levels of miR-218 in
NCI-H157 cells were analyzed by qPCR at 48 h post-transfection
shown in the bar graphs. (D) The expression levels of miR-218 in
XWLC-05 cells were analyzed by qPCR at 48 h post-transfection shown
in the bar graphs. miR-218 abundance was normalized to U6 as a
reference gene (n=3, ***P<0.001, ****P<0.0001 vs. control).
Negative, negative control (transfected with pGpU6/EGFP/Neo-miR-NC
plasmid). The experiment was repeated three times. miR, microRNA;
UTR, untranslated region; qPCR, quantitative PCR. |
Cell growth status in each group
It was observed under the optical microscope that
XWLC-05 and NCI-H157 cells of the negative control group and the
blank control group grew well with normal attachment and normal
morphology. However, the cells of the transfected group were mostly
semi-adherent or of a floating, rounded shape; the number of
adherent cells was significantly reduced and the cell morphology
was also changed. These findings indicated that miR-218
overexpression could affect cell growth (Fig. 3).
Overexpression of miR-218 inhibits
PTEN, BCL-2, BMI-1 and YY1 mRNA expression in NSCLC cells
PTEN, BCL-2, BMI-1 and YY1 mRNA levels
were detected using qPCR after the overexpression of miR-218 for 48
h in XWLC-05 and NCI-H157 cells. The results demonstrated that the
mRNA levels of PTEN and YY1 were significantly
unregulated, while BCL-2 and BMI-1 were significantly
downregulated in miR-218 overexpressing cells (Fig. 4).
 | Figure 4.mRNA expression levels of PTEN,
BCL-2, BMI-1 and YY1 in XWLC-05 and NCI-H157 cells. The mRNA
expression levels of PTEN, BCL-2, BMI-1 and YY1 were detected by
qPCR in 48 h transfected cells. mRNA abundance was normalized to
RPS13 (n=3, **P<0.01, ****P<0.0001 vs. the control). The
experiment was repeated three times. miR, microRNA; qPCR,
quantitative PCR. PTEN, phosphatase and tensin homolog; YY1,
anti-transcriptional repressor protein YY1; BCL-2, B-cell lymphoma
2; BMI-1, BMI1 proto-oncogene, polycomb ring finger. |
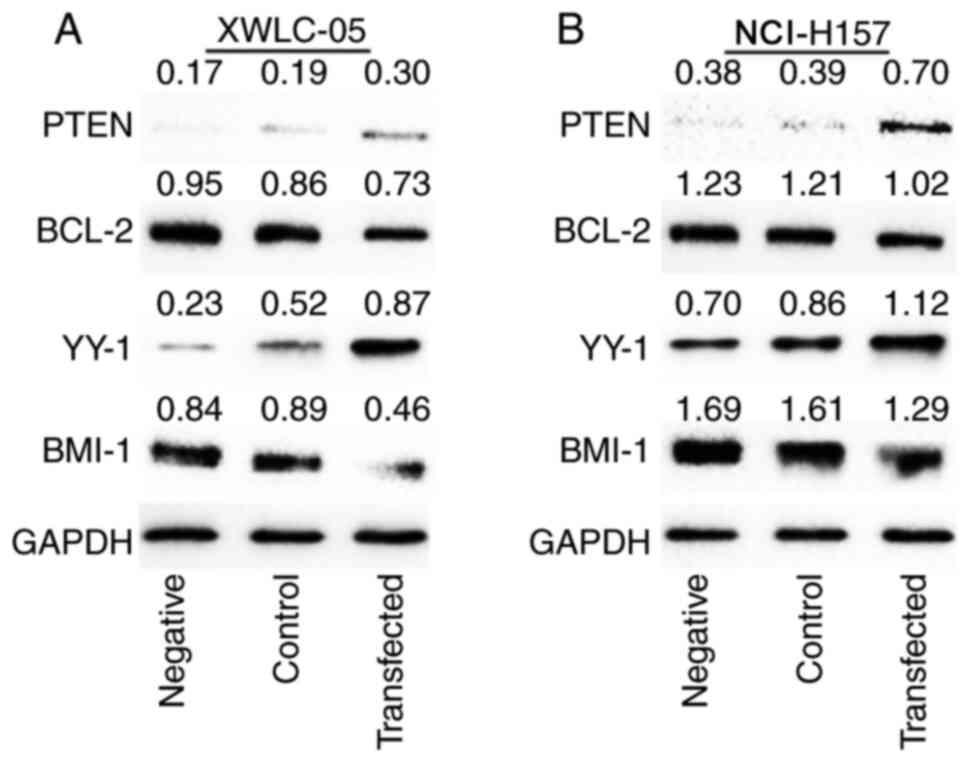
Effect of protein expression on NSCLC
cells transfected with miR-218
As shown in Fig. 5,
the expression of PTEN and YY1 protein levels in XWLC-05 and
NCI-H157 cells were elevated in the transfected group compared with
the negative control group and the blank control group, while the
Bcl-2 and BMI-1 protein expression levels were decreased in the
transfected group compared with the negative control group and the
blank control group. The above results confirmed that miR-218
overexpression could inhibit the expression of oncogenes
BCL-2 and BMI-1 but increase the expression of
tumor-suppression genes PTEN and YY1 in both XWLC-05
and NCI-H157 cell lines.
Effect of miR-218 on NSCLC cell
proliferation
In order to confirm the role of miR-218 on NSCLC
cell proliferation and viability, NCI-H157 and XWLC-05 cells were
transfected with miR-218 overexpression plasmid or empty plasmid.
The cells were incubated for different time periods and the cell
proliferation was assessed by MTT assay. As shown in Fig. 6, the proliferation of both NCI-H157
and XWLC-05 cells transfected with pGpU6/EGFP/Neo-miR-218 was
significantly inhibited relative to the remaining groups
(P<0.01), but the proliferation in the negative control group
demonstrated no significant change compared with the blank control
group (P>0.05).
Role of miR-218 in NSCLC cell
migration ability
The results of the scratch assay demonstrated that
the wound healing ability of XWLC-05 and NCI-H157 cells in the
transfection group was significantly reduced. The wounds of
NCI-H157 cells were fully healed at 30 h in the blank control group
and the negative control group, while the wounds in the transfected
group (141.15±8.12 µm) were not yet healed. Similarly, the wounds
of XWLC-05 cells in both the blank control group and the negative
control group were completely healed at 72 h, while the wounds in
the transfected group were still a certain width (287.41±24.81 µm;
Fig. 7A and B). Furthermore, there
was a significant difference in migration rate at 24 h between the
transfection group and the negative control group (P<0.05;
Fig. 7C and D). These findings
indicated that overexpression of miR-218 weakened the migration
ability of NSCLC cells.
Function of miR-218 in NSCLC cell
invasion
As shown in Fig. 8A and
B, the Transwell invasion assay demonstrated that a large
number of XWLC-05 cells in both the negative control group and the
blank control group passed through the membrane (the average number
of cells in 5 fields was 183.6±4.24 and 187.7±2.08). However, the
number of membrane-passed XWLC-05 cells in the transfection group
(the average number of cells in 5 fields was 91.8±3.42) was
significantly decreased (P<0.01). Furthermore, the transfection
group (the average number of cells in 5 fields was 165.2±9.03)
demonstrated significantly fewer membrane-passed NCI-H157 cells
compared with both the negative control group and the blank control
group (the average number of cells in 5 fields was 207.75±5.24 and
226.75±2.56; P<0.01, P<0.001). The above data confirmed that
miR-218 overexpression reduced NSCLC cell invasion.
Overexpression of miR-218 induces
NSCLC cell apoptosis
The apoptosis rate was measure by flow cytometry. As
shown in Fig. 9A-C, the apoptosis
rate of both XWLC-05 and NCI-H157 cells in the transfection group
was significantly higher compared with the negative control group
and the blank control group (P<0.01). Furthermore,
overexpression of miR-218 in XWLC-05 and NCI-H157 cells resulted in
significant cell cycle arrest in the G2/M phases (Fig. 9D-F). The results of transmission
electron microscopy demonstrated that lung cancer XWLC-05 cells in
the transfection group exhibited apoptotic alterations in
morphology (Fig. 9G). For example,
cell plasma membranes were concentrated; ribosome, mitochondria and
other organelles were aggregated; cell sizes were reduced and cell
structure became denser. There were several apoptotic bodies at
different sizes in the apoptotic cells. The findings indicated that
overexpression of miR-218 induced the apoptosis of XWLC-05 and
NCI-H157 cells.
 | Figure 9.Overexpression of miR-218 induces
G2/M phase cell cycle arrest and apoptosis in NCI-H157 and XWLC-05
cells. (A) Flow cytometry was used to analyze the apoptosis rate of
cells in each group at a qualitative level. (B and C) The
percentage of apoptotic cells in each group. Data represented mean
± SD of three independent repeats. (n=3, **P<0.01 vs. control or
negative control). Negative, negative control (transfected with
pGpU6/EGFP/Neo-miR-NC plasmid). (D) Representative images of cell
cycle distribution detected by flow cytometry. (E and F) The
percentage of cells at various phases of the cell cycle as measured
by flow cytometry. Negative, the negative control group
(transfected with pGpU6/EGFP/Neo-miR-NC plasmid) and data
represented mean ± SD of three independent repeats. (n=3,
**P<0.01, ****P<0.0001 vs. the control or negative control).
(G) Morphological changes of XWLC-05 cells induced by miR-218
overexpression by transmission electron microscopy. Magnification,
×8,000 (bottom) and ×10,000 (top); scale bar, 2 µm. XWLC-05 cells
maintaining lung cancer cell morphology from the blank control
group (top). XWLC-05 cells exhibiting apoptotic cell morphology
from the pGpU6/EGFP/Neo-miR-NC plasmid transfected group at 48 h
post-transfection (bottom). The experiment was repeated three
times. miR, microRNA. |
Discussion
Lung cancer is the leading cause of cancer-related
death worldwide and a high incidence and mortality rate occurs in
Xuanwei County, a county-level city in Yunnan Province, China
(1,2). Therefore, the present study focused on
Xuanwei lung cancer for its regional specificity. This disease has
attracted attention worldwide and a number of studies have been
performed on reducing the incidence and mortality of Xuanwei lung
cancer (5–7,10,59).
However, the survival rate remains low and the precise mechanisms
of lung cancer progression in Xuanwei County remain to be
elucidated (2,60). Therefore, novel strategies for this
regional-specific lung cancer treatment are urgently required.
It is known that the abnormal expression of
microRNAs is associated with carcinogenesis. A number of studies
report that the dysregulations of miR-155 (61), miR-21 (62), miR-32 (63) and miR-34 (64), in addition to miR-218, serve an
important role in the progression of lung cancer (39). The regulation of multiple target
genes by miR-218 has been experimentally validated (65). The present study, for the first time
to the best of the authors' knowledge, investigated the regulatory
mechanisms between miR-218 and BCL-2, BMI, PTEN and
YY1 in XWLC-05 cells.
The present study demonstrated that miR-218
overexpression significantly suppressed cell apoptosis in XWLC-05
cells and a similar result was observed in NCI-H157 cells; this was
consistent with previous studies (66–68).
In order to further investigate the mechanism behind
miR-218-induced apoptosis, the protein and mRNA expression levels
of BCL-2, BMI, and PTEN were examined in XWLC-05 and
NCI-H157 cells following miR-218 transfection. Bioinformatics
analysis demonstrated that BCL-2 was a potential target of
miR-218 and previous findings confirm that BCL-2 can serve
key roles in cell apoptosis (43,44).
The results of the present study demonstrated that overexpression
of miR-218 could induce cell apoptosis in XWLC-05 and NCI-H157
cells partly by reducing the mRNA and protein expression levels of
BCL-2, further emphasizing that BCL-2 expression was
regulated by multiple miRNAs in lung cancer (22). In addition, a previous study notes
that overexpression of miR-218 induces cell apoptosis in colon
cancer by direct regulation of BMI-1 (66). The results of the present study also
revealed that overexpression of miR-218 induced cell apoptosis
partly by decreasing the mRNA and protein expression levels of
BMI-1, while increasing the mRNA and protein expression
levels of PTEN in XWLC-05 and NCI-H157 cells. BMI-1
acts as an oncogene by repressing the tumor-suppressor PTEN
that can exert a critical negative effect on the activity of the
PI3K/AKT pathway (69–71). The findings of the present study
indicated that miR-218 could inhibit BMI-1, while increasing
PTEN, and may inactivate the PI3K/AKT pathway, consequently
inducing apoptosis of lung cancer cells. The present study used the
MTT assay to evaluate the effect of miR-218 on XWLC-05 and NCI-H157
cell proliferation. MTT assay demonstrated that cell proliferation
was significantly inhibited by increasing the expression of
exogenous miR-218 in both XWLC-05 and NCI-H157 cells, which was
consistent with other studies (32,34,41).
The effect of miR-218 on cell cycle progression was further
investigated using flow cytometry. The results demonstrated that
miR-218 overexpression led to a significant increase in the number
of cells accumulating in the G2 phase, suggesting that miR-218
could reduce cell proliferative capacity with G2 cell cycle arrest
in lung cancer. Given the critical function of BMI-1, YY1
and PTEN in cell proliferation, the protein and mRNA
expression levels of BMI-1, YY1 and PTEN were
examined in XWLC-05 and NCI-H157 cells following miR-218
transfection to identify the potential mechanism responsible for
the observed effects of miR-218 on cell growth in lung cancer cells
(69–72). The results indicated that miR-218
could inhibit BMI-1 expression, while elevating PTEN
expression, and may inactivate the PI3K/AKT pathway, thus
inhibiting the proliferation of lung cancer cells. In addition,
miR-218 is a direct target of YY1 and it suppresses the
proliferation of glioma cells by downregulating YY1
expression (72). However, in the
present study overexpression of miR-218-5p significantly increased
the expression of endogenous YY1 resulting in the inhibition
of the proliferation of lung cancer cells. The role and mechanisms
of YY1 in tumorigenesis and development remains
controversial (52). Research has
identified that YY1 and AP1 synergistically induce
the expression of tumor-suppressor gene HLJ1 in lung cancer,
thereby inhibiting lung cancer invasion (73). However, research shows that
YY1 serves an oncogene role in the occurrence and
development of lung cancer (54).
The findings of the present study indicated that miR-218 could
suppress lung cancer progression partly via miR-218-directed
regulation of YY1 expression; YY1 may act as a tumor
suppressor under certain conditions (74). It has been confirmed that
c-MYC works synergistically with BMI-1, which is a
direct target gene of miR-218 (75). Furthermore, c-MYC can
suppress the expression levels of YY1 and c-MYC
function can also be inhibited by YY1 (76,77).
The findings of the present study also indicated that miR-218 might
not directly activate YY1 expression, but indirectly
suppressed c-MYC, resulting in elevated YY1
expression levels, thereby inhibiting the biological
characteristics of malignant tumors.
Next, whether miR-218 contributed to the migration
and invasion ability of XWLC-05 and NCI-H157 cells was examined.
The wound-healing assay and Transwell invasion assay revealed that
ectopic expression of miR-218 markedly repressed the migration and
invasion of XWLC-05 and NCI-H157 cells. However, since cells should
be grown in low-serum or serum-free media during the wound-healing
assay to avoid cell proliferation, further work is needed to
optimize the wound-healing assay with a low concentration of serum.
Furthermore, it was demonstrated that miR-218 repressed the
expression of BMI-1, but increased the expression of
PTEN and YY1 in XWLC-05 and NCI-H157 cells, leading
to decreased cancer migration and invasion. Repression of
BMI-1 was found to suppress cancer cell migration and
invasion, including in lung cancer (47,78).
Various miRNAs have been identified as suppressing BMI-1 to
inhibit tumor invasion (79–81)
and studies reveal that miR-218 functioned as a tumor suppressor
via negatively regulating BMI-1 in glioma (82) and colorectal cancers (29). Consistent with these findings, the
results of the present study demonstrated that miR-218-inhibited
BMI-1 was the key to migration and invasion both in XWLC-05
and NCI-H157 cells. BMI-1 can inhibit PTEN resulting
in activation of the PI3K/AKT pathway, leading to overexpression of
MMP-2, MMP-9 and VEGF and promotion of colon and
liver cancer invasion and metastasis (29,83).
The findings of the present study indicated that miR-218 could
inhibit BMI-1 expression, while indirectly increasing
PTEN expression, thereby inhibiting PI3K-AKT pathway
activity and lung cancer invasion. The exact mechanism of this
requires further study. The present study did not observe any
significant difference in the role of miR-218 and its regulation of
BCL-2, BMI-1, PTEN and YY1 expression in XWLC-05 and
NCI-H157 cells. These results were consistent with our finding
(data not shown) and other clinical sample findings (84,85).
The findings of the present study indicated that miR-218 might
serve a common function in the progression of Xuanwei NSCLC and
other NSCLC, which raises doubts about the current ideas about
these regional-specific diseases.
Thus far, the precise regulatory mechanisms of lung
cancer progression in Xuanwei County have not been fully
understood. The specific epidemiological characteristics in Xuanwei
County led some researchers to believe that there may be unique
molecular mechanisms in the progression of Xuanwei lung cancer
(86,87). For example, some studies confirm
that mutations in the MUC16 gene are observed in 50% of lung
cancer patients residing in Xuanwei and MUC16 participates
in the progression of Xuanwei lung cancer (88,89).
Moreover, miR-144 was remarkably decreased in Xuanwei NSCLC
(90). These findings suggested
that air pollution-related genes such as MUC16 and miR-144
might be critical in Xuanwei lung cancer progression (6,87–91).
However, the results from the present study emphasized that the
dysregulation of miR-218 was also essential for the progression of
both Xuanwei NSCLC and other NSCLC. More importantly, the present
and previous studies indicated that dysregulations of key miRNAs,
including miR-218, miR-21 and miR-34, were the common events both
in the progression of the regional-specific NSCLC and other NSCLC
(22,92–94).
In summary, the results of the present study
confirmed that miR-218 could suppress BCL-2 and BMI-1
expression, while increasing PTEN and YY1 expression,
leading to the suppression of NSCLC progression. Furthermore, the
findings further underline the pivotal roles served by miR-218,
providing new insight into the progression of NSCLC. The possible
regulatory mechanisms of miR-218 are demonstrated in Fig. 10. Further studies will be required
to elucidate the precise mechanisms involved in the downstream
molecules of BCL-2, BMI-1, PTEN and YY1 that could
contribute to miR-218-mediated suppression of lung cancer
progression. Notably, the roles of miR-218 and miR-218-mediated
regulation of BCL-2, BMI-1, PTEN and YY1 expression
in the progression of Xuanwei NSCLC have no significant difference
from other NSCLC. These findings were consistent with our previous
studies and suggest that the roles and regulatory mechanisms of
certain key miRNAs, including miR-218, miR-21 and miR-34a, on gene
expression were similar in the progression of Xuanwei NSCLC and
other NSCLC (22,95). It is difficult to say whether the
high incidence of Xuanwei NSCLC in South China could only be
attributed to scale-specific effects of environmental variables in
the area or specific molecular genetic variation. Further studies
are required on the precise regulatory network involved in the
downstream molecules of BCL-2, BMI-1, PTEN and YY1
that could contribute to miR-218-mediated tumor suppression for the
clinical treatment of Xuanwei NSCLC.
 | Figure 10.Possible regulatory mechanisms
between miR-218 and PTEN, YY1, BCL-2 and BMI-1 in NSCLC cells
transfected with pGpU6/EGFP/Neo-miR-218. At the
post-transcriptional level, PTEN, YY1, BCL-2 and BMI-1 expression
levels were regulated by miR-218. Overexpression of miR-218
activated PTEN and YY1, and repressed BCL-2 and BMI-1 at the mRNA
and protein levels. PTEN, YY1, BCL-2 and BMI-1 may have single or
combined effects on NSCLC cell proliferation, migration, invasion
and apoptosis at the mRNA and protein levels. miR, microRNA; NSCLC,
non-small cell lung cancer; PTEN, anti-phosphatase and tensin
homolog; BMI-1, anti-polycomb complex protein BMI-1; YY1,
anti-transcriptional repressor protein YY1; VEGF, vascular
endothelial growth factor. |
Acknowledgements
The authors thank Dr Xicai Wang (Yunnan Cancer
Hospital and The Third Affiliated Hospital of Kunming Medical
University and Yunnan Cancer Center, Yunnan, China) for their
technical assistance.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 81560380),
Yunnan Medical Discipline Leader Project (grant no. D-201601) and
the Project of Medical and Health Technology Development Program of
Yunnan Province (grant no. 2017NS203).
Availability of data and materials
The datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
QZ, YC, JY and CC designed the experiments. ZX, HL,
LZ, YZ, TW, DC, GL, SL, QY and CY performed the experiments. ZX and
QC analyzed the data. YC, CH, CC and LC interpreted the data. QZ,
YC, CG and LC contributed reagents/materials/analysis tools. YC, QZ
and QC wrote the manuscript. CG made substantial contributions to
the aquisition of data. All authors participated in revising the
manuscript critically for important intellectual content and final
approval of the version to be published. All authors agreed to be
accountable for the work in ensuring that questions related to the
integrity of any part of the work are appropriately investigated
and resolved. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Miller KD, Nogueira L, Mariotto AB,
Rowland JH, Yabroff KR, Alfano CM, Jemal A, Kramer JL and Siegel
RL: Cancer treatment and survivorship statistics, 2019. CA Cancer J
Clin. 69:363–385. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li J, Guo W, Ran J, Tang R, Lin H, Chen X,
Ning B, Li J, Zhou Y, Chen LC, et al: Five-year lung cancer
mortality risk analysis and topography in Xuan Wei: A
spatiotemporal correlation analysis. BMC Public Health. 19:1732019.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cao Y and Gao H: Prevalence and causes of
air pollution and lung cancer in Xuanwei City and Fuyuan County,
Yunnan Province, China. Front Med. 6:217–220. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xiao Y, Shao Y, Yu X and Zhou G: The
epidemic status and risk factors of lung cancer in Xuanwei City,
Yunnan Province, China. Front Med. 6:388–394. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang J, Duan Y, Meng QH, Gong R, Guo C,
Zhao Y and Zhang Y: Integrated analysis of DNA methylation
profiling and gene expression profiling identifies novel markers in
lung cancer in Xuanwei, China. PLoS One. 13:e02031552018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li J, Ran J, Chen LC, Costa M, Huang Y,
Chen X and Tian L: Bituminous coal combustion and Xuan Wei Lung
cancer: A review of the epidemiology, intervention, carcinogens,
and carcinogenesis. Arch Toxicol. 93:573–583. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang Y, Chen K, Zhou Y, Hu Z, Chen S and
Huang Y: Application of serum microRNA-9-5p, 21-5p, and 223-3p
combined with tumor markers in the diagnosis of non-small-cell lung
cancer in Yunnan in southwestern China. OncoTargets Ther.
11:587–597. 2018. View Article : Google Scholar
|
|
8
|
Yan FC, Wang QQ, Ruan YH, Ma LJ, Jia JT,
Jin KW and Chin J: Establishment and biological characteristics of
lung cancer cell line XWLC-05. Ai Zheng. 26:21–25. 2007.PubMed/NCBI
|
|
9
|
Lei J, Li QH, Yang JL, Liu F, Wang L, Xu
WM and Zhao WX: The antitumor effects of oncolytic adenovirus H101
against lung cancer. Int J Oncol. 47:555–562. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xiong G, Chen X, Zhang Q, Fang Y, Chen W,
Li C and Zhang J: RNA interference influenced the proliferation and
invasion of XWLC-05 lung cancer cells through inhibiting aquaporin
3. Biochem Biophys Res Commun. 485:627–634. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang Y, He S, Mei R, Kang Y, Duan J, Wei
R, Xiang C, Wu Y, Lu X, Cai Z, et al: miR 29a suppresses IL 13
induced cell invasion by inhibiting YY1 in the AKT pathway in lung
adenocarcinoma A549 cells. Oncol Rep. 39:2613–2623. 2018.PubMed/NCBI
|
|
12
|
Cai L, Lin S, Girard L, Zhou Y, Yang L, Ci
B, Zhou Q, Luo D, Yao B, Tang H, et al: LCE: An open web portal to
explore gene expression and clinical associations in lung cancer.
Oncogene. 38:2551–2564. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tie J, Pan Y, Zhao L, Wu K, Liu J, Sun S,
Guo X, Wang B, Gang Y, Zhang Y, et al: MiR-218 inhibits invasion
and metastasis of gastric cancer by targeting the Robo1 receptor.
PLoS Genet. 6:e10008792010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Esquela-Kerscher A and Slack FJ: Oncomirs
- microRNAs with a role in cancer. Nat Rev Cancer. 6:259–269. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee RC, Feinbaum RL and Ambros V: The C.
elegans heterochronic gene lin-4 encodes small RNAs with antisense
complementarity to lin-14. Cell. 75:843–854. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pu M, Chen J, Tao Z, Miao L, Qi X, Wang Y
and Ren J: Regulatory network of miRNA on its target: Coordination
between transcriptional and post-transcriptional regulation of gene
expression. Cell Mol Life Sci. 76:441–451. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mestdagh P, Boström AK, Impens F, Fredlund
E, Van Peer G, De Antonellis P, von Stedingk K, Ghesquière B,
Schulte S, Dews M, et al: The miR-17-92 microRNA cluster regulates
multiple components of the TGF-β pathway in neuroblastoma. Mol
Cell. 40:762–773. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Naeli P, Yousefi F, Ghasemi Y,
Savardashtaki A and Mirzaei H: The role of microRNAs in lung
cancer: Implications for diagnosis and therapy. Curr Mol Med.
20:90–101. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Uddin A and Chakraborty S: Role of miRNAs
in lung cancer. J Cell Physiol. April 20–2018.(Epub ahead of
print). View Article : Google Scholar
|
|
21
|
Lin PY, Yu SL and Yang PC: MicroRNA in
lung cancer. Br J Cancer. 103:1144–1148. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu LF, Wu ZP, Chen Y, Zhu QS, Hamidi S and
Navab R: MicroRNA-21 (miR-21) regulates cellular proliferation,
invasion, migration, and apoptosis by targeting PTEN, RECK and
Bcl-2 in lung squamous carcinoma, Gejiu City, China. PLoS One.
9:e1036982014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Deng M, Zeng C, Lu X, He X, Zhang R, Qiu
Q, Zheng G, Jia X, Liu H and He Z: miR-218 suppresses gastric
cancer cell cycle progression through the CDK6/Cyclin D1/E2F1 axis
in a feedback loop. Cancer Lett. 403:175–185. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guan B, Wu K, Zeng J, Xu S, Mu L, Gao Y,
Wang K, Ma Z, Tian J, Shi Q, et al: Tumor-suppressive microRNA-218
inhibits tumor angiogenesis via targeting the mTOR component RICTOR
in prostate cancer. Oncotarget. 8:8162–8172. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jun GJ, Zhong GG and Ming ZS: miR-218
inhibits the proliferation of glioma U87 cells through the
inactivation of the CDK6/cyclin D1/p21Cip1/Waf1 pathway. Oncol
Lett. 9:2743–2749. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tu K, Li C, Zheng X, Yang W, Yao Y and Liu
Q: Prognostic significance of miR-218 in human hepatocellular
carcinoma and its role in cell growth. Oncol Rep. 32:1571–1577.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu B, Tian Y, Li F, Zhao Z, Jiang X, Zhai
C, Han X and Zhang L: Tumor-suppressing roles of miR-214 and
miR-218 in breast cancer. Oncol Rep. 35:3178–3184. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang T, Xu L, Jia R and Wei J: MiR-218
suppresses the metastasis and EMT of HCC cells via targeting
SERBP1. Acta Biochim Biophys Sin (Shanghai). 49:383–391. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang X, Shi H, Tang H, Fang Z, Wang J and
Cui S: miR-218 inhibits the invasion and migration of colon cancer
cells by targeting the PI3K/Akt/mTOR signaling pathway. Int J Mol
Med. 35:1301–1308. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Davidson MR, Larsen JE, Yang IA, Hayward
NK, Clarke BE, Duhig EE, Passmore LH, Bowman RV and Fong KM:
MicroRNA-218 is deleted and downregulated in lung squamous cell
carcinoma. PLoS One. 5:e125602010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu DW, Cheng YW, Wang J, Chen CY and Lee
H: Paxillin predicts survival and relapse in non-small cell lung
cancer by microRNA-218 targeting. Cancer Res. 70:10392–10401. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang Y, Ding L, Hu Q, Xia J, Sun J, Wang
X, Xiong H, Gurbani D, Li L, Liu Y, et al: MicroRNA-218 functions
as a tumor suppressor in lung cancer by targeting IL-6/STAT3 and
negatively correlates with poor prognosis. Mol Cancer. 16:1412017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shi ZM, Wang L, Shen H, Jiang CF, Ge X, Li
DM, Wen YY, Sun HR, Pan MH, Li W, et al: Downregulation of miR-218
contributes to epithelial-mesenchymal transition and tumor
metastasis in lung cancer by targeting Slug/ZEB2 signaling.
Oncogene. 36:2577–2588. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhu K, Ding H, Wang W, Liao Z, Fu Z, Hong
Y, Zhou Y, Zhang CY and Chen X: Tumor-suppressive miR-218-5p
inhibits cancer cell proliferation and migration via EGFR in
non-small cell lung cancer. Oncotarget. 7:28075–28085. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Song L, Li D, Zhao Y, Gu Y, Zhao D, Li X,
Bai X, Sun Y, Zhang X, Sun H, et al: miR-218 suppressed the growth
of lung carcinoma by reducing MEF2D expression. Tumour Biol.
37:2891–2900. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chiu KL, Kuo TT, Kuok QY, Lin YS, Hua CH,
Lin CY, Su PY, Lai LC and Sher YP: ADAM9 enhances CDCP1 protein
expression by suppressing miR-218 for lung tumor metastasis. Sci
Rep. 5:164262015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xie J, Yu F, Li D, Zhu X, Zhang X and Lv
Z: MicroRNA-218 regulates cisplatin (DPP) chemosensitivity in
non-small cell lung cancer by targeting RUNX2. Tumour Biol.
37:1197–1204. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang C, Ge S, Hu C, Yang N and Zhang J:
MiRNA-218, a new regulator of HMGB1, suppresses cell migration and
invasion in non-small cell lung cancer. Acta Biochim Biophys Sin
(Shanghai). 45:1055–1061. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zeng F, Wang Q, Wang S, Liang S, Huang W,
Guo Y, Peng J, Li M, Zhu W and Guo L: Linc00173 promotes
chemoresistance and progression of small cell lung cancer by
sponging miR-218 to regulate Etk expression. Oncogene. 39:293–307.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jin X, Liu X, Zhang Z and Guan Y: lncRNA
CCAT1 acts as a microRNA-218 sponge to increase gefitinib
resistance in NSCLC by targeting HOXA1. Mol Ther Nucleic Acids.
19:1266–1275. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu Z, Lu C, Zhao G, Han X, Dong K, Wang
C, Guan J-Z and Wang Z: Downregulation of miR-218 by nicotine
promotes cell proliferation through targeting CDK6 in non-small
cell lung cancer. J Cell Biochem. 120:18370–18377. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li YJ, Zhang W, Xia H, Zhang BS, Chen P,
Zhao YL and Li J: miR-218 suppresses epithelial-to-mesenchymal
transition by targeting Robo1 and Ecop in lung adenocarcinoma
cells. Future Oncol. 13:2571–2582. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Singh R, Letai A and Sarosiek K:
Regulation of apoptosis in health and disease: The balancing act of
BCL-2 family proteins. Nat Rev Mol Cell Biol. 20:175–193. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Radha G and Raghavan SC: BCL2: A promising
cancer therapeutic target. Biochim Biophys Acta Rev Cancer.
1868:309–314. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hata AN, Engelman JA and Faber AC: The
BCL2 family: Key mediators of the apoptotic response to targeted
anticancer therapeutics. Cancer Discov. 5:475–487. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang M-C, Li C-L, Cui J, Jiao M, Wu T,
Jing LI and Nan K-J: BMI-1, a promising therapeutic target for
human cancer. Oncol Lett. 10:583–588. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Meng X, Wang Y, Zheng X, Liu C, Su B, Nie
H, Zhao B, Zhao X and Yang H: shRNA-mediated knockdown of Bmi-1
inhibit lung adenocarcinoma cell migration and metastasis. Lung
Cancer. 77:24–30. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Siddique HR and Saleem M: Role of BMI1, a
stem cell factor, in cancer recurrence and chemoresistance:
Preclinical and clinical evidences. Stem Cells. 30:372–378. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Gkountakos A, Sartori G, Falcone I, Piro
G, Ciuffreda L, Carbone C, Tortora G, Scarpa A, Bria E, Milella M,
et al: PTEN in lung cancer: Dealing with the problem, building on
new knowledge and turning the game around. Cancers (Basel).
11:11412019. View Article : Google Scholar
|
|
50
|
Álvarez-Garcia V, Tawil Y, Wise HM and
Leslie NR: Mechanisms of PTEN loss in cancer: It's all about
diversity. Semin Cancer Biol. 59:66–79. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Papa A and Pandolfi PP: The PTEN-PI3K axis
in cancer. Biomolecules. 9:1532019. View Article : Google Scholar
|
|
52
|
Sarvagalla S, Kolapalli SP and
Vallabhapurapu S: The two sides of YY1 in cancer: A friend and a
foe. Front Oncol. 9:12302019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wang CC, Tsai MF, Hong TM, Chang GC, Chen
CY, Yang WM, Chen JJW and Yang PC: The transcriptional factor YY1
upregulates the novel invasion suppressor HLJ1 expression and
inhibits cancer cell invasion. Oncogene. 24:4081–4093. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Huang T, Wang G, Yang L, Peng B, Wen Y,
Ding G and Wang Z: Transcription factor YY1 modulates lung cancer
progression by activating lncRNA-PVT1. DNA Cell Biol. 36:947–958.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Liu J, Blackhall F, Seiden-Long I,
Jurisica I, Navab R, Liu N, Radulovich N, Wigle D, Sultan M, Hu J,
et al: Modeling of lung cancer by an orthotopically growing H460SM
variant cell line reveals novel candidate genes for systemic
metastasis. Oncogene. 23:6316–6324. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Li W, Wang W, Ding M, Zheng X, Ma S and
Wang X: MiR-1244 sensitizes the resistance of non-small cell lung
cancer A549 cell to cisplatin. Cancer Cell Int. 16:302016.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Liang CC, Park AY and Guan JL: In vitro
scratch assay: A convenient and inexpensive method for analysis of
cell migration in vitro. Nat Protoc. 2:329–333. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Vermeulen R, Downward GS, Zhang J, Hu W,
Portengen L, Bassig BA, Hammond SK, Wong JYY, Li J, Reiss B, et al:
Constituents of household air pollution and risk of lung cancer
among never-smoking women in Xuanwei and Fuyuan, China. Environ
Health Perspect. 127:970012019. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Chen G, Sun X, Ren H, Wan X, Huang H, Ma
X, Ning B, Zou X, Hu W and Yang G: The mortality patterns of lung
cancer between 1990 and 2013 in Xuanwei, China. Lung Cancer.
90:155–160. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Shao C, Yang F, Qin Z, Jing X, Shu Y and
Shen H: The value of miR-155 as a biomarker for the diagnosis and
prognosis of lung cancer: A systematic review with meta-analysis.
BMC Cancer. 19:11032019. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Bica-Pop C, Cojocneanu-Petric R, Magdo L,
Raduly L, Gulei D and Berindan-Neagoe I: Overview upon miR-21 in
lung cancer: Focus on NSCLC. Cell Mol Life Sci. 75:3539–3551. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zhou B, Yuan W and Li X: Long intergenic
noncoding RNA 319 (linc00319) promotes cell proliferation and
invasion in lung cancer cells by directly downregulating the tumor
suppressor miR-32. Oncol Rese. Aug 11–2017.(Epub ahead of print).
View Article : Google Scholar
|
|
64
|
Zhang L, Liao Y and Tang L: MicroRNA-34
family: A potential tumor suppressor and therapeutic candidate in
cancer. J Exp Clin Cancer Res. 38:532019. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Lu YF, Zhang L, Waye MMY, Fu WM and Zhang
JF: MiR-218 mediates tumorigenesis and metastasis: Perspectives and
implications. Exp Cell Res. 334:173–182. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
He X, Dong Y, Wu CW, Zhao Z, Ng SSM, Chan
FKL, Sung JJY and Yu J: MicroRNA-218 inhibits cell cycle
progression and promotes apoptosis in colon cancer by
downregulating BMI1 polycomb ring finger oncogene. Mol Med.
18:1491–1498. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Hu Y, Xu K and Yagüe E: miR-218 targets
survivin and regulates resistance to chemotherapeutics in breast
cancer. Breast Cancer Res Treat. 151:269–280. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Zarogoulidis P, Petanidis S, Kioseoglou E,
Domvri K, Anestakis D and Zarogoulidis K: miR-205 and miR-218
expression is associated with carboplatin chemoresistance and
regulation of apoptosis via Mcl-1 and Survivin in lung cancer
cells. Cell Signal. 27:1576–1588. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Song LB, Li J, Liao WT, Feng Y, Yu CP, Hu
LJ, Kong QL, Xu LH, Zhang X, Liu WL, et al: The polycomb group
protein Bmi-1 represses the tumor suppressor PTEN and induces
epithelial-mesenchymal transition in human nasopharyngeal
epithelial cells. J Clin Invest. 119:3626–3636. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Choi Y, Zhang J, Murga C, Yu H, Koller E,
Monia BP, Gutkind JS and Li W: PTEN, but not SHIP and SHIP2,
suppresses the PI3K/Akt pathway and induces growth inhibition and
apoptosis of myeloma cells. Oncogene. 21:5289–5300. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Lu XX, Cao LY, Chen X, Xiao J, Zou Y and
Chen Q: PTEN inhibits cell proliferation, promotes cell apoptosis,
and induces cell cycle arrest via downregulating the PI3K/AKT/
hTERT pathway in lung adenocarcinoma A549 cells. BioMed Res Int.
2016:24768422016. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Gao Y, Sun L, Wu Z, Xuan C, Zhang J, You Y
and Chen X: miR-218 inhibits the proliferation of human glioma
cells through downregulation of Yin Yang 1. Mol Med Rep.
17:1926–1932. 2018.PubMed/NCBI
|
|
73
|
Wang CC, Tsai MF, Dai TH, Hong TM, Chan
WK, Chen JJW and Yang PC: Synergistic activation of the tumor
suppressor, HLJ1, by the transcription factors YY1 and activator
protein 1. Cancer Res. 67:4816–4826. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Tan H, Huang S, Zhang Z, Qian X, Sun P and
Zhou X: Pan-cancer analysis on microRNA-associated gene activation.
EBioMedicine. 43:82–97. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Jacobs JJ, Scheijen B, Voncken JW, Kieboom
K, Berns A and van Lohuizen M: Bmi-1 collaborates with c-Myc in
tumorigenesis by inhibiting c-Myc-induced apoptosis via INK4a/ARF.
Genes Dev. 13:2678–2690. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Shrivastava A, Saleque S, Kalpana GV,
Artandi S, Goff SP and Calame K: Inhibition of transcriptional
regulator Yin-Yang-1 by association with c-Myc. Science.
262:1889–1892. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Austen M, Cerni C, Lüscher-Firzlaff JM and
Lüscher B: YY1 can inhibit c-Myc function through a mechanism
requiring DNA binding of YY1 but neither its transactivation domain
nor direct interaction with c-Myc. Oncogene. 17:511–520. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Guo BH, Feng Y, Zhang R, Xu LH, Li MZ,
Kung HF, Song LB and Zeng MS: Bmi-1 promotes invasion and
metastasis, and its elevated expression is correlated with an
advanced stage of breast cancer. Mol Cancer. 10:102011. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Xu L, Li Y, Yan D, He J and Liu D:
MicroRNA-183 inhibits gastric cancer proliferation and invasion via
directly targeting Bmi-1. Oncol Lett. 8:2345–2351. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
He Z, Xia Y, Pan C, Ma T, Liu B, Wang J,
Chen L and Chen Y: Up-regulation of miR-452 inhibits metastasis of
non-small cell lung cancer by regulating BMI1. Cell Physiol
Biochem. 37:387–398. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Guo S, Xu X, Tang Y, Zhang C, Li J, Ouyang
Y, Ju J, Bie P and Wang H: miR-15a inhibits cell proliferation and
epithelial to mesenchymal transition in pancreatic ductal
adenocarcinoma by down-regulating Bmi-1 expression. Cancer Lett.
344:40–46. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Tu Y, Gao X, Li G, Fu H, Cui D, Liu H, Jin
W and Zhang Y: MicroRNA-218 inhibits glioma invasion, migration,
proliferation, and cancer stem-like cell self-renewal by targeting
the polycomb group gene Bmi1. Cancer Res. 73:6046–6055. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Li X, Yang Z, Song W, Zhou L, Li Q, Tao K,
Zhou J, Wang X, Zheng Z, You N, et al: Overexpression of Bmi-1
contributes to the invasion and metastasis of hepatocellular
carcinoma by increasing the expression of matrix metalloproteinase
(MMP) 2, MMP-9 and vascular endothelial growth factor via the
PTEN/PI3K/Akt pathway. Int J Oncol. 43:793–802. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Zhou Y, Wang X, Huang Y, Chen Y, Zhao G,
Yao Q, Jin C, Huang Y, Liu X and Li G: Down-regulated SOX4
expression suppresses cell proliferation, metastasis and induces
apoptosis in Xuanwei female lung cancer patients. J Cell Biochem.
116:1007–1018. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Wang D, Hao T, Pan Y, Qian X and Zhou D:
Increased expression of SOX4 is a biomarker for malignant status
and poor prognosis in patients with non-small cell lung cancer. Mol
Cell Biochem. 402:75–82. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Li R, Liu Y, Wang T, Tang J, Xie L, Yao Z,
Li K, Liao Y, Zhou L, Geng Z, et al: The characteristics of lung
cancer in Xuanwei County: A review of differentially expressed
genes and noncoding RNAs on cell proliferation and migration.
Biomed Pharmacother. 119:1093122019. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Hu Z, Wang X, Yang Y, Zhao Y, Shen Z and
Huang Y: MicroRNA expression profiling of lung adenocarcinoma in
Xuanwei, China: A preliminary study. Medicine (Baltimore).
98:e157172019. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Yu XJ, Yang MJ, Zhou B, Wang GZ, Huang YC,
Wu LC, Cheng X, Wen ZS, Huang JY, Zhang YD, et al: Characterization
of somatic mutations in air pollution-related lung cancer.
EBioMedicine. 2:583–590. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Kanwal M, Ding XJ, Song X, Zhou GB and Cao
Y: MUC16 overexpression induced by gene mutations promotes lung
cancer cell growth and invasion. Oncotarget. 9:12226–12239. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Pan HL, Wen ZS, Huang YC, Cheng X, Wang
GZ, Zhou YC, Wang ZY, Guo YQ, Cao Y and Zhou GB: Down-regulation of
microRNA-144 in air pollution-related lung cancer. Sci Rep.
5:143312015. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Zhou G: Tobacco, air pollution,
environmental carcinogenesis, and thoughts on conquering strategies
of lung cancer. Cancer Biol Med. 16:700–713. 2019.PubMed/NCBI
|
|
92
|
Markou A, Zavridou M and Lianidou ES:
miRNA-21 as a novel therapeutic target in lung cancer. Lung Cancer
(Auckl). 7:19–27. 2016.PubMed/NCBI
|
|
93
|
Li YL, Liu XM, Zhang CY, Zhou JB, Shao Y,
Liang C, Wang HM, Hua ZY, Lu SD and Ma ZL: MicroRNA-34a/EGFR axis
plays pivotal roles in lung tumorigenesis. Oncogenesis. 6:e3722017.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Wu S, Shen W, Pan Y, Zhu M, Xie K, Geng L,
Wang Y, Liang Y, Xu J, Cao S, et al: Genetic variations in key
MicroRNAs are associated with the survival of nonsmall cell lung
cancer. Medicine (Baltimore). 94:e20842015. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Liuxin Z: Role of miR-34a in local lung
cancer cell lines XWLC-05 and YTMLC-90 (unpublished PhD thesis).
Yunnan University; 2018
|