Introduction
Hepatocellular carcinoma (HCC) is a frequent
malignant tumor (1), according to
the latest data from the American Cancer Society, there were 42,030
new cases of liver and intrahepatic bile duct cancer in 2019
(2). Since the early symptoms of
HCC are not obvious, as they are small nodular hypercellular
lesions (3), a notable proportion
of patients are diagnosed with advanced HCC, and few can be treated
via radical resection (1), with
<30% of patients benefiting from curative treatment (4). Previous studies have reported that
while surgical treatment is effective for patients with early HCC,
the prognosis of patients with intermediate and advanced stage HCC
is poor due to the high frequency of metastasis and recurrence
(4), and because surgery,
chemotherapy, radiotherapy and other therapeutic methods are not
effective and can possess toxic side effects (5–7). HCC
caused >0.6 million deaths annually with the highest rates of
death in Eastern and Southeast Asia (8). In the United States, the death rates
from liver cancer increased by 43% (from 7.2 to 10.3 deaths per
100,000) between 2000 and 2016 (9).
These challenges require novel potential biomarkers and targets to
design more powerful treatments.
It has previously been reported that natural
products from plants serve key roles in cancer treatment (10). Radix rehmanniae, a dry root of
Rehmannia glutinosa Libosch, exhibits a number of
pharmacological effects (11),
including anti-inflammatory and antioxidative activities, and they
can reduce blood glucose levels and partially recover nerve damage
(12,13). Catalpol is an iridoid glucoside
compound isolated from the root of Radix rehmanniae that has active
pharmacological functions, such as anti-cerebral ischemia injury,
anti-aging, anti-inflammation and antitumor activity (14–16).
Catalpol also produces cardiovascular protection via the PI3K/AKT
signaling pathway in an ischemia/reperfusion rat model (17). The anti-aging effects of catalpol
are achieved via promoting endogenous antioxidant enzyme
activities, and restoring and improving metabolism failure
(18). The antitumor potential of
catalpol has been confirmed in numerous types of malignant tumors,
including colon, breast and gastric cancer, as well as
osteosarcoma, and a number of molecular mechanisms underlying this
antitumor effect have been proposed (19–21).
For example, catalpol suppresses proliferation, growth and invasion
of CT26 colon cancer via inhibiting inflammation and tumor
angiogenesis (19). Additionally,
catalpol inhibits migration and induces apoptosis of gastric cancer
cells in athymic nude mice (21).
However, to the best of our knowledge, the anticancer effects of
catalpol on HCC are rarely reported.
It has been revealed that microRNAs (miRNA/miR) are
involved in tumor occurrence and progression (22,23).
miR-140-5p is a tumor inhibitor that significantly blocks
migration, invasion and other biological features of tumor cells
(24–26). Moreover, miR-140-5p has been shown
to notably inhibit cell proliferation, migration and invasion in
HCC (27,28). Catalpol can reduce the proliferation
of cancer cells via regulating miRNAs. For example, catalpol
suppresses proliferation and promotes apoptosis of MCF-7 breast
cancer cells via increasing the expression of miR-146a (20). Catalpol attenuates cardiomyocyte
apoptosis in diabetic cardiomyopathy via the nuclear paraspeckle
assembly transcript 1 (Neat1)/miR-140-5p/histone deacetylase 4 axis
(29). However, to the best of our
knowledge, whether catalpol is involved in HCC by regulating
miR-140-5p has not been previously reported. The present study
investigated the role and mechanism of catalpol in HCC cells in
vitro.
Materials and methods
Cell culture
Human HCC cell lines HCCLM3 and Huh7 (both American
Type Culture Collection) were cultured in DMEM (Sigma-Aldrich;
Merck KGaA) supplemented with 10% FBS (Sigma-Aldrich; Merck KGaA)
in an incubator at 37°C with 5% CO2.
Transfection
The 2-O-methyl type inhibitor for miR-140-5p
(Shanghai GenePharma Co., Ltd.), inhibitor control (Shanghai
GenePharma Co., Ltd.) and Lipofectamine® 2000
transfection reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
were used in cell transfection. Lipofectamine 2000 (1 µl) was
diluted in 50 µl Opti-MEM (Invitrogen; Thermo Fisher Scientific,
Inc.), and 30 pmol miR-140-5p inhibitor was diluted in 50 µl
Opti-MEM. Huh7 and HCCLM3 cells (2×105 cells/well) were
inoculated into a 96-well culture plate, added with the diluted
Lipofectamine 2000 and miR-140-5p inhibitor and incubated at 37°C
with 5% CO2 for 48 h. Following this, cells were
collected for subsequent experiments. The cells in the Control
group were untreated, and those in the Inhibitor control group were
treated with transfection agents only. The primer sequences are
presented in Table I.
 | Table I.Sequences used for transfection. |
Table I.
Sequences used for transfection.
| Name | Primer (5′→3′) |
|---|
| MicroRNA-140-5p
inhibitor |
CUACCAUAGGGUAAAACCACUG |
| Inhibitor
control |
UCUACUCUUUCUAGGAGGUUGUGA |
Drug and TGF-β1 treatment
For catalpol (Sigma-Aldrich; Merck KGaA) treatment,
HCCLM3 and Huh7 cells were adjusted to 1×104 cells/ml
density using DMEM, seeded into 96-well plates and treated with
different concentrations of catalpol (0.0, 2.5, 5.0, 10.0, 20.0,
50.0 and 100.0 µM) for 24, 48 and 72 h at 37°C. HCCLM3 and Huh7
cells were collected for cell viability experiments.
In order to observe the effect of catalpol treatment
on morphological changes of TGF-β1-treated cancer cells, HCCLM3 and
Huh7 cells were divided into Control (no treatment), TGF-β1
[treated with 5 ng/ml TGF-β1 (Miltenyi Biotec, Inc.) for 48 h at
37°C] and Catalpol + TGF-β1 (treated with 50 µM catalpol and
stimulated with 5 ng/ml TGF-β1 for 48 h at 37°C) groups.
Morphological observation
HCCLM3 and Huh7 cells (1×104 cells/well)
were seeded onto 24-well plates. The Control group consisted of
untreated cells cultured in DMEM for 48 h at 37°C; the TGF-β1 group
was treated with DMEM containing TGF-β1 (5 ng/ml for 48 h at 37°C);
and the Catalpol + TGF-β1 group were treated with DMEM containing
TGF-β1 (5 ng/ml) and catalpol (50 µM) for 48 h at 37°C. After 48 h,
cells were observed under a light microscope (magnification, ×200;
CKX41; Olympus Corporation).
Cell Counting Kit-8 (CCK-8) assay
A CCK-8 assay was performed to detect viability of
HCCLM3 and Huh7 cells in vitro. Cells (2×103
cells/well) were seeded onto 96-well plates and cultured for 24, 48
and 72 h at 37°C with 5% CO2. Following the incubation,
10 µl CCK-8 (Dojindo Molecular Technologies, Inc.) was added into
each well, followed by a 2-h incubation at 37°C, according to the
manufacturer's instructions. The optical density at 450 nm was
recorded using a microplate reader (Model 680; Bio-Rad
Laboratories, Inc.).
5-Bromo-2-deoxyuridine (BrdU)
assay
A BrdU assay was used to measure the proliferation
of HCCLM3 and Huh7 cells in vitro. Cells were seeded onto
96-well plates (1×104 cells/well), incubated for 48 h at
37°C, and then incubated with BrdU (20 mM) for 4 h at 37°C. Cells
were permeabilized with 0.1% Triton-100 for 10 min at room
temperature, and blocked with 3% FBS at room temperature for 1 h,
and cellular DNA was denatured via 50 units DNase I for 30 min at
37°C. The Alexa Fluor® 488-conjugated anti-BrdU
monoclonal antibody (1:200; cat. no. IC7225G; R&D Systems,
Inc.) was added to cells and incubated at 4°C overnight. The next
day, nuclei were counterstained with DAPI for 10 min at room
temperature, and images were captured under a fluorescence
microscope (magnification, ×200; SteREO Lumar.V12; Zeiss AG).
Wound healing assay
A wound healing assay was performed to detect the
migration of HCCLM3 and Huh7 cells. The treated cells
(1×106 cells/well) were plated on a 6-well plate, and a
straight wound was created using a sterile tip after cell
confluence reached >90%. The cells were cultured in serum-free
DMEM. Floating cells were removed using DMEM at 0 and 24 h, and
images were captured using a light microscope (magnification, ×100;
CKX41; Olympus Corporation) and analyzed via ImageJ software 1.8.0
(National Institutes of Health).
Transwell assay
A Transwell assay (Costar; Corning, Inc.) was used
to determine the invasion ability of HCCLM3 and Huh7 cells. The
Matrigel-precoated (at 37°C for 4 h) upper chamber of the Transwell
inserts (8 µm; Corning, Inc.) containing FBS-free DMEM was added
with the treated cells (2×104 cells/ml) and incubated
for 24 h at 37°C with 5% CO2, while DMEM supplemented
with 10% FBS was added into the lower chamber. The remaining cells
in the upper chamber were removed using a cotton swab, while those
in the lower chamber surface were treated with 4% paraformaldehyde
for 15 min at room temperature and stained with 0.1% gentian violet
for 30 min at room temperature. Images were captured using a light
microscope (magnification, ×200; Digital Microscope VHX-5000;
Keyence Corporation).
Bioinformatics analysis
The target gene of miR-140-5p was detected via
computational analysis performed using TargetScan software (version
7.2; targetscan.org/vert_72), as
previously described (30).
Reverse transcription-quantitative
(RT-q)PCR
RNA was extracted from cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), and purity and concentration were assessed via NanoDrop
2000/2000c (Thermo Fisher Scientific, Inc.). To detect miR-140-5p
expression levels, TaqMan™ MicroRNA Reverse Transcription kit
(Applied Biosystems; Thermo Fisher Scientific, Inc.) was used for
reverse transcription of RNA into cDNA, at 42°C for 30 min and 85°C
for 5 min. qPCR was performed using a Hairpin-it™ miRNA qPCR
Quantitation kit (Shanghai GenePharma Co., Ltd.) and 7500 Fast
Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific,
Inc.) under the following conditions: Initial denaturation at 95°C
for 3 min, followed by 40 cycles at 95°C for 12 sec, and 62°C for
40 sec.
For the detection of mRNA expression levels, RNA (2
mg) was reverse-transcribed into cDNA using a TaqMan RT kit
(Applied Biosystems; Thermo Fisher Scientific, Inc.), at 37°C for
30 min and 85°C for 5 min. RT-qPCR was performed using an ABI 7500
Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific,
Inc.) and SYBR-Green PCR Master Mix kit (Takara Bio, Inc.) at 94°C
for 2 min, followed by 40 cycles at 94°C for 20 sec, 58°C for 20
sec and 72°C for 20 sec. The relative mRNA expression levels were
calculated via the 2−ΔΔCq (31) method. U6 and GAPDH were used as
endogenous controls. Primers are presented in Table II.
 | Table II.Primers used for reverse
transcription-quantitative PCR. |
Table II.
Primers used for reverse
transcription-quantitative PCR.
| Name | Forward
(5′→3′) | Reverse
(5′→3′) |
|---|
|
MicroRNA-140-5p |
GAGTGTCAGTGGTTTTACCCT |
GCAGGGTCCGAGGTATTC |
| U6 |
CGCTTCGGCACATATACTA |
CGCTTCACGAATTTGCGTGTCA |
| Vimentin |
AGAGAACTTTGCCGTTGAAGC |
ACGAAGGTGACGAGCCATT |
| N-cadherin |
GACGGTTCGCCATCCAGAC |
TCGATTGGTTTGACCACGG |
| E-cadherin |
TTGCTACTGGAACAGGGACA |
GTATTGGGAGGAAGGTCTGC |
| GAPDH |
AACTTTGGCATTGTGGAAGG |
ACACATTGGGGGTAGGAACA |
Western blotting
Proteins were lysed using RIPA buffer (Beyotime
Institute of Biotechnology), and the concentration was determined
via Bio-Rad DC Assay kit (Bio-Rad Laboratories, Inc.). The protein
samples (20 µg/lane) were separated on 10% SDS-PAGE (Invitrogen;
Thermo Fisher Scientific, Inc.), and transferred onto PVDF
membranes (EMD Millipore), which were blocked with 5% non-fat dry
milk for 2 h at room temperature. The membranes were then incubated
with vimentin (1:1,000; cat. no. ab92547; Abcam), N-cadherin
(1:1,000; cat. no. ab18203; Abcam), E-cadherin (1:10,000; cat. no.
ab40772; Abcam) and GAPDH (1:2,000; cat. no. ab8245; Abcam) at 4°C
overnight, and subsequently incubated with horseradish peroxidase
(HRP)-conjugated goat anti-mouse IgG H&L (1:2,000; cat. no.
ab205719; Abcam) and HRP-conjugated goat anti-rabbit IgG H&L
(1:2,000; cat. no. ab205718; Abcam) for 1 h at room temperature.
Bands of specific proteins were analyzed via SuperECL Plus
detection reagent (Nanjing KeyGEN Biotech Co., Ltd.) and quantified
using ImageJ Software (version 1.46; National Institutes of
Health). GAPDH was used as an internal control.
Statistical analysis
The data were analyzed and plotted using SPSS
software (version 20.0; IBM Corp.) and presented as the mean ± SD
of three independent repeats. Differences between groups were
analyzed using unpaired Student's t-test or one-way ANOVA followed
by Tukey's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
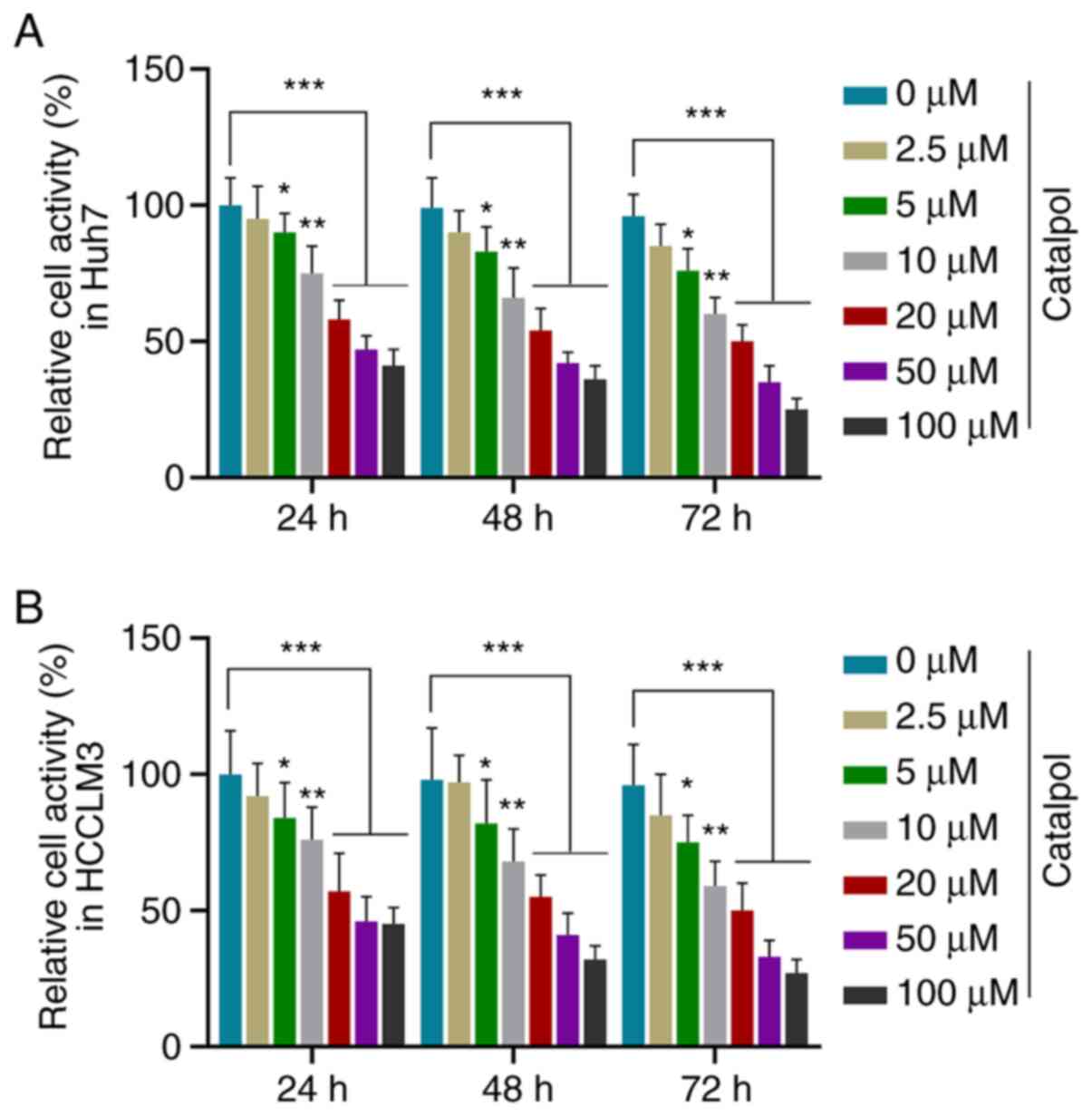
Catalpol suppresses the viability of
HCC cells
In order to investigate the role of catalpol in HCC
cells, Huh7 and HCCLM3 cells were treated with different
concentrations of catalpol (0.0, 2.5, 5.0, 10.0, 20.0, 50.0 and
100.0 µM) to determine the optimal drug concentration for
subsequent experiments. CCK-8 results demonstrated that viability
of Huh7 (Fig. 1A) and HCCLM3
(Fig. 1B) cells was decreased by
different concentrations of catalpol at three time points in a
dose-dependent manner compared with untreated cells (P<0.05).
Notably, the cell viability was decreased by >50% following
treatment with 50 µM catalpol for 48 h. Thus, treatment with 50 µM
catalpol for 48 h was selected for use in further in vitro
functional measurements.
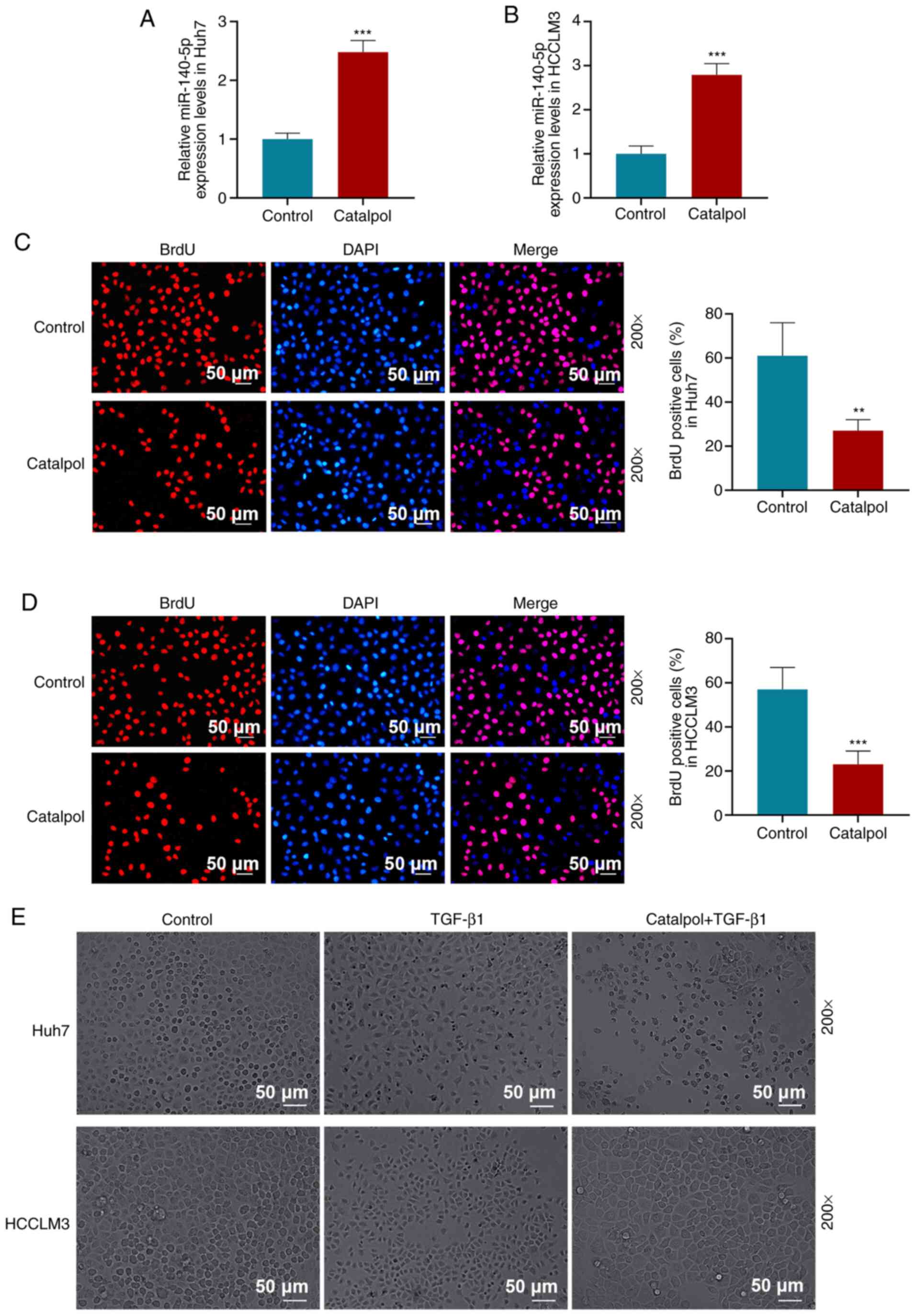
Catalpol inhibits proliferation,
invasion and migration of HCC cells, and increases miR-140-5p
expression
After treating HCC cells with 50 µM catalpol for 48
h, RT-qPCR results demonstrated that miR-140-5p expression levels
in Huh7 (Fig. 2A) and HCCLM3
(Fig. 2B) cells were increased
compared with those in the Control group (P<0.001).
BrdU assay results suggested that 50 µM catalpol
treatment for 48 h decreased the proportion of BrdU-positive cells
compared with the Control group in Huh7 (P<0.01; Fig. 2C) and HCCLM3 cells (P<0.001;
Fig. 2D). The occurrence of EMT in
tumor cells requires the induction of signaling factors, such as
TGF-β1, that promote N-cadherin expression but inhibit E-cadherin
expression (32). TGF-β1 was used
to treat Huh7 and HCCLM3 (Fig. 2E)
cells, and it was observed that, compared with the Control group,
the morphology of Huh7 and HCCLM3 cells in the TGF-β1 group was
altered, indicated by a spindle shape and decreased intercellular
connections. Compared with TGF-β1-treated cells, the number of
spindle cells in the Catalpol + TGF-β1 group was decreased, cell
morphology recovered and intercellular connections were tight.
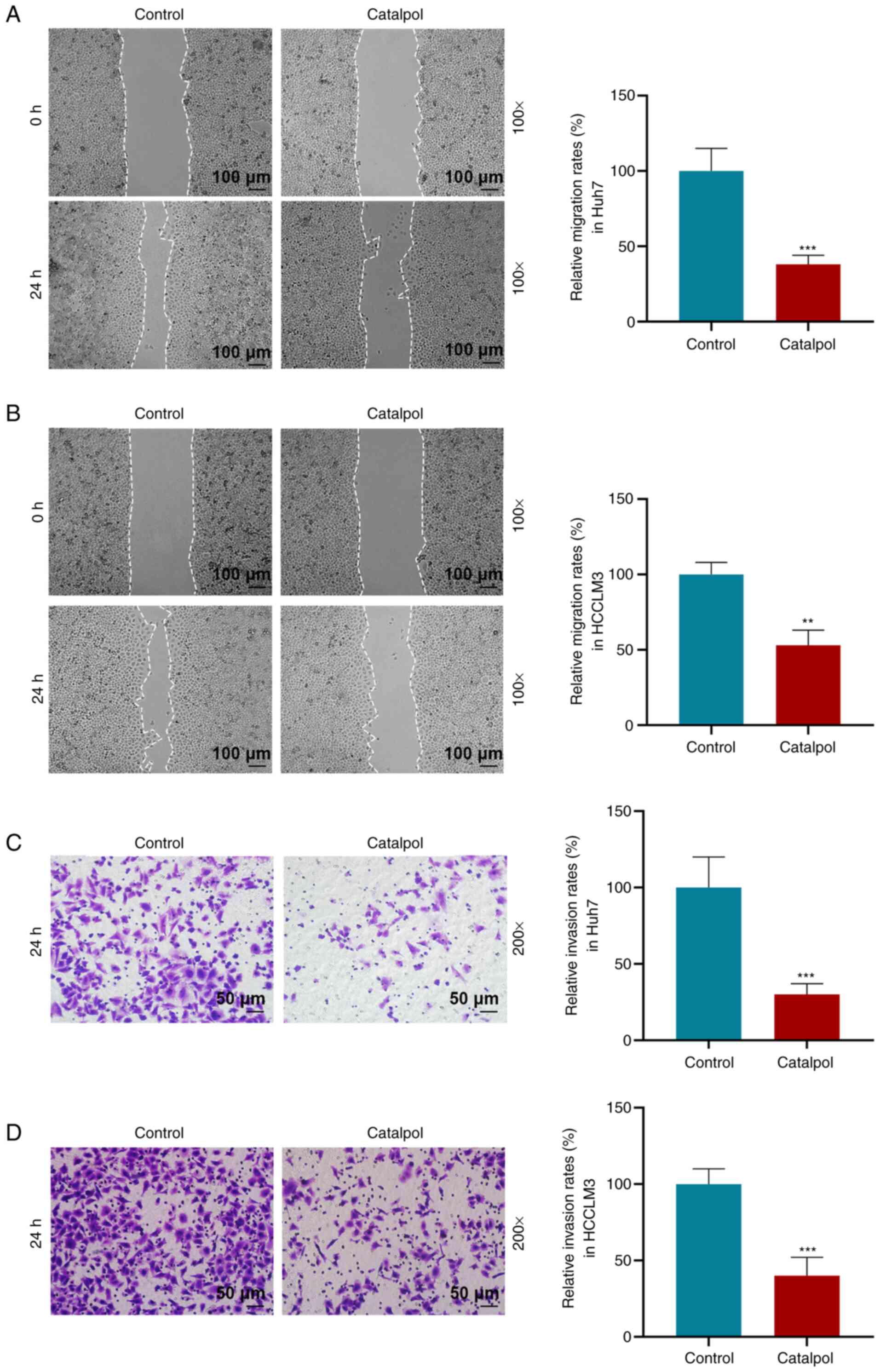
The effects of catalpol (50 µM) on the migration and
invasion of Huh7 and HCCLM3 cells were determined via wound healing
and Transwell assays. Following catalpol treatment, Huh7 and HCCLM3
cells exhibited decreased migration and invasion compared with
those in the Control group (P<0.01 or P<0.001; Fig. 3). Consistent with these results,
RT-qPCR and western blotting identified that catalpol decreased
expression levels of vimentin and N-cadherin in Huh7 cells
(Fig. 4A and B), whereas the
expression of E-cadherin was increased compared with the Control
group (P<0.001). Moreover, vimentin and N-cadherin in HCCLM3
cells (Fig. 4C and D) had low
expression levels and E-cadherin was highly expressed in the
Catalpol group compared with the Control group (P<0.001).
Therefore, it was suggested that catalpol (50 µM) induced
inhibitory effects on cell proliferation, invasion and migration,
as well as EMT, but increased miR-140-5p expression.
Catalpol mediates biological function
of HCC cells via regulating miR-140-5p expression
It was demonstrated that 50 µM catalpol increased
miR-140-5p expression in Huh7 and HCCLM3 cells. Therefore, a
miR-140-5p inhibitor was transfected into HHC cells to investigate
whether catalpol inhibits HCC cell proliferation and metastasis via
regulating miR-140-5p expression. Huh7 and HCCLM3 cells were
transfected with miR-140-5p inhibitor and treated with 50 µM
catalpol for 48 h. RT-qPCR results indicated that, compared with
the Inhibitor control group, miR-140-5p expression levels in Huh7
(Fig. 5A) and HCCLM3 (Fig. 5B) cells were decreased by miR-140-5p
inhibitor (P<0.01).
Following transfection with miR-140-5p inhibitor and
treatment with 50 µM catalpol for 48 h, the CCK-8 results suggested
that the viability of Huh7 (Fig.
5C) and HCCLM3 (Fig. 5D) cells
transfected with miR-140-5p inhibitor control and treated with
catalpol was lower compared with those transfected with miR-140-5p
inhibitor control only (P<0.01). Moreover, the viability of
cells treated with Catalpol + Inhibitor was higher compared with
those treated with Catalpol + Inhibitor control (P<0.001), but
lower compared with the cells transfected with miR-140-5p inhibitor
alone (P<0.05).
Wound healing and Transwell assays were performed to
determine cell migration and invasion. Compared with the Inhibitor
control group, migration of Huh7 (Fig.
6A and B) and HCCLM3 cells (Fig. 6C
and D) in the Catalpol + Inhibitor control group was decreased
(P<0.001), but increased in the Inhibitor group (P<0.001).
Cell migration was higher in the Catalpol + Inhibitor group
compared with the Catalpol + Inhibitor control group, but lower
compared with the Inhibitor group (Fig.
6A-D; P<0.001). Furthermore, the invasion of Huh7 (Fig. 6E and F) and HCCLM3 (Fig. 6G and H) cells in the Catalpol +
Inhibitor control group was decreased (P<0.001), but increased
in Inhibitor group compared with Inhibitor control group
(P<0.05). The invasive ability of cells transfected with
miR-140-5p inhibitor and treated with Catalpol was higher compared
with the Catalpol + Inhibitor control group, but lower compared
with the Inhibitor group (P<0.001). Thus, catalpol treatment
inhibited the viability, invasion and migration of Huh7 and HCCLM3
cells, while the miR-140-5p inhibitor significantly reversed the
effects of catalpol on cells.
Catalpol mediates EMT of HCC cells via
regulating miR-140-5p expression
EMT is a biological process in which the epithelial
phenotype is transformed into a mesenchymal phenotype; during EMT,
the expression of E-cadherin is decreased, and those of vimentin
and N-cadherin are increased (33,34).
In order to determine whether miR-140-5p targets vimentin,
N-cadherin or E-cadherin, bioinformatics was conducted to predict
miR-140-5p target genes, and it was identified that there was no
binding site between them. Thus, miR-140-5p did not target
vimentin, N-cadherin and E-cadherin.
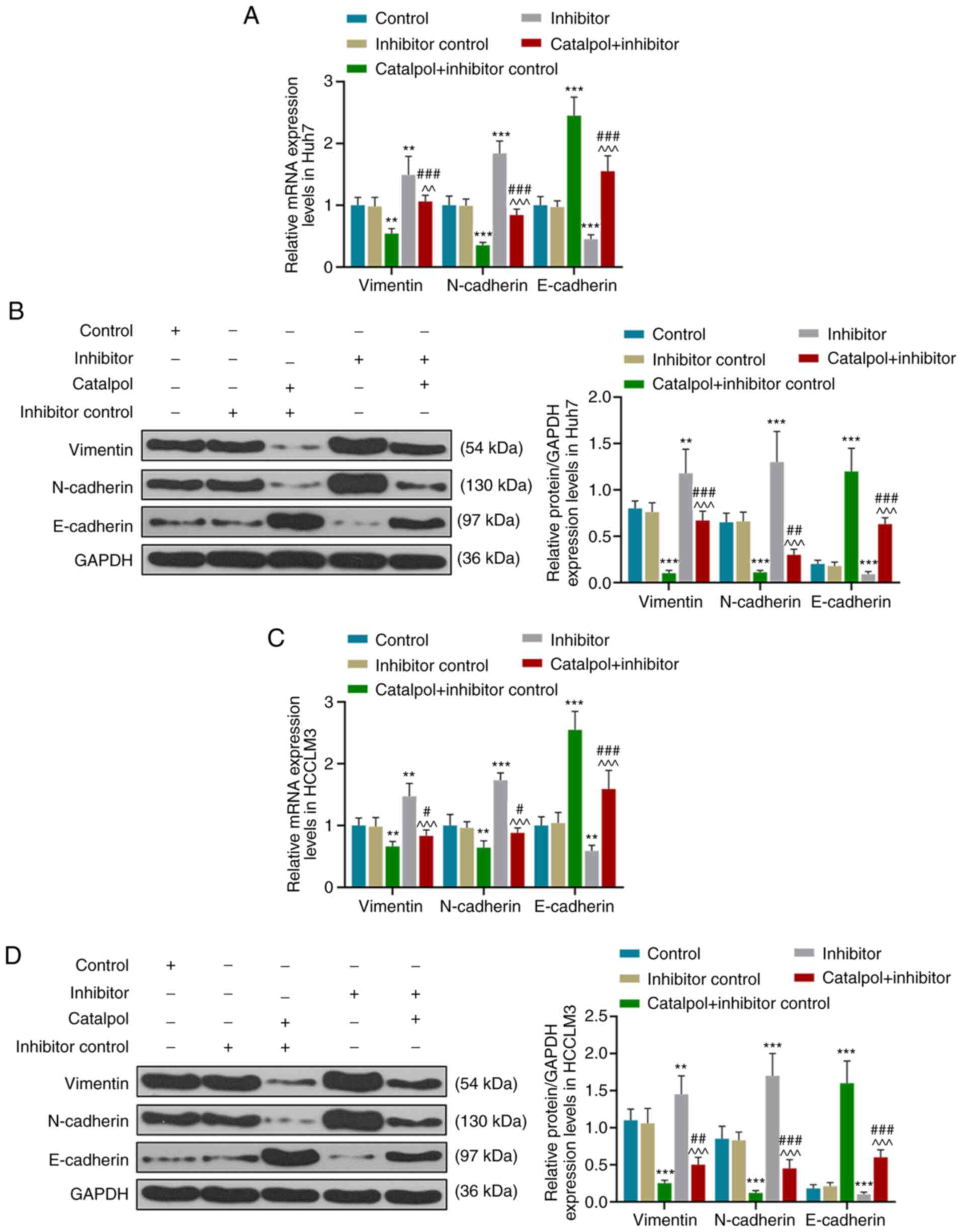
RT-qPCR and western blotting results demonstrated
that for Huh7 cells, transfection with inhibitor control and
treatment with catalpol decreased the expression levels of vimentin
and N-cadherin, and increased those of E-cadherin, compared with
the Inhibitor control group (P<0.001; Fig. 7A and B). However, compared with the
Inhibitor control group, expression levels of vimentin and
N-cadherin in the Inhibitor group were higher, and those of
E-cadherin were lower (P<0.001). The expression levels of
vimentin and N-cadherin in the Catalpol + Inhibitor group were
higher compared with those in the Catalpol + Inhibitor control
group but lower compared with those in the Inhibitor group
(P<0.001). Furthermore, E-cadherin expression levels were lower
compared with those in the Catalpol + Inhibitor control group but
higher compared with the Inhibitor group (P<0.001).
Similar effects on the expression levels of
vimentin, N-cadherin and E-cadherin of HCCLM3 cells were observed
(P<0.05; Fig. 7C and D).
Catalpol significantly inhibited vimentin and N-cadherin expression
levels, but increased E-cadherin expression in Huh7 and HCCLM3
cells, while the miR-140-5p inhibitor produced the opposite effects
and reversed the effects of catalpol on vimentin, N-cadherin and
E-cadherin expression levels. These results indicated that the
miR-140-5p inhibitor promoted EMT in Huh7 and HCCLM3 cells, and
catalpol reversed the promoting effect of miR-140-5p inhibitor on
EMT.
Discussion
HCC is the fourth most common cause of
cancer-related deaths worldwide (35), with a 5-year survival rate of 18%
(36), and thus, there is a need to
identify effective clinical treatment methods (37,38).
Catalpol exhibits pharmacological effects in a number of diseases,
such as ischemia/reperfusion (17)
and diabetes (39), via regulating
the NF-κB, PI3K/AKT and brain-derived neurotrophic factor pathways
(17,40–43).
The present study performed in vitro functional experiments,
and demonstrated that catalpol treatment significantly increased
the expression of miR-140-5p, and effectively inhibited EMT of HCC
cells, as well as cell migration and invasion.
HCC is characterized by insidious onset, rapid
progression, strong invasion and metastasis (44). Malignant tumors are characterized by
uncontrolled spontaneous growth, and their continued invasion and
proliferation are considered to be primary causes of metastasis
(45). In the present study,
catalpol treatment inhibited the proliferation, invasion and
migration of HCC cells, indicating that catalpol exhibited an
anticancer effect on HCC cells. This finding was consistent with
previous studies, which have reported that catalpol inhibits
proliferation of bladder cancer cells by inducing apoptosis via
blocking AKT-mediated anti-apoptotic pathway signaling (46), and that catalpol inhibits the
progression of colorectal cancer by inhibiting tumor angiogenesis
and decreasing the inflammatory response (19).
EMT serves a key role in tumor invasion and
metastasis (47,48). The decrease and loss of E-cadherin
expression is a significant hallmark in the occurrence of EMT in
cancer cells, whereas the presence of vimentin and N-cadherin
expression levels are indicative of enhanced cell migration and
invasion (49,50). The present study demonstrated that
catalpol treatment increased E-cadherin expression but decreased
vimentin and N-cadherin expression levels, and inhibited EMT of HCC
cells, thus inhibiting tumor metastasis. Morphological observation
further identified that catalpol treatment inhibited EMT in HCC. In
the current study, TGF-β1 was used to treat HCC cells; following
treatment, cell morphology was altered, as indicated by the change
in spindle shape, and cells lost their intercellular interactions.
However, catalpol significantly decreased the morphological changes
induced by TGF-β1, indicating that catalpol exhibited a strong
inhibitory effect on EMT. In line with these results, Wang et
al (51) reported that catalpol
inhibits cell proliferation via inhibiting EMT and promoting
apoptosis of osteosarcoma cells.
A number of miRNAs, such as miR-139-5p (52), miR-21 (53), miR-487a (54) and miR-140-5p (27), have been reported to affect the
proliferation and metastasis of HCC cells. In the present study,
in vitro functional tests were performed and it was observed
that decreasing miR-140-5p expression promoted cell migration,
invasion and occurrence of EMT. These results were consistent with
previous studies, which identified miR-140-5p as a tumor inhibitor
(27,55). Zhao et al (56) revealed that catalpol inhibited cell
proliferation, invasion and migration via regulating
miR-22-3p/metastasis associated 1 family member 3 signaling in HCC.
The present study also investigated the effects of catalpol on the
expression levels of miR-140-5p and found that catalpol treatment
significantly upregulated miR-140-5p expression. Therefore, it was
hypothesized that catalpol may serve an anticancer role via
mediating miR-140-5p expression. As miR-140-5p exhibits inhibitory
effects on cancer progression, knockdown of miR-140-5p expression
decreased the inhibitory effect of catalpol on HCC progression,
indicating that the anticancer effect of catalpol on HCC cells may
be achieved via increasing expression of miR-140-5p. However, there
are certain limitations in the present study; for example, further
investigation is required to determine whether miR-140-5p directly
or indirectly reverses the anticancer effects of catalpol, and to
identify the target gene for miR-140-5p. In addition, the results
of the study require verification via in vivo experiments.
Bioinformatic analysis of miR-140-5p targets followed by luciferase
reporter assays to test the interaction between catalpol and
miR-140 is also required.
In conclusion, the present study demonstrated that
catalpol at different concentrations exhibited anticancer effects,
which inhibited proliferation and migration of HCC cells, as well
as the EMT. Moreover, the present results suggested that catalpol
increased miR-140-5p expression, and that miR-140-5p knockdown
decreased the antitumor ability of catalpol. Thus, the present
study provides further understanding for novel approaches for the
treatment of HCC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Science and
Technology Development Project of Hangzhou (grant no.
20163501Y17).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LW and HL conceptualized and designed the study. SC,
XW, XC and FW collected, analyzed and interpreted the data. LW and
HL drafted and revised the manuscript. All authors agreed to be
accountable for all aspects of the work in ensuring that questions
related to the accuracy or integrity of the work are appropriately
investigated and resolved. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
HCC
|
hepatocellular carcinoma
|
|
CCK-8
|
Cell Counting Kit-8
|
|
RT-qPCR
|
reverse transcription-quantitative
PCR
|
References
|
1
|
Intaraprasong P, Siramolpiwat S and
Vilaichone RK: Advances in management of hepatocellular carcinoma.
Asian Pac J Cancer Prev. 17:3697–3703. 2016.PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sakamoto M: Pathology of early
hepatocellular carcinoma. Hepatol Res. 37 (Suppl 2):S135–S138.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wei PL, Huang CY and Chang YJ: Propyl
gallate inhibits hepatocellular carcinoma cell growth through the
induction of ROS and the activation of autophagy. PLoS One.
14:e02105132019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Deng GL, Zeng S and Shen H: Chemotherapy
and target therapy for hepatocellular carcinoma: New advances and
challenges. World J Hepatol. 7:787–798. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fang F, Yang L, Tao Y and Qin W: FBI-1
promotes cell proliferation and enhances resistance to chemotherapy
of hepatocellular carcinoma in vitro and in vivo. Cancer.
118:134–146. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Moriguchi M, Umemura A and Itoh Y: Current
status and future prospects of chemotherapy for advanced
hepatocellular carcinoma. Clin J Gastroenterol. 9:184–190. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Clark T, Maximin S, Meier J, Pokharel S
and Bhargava P: Hepatocellular carcinoma: Review of epidemiology,
screening, imaging diagnosis, response assessment, and treatment.
Curr Probl Diagn Radiol. 44:479–486. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu J: Trends in liver cancer mortality
among adults aged 25 and over in the United States, 2000–2016. NCHS
Data Brief. 1–8. 2018.
|
|
10
|
Liao CY, Lee CC, Tsai CC, Hsueh CW, Wang
CC, Chen IH, Tsai MK, Liu MY, Hsieh AT, Su KJ, et al: Novel
investigations of flavonoids as chemopreventive agents for
hepatocellular carcinoma. Biomed Res Int. 2015:8405422015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu C, Ma R, Wang L, Zhu R, Liu H, Guo Y,
Zhao B, Zhao S, Tang J, Li Y, et al: Rehmanniae radix in
osteoporosis: A review of traditional Chinese medicinal uses,
phytochemistry, pharmacokinetics and pharmacology. J
Ethnopharmacol. 198:351–362. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim SH, Yook TH and Kim JU: Rehmanniae
radix, an effective treatment for patients with various
inflammatory and metabolic diseases: Results from a review of
Korean publications. J Pharmacopuncture. 20:81–88. 2017.PubMed/NCBI
|
|
13
|
Lee B, Shim I, Lee H and Hahm DH:
Rehmannia glutinosa ameliorates scopolamine-induced learning and
memory impairment in rats. J Microbiol Biotechnol. 21:874–883.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yuan CX, Chu T, Liu L, Li HW, Wang YJ, Guo
AC and Fan YP: Catalpol induces oligodendrocyte precursor
cell-mediated remyelination in vitro. Am J Transl Res. 7:2474–2481.
2015.PubMed/NCBI
|
|
15
|
Liu YR, Lei RY, Wang CE, Zhang BA, Lu H,
Zhu HC and Zhang GB: Effects of catalpol on ATPase and amino acids
in gerbils with cerebral ischemia/reperfusion injury. Neurol Sci.
35:1229–1233. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li MY, Cheng XR, Zhou WX and Zhang YX: The
effects of catalpol on Alzheimer's disease: Research advances.
Journal of International Pharmaceutical Research. 43:199–204.
2016.
|
|
17
|
Zhu J, Chen X, Wang H and Yan Q: Catalpol
protects mice against renal ischemia/reperfusion injury via
suppressing PI3K/Akt-eNOS signaling and inflammation. Int J Clin
Exp Med. 8:2038–2044. 2015.PubMed/NCBI
|
|
18
|
Zhang X, Zhang A, Jiang B, Bao Y, Wang J
and An L: Further pharmacological evidence of the neuroprotective
effect of catalpol from rehmannia glutinosa. Phytomedicine.
15:484–490. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhu P, Wu Y, Yang A, Fu X, Mao M and Liu
Z: Catalpol suppressed proliferation, growth and invasion of CT26
colon cancer by inhibiting inflammation and tumor angiogenesis.
Biomed Pharmacother. 95:68–76. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu C, Wu F, Liu Y and Meng C: Catalpol
suppresses proliferation and facilitates apoptosis of MCF-7 breast
cancer cells through upregulating microRNA-146a and downregulating
matrix metalloproteinase-16 expression. Mol Med Rep. 12:7609–7614.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang ZH and Sheng HZ: Catalpol inhibits
migration and induces apoptosis in gastric cancer cells and in
athymic nude mice. Biomed Pharmacother. 103:1708–1719. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Adams BD, Kasinski AL and Slack FJ:
Aberrant regulation and function of microRNAs in cancer. Curr Biol.
24:R762–R776. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Seven M, Karatas OF, Duz MB and Ozen M:
The role of miRNAs in cancer: From pathogenesis to therapeutic
implications. Future Oncol. 10:1027–1048. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fang Z, Yin S, Sun R, Zhang S, Fu M, Wu Y,
Zhang T, Khaliq J and Li Y: miR-140-5p suppresses the
proliferation, migration and invasion of gastric cancer by
regulating YES1. Mol Cancer. 16:1392017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhai H, Fesler A, Ba Y, Wu S and Ju J:
Inhibition of colorectal cancer stem cell survival and invasive
potential by hsa-miR-140-5p mediated suppression of Smad2 and
autophagy. Oncotarget. 6:19735–19746. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lan H, Chen W, He G and Yang S: miR-140-5p
inhibits ovarian cancer growth partially by repression of PDGFRA.
Biomed Pharmacother. 75:117–122. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yan X, Zhu Z, Xu S, Yang LN, Liao XH,
Zheng M, Yang D, Wang J, Chen D, Wang L, et al: MicroRNA-140-5p
inhibits hepatocellular carcinoma by directly targeting the unique
isomerase Pin1 to block multiple cancer-driving pathways. Sci Rep.
7:459152017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li C, Zhou D, Hong H, Yang S, Zhang L, Li
S, Hu P, Ren H, Mei Z and Tang H: TGFβ1-miR-140-5p axis mediated
up-regulation of Flap endonuclease 1 promotes
epithelial-mesenchymal transition in hepatocellular carcinoma.
Aging (Albany NY). 11:5593–5612. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zou G, Zhong W, Wu F, Wang X and Liu L:
Catalpol attenuates cardiomyocyte apoptosis in diabetic
cardiomyopathy via Neat1/miR-140-5p/HDAC4 axis. Biochimie.
165:90–99. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Agarwal V, Bell GW, Nam JW and Bartel DP:
Predicting effective microRNA target sites in mammalian mRNAs.
ELife. 4:e050052015. View Article : Google Scholar
|
|
31
|
Rao X, Huang X, Zhou Z and Lin X: An
improvement of the 2^(-delta delta CT) method for quantitative
real-time polymerase chain reaction data analysis. Biostat
Bioinforma Biomath. 3:71–85. 2013.PubMed/NCBI
|
|
32
|
Mikaelian I, Malek M, Gadet R, Viallet J,
Garcia A, Girard-Gagnepain A, Hesling C, Gillet G, Gonzalo P,
Rimokh R and Billaud M: Genetic and pharmacologic inhibition of
mTORC1 promotes EMT by a TGF-β-independent mechanism. Cancer Res.
73:6621–6631. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gheldof A and Berx G: Cadherins and
epithelial-to-mesenchymal transition. Prog Mol Biol Transl Sci.
116:317–336. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yilmaz M and Christofori G: EMT, the
cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev.
28:15–33. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Villanueva A: Hepatocellular carcinoma. N
Engl J Med. 380:1450–1462. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jemal A, Ward EM, Johnson CJ, Cronin KA,
Ma J, Ryerson B, Mariotto A, Lake AJ, Wilson R, Sherman RL, et al:
Annual report to the nation on the status of cancer, 1975–2014,
featuring survival. J Natl Cancer Inst. 109:djx0302017. View Article : Google Scholar
|
|
37
|
Liang D, Xue H, Yu Y, Lv F, You W and
Zhang B: Elevated expression of UHRF1 predicts unfavorable
prognosis for patients with hepatocellular carcinoma. Int J Clin
Exp Pathol. 8:9416–9421. 2015.PubMed/NCBI
|
|
38
|
Reig M, da Fonseca LG and Faivre S: New
trials and results in systemic treatment of HCC. J Hepatol.
69:525–533. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bai Y, Zhu R, Tian Y, Li R, Chen B, Zhang
H, Xia B, Zhao D, Mo F, Zhang D and Gao S: Catalpol in diabetes and
its complications: A review of pharmacology, pharmacokinetics, and
safety. Molecules. 24:33022019. View Article : Google Scholar
|
|
40
|
Lu J, Wang Y, Zhao W, Li N, Li H, Lu J,
Zeng W, Bao S and Bai Y: Effects of catalpol, L-shikonin and
paeonol extracted from radix rehmanniae, radix arnebiae and cortex
moutan on KGF-induced HaCaT cell proliferation. Zhonghua Yi Xue Za
Zhi. 94:1265–1269. 2014.(In Chinese). PubMed/NCBI
|
|
41
|
Wang JH, Zou L, Wan D, Huifeng Z, Wang Y
and Qin L: Review of catalpol's pleiotropic signaling pathways.
Chin Pharmacol Bull. 31:1189–1194. 2015.
|
|
42
|
Wan D, Xue L, Zhu H and Luo Y: Catalpol
induces neuroprotection and prevents memory dysfunction through the
cholinergic system and BDNF. Evid Based Complement Alternat Med.
2013:1348522013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhou J, Xu G, Ma S, Li F, Yuan M, Xu H and
Huang K: Catalpol ameliorates high-fat diet-induced insulin
resistance and adipose tissue inflammation by suppressing the JNK
and NF-κB pathways. Biochem Biophys Res Commun. 467:853–858. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cai Z, Xu K, Li Y, Lv Y, Bao J and Qiao L:
Long noncoding RNA in liver cancer stem cells. Discov Med.
24:87–93. 2017.PubMed/NCBI
|
|
45
|
Yang JY, Li D, Zhang Y, Guan BX, Gao P,
Zhou XC and Zhou CJ: The expression of MCM7 is a useful biomarker
in the early diagnostic of gastric cancer. Pathol Oncol Res.
24:367–372. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Jin D, Cao M, Mu X, Yang G, Xue W, Huang Y
and Chen H: Catalpol inhibited the proliferation of T24 human
bladder cancer cells by inducing apoptosis through the blockade of
Akt-mediated anti-apoptotic signaling. Cell Biochem Biophys.
71:1349–1356. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Brabletz T: EMT and MET in metastasis:
Where are the cancer stem cells? Cancer Cell. 22:699–701. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Heerboth S, Housman G, Leary M, Longacre
M, Byler S, Lapinska K, Willbanks A and Sarkar S: EMT and tumor
metastasis. Clin Transl Med. 4:62015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
De Craene B and Berx G: Regulatory
networks defining EMT during cancer initiation and progression. Nat
Rev Cancer. 13:97–110. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Tiwari N, Gheldof A, Tatari M and
Christofori G: EMT as the ultimate survival mechanism of cancer
cells. Semin Cancer Biol. 22:194–207. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wang L and Xue GB: Catalpol suppresses
osteosarcoma cell proliferation through blocking
epithelial-mesenchymal transition (EMT) and inducing apoptosis.
Biochem Biophys Res Commun. 495:27–34. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wu J, Zhang T, Chen Y and Ha S: MiR-139-5p
influences hepatocellular carcinoma cell invasion and proliferation
capacities via decreasing SLITRK4 expression. Biosci Rep.
40:BSR201932952020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wang Y, Zhang P, Yuan M and Li X:
Overexpression of miRNA-21 promotes the proliferation and invasion
in hepatocellular carcinoma cells via suppressing SMAD7. Technol
Cancer Res Treat. 18:15330338198786862019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kong Q, Liang C, Jin Y, Pan Y, Tong D,
Kong Q and Zhou J: The lncRNA MIR4435-2HG is upregulated in
hepatocellular carcinoma and promotes cancer cell proliferation by
upregulating miRNA-487a. Cell Mol Biol Lett. 24:262019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lv J, Fan HX, Zhao XP, Lv P, Fan JY, Zhang
Y, Liu M and Tang H: Long non-coding RNA Unigene56159 promotes
epithelial-mesenchymal transition by acting as a ceRNA of
miR-140-5p in hepatocellular carcinoma cells. Cancer Lett.
382:166–175. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhao L, Wang Y and Liu Q: Catalpol
inhibits cell proliferation, invasion and migration through
regulating miR-22-3p/MTA3 signalling in hepatocellular carcinoma.
Exp Mol Pathol. 109:51–60. 2019. View Article : Google Scholar : PubMed/NCBI
|