Introduction
Allergic rhinitis (AR) is an allergic inflammatory
reaction of the nasal mucosa, and is a major health concern due to
its serious impact on quality of life and work efficiency (1). AR is a global public health disease,
affecting 10–40% of the population, particularly children (2). The emergence of AR symptoms is closely
associated with the infiltration and activation of eosinophils,
basophils, mast cells and CD4+ T helper (Th)2 cells, as
well as the local release of various inflammatory mediators, which
result in hyperresponsiveness, hypersecretion and remodeling of the
nasal mucosa (3).
MicroRNAs (miRNAs/miRs) are a class of
evolutionarily conserved endogenous non-coding RNAs, 18–25
nucleotides in length, which can negatively regulate gene
expression at the post-transcriptional level by binding to target
mRNA, in order to block mRNA translation or induce mRNA cleavage
(4). Furthermore, miRNAs are key
mediators of a variety of biological processes, including cell
cycle progression, proliferation, apoptosis, angiogenesis, tumor
progression and inflammation (4,5).
Previous studies have reported that miR-31 may have a vital role in
tumor immune response, autoimmune diseases and asthma (6,7).
Notably, van der Heide et al (8) demonstrated that miR-31 not only
promoted the transformation of CD4+ T cells to a Th1
phenotype, but also mediated the transcription of Th1 cytokines,
prevented excessive transcription of Th2 cytokines, and maintained
the Th1/Th2 balance. Recently, miR-31 has been reported to be
involved in the occurrence and development of ulcerative colitis
via regulating the expression of thymic stromal lymphopoietin
(TSLP), a representative Th2-polarizing cytokine (9). Therefore, it was hypothesized that
miR-31 might have therapeutic effects in the treatment of AR.
Previous studies have reported that upregulation of
miR-31 can reduce the expression of interleukin (IL)-13 receptor α1
chain (IL-13Rα1) and block IL-13-dependent phosphorylation of
signal transducer and activator of transcription 6 (STAT6) in gut
epithelium cell lines (10,11). As a typical Th2 cytokine, IL-13 was
shown to be a key mediator of immunoglobulin E (IgE)-mediated
allergic diseases via activating IL-13Rα1 and promoting STAT6
phosphorylation (12). Furthermore,
Ramalingam et al (13)
reported that IL-13Rα1-deficient mice failed to develop
allergen-induced airway hyperresponsiveness and mucus
hypersecretion.
Based on the aforementioned findings, it was
hypothesized that miR-31 could regulate AR by suppressing
IL-13-induced nasal epithelial inflammatory responses. To the best
of our knowledge, no previous study has reported the relationship
between AR and miR-31, and whether miR-31 regulates AR progression
remains unclear. The present study established a mouse model of AR.
Subsequently, AR mice were treated with a miR-31 agomir and a
series of in vitro experiments were performed to verify the
hypothesis. The present findings suggested that miR-31 could serve
as a novel therapeutic target for the treatment of AR. To the best
of our knowledge, the present study was the first to identify the
role of miR-31 in AR and to reveal the effects of miR-31 on human
nasal epithelial cells (NECs). Notably, miR-31 may have similar
effects on chronic inflammatory diseases of the airway, such as
chronic rhinosinusitis, asthma and chronic obstructive pulmonary
disease.
Materials and methods
Collection of human nasal epithelial
samples
Nasal epithelial samples were collected from 10
patients with AR (age range, 18–60 years; mean age, 33.9 years) and
10 healthy subjects (age range, 20–58 years; mean age, 35.1 years)
for reverse transcription-quantitative PCR (RT-qPCR). Patients were
recruited at the Renmin Hospital of Wuhan University (Wuhan, China)
between May 2019 and July 2019. All research subjects provided
written informed consent. AR was mainly triggered by common nasal
allergy triggers, such as dust mites, pollen and pet dander. AR
diagnosis and treatment were carried out by physicians. Briefly, AR
was diagnosed based on: i) Whether the patients had AR symptoms for
>3 years; ii) nasal endoscopic examinations demonstrating pale
and edematous nasal mucosa and watery nasal discharge; and iii)
whether the patients had a positive response to the allergen test
after serum antigen-specific IgE measurements (14). The exclusion criteria included acute
or chronic nasal infection, and other severe systemic diseases.
Patients were experiencing an allergic episode at the time of
recruitment and had come to the hospital for medical assistance.
Notably, no patient included in the present study had been treated
with specific immunotherapy, or received antibiotics, steroids,
antihistamines or immune drugs in the 4 weeks prior to recruitment
(15,16). The epithelial samples were gently
scraped from the surface of the inferior nasal turbinate using a
plastic curette and were quickly stored at −80°C. The study
protocol complied with the Declaration of Helsinki and was approved
by the Ethical Committee of Renmin Hospital of Wuhan University
(approval no. WDRY2018-K020).
Animals
A total of 40 clean specific pathogen-free grade
female C57BL/6 mice (weight, 20–25 g; age, 6–8 weeks) were
purchased from Beijing Weitong Lihua Experimental Animal Technology
Co., Ltd. [license no. SCXK (J) 2016-0006]. The mice were housed in
the Animal Experiment Center of the Renmin Hospital of Wuhan
University [license no. SYXK (E) 2015-0027] at room temperature
(18-22°C) with a 12/12 h light/dark cycle and moderate humidity
(50–60%). The mice were reared in microisolator cages, and received
free access to food and water. All mice were acclimated for a
minimum of 1 week before experimentation. All protocols for animal
care and handling were approved by the Institutional Animal Care
and Use Committee of Renmin Hospital of Wuhan University (license
no. WDRM-20190607).
AR model and intranasal administration
of miR-31
The AR murine model was established as previously
described (17) with minor
modifications (the mice were intranasally challenged on days
21–28). The miR-31 agomir was purchased from Guangzhou RiboBio Co.,
Ltd. Briefly, female mice were randomly divided into four
experimental groups: Control, AR, miR-31 agomir (miR-31) and miR
negative control (miR-NC) groups (n=10 mice/group) (18,19).
In the AR group, mice were sensitized by intraperitoneal injection
of 100 μg ovalbumin (OVA, grade V; Sigma-Aldrich; Merck KGaA) and 5
mg Al(OH)3 (Sigma-Aldrich; Merck KGaA) in 100 µl normal
saline on days 0, 7 and 14, and were then challenged daily with OVA
solution (100 mg/ml in normal saline; 20 µl/mouse) into the
nostrils on days 21–28. In the control group, mice were injected
with the same dose of normal saline plus Al(OH)3 without
OVA, and were then challenged with normal saline on days 21–28. In
the miR-31 and miR-NC groups, mice were sensitized by
intraperitoneal injection of 100 µg OVA and 5 mg Al(OH)3
in 100 µl normal saline on days 0, 7 and 14, followed by daily
intranasal administration of miR-NC or miR-31 agomir (25
µg/nostril) 30 min before OVA challenge on days 21–28. A total of
24 h after the last challenge, all mice were sacrificed. The nasal
mucosal tissues were collected for histological analysis,
immunohistochemistry and RT-qPCR. Blood samples were collected from
the angular vein of the mice at the time of death. The serum was
harvested from blood samples by centrifugation at 4°C for 20 min at
600 × g, and was stored at −80°C for ELISA.
Evaluation of allergic symptoms
Within 10 min after the last OVA challenge, the
frequency of nasal rubbing episodes and sneezing was counted for
each mouse to quantitatively evaluate the symptoms of AR. If mice
scratched their nose once it was defined as a single rub. To assure
accurate counting of sneezes and incidences of rubbing, each mouse
was observed for 10 min and its symptoms were recorded by three
observers blinded to the experimental groups. The mean of the
observations was used as the final result.
Histological analysis
The nasal mucosal tissues were removed from mice and
fixed in 4% paraformaldehyde for 24 h at room temperature. Tissue
samples were then dehydrated, embedded in paraffin and cut into 4–5
µm sections. The sections were stained with hematoxylin and eosin
(H&E) and periodic acid-Schiff (PAS) for 14 days at 26°C. The
total numbers of eosinophils and goblet cells were counted in four
randomly selected fields under a light microscope at ×400
magnification.
Immunohistochemistry
Paraffin-embedded sections were dewaxed and
endogenous peroxidase activity was blocked with 3%
H2O2 for 15 min at room temperature. Sections
were then incubated with rabbit polyclonal antibodies against
IL-13Rα1 (1:1,000; cat. no. AF9095; Affinity Biosciences), STAT6
(1:1,000; cat. no. 5397; Cell Signaling Technology, Inc.) and
phosphorylated (p)-STAT6 (1:1,000; cat. no. 56554; Cell Signaling
Technology, Inc.) overnight at 4°C, washed, incubated with goat
anti-rabbit secondary antibody (1:2,000; cat. no. SA00001-2;
ProteinTech Group, Inc.) for 30 min at room temperature, and washed
again. Subsequently, samples were treated with 3,3-diaminobenzidine
solution for 5 min at room temperature and counterstained with
hematoxylin for 30 sec at room temperature. Positive
immunoreactivity was detected by the presence of brown staining.
The treated specimens were observed and analyzed under a light
microscope (Olympus Corporation). Semi-quantitative analysis was
performed based on the staining intensity as follows: i) 0,
colorless; ii) 1, pale yellow; iii) 2, brownish yellow; and iv) 3,
dark brown.
Cell culture and treatment
Human NECs were purchased from Beina Biological
Company (cat. no. BNCC340481) and cultured in Dulbecco's modified
Eagle's medium supplemented with 10% fetal bovine serum (both
Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin/streptomycin at 37°C in a humidified incubator
containing 5% CO2. NECs were divided into four
experimental groups: NECs, NECs + IL-13, NECs-miR-31 + IL-13 and
NECs-miR-NC + IL-13 groups. The cells were cultured in medium at
70–80% density. miR-31 mimic and miR-NC were purchased from
Guangzhou RiboBio Co., Ltd. miR-31 mimic (50 nmol/l; sense,
5′-AGGCAAGAUGCUGGCAUAGCUG-3′ and antisense,
3′-UCCGUUCUACCGUAUCGAC-5′) and miR-NC oligonucleotides (50 nmol/l;
sense, 5′-UUUGUACUACACAAAAGUACUG-3′ and antisense,
3′-AAACAUGAUGUGUUUUCAUGAC-5′) were transfected into NECs using
riboFECT™ CP Transfection kit (Guangzhou RiboBio Co., Ltd.) for 48
h at 37°C. After transfection, NECs were stimulated with IL-13 (50
ng/ml; cat. no. 200–13; PeproTech, Inc.) for 24 h at 37°C. The
supernatants were then collected for ELISA, and cell pellets for
western blot analysis.
RT-qPCR
Total RNA (including miRNAs) was extracted from the
isolated human/mouse nasal mucosal samples and human NECs using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol. A total of 1 µg
RNA was reverse transcribed into cDNA using SuperScript III Reverse
Transcriptase (Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C
for 15 min and at 85°C for 5 sec. The synthesized cDNA was then
amplified using Hieff® qPCR SYBR-Green Master Mix (No
Rox; Shanghai Yeasen Biotechnology Co., Ltd.) on a CFX96 Touch
Real-Time Detection system (Bio-Rad Laboratories, Inc.). The
thermocycling conditions were as follows: 95°C for 10 min, followed
by 40 cycles at 95°C for 30 sec and 60°C for 1 min. U6 was used as
the endogenous reference for miR-31. All primers were synthesized
by Invitrogen; Thermo Fisher Scientific, Inc. For human samples,
the primers used were as follows (5′ to 3′): miR-31, forward
CTAGGCAAGATGCTGGCATAG, reverse, CTC AACTGGTGTCGTGGAGTC; and U6,
forward, CTCGCTTC GGCAGCACAT and reverse, AACGCTTCACGAATTTG CGT.
For mouse samples, the primers used were as follows (5′ to 3′):
miR-31, forward, CTCGGATCCTGTGCATAACTG CCTTCA, reverse,
CACAAGCTTGAAGTCAGGGCGAG ACAGAC; and U6, forward, CTCGCTTCGGCAGCACA
and reverse, AACGCTTCACGAATTTGCGT. Each assay was run in triplicate
and relative gene expression was calculated using the
2−ΔΔCq method (18).
ELISA
The concentrations of total IgE, interferon (IFN)-γ
(cat. no. DIF50C), IL-12 (cat. no. SM1270), IL-4 (cat. no. M4000B),
IL-5 (cat. no. M5000) and IL-13 (cat. no. M1300CB) in mouse serum
were determined using mouse ELISA kits (all purchased from R&D
Systems, Inc.). The levels of granulocyte-macrophage
colony-stimulating factor (GM-CSF; cat. no. DGM00; R&D Systems,
Inc.), eotaxin (cat. no. DTX00; R&D Systems, Inc.) and mucin
5AC [MUC5AC; cat. no. ELK2098; ELK (Wuhan) Biotechnology Co., Ltd.]
in the culture supernatants were measured using human ELISA kits.
ELISA kits were used according to the manufacturer's
instructions.
Western blotting
NECs were homogenized and lysed in RIPA lysis buffer
(Sigma-Aldrich; Merck KGaA) with protease and phosphatase
inhibitors. Protein concentration was determined using BCA reagent
(Beyotime Institute of Biotechnology). Protein samples (40 µg) were
separated by SDS-PAGE on 12% gel, and subsequently transferred onto
polyvinylidene difluoride membranes. The membranes were then
blocked in 5% low-fat milk for 1 h at room temperature, following
which the membranes were incubated with primary antibodies at 4°C
overnight, followed by incubation with a goat anti-rabbit secondary
antibody (1:10,000; cat. no. 926-32211; LI-COR Biosciences) at room
temperature for 2 h. The membranes were washed with TBS with 0.1%
Tween-20 and scanned using an Odyssey infrared scanning device
(LI-COR Biosciences). The Odyssey® CLx Imaging System
(LI-COR Biosciences) was used to detect the expression level of
each protein. Rabbit anti-mouse TSLP (1:1,000), STAT6 (1:1,000),
p-STAT6 (1:1,000) and GAPDH (1:1,000) antibodies were purchased
from Cell Signaling Technology, Inc. Rabbit anti-mouse IL-13Rα1
(1:1,000) antibodies was purchased from Affinity Biosciences. Band
intensities (gray values) were measured with ImageJ 1.52 (National
Institutes of Health), and GAPDH was used as the internal
reference.
Statistical analysis
All data analyses were performed with SPSS 23.0
software (IBM Corp.) and GraphPad Prism 6.0 software (GraphPad
Software, Inc.). Descriptive analysis, normality test and
homogeneity test of variance were performed for all data sets. The
Kolmogorov-Smirnov test and Bartlett test were used to test
normality and homogeneity of variance, respectively. Data with
normal distribution were presented as mean ± standard deviation.
Multiple groups were compared using one-way analysis of variance
followed by Tukey's post hoc test, and two groups were compared
using the Student's t-test. Data in Fig. 4 are presented as the median (IQR),
and differences among groups were compared using Kruskal-Wallis
followed by a Dunn's post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
 | Figure 4.Expression of IL-13Rα1, p-STAT6 and
STAT6 in nasal mucosal tissues. IL-13Rα1, p-STAT6 and STAT6
expression was detected by immunohistochemical staining. Nasal
mucosal tissues from different groups were subjected to single
staining using IL-13Rα1, p-STAT6 and STAT6 antibodies. (A)
Immunohistochemical staining image (original magnification, ×400).
(B-D) Immunohistochemical staining scores. (E) Relative expression
level of p-STAT6/STAT6. All data are presented as the median (IQR)
(n=6/group). Differences among groups were compared using
Kruskal-Wallis followed by a Dunn's post hoc test. *P<0.05. AR,
allergic rhinitis; miR-31, microRNA-31; NC, negative control; Ctrl,
control; IL-13Rα1, interleukin-13 receptor α1 chain; STAT6, signal
transducer and activator of transcription 6; p-STAT6,
phosphorylated-STAT6. |
Results
miR-31 is significantly downregulated
in nasal mucosa from patients with AR and AR mice
As shown in Fig. 1A,
the expression levels of miR-31 in nasal mucosa were significantly
downregulated in patients with AR compared with those in healthy
individuals. In addition, the expression levels of miR-31 were also
significantly downregulated in the in the nasal mucosa of AR mice
compared with those in control mice, thus confirming the finding
that miR-31 expression was markedly downregulated under AR
conditions (Fig. 1B).
miR-31 alleviates allergic symptoms,
and reduces nasal eosinophil infiltration and goblet cell
hyperplasia in mice
The present study assessed the effect of intranasal
administration of miR-31 on AR symptoms in mice (Fig. 2A). To confirm whether exogenous
miR-31 entered mouse nasal mucosal tissues, the expression levels
of miR-31 were detected in nasal mucosal tissues. The results
revealed that intranasal administration with miR-31 agomir
significantly upregulated the expression levels of miR-31 in nasal
mucosal tissues. Intranasal administration with miR-NC had no
influence on the expression of miR-31 (Fig. 2B). As shown in Fig. 2C and D, the number of sneezes and
nose rubbing events were significantly increased in OVA-induced
mice compared with those in control mice. In addition, in the AR
and miR-NC groups, both sneezing and nasal rubbings were observed
more frequently than those in the miR-31 group. Therefore, these
data demonstrated that intranasal administration of miR-31
significantly alleviated allergic symptoms in AR mice.
The present study also analyzed the pathological
changes in the nasal mucosal tissues of mice by histological
analysis. OVA-induced mice exhibited histopathological features of
AR, including cilia shedding, numerous eosinophils, goblet cell
hyperplasia and monocyte recruitment in the nasal mucosa. The
number of eosinophils and goblet cells were markedly higher in
OVA-induced mice compared with those in control mice. By contrast,
miR-31 agomir-treated mice displayed fewer eosinophils and goblet
cells than mice in the AR and miR-NC groups. No significant
differences in AR histopathological features were observed between
the AR and miR-NC groups (Fig. 3).
These findings suggested that miR-31 agomir exhibited
anti-inflammatory activity to reduce eosinophil infiltration and
goblet cell hyperplasia in the nasal mucosa.
miR-31 inhibited IL-13Rα1 expression
and STAT6 phosphorylation in vivo
The present study evaluated the protein expression
levels of IL-13Rα1, p-STAT6 and STAT6 in nasal mucosal tissues by
immunohistochemical analysis. The results revealed that
overexpression of miR-31 inhibited the expression of IL-13Rα1 and
phosphorylation of STAT6 in vivo (Fig. 4).
miR-31 decreases serum levels of total
IgE in AR mice
To investigate the effect of miR-31 on total IgE
levels, the levels of serum total IgE were measured by ELISA. As
shown in Fig. 5A, total IgE levels
were markedly higher in OVA-induced mice compared with those in
control mice. Following miR-31 treatment, OVA-induced mice
exhibited significantly reduced levels of total IgE. These data
demonstrated that miR-31 may regulate the levels of total IgE in AR
mice.
 | Figure 5.Effects of miR-31 on the production
of OVA-specific IgE and inflammatory cytokines. Overexpression of
miR-31 affected the secretion of (A) OVA-specific IgE, and the
inflammatory cytokines, (B) IL-4, (C) IL-5, (D) IL-13, (E) IFN-γ
and (F) IL-12, in the serum of AR mice. ELISA was performed to
detect the protein concentrations of these cytokines. Data are
presented as the mean ± SD of 10 mice in each group. *P<0.05.
AR, allergic rhinitis; miR-31, microRNA-31; NC, negative control;
Ctrl, control; IL, interleukin; IgE, immunoglobulin E; IFN-γ,
interferon-γ; OVA, ovalbumin. |
miR-31 decreases serum cytokine
concentrations in AR mice
To assess the effect of miR-31 on AR-associated
inflammation, the serum levels of several inflammatory cytokines
were determined by ELISA. As shown in Fig. 5B-D, the levels of the Th2 cytokines,
IL-4, IL-5 and IL-13, were significantly increased in AR mice
compared with those in control mice, and administration of
exogenous miR-31 significantly decreased their levels. Conversely,
the reduced levels of the Th1 cytokines IFN-γ and IL-12 in AR mice
were partially corrected following miR-31 agomir treatment
(Fig. 5E and F). These results
suggested that miR-31 may exert an anti-AR role via regulating the
secretion of inflammatory cytokines.
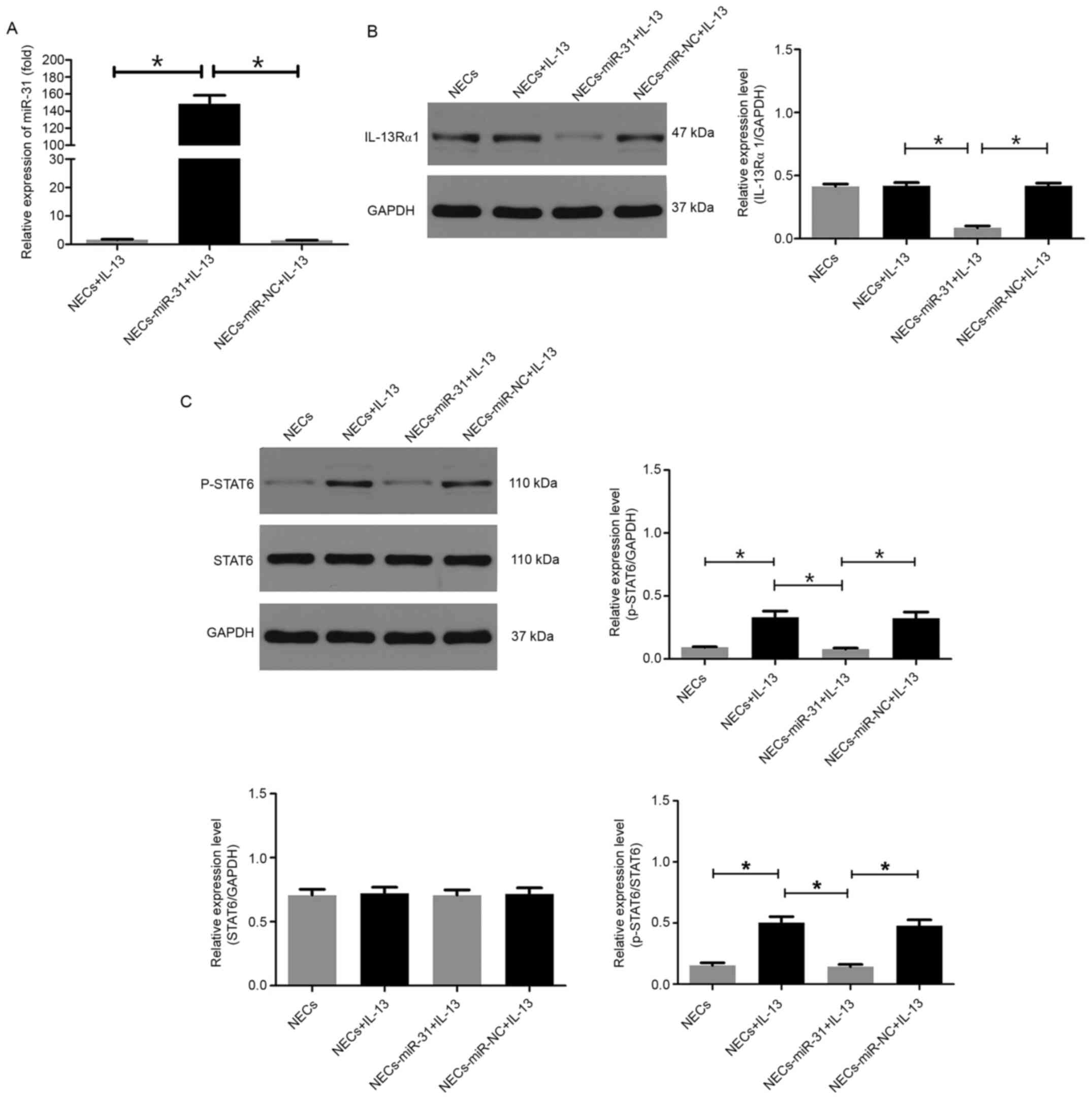
miR-31 inhibits IL-13Rα1 expression
and STAT6 phosphorylation in human NECs
Gain-of-function experiments were performed in human
NECs using miR-31 mimics. To confirm whether exogenous miR-31
entered human NECs, the mRNA expression levels of miR-31 were
detected in each experimental group. The results revealed that
transfection with miR-31 mimics significantly upregulated the
expression of miR-31 in NECs (Fig.
6A). To confirm whether miR-31 can inhibit IL-13Rα1 expression
and STAT6 phosphorylation in human NECs, western blotting was
performed on protein samples prepared from NECs in each treatment
group. The results demonstrated that overexpression of miR-31
significantly suppressed IL-13Rα1 expression (Fig. 6B) and had an inhibitory effect on
STAT6 phosphorylation (Fig.
6C).
miR-31 suppresses IL-13-induced
expression of TSLP, GM-CSF, eotaxin and MUC5AC in human NECs
To confirm whether miR-31 regulates the synthesis of
inflammatory cytokines and MUC5AC protein in IL-13-stimulated NECs,
TSLP protein expression levels were measured in the cell pellets,
and GM-CSF, eotaxin and MUC5AC levels were measured in the culture
supernatants of each group. The results demonstrated that the
protein expression levels of TSLP, and the levels of GM-CSF,
eotaxin and MUC5AC were significantly increased in NECs following
IL-13 stimulation for 24 h, but were significantly decreased in
miR-31-overexpressing NECs compared with those in the cells treated
with IL-13 only. In addition, no obvious differences were observed
between the IL-13-stimulated and miR-NC groups (Fig. 7).
 | Figure 7.Effects of miR-31 overexpression on
the production of inflammatory cytokines and MUC5AC protein in
IL-13-stimulated NECs. (A) Protein expression levels of TSLP were
measured by western blotting (n=3). GAPDH was used as the internal
control. The protein levels of (B) GM-CSF, (C) eotaxin and (D)
MUC5AC were measured by ELISA (n=10). miR-31 overexpression
suppressed IL-13-induced expression of TSLP, GM-CSF, eotaxin and
MUC5AC in human NECs. All data are presented as the mean ± SD.
*P<0.05. NECs, nasal epithelial cells; miR-31, microRNA-31; NC,
negative control; IL-13, interleukin-13; TSLP, thymic stromal
lymphopoietin; GM-CSF, granulocyte-macrophage colony-stimulating
factor; MUC5A, mucin 5AC. |
Discussion
AR is a chronic inflammatory disease of the nasal
mucosa involving a variety of immune cells, cytokines and
inflammatory mediators. Routine AR treatments include
antihistamines, antileukotrienes agents and glucocorticoid-based
drugs, which only provide short-term relief from AR symptoms
(19,20). In addition, some patients do not
respond to these pharmacotherapies. Therefore, it is important to
improve understanding of the pathogenesis of AR and to develop
improved treatment strategies. Increasing evidence has suggested
that AR development is a genetic manipulation process, and miRNAs
have been shown to act as key regulators in allergic diseases
(21). As suppressors of gene
expression at the post-transcriptional level, miRNAs have been
reported to serve a vital role in the development and activity of
innate and adaptive immune systems, which are involved in
inflammation, and hence are considered promising diagnostic and
prognostic biomarkers and treatment targets (22).
A previous study reported that miR-31 may have an
important role in various inflammation-related disorders, such as
psoriasis, systemic lupus erythematosus, inflammatory bowel disease
and autoimmune encephalomyelitis (23). miR-31 was previously revealed to be
overexpressed in the keratinocytes of patients with psoriasis, and
was shown to increase the expression of cytokines and chemokines,
leading to chronic inflammation (24). Moreover, miR-31 has been reported to
regulate the function of T cells in patients with systemic lupus
erythematosus (7). Whiteoak et
al (9) demonstrated that TSLP
served a role in promoting mucosal healing and regulating
inflammation in ulcerative colitis, whereas miR-31 could inhibit
this effect. However, little is known about the role of miR-31 in
AR.
The present study demonstrated that the expression
levels of miR-31 were significantly lower in the nasal mucosa of
patients with AR and AR mice compared with those in the control
groups, thus suggesting that miR-31 negatively regulated
pro-allergic properties in AR. The results of in vivo
experiments revealed that treatment of mice with a miR-31 agomir
significantly alleviated allergic symptoms, such as sneezing and
nasal rubbing, suggesting its potential in AR treatment. It has
been reported that IgE-mediated immune responses, inflammatory cell
disorders and Th1/Th2 imbalance-induced abnormal cytokine
production are closely related to the occurrence of AR, and there
is a critical link between them with regards to AR pathogenesis
(25). The present results also
demonstrated that overexpression of miR-31 effectively ameliorated
AR pathology through inhibition of local inflammatory responses,
including eosinophil infiltration and goblet cell hyperplasia. In
addition, consistent with previous findings, serum OVA-specific IgE
concentration was decreased, and the secretion of anti-inflammatory
cytokines (IFN-γ and IL-12) mainly produced by Th1 cells
(correcting the Th1/Th2 imbalance) were increased following
intranasal administration of miR-31 (19). As the nose has an abundant supply of
blood and good lymphatic circulation (26), nasal administration was used in the
present study for quick absorption into systemic circulation. The
systemic immune change may be caused by blood circulation or
lymphatic circulation after the intranasal administration of the
miRNA; however, the mechanism needs to be further studied. Taken
together, these findings suggested that miR-31 may have a
therapeutic effect on an experimental AR model.
Previous studies have demonstrated that NECs are not
only effector cells in AR, but also have an important role in the
development of airway allergic inflammation, and are involved in
the production of GM-CSF and polarization of allergen-driven Th2
lymphocytes (27,28). In previous studies, NECs have been
shown to be a rich source of proinflammatory cytokines, such as
tumor necrosis factor-α, eotaxin, GM-CSF, IL-6 and IL-8, which
serve a central role in airway host defense, asthma and AR
(29,30). IL-13 is a typical Th2-related
cytokine that promotes airway hyperresponsiveness, inflammation,
mucus production and fibrosis, and participates in allergic
reactions in each stage of attack (16). Recent studies have shown that IL-13
and IL-13-induced signaling pathways in AR are closely associated
with increased mucus production and secretion of inflammatory
mediators in airway epithelial cells (16,31).
Furthermore, it has been demonstrated that IL-13 may induce
pathological responses in airway allergic diseases, such as
eosinophil recruitment, mucus cell metaplasia, subepithelial
fibrosis and smooth muscle hypertrophy (30,32).
Hence, based on these findings, IL-13-treated NECs were used to
induce an AR-like phenotype in vitro.
A previous study revealed that miR-31 has an
important role in limiting IL-13 signaling in ulcerative colitis
(11). Therefore, blocking the
IL-13 signaling pathway using miR-31 mimics may be a beneficial
approach for controlling the pathological process of allergic
airway diseases. IL-13 signaling is initiated by binding with
IL-13Rα1, which forms a receptor complex with IL-4Rα and mediates
signal transduction through eliciting phosphorylation of STAT6
(33,34). In addition, IL-13Rα1 is a critical
signaling molecule and is essential for the development of
allergen-induced airway inflammation (13). STAT6, an important signaling
molecule for IL-13-induced inflammatory response, is usually
expressed in airway epithelial cells and has an important role in
the development of allergic airway inflammation (35). It has also been reported that
activation of STAT6 is critical for IL-13-induced TSLP, eotaxin and
MUC5AC expression in epithelial cells during OVA-induced airway
inflammation (36,37). Therefore, the present study
performed western blotting to detect the protein expression levels
of IL-13Rα1 and p-STAT6. Transfection with miR-31 mimics
significantly decreased IL-13Rα1 expression and STAT6
phosphorylation compared with in cells transfected with miR-NC,
suggesting that miR-31 may inhibit IL-13 signaling in NECs. To the
best of our knowledge, this is the first study reporting the
effects of miR-31 on human NECs.
TSLP, an epithelium-derived cytokine, is regarded as
a novel pro-allergic molecule. TSLP can strongly activate dendritic
cells through interaction with the TSLP receptor to induce an
inflammatory Th2-type response, which is essential for initiating
allergic inflammation (38). It has
been demonstrated that IL-13 can induce TSLP production in the
nasal epithelium in a STAT6-dependent manner, which is critical for
the development of IL-13-driven allergic inflammation in the nasal
mucosa (37). As pro-inflammatory
cytokines in allergic airway inflammation, GM-CSF and eotaxin are
synthesized and released by airway epithelial cells, infiltrating
leukocytes and fibroblasts in response to allergens and
inflammatory mediators (39,40).
GM-CSF can stimulate the differentiation of hematopoietic stem
cells into granulocytes and monocytes, and support eosinophil
survival (41). Eotaxins are a
family of CC chemokines which recruit immune cells, such as Th2
cells, eosinophils, mast cells and basophils to inflammatory sites
in allergic diseases (31).
Additionally, MUC5AC is a glycoprotein belonging to the superfamily
of mucins. Mucus hypersecretion is a common feature of AR (42). The present results revealed that
miR-31 overexpression significantly decreased the protein
expression levels of TSLP in NECs, and the levels of GM-CSF,
eotaxin and MUC5AC secreted by NECs following IL-13 stimulation.
These data suggested that the anti-inflammatory effect of miR-31 on
AR may be mediated by suppressing inflammatory responses in the
nasal epithelium via inhibiting IL-13-induced inflammatory cytokine
and mucus production.
However, this study has several limitations.
Firstly, although scraping and collection of human nasal epithelial
cells is not particularly invasive, this action often makes
patients uncomfortable, thus limiting the clinical sample size of
the present study. In future studies, more patients and volunteers
will be recruited. Secondly, the dosage of miR-31 agomir was chosen
based a previous study protocol (43). In future, the optimal dosage of
miR-31 agomir should be tested to determine the most effective
dosage. Thirdly, although IL-13-induced inflammatory response in
the nasal epithelium is a crucial part of AR pathology, other
pathways may be involved in miR-31-mediated effects in AR, which
requires further investigation.
In conclusion, the present study demonstrated the
suppressive effects of intranasal administration of miR-31 on
allergic airway responses in an OVA-induced mouse AR model. The
results demonstrated that miR-31 could alleviate allergic symptoms
and suppress IL-13 signaling in nasal mucosal. Furthermore, these
data indicated that miR-31 might exert its anti-inflammatory
effects on AR through inhibiting IL-13-induced inflammatory
cytokine and mucus production in NECs. Hence, miR-31 may serve as a
potential therapeutic target for AR.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural
Science Foundation of China (grant nos. 81371070 and 81770986) and
the Wuhan Science and Technology Bureau Scientific Research Project
(grant no. 2016060101010037)
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FZ conceived and designed the current study,
performed the experiments and wrote the manuscript. PL and HL
analyzed the data. WC and ZG contributed to study design and
manuscript preparation. YX conceived the study and critically
reviewed the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from all the
patients and the research protocols were approved by the Ethics
Committee of Renmin Hospital of Wuhan University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Brożek JL, Bousquet J, Agache I, Agarwal
A, Bachert C, Bosnic-Anticevich S, Brignardello-Petersen R,
Canonica GW, Casale T, Chavannes NH, et al: Allergic rhinitis and
its impact on asthma (ARIA) guidelines-2016 revision. J Allergy
Clin Immunol. 140:950–958. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Katelaris CH, Lee BW, Potter PC, Maspero
JF, Cingi C, Lopatin A, Saffer M, Xu G and Walters RD: Prevalence
and diversity of allergic rhinitis in regions of the world beyond
Europe and North America. Clin Exp Allergy. 42:186–207. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Scadding G: Cytokine profiles in allergic
rhinitis. Curr Allergy Asthma Rep. 14:4352014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Almeida MI, Reis RM and Calin GA: MicroRNA
history: Discovery, recent applications, and next frontiers. Mutat
Res. 717:1–8. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li L, Hui Y and Xing C: MicroRNA-31
affects the expression of asthma-related cytokines via regulation
of CD44. Int J Clin Exp Med. 9:112016.
|
|
7
|
Fan W, Liang D, Tang Y, Qu B, Cui H, Luo
X, Huang X, Chen S, Higgs BW, Jallal B, et al: Identification of
microRNA-31 as a novel regulator contributing to impaired
interleukin-2 production in T cells from patients with systemic
lupus erythematosus. Arthritis Rheum. 64:3715–3725. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
van der Heide V, Möhnle P, Rink J, Briegel
J and Kreth S: Down-regulation of MicroRNA-31 in CD4+ T
cells contributes to immunosuppression in human sepsis by promoting
TH2 skewing. Anesthesiology. 124:908–922. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Whiteoak SR, Claridge A, Balendran CA,
Harris RJ, Gwiggner M, Bondanese VP, Erlandsson F, Hansen MB,
Cummings JR and Sanchez-Elsner T: MicroRNA-31 targets thymic
stromal lymphopoietin in mucosal infiltrated CD4+ T
cells: A Role in Achieving Mucosal Healing in Ulcerative Colitis?
Inflamm Bowel Dis. 24:2377–2385. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gwiggner M, Claridge A, Cummings F and
Sanchez-Elsner T: OC-162 the role of micrornas miR-31 and miR-155
in the deregulation of the IL-13 pathway in ulcerative colitis.
Gut. 61 (Suppl 2):A69–A70. 2012. View Article : Google Scholar
|
|
11
|
Gwiggner M, Martinez-Nunez RT, Whiteoak
SR, Bondanese VP, Claridge A, Collins JE, Cummings JR and
Sanchez-Elsner T: MicroRNA-31 and MicroRNA-155 Are Overexpressed in
ulcerative colitis and regulate IL-13 signaling by targeting
interleukin 13 receptor α-1. Genes (Basel). 9:852018. View Article : Google Scholar
|
|
12
|
Niu Y, Murata T, Watanabe K, Kawakami K,
Yoshimura A, Inoue J, Puri RK and Kobayashi N: MIP-T3 associates
with IL-13Ralpha1 and suppresses STAT6 activation in response to
IL-13 stimulation. FEBS Lett. 550:139–143. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ramalingam TR, Pesce JT, Sheikh F, Cheever
AW, Mentink-Kane MM, Wilson MS, Stevens S, Valenzuela DM, Murphy
AJ, Yancopoulos GD, et al: Unique functions of the type II
interleukin 4 receptor identified in mice lacking the interleukin
13 receptor alpha1 chain. Nat Immunol. 9:25–33. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cheng L, Chen J, Fu Q, He S, Li H, Liu Z,
Tan G, Tao Z, Wang D, Wen W, et al: Chinese society of allergy
guidelines for diagnosis and treatment of allergic rhinitis.
Allergy Asthma Immunol Res. 10:300–353. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tan L, Qiu T, Xiang R, Cao C, Deng Y, Tao
Z and Xu Y: Down-regulation of Tet2 is associated with Foxp3 TSDR
hypermethylation in regulatory T cell of allergic rhinitis. Life
Sci. 241:1171012020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang L, Lv Q, Song X, Jiang K and Zhang J:
ADRB2 suppresses IL-13-induced allergic rhinitis inflammatory
cytokine regulated by miR-15a-5p. Hum Cell. 32:306–315. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xiang R, Xu Y, Zhang W, Kong YG, Tan L,
Chen SM, Deng YQ and Tao ZZ: Semaphorin 3A inhibits allergic
inflammation by regulating immune responses in a mouse model of
allergic rhinitis. Int Forum Allergy Rhinol. 9:528–537. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−ΔΔC(T)) method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang J, Cui Z, Liu L, Zhang S, Zhang Y,
Zhang Y, Su H and Zhao Y: MiR-146a mimic attenuates murine allergic
rhinitis by downregulating TLR4/TRAF6/NF-κB pathway. Immunotherapy.
11:1095–1105. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu HJ, Zhang AF, Zhao N and Li XZ: Role
of miR-146a in enforcing effect of specific immunotherapy on
allergic rhinitis. Immunol Invest. 45:1–10. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Singh PB, Pua HH, Happ HC, Schneider C,
von Moltke J, Locksley RM, Baumjohann D and Ansel KM: MicroRNA
regulation of type 2 innate lymphoid cell homeostasis and function
in allergic inflammation. J Exp Med. 214:3627–3643. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lu TX and Rothenberg ME: Diagnostic,
functional, and therapeutic roles of microRNA in allergic diseases.
J Allergy Clin Immunol. 132:3–13, quiz 14. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stepicheva NA and Song JL: Function and
regulation of microRNA-31 in development and disease. Mol Reprod
Dev. 83:654–674. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu N, Meisgen F, Butler LM, Han G, Wang
XJ, Söderberg-Nauclér C, Ståhle M, Pivarcsi A and Sonkoly E:
MicroRNA-31 is overexpressed in psoriasis and modulates
inflammatory cytokine and chemokine production in keratinocytes via
targeting serine/threonine kinase 40. J Immunol. 190:678–688. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu S, Han B, Liu S, Wang H, Zhuang W,
Huang Y and Zhang R: Derp1-modified dendritic cells attenuate
allergic inflammation by regulating the development of T helper
type1(Th1)/Th2 cells and regulatory T cells in a murine model of
allergic rhinitis. Mol Immunol. 90:172–181. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cunha S, Amaral MH, Lobo JMS and Silva AC:
Lipid nanoparticles for nasal/intranasal drug delivery. Crit Rev
Ther Drug Carrier Syst. 34:257–282. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shimizu S, Kouzaki H, Ogawa T, Takezawa K,
Tojima I and Shimizu T: Eosinophil-epithelial cell interactions
stimulate the production of MUC5AC mucin and profibrotic cytokines
involved in airway tissue remodeling. Am J Rhinol Allergy.
28:103–109. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang Y, Bai C, Li K, Adler KB and Wang X:
Role of airway epithelial cells in development of asthma and
allergic rhinitis. Respir Med. 102:949–955. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Adam E, Coulon L and Jaumotte E: The house
dust mite protease allergen Der p1 induced IL-8 production in human
airway epithelial cells through activation of ERK1/2
mitogen-activated protein kinase and AP-1 signaling pathways. J
Allergy Clin Immunol. 113:S339. 2004. View Article : Google Scholar
|
|
30
|
Matsukura S, Stellato C, Georas SN,
Casolaro V, Plitt JR, Miura K, Kurosawa S, Schindler U and
Schleimer RP: Interleukin-13 upregulates eotaxin expression in
airway epithelial cells by a STAT6-dependent mechanism. Am J Respir
Cell Mol Biol. 24:755–761. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shi J, Luo Q, Chen F, Chen D, Xu G and Li
H: Induction of IL-6 and IL-8 by house dust mite allergen Der p1 in
cultured human nasal epithelial cells is associated with
PAR/PI3K/NFkappaB signaling. ORL J Otorhinolaryngol Relat Spec.
72:256–265. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bartel S, La Grutta S, Cilluffo G,
Perconti G, Bongiovanni A, Giallongo A, Behrends J, Kruppa J,
Hermann S, Chiang D, et al: Human airway epithelial extracellular
vesicle miRNA signature is altered upon asthma development.
Allergy. 75:346–356. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
O'Shea JJ, Gadina M and Schreiber RD:
Cytokine signaling in 2002: New surprises in the Jak/Stat pathway.
Cell. 109 (Suppl 1):S121–S131. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kuperman D, Schofield B, Wills-Karp M and
Grusby MJ: Signal transducer and activator of transcription factor
6 (Stat6)-deficient mice are protected from antigen-induced airway
hyperresponsiveness and mucus production. J Exp Med. 187:939–948.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Urban JF Jr, Noben-Trauth N, Donaldson DD,
Madden KB, Morris SC, Collins M and Finkelman FD: IL-13,
IL-4Ralpha, and Stat6 are required for the expulsion of the
gastrointestinal nematode parasite Nippostrongylus
brasiliensis. Immunity. 8:255–264. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Miyata M, Nakamura Y, Shimokawa N, Ohnuma
Y, Katoh R, Matsuoka S, Okumura K, Ogawa H, Masuyama K and Nakao A:
Thymic stromal lymphopoietin is a critical mediator of IL-13-driven
allergic inflammation. Eur J Immunol. 39:3078–3083. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang X, Li Y, Luo D, Wang X, Zhang Y, Liu
Z, Zhong N, Wu M and Li G: Lyn regulates mucus secretion and MUC5AC
via the STAT6 signaling pathway during allergic airway
inflammation. Sci Rep. 7:426752017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Takai T: TSLP expression: Cellular
sources, triggers, and regulatory mechanisms. Allergol Int.
61:3–17. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Teng Y, Zhang R, Liu C, Zhou L, Wang H,
Zhuang W, Huang Y and Hong Z: miR-143 inhibits
interleukin-13-induced inflammatory cytokine and mucus production
in nasal epithelial cells from allergic rhinitis patients by
targeting IL13Rα1. Biochem Biophys Res Commun. 457:58–64. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Matsuwaki Y, Wada K, White T, Moriyama H
and Kita H: Alternaria fungus induces the production of GM-CSF,
interleukin-6 and interleukin-8 and calcium signaling in human
airway epithelium through protease-activated receptor 2. Int Arch
Allergy Immunol. 158 (Suppl 1):19–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Daffern PJ, Jagels MA, Saad JJ, Fischer W
and Hugli TE: Upper airway epithelial cells support eosinophil
survival in vitro through production of GM-CSF and prostaglandin
E2: Regulation by glucocorticoids and TNF-alpha. Allergy Asthma
Proc. 20:243–253. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tomazic PV, Darnhofer B and
Birner-Gruenberger R: Nasal mucus proteome and its involvement in
allergic rhinitis. Expert Rev Proteomics. 17:191–199. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang XH, Zhang YN, Li HB, Hu CY, Wang N,
Cao PP, Liao B, Lu X, Cui YH and Liu Z: Overexpression of miR-125b,
a novel regulator of innate immunity, in eosinophilic chronic
rhinosinusitis with nasal polyps. Am J Respir Crit Care Med.
185:140–151. 2012. View Article : Google Scholar : PubMed/NCBI
|