Introduction
Acute kidney injury (AKI) is a common clinical
disease that is caused by multiple etiologies and has a complex
pathophysiological process (1). The
morbidity and mortality rates for AKI are high among adults and
children, thus, resulting in substantial hospitalization costs
(2,3). A meta-analysis of global incidence of
AKI revealed incidence rates of AKI were 21.6% in adults and 33.7%
in children and AKI-associated mortality rates were 23.9% in adults
and 13.8% in children (4).AKI
occurs in ~20% of hospitalized patients, 10% of whom require renal
replacement therapy, and in as much as 50% of patients in intensive
care units, thus significantly increasing the length and cost of
hospitalization (5). AKI is
characterized by a sharp decrease in glomerular filtration rate in
the short term, which is accompanied by an increase in serum
creatinine, oliguria or both; these changes can lead to chronic
kidney disease or end-stage renal disease (6). Due to the variability of renal injury,
the lack of reliable early biomarkers and the heterogeneity of
pathways involved in pathophysiology, there are currently no
effective means of prevention and intervention except conservative
treatment and renal replacement therapy (7). Thus, identifying novel treatment
regimens and drugs for AKI is particularly important. The past
decade has seen a wealth of research expanding the traditional view
of necrosis. Recent research has challenged traditional views of
cell death by identifying novel pathways in which cells die in a
regulated manner, but exhibit the morphologic features of necrosis
(7–9). This regulated necrosis takes several
forms: Necroptosis, ferroptosis, pyroptosis, mitochondrial
permeability transition-driven necrosis and parthanatos (10). Necroptosis and ferroptosis are the
most studied forms of regulated necrosis occurring in the kidney
(11). A previous study
demonstrated that inhibiting ferroptosis effectively ameliorates
AKI (12).
Ferropotosis is a new type of regulatory cell death,
which was first proposed by Dixon et al in 2012 (13). This type of cell death is
iron-dependent and is accompanied by massive iron accumulation and
lipid peroxidation during cell death (13). Ferroptosis serves an important
regulatory role in the occurrence and development of several
diseases, including tumors, neurological diseases and AKI (14). In an in vivo study,
ferrostatin 1 or 16–86 was administered 15 min before ischemia in
mice with severe ischemia reperfusion injury (IRI)-AKI, and renal
tissue damage, serum creatinine and urea all decreased in the mice
48 h after ischemia, thus, suggesting that ferroptosis has a
crucial role in the pathogenesis of IRI (15). Therefore, regulating iron
homeostasis, resisting lipid peroxidation and inhibiting
ferroptosis may provide a promising therapeutic strategy for
AKI.
Pachymic acid (PA), a lanostane-type triterpenoid
from Poria cocos, has several pharmacological effects, such as
antitumor, anti-inflammatory, antioxidative, hypoglycemic, sedative
and hypnotic activities (16–20). A
previous study has reported that PA can improve renal injury during
sepsis in rats by inhibiting inflammatory responses and activating
the nuclear factor erythroid derived 2 like 2 (NRF2)/heme oxygenase
1 (HO-1) pathway through antioxidant stress (21). In addition, Poricoic acid A
effectively enhances the inhibition of melatonin in the transition
from AKI to chronic kidney disease following renal
ischemia/reperfusion injury (22).
PA protects against AKI, however, this protective mechanism remains
to be further investigated. PA has been reported to activate NRF2
in a study of renal injury in sepsis (21). Notably, the first two
ferroptosis-inducing agents (RSL-3 and erastin) identified in
ferroptosis studies initiate the ferroptotic cascade via inhibition
of glutathione peroxidase 4 (GPX4) and the cystine/glutamate
transporter system (xC-/xCT), respectively, both of which are
downstream targets of NRF2 (23).
PA is assumed to regulate its downstream proteins, GPX4 and xCT by
activating NRF2, and to interfere with ferroptosis in AKI. The
present study aimed to investigate the effect of PA on ferroptosis
in mice with ischemia-reperfusion kidney injury, and determine its
underlying molecular mechanism of action to provide novel ideas for
the treatment of clinical AKI.
Materials and methods
Reagents and animals
PA (white crystalline powder with purity ≥99.9%) was
purchased from MedChemExpress (cat. no. HY-N0371) and stored away
from light at 4°C. A total of 30 C57BL/6 male mice (8–10 weeks;
20–25 g body weight) were purchased from Chongqing Medical
University (Chongqing, China). The housing conditions were as
follows: 12 h light/12 h dark, ~60% humidity, 25°C, free access to
drinking water and food and bedding replacement every 3 days; the
drinking water and bedding materials were disinfected before use.
All animal experiments were performed according to the Guide for
the Care and Use of Laboratory Animals, issued by the National
Institutes of Health in 1996 (24).
The present study was approved by the Institutional Animal Care and
Use Committee of Chongqing Medical University (Chongqing, China;
approval no. 2018–019).
IRI-AKI model
Mice were fed under the same conditions for 1 week
to produce the model of renal ischemia reperfusion injury, as
previously described (25). The
mice were anesthetized with 50–60 mg/kg of pentobarbital sodium
(cat. no. P3761; Sigma-Aldrich; Merck KGaA) by intraperitoneal
injection; the skin at the surgical area was wiped with 70%
alcohol. The incision was positioned at the left and right sides of
the spine (0.5 cm), and the incision length was 1–1.5 cm along the
back. The kidneys were subsequently pulled out from the incision to
expose the renal pedicle. A microaneurysm clip was used to clamp
the pedicle to block the blood flow to the kidney and induce renal
ischemia. Complete ischemia was indicated by a change in the color
of the kidney from red to dark purple within a few seconds. After
40 min of ischemia, the microaneurysm clips were released to allow
each kidney to start reperfusion, which was indicated by the change
of the kidney color to red. The muscle skin was sutured after the
kidney color returned to normal. The entire renal ischemia
procedure was completed on a thermostatic pad, and the body
temperature of the mice was kept constant at 36.5–37°C. A total of
0.8 ml warm sterile saline was administered intraperitoneally to
each mouse. Each animal was placed on a heating pad until it
regained full consciousness and was subsequently returned to its
cage. The mice were sacrificed via cervical dislocation 24 h after
surgery, and the blood was collected from the orbital sinus.
One-third of the renal tissue was used for histopathological
analyses, and the remaining two-thirds were quickly stored at
−80°C.
Animal groups
C57BL/6 mice were divided into five groups
(n=6/group) according to a random number table, as follows: Sham
group (Sham), model group (IRI), low dose group
(IRI+PAL), moderate dose group (IRI+PAM) and
high dose group (IRI+PAH). Animals in the three
experimental treatment groups received intraperitoneal injection
with PA at dosages of 5, 10 and 20 mg/kg.bw for 3 days, before the
model was induced. The sham and IRI groups were injected with the
same volume dimethyl sulfoxide for 3 days before model induction.
The modeling method was performed as previously described (25). The bilateral renal pedicels of the
mice in the sham group were exposed and treated as aforementioned,
but were not clamped. The surgical incision was sutured after 40
min.
Serum biochemical analysis
The mice were sacrificed 24 h post-surgery, and
0.8–1 ml blood was collected from the orbital sinus, allowed to
rest for 30 min and centrifuged at 900 × g for 10 min at 4°C. The
upper serum layer was collected, packed and stored at −80°C. Serum
creatinine (Scr; cat. no. c011-2-1) and blood urea nitrogen (BUN;
cat. no. c013-2-1) were assessed using reagent kits, according to
the manufacturer's instructions (Nanjing Jiancheng Bioengineering
Institute).
Histopathological examination
At 24 h post-IRI, portions of the kidney tissues
were fixed in 10% neutral-buffered formalin for 24–48 h at room
temperature, processed routinely by embedding in paraffin. The
paraffin-fixed tissue specimens were sliced into 4 µm thick
sections, which were mounted on glass slides and stained with
hematoxylin for 10 min at room temperature and eosin for 3 min at
room temperature. A BX51 light microscope (Olympus Corporation) was
used to detect the renal histopathological changes (magnification,
×200 and ×400). The Paller score was used to grade renal tubular
necrosis in renal IRI (26). A
total of 10 non-overlapping areas were randomly selected under high
magnification using an optical light microscope (magnification,
×400), and 10 renal tubules were randomly selected in each field of
vision. The renal pathology sections of three mice in each
experimental group were randomly selected for observation. A total
of 10 visual fields were randomly selected for each pathological
section, and 10 renal tubules were scored in each visual field. A
total of 100 tubules was observed for each mouse, giving a total of
300 renal tubules observed in each group. The higher the score, the
more serious the degree of damage of renal tubules.
Transmission electron microscopy
(TEM)
Renal tissue was collected 24 h post-surgery.
Following anesthesia, 1 mm3 renal tissue was removed
in vivo and soaked in tissue fixation solution composed of
2.5% glutaraldehyde and phosphoric acid buffer solution (cat. no.
G7776; Sigma-Aldrich; Merck KGaA) for 2 h at 4°C, dehydrated in
gradual ethanol (50–100%) and propylene oxide, embedded in Epon 812
(cat. no. GP18010; Beijing Zhongjingkeyi Technology Co., Ltd.) for
1 h at 35°C and solidified for 24 h at 60°C. Ultrathin sections
(thickness, 50–70 nm) were placed onto 200-mesh copper grids and
stained with 2% uranyl acetate for 30 min at room temperature and
0.04% lead citrate for 15 min at room temperature. The samples were
viewed under an electron microscope (JEM-1400 plus, Japan Electron
Optics Laboratory Co., Ltd.; magnification, ×5,000 and ×10,000).
Images were obtained using a SlowScan charge-coupled device camera
and iTEM analySIS5.0 software (Olympus Soft Imaging Solutions
GmbH).
Antioxidant glutathione (GSH) and
lipid peroxidation malondialdehyde (MDA) detection
Kidney tissue (50–100 mg) was collected, and
physiological saline solution (cat. no. B020; Nanjing Jiancheng
Bioengineering Institute) was added to the tissue at a 1:9 weight
(g):volume (ml) ratio. The tissue was homogenized, to produce 10%
tissue homogenate, which was centrifuged at 13,800 × g for 15 min
at 4°C. The supernatant was removed and the total protein content
of the supernatant was determined using the bicinchoninic acid
method (21). GSH (cat. no.
A006-2-1) and MDA (cat. no. A003-2-2) were assessed using the
reagent kits, according to the manufacturer's instructions (Nanjing
Jiancheng Bioengineering Institute).
Western blotting
Total protein was extracted from mouse kidney
tissues using RIPA cell lysis buffer (cat. no. W062-1-1; Nanjing
Jiancheng Bioengineering Institute) and the protein concentrations
were determined via BCA assay (cat. no. A045-4-2; Nanjing Jiancheng
Bioengineering Institute). Equal amounts of proteins (50 µg) were
separated via SDS-PAGE (Cox-2, 10.0; NRF2, 10.0; xCT, 12.5; HO-1,
12.5; GPx4, 15.0%) and transferred onto PVDF membranes (GE
Healthcare Life Sciences). The membranes were subsequently blocked
with 5% skimmed milk powder for 1 h at room temperature, and
incubated with primary antibodies against the following:
Cyclooxygenase 2 (COX-2; 1:1,000; cat. no. ab179800; Abcam), GPX4
(1:5,000; cat. no. ab125066; Abcam), xCT (1:2,000; cat. no.
ab175186; Abcam), HO-1 (1:1,000 cat. no. 101147; Gentex) and NRF2
(1:1,000; cat. no. D1Z9C; CST) overnight at 4°C. Membranes were
subsequently washed with TBST (0.1% Tween-20) three times.
Following the primary incubation, membranes were incubated with
horseradish peroxidase-conjugated secondary antibodies (1:10,000;
40295G; BIOSS) for 1h at room temperature. The membranes were
re-washed three times with TBST and visualized with BeyoECLPlus
(cat. no. P00185; Beyotime Institute of Biotechnology), using a
Chemi Doc Imaging System (Bio-Rad Laboratories, Inc.) The intensity
of protein bands was determined using ImageJ v1.8.0 software
(National Institutes of Health). All experiments were performed in
triplicate.
Reverse transcription quantitative
(RT-q)PCR
Total RNA was extracted from the kidneys of mice in
different groups using TRIzol® reagent (Takara Bio,
Inc.) and cDNA was synthetized using the PrimeScript RT Reagent kit
with gDNA Eraser (cat. no. RR047A; Takara Biotechnology Co., Ltd.).
Relative levels of the target genes were determined via qPCR using
Ultra SYBR Mixture (Takara Biotechnology Co., Ltd.). RT-qPCR was
performed in a 25 µl volume with 2 µl cDNA, 400 nM each sense and
antisense primer and 12.5 µl Brilliant SYBR Green QPCR Master Mix
(Takara Bio, Inc.) on an ABI PRISM 7000 system (Applied Biosystems;
Thermo Fisher Scientific, Inc.). The reaction was performed for 40
cycles of denaturation at 95°C for 30 sec, annealing at 53°C for 30
sec and extension at 72°C for 10 sec. The following primer
sequences were used for qPCR: GPX4 forward,
5′-GCCTGGATAAGTACAGGGGTT-3′ and reverse,
5′-CATGCAGATCGACTAGCTGAG-3′; NRF2 forward,
5′-TCTTGGAGTAAGTCGAGAAGTGT-3′ and reverse,
5′-GTTGAAACTGAGCGAAAAAGGC-3′; xCT forward,
5′-GGCACCGTCATCGGATCAG-3′ and reverse, 5′-CTCCACAGGCAGACCAGAAAA-3′;
COX-2 forward, 5′-TGAGCAACTATTCCAAACCAGC-3′ and reverse,
5′-GCACGTAGTCTTCGATCACTATC-3′; HO-1 forward,
5′-AAGCCGAGAATGCTGAGTTCA-3′ and reverse,
5′-GCCGTGTAGATATGGTACAAGGA-3′; and β-actin forward,
5′-GGCTGTATTCCCCTCCATCG-3′ and reverse,
5′-CCAGTTGGTAACAATGCCATGT-3′.
An average value of gene expression after β-actin
normalization was used as a calibrator to determine the relative
levels of the target genes. Relative expression levels of the
target genes were calculated using the 2−ΔΔCq method
(27).
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 7.0 (GraphPad Software, Inc.) and SPSS 23 software (IBM
Corp.). Data are presented as the mean ± standard deviation of
three independent repeats, unless stated otherwise. One-way ANOVA
followed by Tukey's post hoc test were used to compare differences
between multiple groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
PA decreases renal injury in mice with
IRI
Scr and BUN are two important indicators of renal
function (5). The levels of Scr and
BUN were significantly higher in the IRI group compared with those
in the sham group (P<0.05; Fig. 1A
and B). Treatment with mid-dose and high-dose PA significantly
mitigated the increases in serum creatinine and urea compared with
the IRI group (P<0.05); however, the levels of serum creatinine
and urea did not significantly differ between the light-dose PA
treatment group and the IRI group (P>0.05; Fig. 1A and B).
 | Figure 1.PA alleviates renal injury following
ischemia reperfusion. (A) BUN and (B) serum creatinine were
measured in mice in the Sham, IRI, IRI+PAL,
IRI+PAM and IRI+PAH groups (40 min renal
ischemia followed by 24 h of reperfusion; n=6/group). (C) Paller
scores were used to grade renal tubular injury in the Sham, IRI,
IRI+PAL, IRI+PAM and IRI+PAH
groups (n=3/group). (D) Hematoxylin and eosin images of the kidneys
from mice in the Sham, IRI, IRI+PAL,
IRI+PAM and IRI+PAH group. Scale bar, 50 µm.
Data are presented as the mean ± standard deviation. *P<0.05 vs.
sham group; #P<0.05 vs. IRI group. PA, pachymic acid;
BUN, blood urea nitrogen; IRI, ischemia reperfusion injury;
L, low dose group; M, moderate dose group;
H, high dose group. |
As presented in Fig.
1D, H&E staining of kidney tissues in the sham group
demonstrated no significant changes in renal tissue structure.
Conversely, the IRI group demonstrated edema and loss of renal
tubular epithelial cells, tubular dilation, interstitial
inflammation, inflammatory cell infiltration, collagen deposition
and deposition tube necrosis material (Fig. 1D). The Paller score, an indicator of
renal tissue injury (26), was
significantly higher in the IRI groups compared with the sham group
(P<0.05; Fig. 1C). In
particular, moderate-dose and high-dose PA therapy significantly
ameliorated these lesions, and the Paller score was significantly
lower compared with that in the IRI group (P<0.05); however,
there was no significant difference between the IRI model group and
the low-dose group (P>0.05; Fig.
1C).
Effects of PA on the morphological
changes in mitochondria in the renal tissue of mice following renal
IRI
TEM demonstrated no significant changes in the
mitochondrial structure of the renal tissue in the sham group. In
the model group, ferroptosis-associated mitochondrial changes, such
as decreased mitochondrial volume, increased membrane density, and
decreased or absent mitochondrial cristae, were observed in renal
tissue (Fig. 2A). Compared with
those in the model group, the mitochondria of the moderate-dose
group mainly exhibited edema, and the specific changes
characteristic of ferroptosis were rare (Fig. 2A). However, in the high-dose PA
group, no characteristic changes of ferroptosis were observed in
the mitochondria of the renal tissue, and only mild edema exhibited
(Fig. 2A). The changes in the
mitochondria in the low-dose group were the same as those in the
model group (Fig. 2A).
 | Figure 2.PA decreases ferroptosis in IRI-acute
kidney injury. (A) Transmission electron microscopy images in the
Sham, IRI, IRI+PAL, IRI+PAM and
IRI+PAH groups (40 min renal ischemia followed by 24 h
of reperfusion). Magnification, ×20,000; scale bar, 1 µm. The black
arrows indicate that the mitochondrial volume decreased, membrane
density increased and decreased, or disappeared mitochondrial
cristae were observed in renal tissue. The blue arrows indicate
mitochondrial edema. The levels of (B) GSH and (C) MDA in renal
tissues were assessed (n=6/group). Data are presented as the mean ±
standard deviation. *P<0.05 vs. sham group;
#P<0.05 vs. IRI group. PA, pachymic acid; IRI,
ischemia reperfusion injury; L, low dose group;
M, moderate dose group; H, high dose group;
GSH, glutathione; MDA, malondialdehyde. |
Effects of PA on GSH and MDA
expression in renal IRI
GSH is a necessary cofactor for GPX4 function. GSH
synthesis directly affects GPX4 activity. MDA is a product of lipid
oxidation, which reflects the degree of intracellular lipid
peroxidation and indirectly reflects the occurrence of ferroptosis
(28,29). As presented in Fig. 2B, the kidney tissues had
significantly lower levels of GSH in the IRI group compared with
the sham group. Among the drug treatment groups, GSH expression was
significantly higher in the IRI+PAM and
IRI+PAH groups compared with the IRI model group
(P<0.05). However, GSH expression did not significantly differ
between the IRI+PAL and IRI groups (P>0.05).
Conversely, as presented in Fig.
2C, the tissue lipid peroxidation was greater (based on MDA
levels) in the IRI group compared with the sham group. Among the
drug treatment groups, MDA levels were significantly lower in the
IRI+PAM and IRI+PAH groups compared with the
IRI group (P<0.05). Notably, no significant differences were
observed in the levels of MDA between the IRI+PAL and
IRI groups (P>0.05).
PA regulates the expression of
ferroptosis-related proteins in renal IRI
In the present study, the expression levels of the
ferroptosis-related proteins GPX4, xCT and HO-1 were detected via
western blotting (Fig. 3A and B)
and RT-qPCR (Fig. 3C) analyses. The
results demonstrated that the protein levels of GPX4, xCT and HO-1
were significantly downregulated in the IRI group compared with the
sham group (P<0.05) (Fig. 3A and
B). Similar results were demonstrated following RT-qPCR
analysis. In addition, treatment with moderate-dose and high-dose
PA significantly increased the protein and mRNA expression levels
of GPX4, xCT and HO-1 compared with the IRI group (P<0.05).
However, no significant differences in protein and mRNA expression
levels of GPX4, xCT and HO-1 were observed between the IRI and
low-dose PA treatment groups (P>0.05; Fig. 3B and C).
 | Figure 3.PA increases the expression levels
the ferroptosis related proteins, GPX4, xCT and HO-1. (A) Western
blotting was performed to detect the expression levels of the
ferroptosis related proteins, GPX4, xCT and HO-1 in IRI-acute
kidney injury mice (n=3). (B) Relative expression of gray values
for GPX4, xCT and HO-1. (C) Reverse transcription-quantitative PCR
analysis was performed to detect the mRNA expression levels of the
ferroptosis related proteins (n=3). Data are presented as the mean
± standard deviation. *P<0.05 vs. sham group;
#P<0.05 vs. IRI group. PA, pachymic acid; GPX4,
glutathione peroxidase 4; xCT, glutamate transporter system; HO-1,
heme oxygenase 1; IRI, ischemia reperfusion injury; L,
low dose group; M, moderate dose group; H,
high dose group. |
The results demonstrated that protein and mRNA
levels of the ferroptosis-associated lipid peroxidation protein,
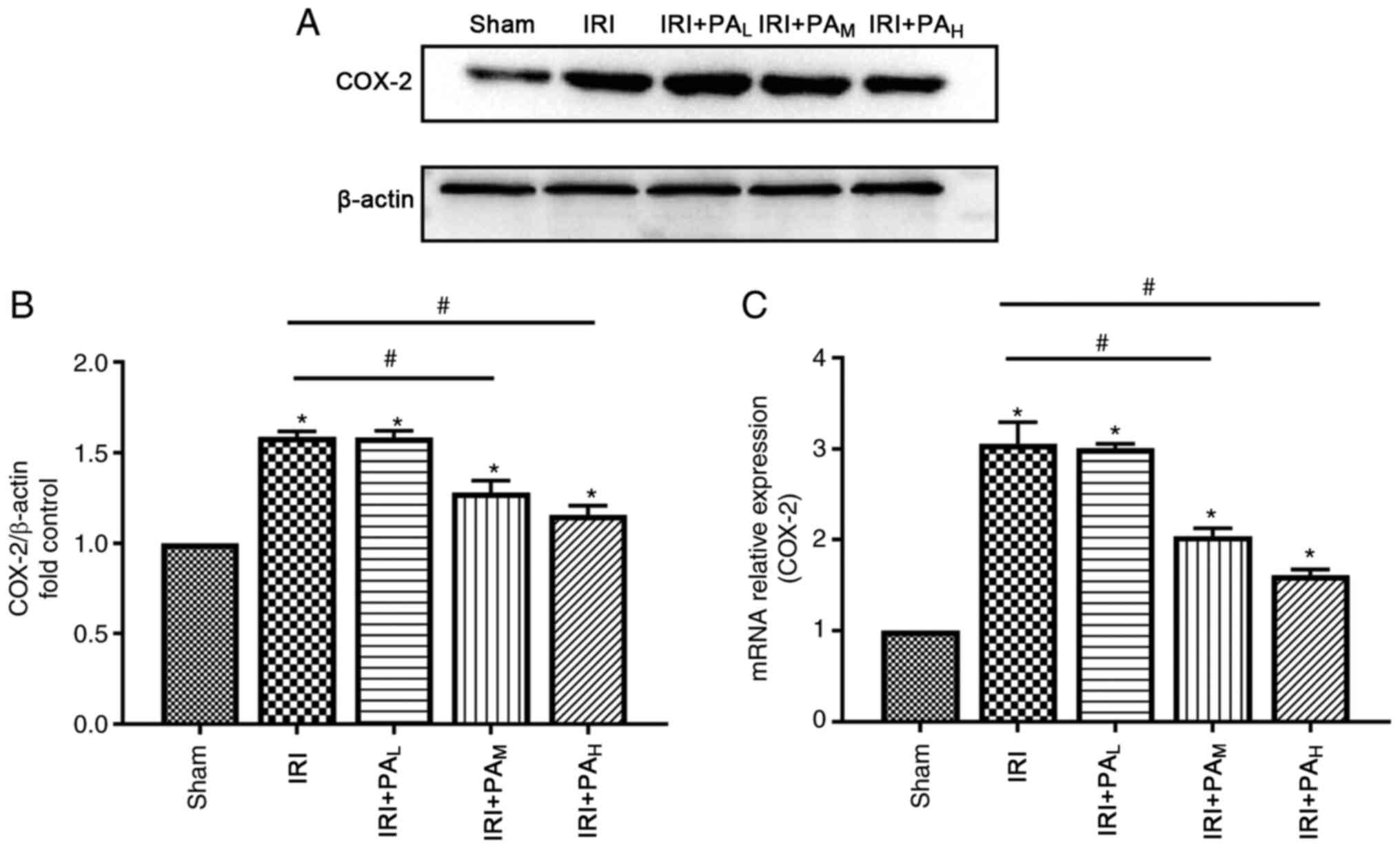
COX-2 were significantly upregulated during IRI (Fig. 4). Treatment with moderate-dose and
high-dose PA significantly decreased COX-2 expression compared with
the IRI group (P<0.05), and no significant differences were
observed between the IRI and low-dose PA treatment groups
(P>0.05; Fig. 4).
PA promotes activation of the NRF2
signaling pathway in renal IRI
To investigate the effect of PA on the NRF2
signaling pathway, the protein and mRNA levels of NRF2 were
assessed (Fig. 5). The results
demonstrated that NRF2 expression was significantly lower in the
IRI group compared with the sham group. In addition, NRF2
expression was significantly higher in the IRI+PAM and
IRI+PAH groups compared with the IRI group (P<0.05);
however, no significant differences were observed between the
IRI+PAL and IRI groups (P>0.05). Furthermore,
treatment with PA also increased NRF2 expression in a
dose-dependent manner (Fig. 5).
Discussion
Previous studies have demonstrated that PA
ameliorates renal injury in AKI and chronic kidney disease via
anti-inflammatory and antioxidative stress (21,22).
However, whether PA inhibits ferroptosis has not yet been
investigated. To the best of our knowledge, the present study was
the first to investigate the effect of PA on ferroptosis in
IRI-AKI. In the present study, bilateral renal pedicle clamping in
mice for 40 min followed by reperfusion for 24 h was used to
successfully establish IRI and induce AKI. The results demonstrated
that Scr and BUN levels were significantly elevated in the model
group, and renal tubules were significantly damaged, based on
H&E staining and the Paller score. Thus, the IRI-AKI model was
constructed. Notably, administration of PA significantly decreased
the IRI enhanced Scr and BUN serum levels, and alleviated kidney
injury in a dose-dependent manner.
GPX4 serves an important role in ferroptosis
(30). Small molecules, such as
erastin, RSL-3 and RSL-5 (13),
through different signaling pathways, directly or indirectly
inhibit GPX4 activity, thus decreasing cell antioxidant capacity,
causing reactive oxygen species overload and membrane lipid
peroxidation reactions, and ultimately damaging cell membrane
integrity and causing cell ferroptosis (13). In GPX4 knockout mice, GPX4
deficiency leads to spontaneous acute renal failure and an
increased rate of early mortality, whereas ferroptosis of renal
tubular epithelial cells is the main cause of renal failure in GPX4
knockout mice (30). Thus,
increasing GPX4 activity effectively inhibits ferroptosis. The
synthesis of GSH, a cofactor necessary for GPX4 function, directly
increases GPX4 activity. GSH synthesis is influenced by cystine.
The transport of cystine from the extracellular to the
intracellular space is largely dependent on the cystine/glutamate
reverse transporter (system xc-) on the cell membrane. This
transporter is a heterodimer composed of two subunits, SLC7A11
(xCT) and SLC3A2. It mainly transports glutamate to the
extracellular space and mediates cystine entry into the cell
(28). Inhibiting the activity of
system xc- affects the synthesis of GSH by inhibiting the
absorption of cystine, thereby leading to inactivity of GPX4, a
decrease in cell antioxidant capacity, accumulation of lipid
reactive oxygen species, and ultimately oxidative damage and
ferroptosis (31). Yang et
al (32) confirmed that
prostaglandin-endoperoxide synthase 2 (PTGS2), encoding COX-2, is a
suitable marker for the lipid peroxidation that occurs during
GPX4-regulated ferroptosis. Thus, PTGS2 upregulation has been
suggested to be a downstream marker of ferroptosis. Enhancing
system xc-, promoting the absorption of cystine, increasing the
production of GSH and increasing the activity of GPX4 may inhibit
ferroptosis and decrease renal injury (32,33).
The results of the present study demonstrated that the system xc-
activity decreased, GSH synthesis decreased, GPX4 activity
decreased, MDA and Cox-2 expression increased, and ferroptosis
occurred in IRI-AKI mice. In addition, treatment with PA
significantly increased the expression levels of GSH, xCT and GPX4,
and decreased the expression levels of MDA and Cox-2, thus
suggesting that treatment with moderate and high doses of PA
enhances system xc- activity, promotes the absorption of cystine,
increases GSH production, increases GPX4 activity and inhibits
ferroptosis.
NRF2, a stress-inducible transcription factor, has
emerged as a key regulator of both lipid peroxidation and
ferroptosis (23). Notably, a
number of the proteins and enzymes (such as GPX4, xCT and the
glutamate-cysteine ligase catalytic and modifier subunits)
responsible for preventing lipid peroxidation, and thus initiation
of ferroptosis, are NRF2 target genes (23). NRF2 serves a key role in regulating
iron/heme metabolism (34).
Following activation of NRF2, NRF2 upregulates iron metabolism
related proteins involved in ferroptosis, such as ferritin
(Ferritin light chain and Ferritin heavy polypeptide 1), the
intracellular iron storage proteins (35), iron chelatase and HO-1 (36). Adedoyin et al (37) reported that HO-1 in proximal renal
tubular epithelial cells can resist ferroptosis induced by Erastin
in AKI, thus, decreasing AKI. In addition, NRF2 regulates proteases
involved in GSH synthesis and metabolism, such as
glutamine-cysteine ligase catalytic and regulatory subunits,
glutathione synthase and a subunit of the cystine/glutamate
transporter xCT (38–40). GPX4 is a target regulatory gene of
NRF2 (41). Li et al
(42) demonstrated that
pretreatment with Roxadustat (FG-4592) attenuates folic
acid-induced kidney injury through anti-ferroptosis via the
Akt/GSK-3β/NRF2 pathway. The results of the present study
demonstrated that administration of moderate and high doses of PA
directly or indirectly promoted the activation of the NRF2
signaling pathway, upregulated the expression levels of the
downstream ferroptosis regulated proteins, GPX4, xCT and HO-1,
increased GSH levels in renal tissue, and decreased the levels of
the ferroptosis-associated lipid peroxidation protein, COX-2 and
the lipid oxidation indicator, MDA.
Taken together, the results of the present study
suggest that PA may improve renal function and decrease renal
injury in IRI-AKI mice. This protective effect may be associated
with the inhibition of ferroptosis in the kidneys through direct or
indirect activation of NRF2, and upregulation of the downstream
ferroptosis-related proteins, GPX4, xCT and HO-1 (Fig. 6). However, the present study is not
without limitations. The effect of PA on ferroptosis has not been
extensively studied. Thus, prospective studies should use erastin,
a selective inhibitor of system xc-, to inhibit the activity of xCT
in mice, and subsequently verify whether inhibition of xCT induces
ferrroptosis in mouse kidney cells, thus leading to AKI, and
whether PA inhibits ferroptosis mainly through the xCT-GPX4
pathway. Further mechanistic studies must be performed in
vitro. In addition, PA should be used to treat renal tubular
epithelial cells to verify the effect of PA on ferroptosis in
vitro.
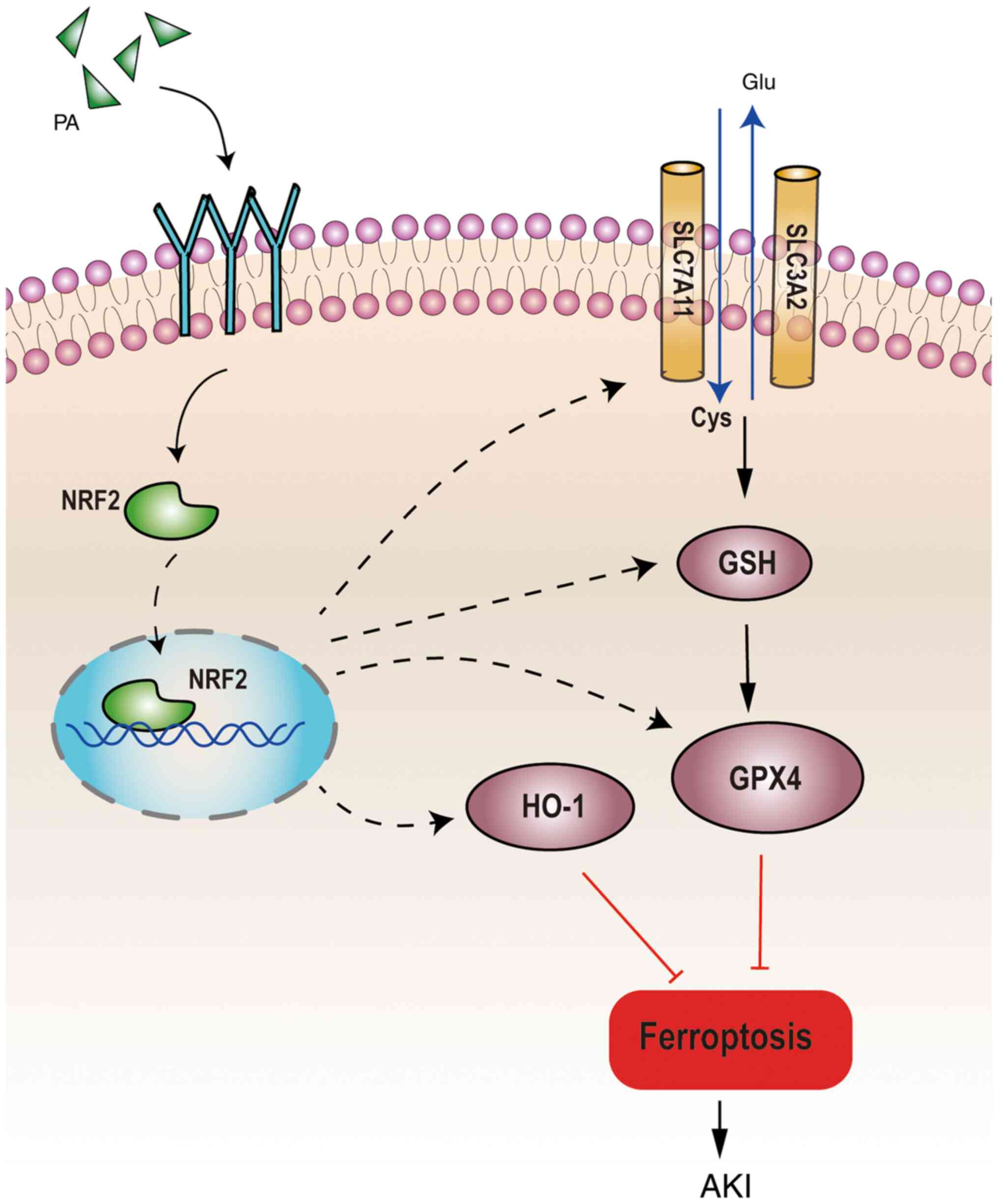 | Figure 6.Schematic diagram of proposed
molecular mechanisms for PA regulating ferroptosis in IRI-AKI. PA
can improve renal function and decrease renal injury in IRI-AKI
mice by activating the NRF2 signaling pathway, GSH and downstream
ferroptosis-related proteins (GPX4, xCT and HO-1) to inhibit
ferroptosis in the kidney. PA, pachymic acid; IRI, ischemia
reperfusion injury; AKI, acute kidney injury; NRF2, nuclear factor
erythroid derived 2 like 2; GSH, glutathione; GPX4, glutathione
peroxidase 4; HO-1, heme oxygenase 1; xCT, glutamate transporter
system; Glu, glutamic acid; Cys, cysteine. |
Acknowledgements
The authors would like to thank Professor Ling Zhang
(Department of Nephrology, The Second Affiliated Hospital,
Chongqing Medical University) for help designing the study and
guiding the animal model construction.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81873604
and 81000299), the Venture and Innovation Support Program for
Chongqing Overseas Returnees (grant no. cx2018038), the Fourth
Batch of Chongqing Young and Middle-aged Medical TopTalent Fund
[grant no. (2018)230] and the Kuanren Talents Program of The Second
Affiliated Hospital of Chongqing Medical University (grant no.
KY2019G008).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GPJ and YJL designed the present study, performed
most of the experiments, and drafted the initial manuscript. XJZ
and LLH analyzed the data. XHL helped analyze and interpret the
data, and revised and corrected the manuscript for important
intellectual content. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All animal experiments were performed according to
the Guide for the Care and Use of Laboratory Animals, issued by the
National Institutes of Health in 1996. The present study was
approved by the Institutional Animal Care and Use Committee of
Chongqing Medical University (Chongqing, China; approval no.
2018-019).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AKI
|
acute kidney injury
|
|
BUN
|
blood urea nitrogen
|
|
COX-2
|
cyclooxygenase 2
|
|
GPX4
|
glutathione peroxidase 4
|
|
GSH
|
glutathione
|
|
H&E
|
hematoxylin and eosin
|
|
HO-1
|
heme oxygenase 1
|
|
IRI
|
ischemia reperfusion injury
|
|
MDA
|
malondialdehyde
|
|
NRF2
|
nuclear factor erythroid derived 2
like 2
|
|
PA
|
pachymic acid
|
|
Scr
|
serum creatinine
|
|
SLC7A11
|
solute carrier family 7 (cationic
amino acid transporter, y+ system) member 11
|
References
|
1
|
Hoste EAJ, Kellum JA, Selby NM, Zarbock A,
Palevsky PM, Bagshaw SM, Goldstein SL, Cerdá J and Chawla LS:
Global epidemiology and outcomes of acute kidney injury. Nat Rev
Nephrol. 14:607–625. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kaddourah A, Basu RK, Bagshaw SM and
Goldstein SL; AWARE Investigators, : Epidemiology of acute kidney
injury in critically Ill children and young adults. N Engl J Med.
376:11–20. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chertow GM, Burdick E, Honour M, Bonventre
JV and Bates DW: Acute kidney injury, mortality, length of stay,
and costs in hospitalized patients. J Am Soc Nephrol. 16:3365–3370.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Susantitaphong P, Cruz DN, Cerda J,
Abulfaraj M, Alqahtani F, Koulouridis I and Jaber BL; Acute Kidney
Injury Advisory Group of the American Society of Nephrology, :
World incidence of AKI: A meta-analysis. Clin J Am Soc Nephrol.
8:1482–1493. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ronco C, Bellomo R and Kellum JA: Acute
kidney injury. Lancet. 394:1949–1964. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
James MT, Bhatt M, Pannu N and Tonelli M:
Long-term outcomes of acute kidney injury and strategies for
improved care. Nat Rev Nephrol. 16:193–205. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Han SJ and Lee HT: Mechanisms and
therapeutic targets of ischemic acute kidney injury. Kidney Res
Clin Pract. 38:427–440. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Priante G, Gianesello L, Ceol M, Del Prete
D and Anglani F: Cell death in the kidney. Int J Mol Sci.
20:35982019. View Article : Google Scholar
|
|
9
|
Martin-Sanchez D, Poveda J,
Fontecha-Barriuso M, Ruiz-Andres O, Sanchez-Niño MD, Ruiz-Ortega M,
Ortiz A and Sanz AB: Targeting of regulated necrosis in kidney
disease. Nefrologia. 38:125–135. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kers J, Leemans JC and Linkermann A: An
overview of pathways of regulated necrosis in acute kidney injury.
Semin Nephrol. 36:139–152. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pefanis A, Ierino FL, Murphy JM and Cowan
PJ: Regulated necrosis in kidney ischemia-reperfusion injury.
Kidney Int. 96:291–301. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hu Z, Zhang H, Yang SK, Wu X, He D, Cao K
and Zhang W: Emerging role of ferroptosis in acute kidney injury.
Oxid Med Cell Longev. 2019:80106142019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: An iron-dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li J, Cao F, Yin HL, Huang ZJ, Lin ZT, Mao
N, Sun B and Wang G: Ferroptosis: Past, present and future. Cell
Death Dis. 11:882020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Linkermann A, Skouta R, Himmerkus N, Mulay
SR, Dewitz C, De Zen F, Prokai A, Zuchtriegel G, Krombach F, Welz
PS, et al: Synchronized renal tubular cell death involves
ferroptosis. Proc Natl Acad Sci USA. 111:16836–16841. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ling H, Zhang Y, Ng KY and Chew EH:
Pachymic acid impairs breast cancer cell invasion by suppressing
nuclear factor-κB-dependent matrix metalloproteinase-9 expression.
Breast Cancer Res Treat. 126:609–620. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li FF, Yuan Y, Liu Y, Wu QQ, Jiao R, Yang
Z, Zhou MQ and Tang QZ: Pachymic acid protects H9c2 cardiomyocytes
from lipopolysaccharide-induced inflammation and apoptosis by
inhibiting the extracellular signal-regulated kinase 1/2 and p38
pathways. Mol Med Rep. 12:2807–2813. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li JY, Wu HX and Yang G: Pachymic acid
improves survival and attenuates acute lung injury in septic rats
induced by cecal ligation and puncture. Eur Rev Med Pharmacol Sci.
21:1904–1910. 2017.PubMed/NCBI
|
|
19
|
Li TH, Hou CC, Chang CL and Yang WC:
Anti-hyperglycemic properties of crude extract and triterpenes from
poria cocos. Evid Based Complement Alternat Med. 2011:1284022011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shah VK, Choi JJ, Han JY, Lee MK, Hong JT
and Oh KW: Pachymic acid enhances pentobarbital-induced sleeping
behaviors via GABAA-ergic systems in mice. Biomol Ther (Seoul).
22:314–320. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cai ZY, Sheng ZX and Yao H: Pachymic acid
ameliorates sepsis-induced acute kidney injury by suppressing
inflammation and activating the Nrf2/HO-1 pathway in rats. Eur Rev
Med Pharmacol Sci. 21:1924–1931. 2017.PubMed/NCBI
|
|
22
|
Chen DQ, Feng YL, Chen L, Liu JR, Wang M,
Vaziri ND and Zhao YY: Poricoic acid A enhances melatonin
inhibition of AKI-to-CKD transition by regulating Gas6/AxlNFκB/Nrf2
axis. Free Radic Biol Med. 134:484–497. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dodson M, Castro-Portuguez R and Zhang DD:
NRF2 plays a critical role in mitigating lipid peroxidation and
ferroptosis. Redox Biol. 23:1011072019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
National Research Council (US) Institute
for Laboratory Animal Research, . Guide for the Care and Use of
Laboratory Animals. Washington (DC): National Academies Press (US);
1996, simplehttps://www.ncbi.nlm.nih.gov/books/NBK232589/doi:
10.17226/5140.
|
|
25
|
Wei Q and Dong Z: Mouse model of ischemic
acute kidney injury: Technical notes and tricks. Am J Physiol Renal
Physiol. 303:F1487–F1494. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Paller MS, Hoidal JR and Ferris TF: Oxygen
free radicals in ischemic acute renal failure in the rat. J Clin
Invest. 74:1156–1164. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xie Y, Hou W, Song X, Yu Y, Huang J, Sun
X, Kang R and Tang D: Ferroptosis: Process and function. Cell Death
Differ. 23:369–379. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Su L, Jiang X, Yang C, Zhang J, Chen B, Li
Y, Yao S, Xie Q, Gomez H, Murugan R and Peng Z: Pannexin 1 mediates
ferroptosis that contributes to renal ischemia/reperfusion injury.
J Biol Chem. 294:19395–19404. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Friedmann Angeli JP, Schneider M, Proneth
B, Tyurina YY, Tyurin VA, Hammond VJ, Herbach N, Aichler M, Walch
A, Eggenhofer E, et al: Inactivation of the ferroptosis regulator
Gpx4 triggers acute renal failure in mice. Nat Cell Biol.
16:1180–1191. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dixon SJ, Patel DN, Welsch M, Skouta R,
Lee ED, Hayano M, Thomas AG, Gleason CE, Tatonetti NP, Slusher BS
and Stockwell BR: Pharmacological inhibition of cystine-glutamate
exchange induces endoplasmic reticulum stress and ferroptosis.
Elife. 3:e025232014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang WS, SriRamaratnam R, Welsch ME,
Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji
AF, Clish CB, et al: Regulation of ferroptotic cancer cell death by
GPX4. Cell. 156:317–331. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cao JY and Dixon SJ: Mechanisms of
ferroptosis. Cell Mol Life Sci. 73:2195–2209. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kerins MJ and Ooi A: The roles of NRF2 in
modulating cellular iron homeostasis. Antioxid Redox Signal.
29:1756–1773. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Harada N, Kanayama M, Maruyama A, Yoshida
A, Tazumi K, Hosoya T, Mimura J, Toki T, Maher JM, Yamamoto M and
Itoh K: Nrf2 regulates ferroportin 1-mediated iron efflux and
counteracts lipopolysaccharide-induced ferroportin 1 mRNA
suppression in macrophages. Arch Biochem Biophys. 508:101–109.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Alam J, Stewart D, Touchard C, Boinapally
S, Choi AM and Cook JL: Nrf2, a Cap'n'Collar transcription factor,
regulates induction of the heme oxygenase-1 gene. J Biol Chem.
274:26071–26078. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Adedoyin O, Boddu R, Traylor A, Lever JM,
Bolisetty S, George JF and Agarwal A: Heme oxygenase-1 mitigates
ferroptosis in renal proximal tubule cells. Am J Physiol Renal
Physiol. 314:F702–F714. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chan JY and Kwong M: Impaired expression
of glutathione synthetic enzyme genes in mice with targeted
deletion of the Nrf2 basic-leucine zipper protein. Biochim Biophys
Acta. 1517:19–26. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang H, Magilnick N, Lee C, Kalmaz D, Ou
X, Chan JY and Lu SC: Nrf1 and Nrf2 regulate rat glutamate-cysteine
ligase catalytic subunit transcription indirectly via NF-kappaB and
AP-1. Mol Cell Biol. 25:5933–5946. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sasaki H, Sato H, Kuriyama-Matsumura K,
Sato K, Maebara K, Wang H, Tamba M, Itoh K, Yamamoto M and Bannai
S: Electrophile response element-mediated induction of the
cystine/glutamate exchange transporter gene expression. J Biol
Chem. 277:44765–44771. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Salazar M, Rojo AI, Velasco D, de Sagarra
RM and Cuadrado A: Glycogen synthase kinase-3beta inhibits the
xenobiotic and antioxidant cell response by direct phosphorylation
and nuclear exclusion of the transcription factor Nrf2. J Biol
Chem. 281:14841–14851. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li X, Zou Y, Xing J, Fu YY, Wang KY, Wan
PZ and Zhai XY: Pretreatment with roxadustat (FG-4592) attenuates
folic acid-induced kidney injury through antiferroptosis via
Akt/GSK-3β/Nrf2 pathway. Oxid Med Cell Longev.
2020:62869842020.PubMed/NCBI
|