Introduction
Lung cancer is the leading cause of cancer-related
mortality worldwide, accounting for an estimated 1.6 million deaths
each year (1,2). Non-small cell lung cancer (NSCLC) is
the predominant subtype of lung cancer, accounting for 85% of all
cases, of which lung squamous cell carcinoma and lung
adenocarcinoma are the most common histopathological subtypes
(3,4). Although significant progress has been
made in the development of treatment strategies in previous years,
including surgery, radiotherapy, chemotherapy and targeted
treatment, the prognosis of NSCLC remains unsatisfactory, and the
5-year overall survival rate is only 16% for all stages (5,6). Thus,
elucidating the molecular mechanisms underlying NSCLC development
and progression is crucial for improving the treatment of
NSCLC.
Glutathione S-transferases (GSTs) are enzymes that
can combine with glutathione and various endogenous and exogenous
metabolites during biotransformation (7). In humans, seven cytoplasmic GST
classes have been identified, including α, μ, σ, π, τ, ζ and ω
(8). GST ω 1 (GSTO1), a member of
the ω class of GSTs, has been reported to be associated with
several types of cancer. For example, a previous study revealed
that polymorphisms in GSTO1 increased the risk of hepatocellular
carcinoma (9). In addition,
accumulating evidence has reported the relationship between GSTO1
gene polymorphisms and NSCLC (10–12).
For instance, Bulus et al (13) demonstrated that GSTO1 expression
levels in colon cancer cells were significantly upregulated
compared with in normal colon epithelial cells. Chuang et al
(14) illustrated that the
expression levels of GSTO1 were upregulated in human bladder cancer
cells. Moreover, GSTO1 has been revealed to contribute to cell
growth, death, migration and invasion in several types of cancer
cell (7,15–17).
For example, Wang et al (15) reported that GSTO1 was upregulated in
cutaneous malignant melanoma (CMM) tissues and cells, where it
contributed to CMM cell growth, migration and invasion. Piaggi
et al (17) identified that
GSTO1 overexpression was associated with protection against
cisplatin-induced apoptosis. However, it remains unclear whether
GSTO1 may be involved in the pathogenesis of NSCLC. Thus, the aim
of present study was to investigate the role of GSTO1 in NSCLC and
to determine the potential molecular mechanism.
Materials and methods
Cell culture
NSCLC cell lines (A549, H2122, H292, H1299 and H460)
and normal human lung epithelial cells (BEAS-2B) were purchased
from The Cell Bank of Type Culture Collection of the Chinese
Academy of Sciences. All cells were maintained in RPMI-1640 medium
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml
penicillin and 100 µg/ml streptomycin in an incubator at 37°C with
a 5% CO2 atmosphere. Cell lines in the logarithmic
growth phase were selected for subsequent experiments.
Cell transfection
Cell transfections were performed using
Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol.
Untransfected cells were used as a blank control. Briefly,
4×105 H2122 or A549 cells were plated into a six-well
plate and cultured to 90–95% confluence. The overexpression of
GSTO1 in NSCLC cells was induced through transfection with a
pcDNA3.1 plasmid (4 µg; Invitrogen; Thermo Fisher Scientific, Inc.)
carrying the GSTO1 cDNA insert, with an empty vector (4 µg) as the
negative control (pcDNA3.1-NC). The knockdown of GSTO1 in NSCLC
cells was induced using 2 µg short hairpin RNA (shRNA; Thermo
Fisher Scientific, Inc.) against GSTO1, using stable non-specific
shRNA (2 µg) as the NC (shRNA-NC). The shRNA-NC sequence was
5′-CCGGCCTAAGGTTAAGTCGCCCTCGCTCGAGCGAGGGCGACTTAACCTTAGGTTTTTC-3′
and the shRNA-GSTO1 sequence was 5′-GCTGGAAGCAATGAAGTTA-3′. Cells
were transfected for 24 h at 37°C in an atmosphere containing 5%
CO2 to obtain stably transfected cells for future use.
At 48 h post-transfection, the overexpression and knockdown of
GSTO1 was confirmed using reverse transcription-quantitative PCR
(RT-qPCR) and western blotting.
RT-qPCR
Total RNA was extracted from cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). Total RNA was reverse transcribed into cDNA using a
RevertAid First Strand cDNA Synthesis kit (Thermo Fisher
Scientific, Inc.), which was conducted according to the
manufacturer's protocol. qPCR analysis was subsequently performed
using a SYBR Green RT-PCR kit (Takara Bio, Inc.) on an ABI 7500
Fast Real-Time PCR system (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The following thermocycling conditions were used
for qPCR: Initial denaturation for 10 min at 95°C; followed by 40
cycles of denaturation at 95°C for 15 sec and annealing/extension
at 55°C for 45 sec. The following primer sequences were used for
the qPCR: GSTO1 forward, 5′-AGCATACCCAGGGAAGAAG-3′ and reverse,
5′-CTGCCATCCACAGTTTCAG-3′; and β-actin forward,
5′-CATCGTCCACCGCAAATGCTTC-3′ and reverse,
5′-AACCGACTGCTGTCACCTTCAC-3′. Expression levels were quantified
using the 2−∆∆Cq method (18). β-actin was used as the endogenous
control and expression levels were normalized to β-actin.
Western blotting
Total protein was extracted from cells using RIPA
lysis buffer (Beyotime Institute of Biotechnology). Total protein
was quantified using a Bradford assay and 25 µg total protein/lane
was separated via SDS-PAGE on 10% gels. The separated proteins were
subsequently transferred onto PVDF membranes (EMD Millipore) and
blocked with 5% non-fat milk in TBS with 0.05% Tween-20 for 30 min
at room temperature. The membranes were then incubated with the
following primary antibodies at 4°C overnight: Anti-GSTO1 (1:500;
cat. no. ab129106; Abcam), anti-Bax (1:500; cat. no. ab182733;
Abcam), anti-caspase 3 (1:500; cat. no. ab44976; Abcam), anti-JAK
(1:500; cat. no. ab47435; Abcam), anti-phosphorylated (p)-JAK
(1:500; cat. no. ab138005; Abcam), anti-STAT3 (1:500; cat. no.
ab119352; Abcam), anti-p-STAT3 (1:500; cat. no. ab30647; Abcam) and
anti-β-actin (1:500; cat. no. ab8227; Abcam). Following the primary
antibody incubation, the membranes were incubated with anti-rabbit
HRP-conjugated secondary IgG antibody (1:50,000; cat. no. ab205718;
Abcam) or anti-mouse HRP-conjugated secondary IgG antibody
(1:5,000; cat. no. ab205719; Abcam) for 1 h at room temperature.
Protein bands were visualized using enhanced chemiluminescence
(Pierce; Thermo Fisher Scientific, Inc.). Several X-ray films were
analyzed to verify the linear range of the chemiluminescence
signals and densitometric analysis was performed using ImageJ
software (version 1.41; National Institutes of Health).
Cell Counting Kit-8 (CKK-8) assay
Cell proliferation was analyzed using CCK-8 solution
(Beyotime Institute of Biotechnology), according to the
manufacturer's protocol. Briefly, cells
(1×104−105 cells/well) were seeded into a
96-well plate with the culture medium and incubated in a
CO2 incubator at 37°C for 24 h. Subsequently, 10 µl
CCK-8 solution was added to each well and incubated for another 2 h
at 37°C. Finally, the absorbance was measured at 450 nm using a
microplate reader.
Cell migration and invasion
assays
Cell migration and invasion were analyzed using
Transwell plates. For the migration assay, 1.0×105 cells
in 200 µl serum-free RPMI-1640 medium were plated into the upper
chambers of 8-µm Transwell plates. For the invasion assays,
1.0×105 cells were plated into the upper chamber of
Transwell plates precoated at 37°C for 4–5 h with Matrigel. The
lower chambers for both assays were filled with RPMI-1640 medium
supplemented with 20% FBS. After 24 h of incubation at 37°C,
non-invasive or non-migratory cells were removed from the upper
chambers, and invasive or migratory cells in the lower chamber were
fixed with 100% methanol for 30 min at room temperature. Finally,
cells were stained with 0.1% crystal violet for 30 min at room
temperature, and then visualized and counted using an inverted
fluorescence microscope (Olympus Corporation; magnification, ×100)
with ImageJ software (version 1.8; National Institutes of
Health).
Flow cytometric analysis of
apoptosis
Cell apoptosis was analyzed using an Annexin
V-FITC/propidium iodide (PI) apoptosis kit (BD Biosciences).
Briefly, 1×105 cells were stained with Annexin V-FITC
and PI, according to the manufacturer's protocol. Apoptotic cells
were subsequently analyzed with a BD FACSAria™ Fusion flow
cytometer (BD Biosciences) using ModFit software version 3.2 (BD
Biosciences). The apoptotic rate was calculated as the percentage
of early and late apoptotic cells.
Statistical analysis
All statistical analyses were performed using SPSS
version 22.0 software (IBM Corp.). Data from each experiment are
presented as the mean ± SD of three independent experiments.
Statistical differences between ≥3 groups were determined by
one-way ANOVA followed by a Tukey's post hoc test for multiple
comparisons. P<0.05 was considered to indicate a statistically
significant difference.
Results
GSTO1 promotes the proliferation of
NSCLC cells
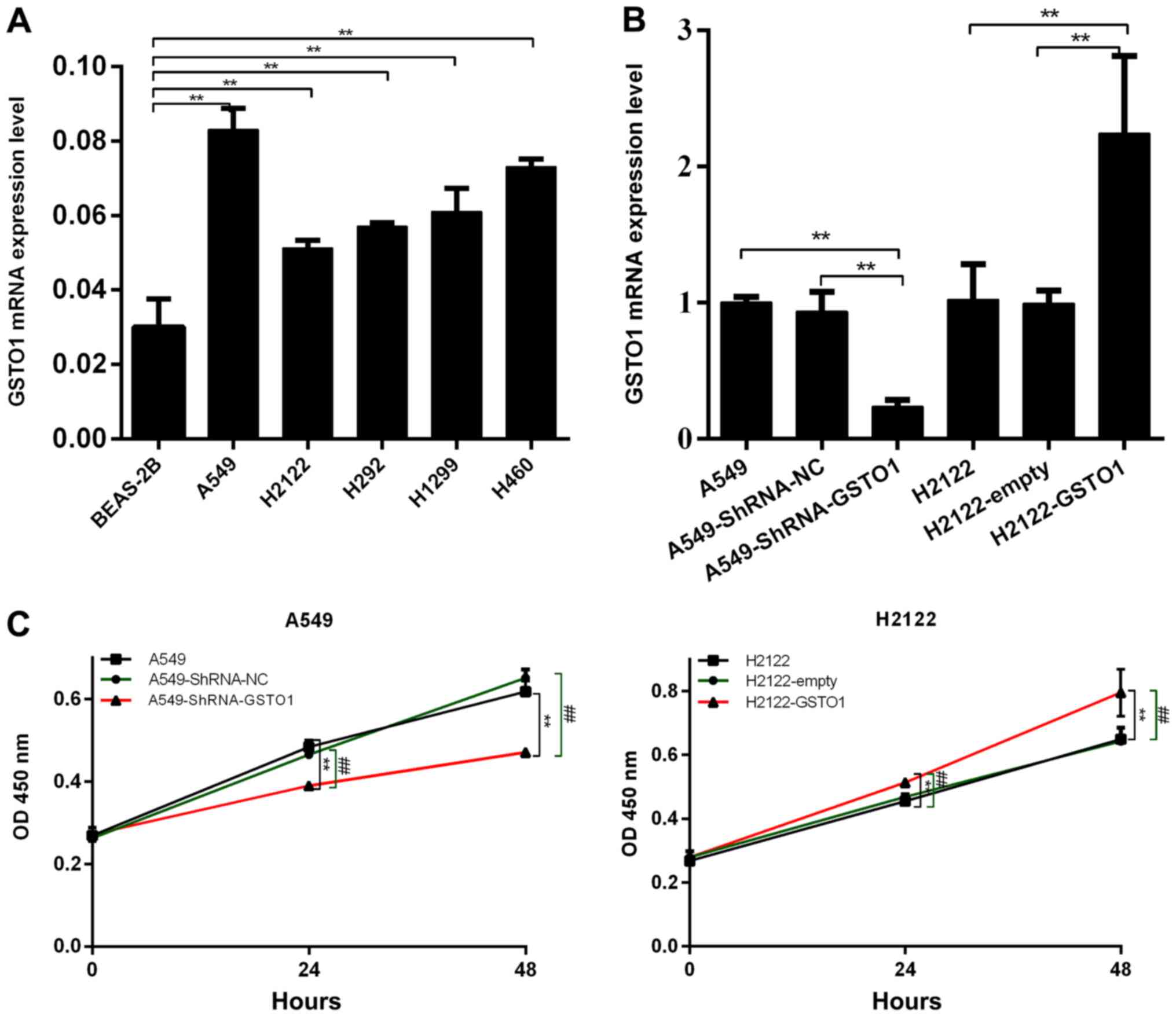
To determine the function of GSTO1 in NSCLC cell
lines, GSTO1 expression levels in several NSCLC cell lines were
analyzed (Fig. 1A). As the
expression levels of GSTO1 were the lowest in the H2122 cell line
compared with BEAS-2B cells (P<0.01), these cells were selected
to construct GSTO1-overexpressing cells. GSTO1 expression levels
were knocked down using shRNA in the A549 cell line, as this cell
line expressed the highest levels of GSTO1 compared with BEAS-2B
cells (P<0.01). RT-qPCR was subsequently used to confirm the
transfection efficiency through analyzing GSTO1 expression levels
in A549 and H2122 cells following the transfections. As shown in
Fig. 1B, GSTO1 expression levels
were significantly downregulated in A549 cells transfected with
shRNA-GSTO1 compared with those in the shRNA-NC and control groups
(P<0.01), whereas GSTO1 expression levels were significantly
upregulated in H2122 cells transfected with pcDNA3.1-GSTO1 compared
with pcDNA3.1-empty and H2122 control cells (P<0.01).
CCK-8 assays were used to investigate
the effect of GSTO1 on the proliferation of NSCLC cells
As shown in Fig. 1C,
the proliferative ability of A549 cells transfected with
shRNA-GSTO1 was significantly inhibited compared with cells
transfected with shRNA-NC (P<0.01). Conversely, the
proliferative ability was significantly increased in cells
transfected with pcDNA3.1-GSTO1 compared with pcDNA3.1-empty-
transfected cells (P<0.01). These results indicated that GSTO1
may promote the proliferation of NSCLC cells.
GSTO1 promotes the migration and
invasion of NSCLC cells
Transwell assays were used to analyze the effect of
GSTO1 on the migratory and invasive abilities of NSCLC cells. The
knockdown of GSTO1 in A549 cells by shRNA significantly decreased
the cell migratory and invasive abilities compared with the
shRNA-NC and control groups (P<0.01), whereas overexpression of
GSTO1 with pcDNA3.1-GSTO1 significantly increased the migratory and
invasive abilities of H2122 cells compared with the
pcDNA3.1-empty-transfected and control cells (P<0.01) (Fig. 2A and B). These results indicated
that GSTO1 may promote the migration and invasiveness of NSCLC
cells.
GSTO1 inhibits the apoptosis of NSCLC
cells
To further confirm the role of GSTO1 in NSCLC cells,
the effects of GSTO1 knockdown or overexpression on apoptosis were
analyzed using flow cytometry. The knockdown of GSTO1 with shRNA
significantly promoted the apoptosis of A549 cells compared with
the shRNA-NC and control groups (P<0.01; Fig. 3A). Following successful transfection
with pcDNA3.1-GSTO1, the overexpression of GSTO1 was discovered to
significantly inhibit the apoptosis of H2122 cells compared with
the pcDNA3.1-empty and control groups (P<0.01). Meanwhile, the
genetic knockdown of GSTO1 significantly upregulated the expression
levels of Bax and caspase 3 compared with the shRNA-NC and control
groups (P<0.01), whereas the overexpression of GSTO1
significantly downregulated the expression levels of Bax and
caspase 3 compared with the pcDNA3.1-empty and control groups
(P<0.01) (Fig. 3B). These
results indicated that GSTO1 may inhibit the apoptosis of NSCLC
cells.
GSTO1 activates the phosphorylation of
JAK and STAT3
To determine the possible mechanisms underlying the
GSTO1-mediated increases in the aggressive phenotypes observed in
NSCLC cells, the effects of GSTO1 on JAK and STAT3 expression
levels were analyzed. The results revealed that the genetic
knockdown of GSTO1 in A549 cells significantly decreased the
phosphorylation levels of JAK and STAT3 compared with the shRNA-NC
and control groups (P<0.01), whereas pcDNA3.1-GSTO1-transfected
H2122 cells had significantly increased phosphorylation levels of
JAK and STAT3 compared with pcDNA3.1-empty-transfected and control
cells (P<0.01) (Fig. 3B). These
results indicated that GSTO1 may promote aggressive phenotypes in
NSCLC cells via activation of the JAK/STAT3 signaling pathway.
Discussion
Despite recent advances in the treatment of NSCLC,
the long-term survival rate of NSCLC remains low, with a 5-year
overall survival rate of 16% (5,6,19,20).
Therefore, further investigations into the mechanism underlying
NSCLC development and progression are essential for improving the
treatment of NSCLC. The expression levels of GSTO1 were previously
discovered to be upregulated in various types of cancer, including
lymphoma, melanoma and colorectal cancer (7,16,21).
However, to the best of our knowledge, the role of GSTO1 in NSCLC
has not been investigated. The present study aimed to investigate
the role of GSTO1 in NSCLC and to determine the potential molecular
mechanism.
To determine the relationship between GSTO1 and
NSCLC, a series of experiments was performed to investigate the
effect of GSTO1 on NSCLC in vitro. The current study first
verified the function of GSTO1 in NSCLC cell lines. The results
demonstrated that GSTO1 overexpression significantly promoted the
proliferation, migration and invasion, and inhibited the apoptosis
of NSCLC cells, whereas knockdown of GSTO1 exerted the opposite
effects. Similarly, Wang et al (15) reported that silencing GSTO1 could
inhibit the growth and aggressiveness of CMM cells, promote cell
cycle arrest and increase cell apoptosis. In addition, Piaggi et
al (17) revealed that GSTO1
overexpression was associated with the protection against
cisplatin-induced apoptosis. These results indicated that GSTO1 may
serve as an oncogene in NSCLC.
To further investigate the possible mechanisms of
the GSTO1-mediated increase in the aggressive phenotypes observed
in NSCLC cells, the effects of GSTO1 on JAK and STAT3 were
investigated. STAT3 is an oncogene, which is known to promote the
proliferation, motility, progression and survival of cancer cells
(22). STAT3 is mainly located in
the cytoplasm, where it can be phosphorylated by JAK-mediated
tyrosine phosphorylation following the stimulation by cytokines
(23). p-STAT3 subsequently
translocates into the nucleus where it acts as transcription
factors for numerous genes involved in cellular apoptosis and
proliferation (24,25). Persistent activation of the
JAK/STAT3 signaling pathway has been observed in various types of
cancer, including NSCLC (26). In
the present study, GSTO1 overexpression was revealed to
significantly increase the phosphorylation levels of JAK and STAT3.
This finding indicated that GSTO1 may promote aggressive phenotypes
in NSCLC cells via activation of the JAK/STAT3 signaling pathway.
Future research should aim to determine the possible mechanism of
the GSTO1- mediated increase in aggressive phenotypes through Gene
Ontology functional term and Kyoto Encyclopedia of Genes and
Genomes signaling pathway enrichment analyses.
In conclusion, the findings of the present study
indicated that GSTO1 may promote the proliferation, migration and
invasion, and inhibit the apoptosis of NSCLC cells via promoting
the phosphorylation of JAK and STAT3. These findings may provide
crucial molecular insights into NSCLC pathogenesis and further
provide a theoretical basis for NSCLC treatment.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KW and WJ conceived and designed the study; KW and
FLZ performed the experiments; KW analyzed and interpreted the
data; KW drafted the manuscript; and WJ provided administrative
support. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
GSTO1
|
glutathione S-transferase ω 1
|
|
NSCLC
|
non-small cell lung cancer
|
|
GST
|
glutathione S-transferase
|
|
CMM
|
cutaneous malignant melanoma
|
|
NC
|
negative control
|
|
RT-qPCR
|
reverse transcription-quantitative
PCR
|
References
|
1
|
Herbst RS, Morgensztern D and Boshoff C:
The biology and management of non-small cell lung cancer. Nature.
553:446–454. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Siegel RL and Jemal A: Lung
cancer statistics. Lung Cancer and Personalized medicine. Ahmad A
and Gadgeel S: Springer; Cham: pp. 1–19. 2016, View Article : Google Scholar
|
|
3
|
Yang Z, He J, Gao P, Niu Y, Zhang J, Wang
L, Liu M, Wei X, Liu C, Zhang C, et al: miR-769-5p suppressed cell
proliferation, migration and invasion by targeting TGFBR1 in
non-small cell lung carcinoma. Oncotarget. 8:113558–113570. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu XL, Gong Y and Zhao DP: Elevated PHD2
expression might serve as a valuable biomarker of poor prognosis in
lung adenocarcinoma, but no lung squamous cell carcinoma. Eur Rev
Med Pharmacol Sci. 22:8731–8739. 2018.PubMed/NCBI
|
|
5
|
Laskin JJ and Sandler AB: State of the art
in therapy for non-small cell lung cancer. Cancer Invest.
23:427–442. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
She K, Huang J, Zhou H, Huang T, Chen G
and He J: lncRNA-SNHG7 promotes the proliferation, migration and
invasion and inhibits apoptosis of lung cancer cells by enhancing
the FAIM2 expression. Oncol Rep. 36:2673–2680. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Manupati K, Debnath S, Goswami K, Bhoj PS,
Chandak HS, Bahekar SP and Das A: Glutathione S-transferase omega 1
inhibition activates JNK-mediated apoptotic response in breast
cancer stem cells. FEBS J. 286:2167–2192. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Whitbread AK, Masoumi A, Tetlow N, Schmuck
E, Coggan M and Board PG: Characterization of the omega class of
glutathione transferases. Methods Enzymol. 401:78–99. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chirila DN, Chirila MD, Micu BV, et al:
The risk of gastric cancer in patients with glutathione
s-transferases (GSTS) gene polymorphisms. Hum Vet Med. 10:104–110.
2018.
|
|
10
|
Ada TG, Ada AO, Kunak SC, Alpar S, Gulhan
M and Iscan M: Association between glutathione S-transferase omega
1 A140D polymorphism in the Turkish population and susceptibility
to non-small cell lung cancer. Arh Hig Rada Toksikol. 64:61–67.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu YT, Wang J, Yin R, Qiu MT and Xu L,
Wang J and Xu L: Genetic polymorphisms in Glutathione S-transferase
Omega (GSTO) and cancer risk: A meta-analysis of 20 studies. Sci
Rep. 4:65782014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Karacaoğlan V, Ada AO, Bilgen S, Çetinkaya
GT, Soydaş E, Kunak CS, Alpar SM, Gülhan M and Işcan M:
Xenobiotic/drug metabolizing enzyme and TP53 polymorphisms and
clinical outcome in advanced nonsmall cell lung cancer patients.
Turk J Med Sci. 47:554–562. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bulus H, Oguztuzun S, Guler Simsek G,
Kilic M, Oguz Ada A, Göl S, Kaya Kocdogan A, Kaygın P, Bozer B and
Iscan M: Expression of CYP and GST in human normal and colon tumor
tissues. Biotech Histochem. 94:1–9. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chuang JJ, Dai YC, Lin YL, Chen YY, Lin
WH, Chan HL and Liu YW: Downregulation of glutathione S-transferase
M1 protein in N-butyl-N-(4-hydroxybutyl)nitrosamine-induced mouse
bladder carcinogenesis. Toxicol Appl Pharmacol. 279:322–330. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang LK, Yue HL, Peng XJ and Zhang SJ:
GSTO1 regards as a meritorious regulator in cutaneous malignant
melanoma cells. Mol Cell Probes. 48:1014492019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Radic T, Coric V, Bukumiric Z,
Pljesa-Ercegovac M, Djukic T, Avramovic N, Matic M, Mihailovic S,
Dragicevic D, Dzamic Z, et al: GSTO1*CC Genotype (rs4925) Predicts
Shorter Survival in Clear Cell Renal Cell Carcinoma Male Patients.
Cancers (Basel). 11:20382019. View Article : Google Scholar
|
|
17
|
Piaggi S, Raggi C, Corti A, Pitzalis E,
Mascherpa MC, Saviozzi M, Pompella A and Casini AF: Glutathione
transferase omega 1-1 (GSTO1-1) plays an anti-apoptotic role in
cell resistance to cisplatin toxicity. Carcinogenesis. 31:804–811.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ding Y, Lu Y, Xie X, Sheng B and Wang Z:
Silencing TRIM37 inhibits the proliferation and migration of
non-small cell lung cancer cells. RSC Advances. 8:36852–36857.
2018. View Article : Google Scholar
|
|
20
|
Besse B, Charrier M, Lapierre V, Dansin E,
Lantz O, Planchard D, Le Chevalier T, Livartoski A, Barlesi F,
Laplanche A, et al: Dendritic cell-derived exosomes as maintenance
immunotherapy after first line chemotherapy in NSCLC.
OncoImmunology. 5:e10710082015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li L, Zhao Y, Cao R, Li L, Cai G, Li J, Qi
X, Chen S and Zhang Z: Activity-based protein profiling reveals
GSTO1 as the covalent target of piperlongumine and a promising
target for combination therapy for cancer. Chem Commun (Camb).
55:4407–4410. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tang SN, Fu J, Shankar S and Srivastava
RK: EGCG enhances the therapeutic potential of gemcitabine and
CP690550 by inhibiting STAT3 signaling pathway in human pancreatic
cancer. PLoS One. 7:e310672012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang T, Ma L, Wu P, Li W, Li T, Gu R, Dan
X, Li Z, Fan X and Xiao Z: Gallic acid has anticancer activity and
enhances the anticancer effects of cisplatin in non small cell lung
cancer A549 cells via the JAK/STAT3 signaling pathway. Oncol Rep.
41:1779–1788. 2019.PubMed/NCBI
|
|
24
|
Wen W, Liang W, Wu J, Kowolik CM, Buettner
R, Scuto A, Hsieh MY, Hong H, Brown CE, Forman SJ, et al: Targeting
JAK1/STAT3 signaling suppresses tumor progression and metastasis in
a peritoneal model of human ovarian cancer. Mol Cancer Ther.
13:3037–3048. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou F, Cheng L, Qiu LX, Wang MY, Li J,
Sun MH, Yang YJ, Wang JC, Jin L, Wang YN, et al: Associations of
potentially functional variants in IL-6, JAKs and STAT3 with
gastric cancer risk in an eastern Chinese population. Oncotarget.
7:28112–28123. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang CL, Liu YY, Ma YG, Xue YX, Liu DG,
Ren Y, Liu XB, Li Y and Li Z: Curcumin blocks small cell lung
cancer cells migration, invasion, angiogenesis, cell cycle and
neoplasia through Janus kinase-STAT3 signalling pathway. PLoS One.
7:e379602012. View Article : Google Scholar : PubMed/NCBI
|