Introduction
Melanoma is a group of severe malignant tumors that
develop from transformation of melanocytes and grow on human skin,
in the mouth, eyes and intestinal tissues (1,2).
Melanoma is the fifth and sixth most common of the human cancers
affecting male and female patients respectively, according to
recent epidemiological investigations. Globally new melanoma cases
occur in >0.2 million individuals each year, an incidence that
has been continuously on the rise during the past years (3,4).
Patients with melanoma at advanced stages who are not suitable for
surgery, chemotherapy nor immunotherapy are commonly treated with
drugs such as temozolomide and dacarbazine, but the efficiency of
this treatment is often impaired by drug resistance (5,6). The
development of novel anti-melanoma drugs critically depends on the
complete understanding of the molecular pathogenic mechanisms in
melanoma.
EPHA7 (ephrin receptor A7) is a member of the Eph
family of receptor tyrosine kinases, which are expressed in
erythropoietin-producing human hepatocellular carcinoma cells
(7). They are commonly known to be
involved in embryonic development, angiogenesis, development of
nervous and vascular systems, and a number of human pathogenic
conditions, including numerous types of tumor, inflammatory
diseases and neurological diseases (8,9).
Alteration of EPHA7 expression has been identified to be closely
associated with the development of several types of human cancer
over the past decade, such as colon and prostate cancer. For
instance, high expression of EPHA7 is associated with adverse
results among patients with primary and recurrent glioblastoma
multiforme (10). By contrast,
expression of the EPHA7 is markedly downregulated in colorectal
cancer tissues, presumably caused by hypermethylation in the 5′CpG
island of EPHA7 (9). EPHA7 is
substantially mutated in patients with melanoma with frequent
mutations recorded in tumor samples, as demonstrated by a
meta-analysis of somatic mutations on the basis of next-generation
sequencing (11). Nevertheless, the
expressional state, regulating mechanism and functions of EPHA7 in
melanoma remain to be elucidated.
MicroRNAs (miRNAs/miRs) are a large group of
non-coding RNA molecules, commonly with 22 nucleotides, which
target expression levels of genes by directly binding with the
3′-untranslated region (UTR) of functional genes (12). The post-transcriptional gene
expression regulation by miRNAs is extensively associated with
various biological processes and human disorders including several
types of cancer, such as gastric, liver and breast cancer (12–14).
Certain miRNAs are also known to modulate the function of EPHA7.
For instance, miR-944 targets EPHA7 to influence the progression of
lung cancer cells (15). In
addition, numerous miRNAs serve crucial roles in regulating the
expression of key genes associated with melanoma pathogenesis and
drug responses (16,17). miR-18a-5p acts has been demonstrated
to be associated with cancer development and progression (18). The high levels of miR-18a-5p
expression in non-small cell lung cancer (NSCLC) tissues promote
proliferation and migration, and suppress apoptosis of cancerous
cells by targeting interferon regulatory factor 2 gene expression
(19). The expression of miR-18a-5p
was reported to be markedly elevated in melanoma cell lines
compared with normal human epidermal melanocytes, suggesting
pathogenic roles for miR-18a-5p in the progression of melanoma
(20). However, the pathogenic
roles of high expression levels of miR-18a-5p in melanoma cells and
the underlying molecular mechanisms, require further
investigation.
The present study performed extensive analysis of
miR-18a-5p expression and its cellular functions associated with
melanoma development and progression using both clinical tissues
and cellular models. EPHA7 was confirmed to be a target gene of
miR-18a-5p in melanoma cells. The findings of the present study
revealed novel molecular mechanisms through which melanoma
pathogenesis is promoted, which may support miRNA-based melanoma
diagnosis and treatment.
Materials and methods
Tissue collection and cell
culture
Melanoma and normal skin tissues were collected from
20 patients diagnosed with malignant melanoma who underwent
surgical treatment at the Department of Dermatology of the
Guangzhou First People's Hospital (Guangdong, China) between July
2018 and November 2018; information about the stage and clinical
status of the patient are presented in Table I. All patients provided written
consent prior to the surgery and the entire study was approved by
the Medical Ethics Committee of the Guangzhou First People's
Hospital (approval no. K-2019-135-01). The present study was
performed in accordance with the Declaration of Helsinki (21). The American Type Culture Collection
supplied the normal human epidermal melanocyte PIG1 and melanoma
cell lines WM266-4, A375, VMM5A and A2058. The cells were cultured
in Dulbecco's modified Eagle's medium (DMEM; Thermo Fisher
Scientific, Inc.) with 10% fetal bovine serum (Thermo Fisher
Scientific, Inc.) and 1% penicillin or streptomycin at 37°C in a
humidified chamber with 5% CO2.
 | Table I.Histological subtypes of cutaneous
melanoma. |
Table I.
Histological subtypes of cutaneous
melanoma.
|
|
|
| Sex (case no.) | In situ or
invasive (case no.) |
| Localization of the
primary lesion (case no.) |
|---|
|
|
|
|
|
|
|
|
|---|
| Histological
Subtype | Patients, n
(%) | Age of onset,
median (years) | Male | Female | In situ | Invasive | Course of disease,
median (months) | Head and Neck | Trunk | Limbs | Hands | Feet |
|---|
| LMM | 3 (15.00) | 42 | 2 | 1 | 1 | 2 | 36 | 2 | 1 | 0 | 0 | 0 |
| SSM | 2 (10.00) | 52 | 1 | 1 | 1 | 1 | 30 | 1 | 1 | 0 | 0 | 0 |
| ALM | 13 (65.00) | 56 | 7 | 6 | 7 | 6 | 36 | 0 | 0 | 1 | 3 | 9 |
| NM | 1 (5.00) | 9 | 0 | 1 | 0 | 1 | 24 | 0 | 0 | 1 | 0 | 0 |
| Metastatic MM | 1 (5.00) | 45 | 1 | 0 | 0 | 0 | 28 | 0 | 1 | 0 | 0 | 0 |
| DM | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 20 | 51 | 11 | 9 | 9 | 10 | 34 | 3 | 3 | 2 | 3 | 9 |
Reverse transcription-quantitative
(RT-q) PCR
To examine the relative expression levels of miRNA
and mRNAs, total RNA was extracted from the skin tissues or
cultured cells with TRIzol® (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocols. A
NanoDrop spectrophotometer (Thermo Fisher Scientific, Inc.) was
used to record the concentration and quality of RNA. On assessing
the RNA quality and concentration using a NanoDrop
spectrophotometer (Thermo Fisher Scientific, Inc.), equal volumes
of RNA samples (3.0 µg) were collected from each group for cDNA
library synthesis with the Bestar™ qPCR RT kit (cat. no. 2220; DBI
Bioscience), according to the manufacturer's protocol, at 37°C for
1 min, 50°C for 60 min and 70°C for 15 min. Then, relative levels
of miRNA or mRNA were measured by RT-qPCR using the Bestar™ qPCR
MasterMix kit (cat. no. 2043; DBI Bioscience), according to the
following procedure: Pre-denaturation at 95°C for 2 min, with 42
cycles of denaturation at 94°C for 20 sec; annealing at 58°C for 20
sec and extension at 72°C for 20 sec. The maximum cycle number
considered for gene expression is 42. U6 and GAPDH were used as the
internal standard for quantitation of miRNA and EPHA7 expression,
respectively. Expression levels were assessed by the standard
2−ΔΔCq method (22). The
experiment was repeated three times. The primers used for RT-qPCR
are listed in Table II.
 | Table II.Sequences of primers used for reverse
transcription-quantitative PCR. |
Table II.
Sequences of primers used for reverse
transcription-quantitative PCR.
| Gene ID | Primer sequence
(5′→3′) | Product length
(bp) |
|---|
| GAPDH | Forward:
TGTTCGTCATGGGTGTGAAC | 154 |
|
| Reverse:
ATGGCATGGACTGTGGTCAT |
|
| EPHA7 | Forward:
GTGAAGATGGGTATTACAGGGC | 187 |
|
| Reverse:
CAACTGCACCGCTTACACAAT |
|
| U6 | Forward:
CTCGCTTCGGCAGCACA | 96 |
|
| Reverse:
AACGCTTCACGAATTTGCGT |
|
| hsa-miR-18a-5p | Forward:
ACACTCCAGCTGGGTAAGGTGCATCTAGTGCAG | 327 |
|
| Reverse:
CTCAACTGGTGTCGTGGA |
|
|
| Reverse
transcription: CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGCTATCTGC |
|
Cell transfection
To determine the effect of miR-18a-5p expression in
melanoma cells, the miR-18a-5p mimics
(5′-UAAGGUGCAUCUAGUGCAGAUAG-3′), miR-18a-5p inhibitors
(5′-CCAGAAGGAGCACUUAGGGCAGU-3′) and negative control (NC;
5′-CAGUACUUUUGUGUAGUACAA-3′) were produced and supplied by Shanghai
GenePharma Co., Ltd. To determine the overexpression of EPHA7 in
melanoma cells, full-length genomic sequences of EPHA7 were
amplified using EPHA7-F1 (5′-CGGATCCATGGTTTTTCAAACTCGGTACCCTTC-3′)
and EPHA7-R1 (5′-CCCTCGAGTCACACTTGAATGCCAGTTCCATGT-3′), which were
then ligated with the pcDNA3.0 plasmid (Vipotion Biotechnology Co.,
Ltd.). The miRNA mimics, inhibitors and recombinant plasmids were
introduced into the cultured melanoma cells using
Lipofectamine® 2000 transfection reagent (cat. no.
11668019; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocols. The quantity of all mimics or inhibitors
used was 100 nM. Alterations of miRNA and EPHA7 expression levels
were confirmed by RT-qPCR following 48 h of transfection at
37°C.
Colony formation assay
Melanoma cell proliferation rate was evaluated by
performing a colony formation assay. Following cell transfection,
WM266 and A375 cells were resuspended in DMEM and seeded in 6-well
plates (100 cells/well). These were cultured under normal
conditions for another 2 weeks at 37°C. The cells were then fixed
using 4% paraformaldehyde for 12 min at 37°C and stained using 0.1%
crystal violet for 30 min at room temperature. Cell colonies were
defined as >50 cells and counted under a fluorescent microscope
(magnification, ×400) in three randomly selected fields of view for
cell proliferation comparison. For statistical analysis, the
experiments were performed in triplicate.
Cell counting Kit-8 (CCK-8) assay
The CCK-8 (Dojindo Molecular Technologies, Inc.)
assay was performed to assess melanoma cell proliferation,
according to the manufacturer's protocols. The cell suspensions
(4,000 cells/well; 100 µl) were seeded in 96-well plates and
maintained at 37°C in a humidified chamber under normal conditions
for 24, 48 and 72 h. Thereafter, the cells were incubated using 10
µl CCK-8 solution for 4 h and absorbance measured at 450 nm
(OD450) with a microplate reader. Cell proliferation was
analyzed on the basis of OD450 values from ≥3
replicates.
Flow cytometry
Flow cytometry was performed to evaluate cell early
apoptosis using the FITC Annexin V Apoptosis Detection kit
(BioLegend, Inc.), according to the manufacturer's protocol.
Melanoma cells cultured in 6-well plates were rinsed three times
using cell staining buffer, resuspended in Annexin V Binding Buffer
(5×106 cells/ml) and incubated using 5 µl FITC Annexin V
and 7-AAD Viability Staining Solution for 15 min at room
temperature in the dark. Finally, the cells were mixed with Annexin
V Binding Buffer and analyzed with a FACScan flow cytometry system
(BD Biosciences) and FlowJo software (version 10; FlowJo LLC). Flow
cytometry analysis experiments were repeated ≥3 times.
Hoechst staining
Apoptosis of WM266 and A375 cells was also evaluated
using Hoechst 33258 reagents (cat. no. C1011; Beyotime Institute of
Biotechnology) according to the manufacturer's protocols. Melanoma
cells (1×105 cells/well) grown on cell slides were mixed
with the fixation solution at 4°C for 12 min, rinsed three times
using PBS, stained with Hoechst 33258 for 5–8 min at 37°C with
agitation, rinsed again with PBS for 3 min at room temperature and
eventually observed in three randomly selected fields of view at a
wavelength of 460 nm using fluorescence microscopy (magnification,
×400); ≥3 replicates of the experiments were performed.
Dual luciferase reporter assay
Dual-Luciferase reporter assay was performed to
validate the binding of miRNA 18a-5p with the 3′-UTR region of
EPHA7 in WM266 and A375 cells. The wild-type (WT) and mutated (MUT)
3′-UTR regions of EPHA7 were ligated separately with the Psi-CHECK2
plasmids (Promega Corporation), which were then transfected into
WM266 and A375 cells (1×105 cells/well) using
Lipofectamine 2000 as aforementioned, together with miRNA 18a-5p
mimics or NC as designated. The cells were cultured under normal
conditions at 37°C for 48 h, followed by rinsing three times with
PBS and incubation with passive lysis buffer. A GloMax®
Bioluminescence detector (Promega Corporation) was used to measure
the luciferase activity of cell lysates. The luciferase activity
was normalized to Renilla luciferase activity.
Western blot analysis
Cell lysis buffer (cat. no. P0013; Beyotime
Institute of Biotechnology) was used to extract total proteins from
cultured cells for western blotting and immunoprecipitation
according to the manufacturer's protocols. The concentration of
protein was measured by a spectrophotometer at 280 nm using the BCA
method. Total protein (~25 µg) collected from each group was boiled
at 100°C for 5 min, separated by 10% SDS-PAGE and blotted onto
polyvinylidene difluoride membranes. Thereafter, the membranes were
blocked with 5% lipid-free liquid milk for 1–2 h at room
temperature, and then incubated with primary antibodies overnight
at 4°C and secondary antibodies for 2 h each at room temperature,
and eventually developed with the Pierce ECL Western Blotting
Substrate (Thermo Fisher Scientific, Inc.), and analyzed using
ImageJ software (v1.8.0; National Institutes of Health). The
following antibodies were used: anti-pro caspase-3 (1:800; cat. no.
MA1-41163; Invitrogen; Thermo Fisher Scientific, Inc.), anti-pro
caspase-9 (1:1,000; cat. no. MA5-32,431; Invitrogen; Thermo Fisher
Scientific, Inc.), anti-cleaved caspase-3 (1:500; cat. no. ab2302;
Abcam), anti-cleaved caspase-9 (1:500; cat. no. ab219590; Abcam),
anti-autophagy marker light chain 3-I/II (LC3I/II; cat. no. 8899;
CST Biological Reagents Co., Ltd.), anti-p62 (1:1,000; cat. no.
ab109012; Abcam), anti-GAPDH antibodies (1:1,000; cat. no. ab9484;
Abcam), goat anti-rabbit IgG (1:800; cat. no. ab205718; Abcam) and
goat anti-mouse IgG (1:800; cat. no. ab205719; Abcam).
Statistical analysis
Data from ≥3 replicates of all experiments are
presented as the mean ± standard deviation, and were analyzed using
SPSS 20.0 (IBM Corp.). Differences between 2 groups were assessed
by an unpaired Student's t-test, while differences between ≥2
groups were analyzed using a one-way analysis of variance, with a
Tukey's post hoc test. P<0.05 was considered to indicate a
statistically significant difference. Pearson's correlation
analysis was used to analyze the correlation between miR-18a-5p and
EPHA7 expression levels.
Results
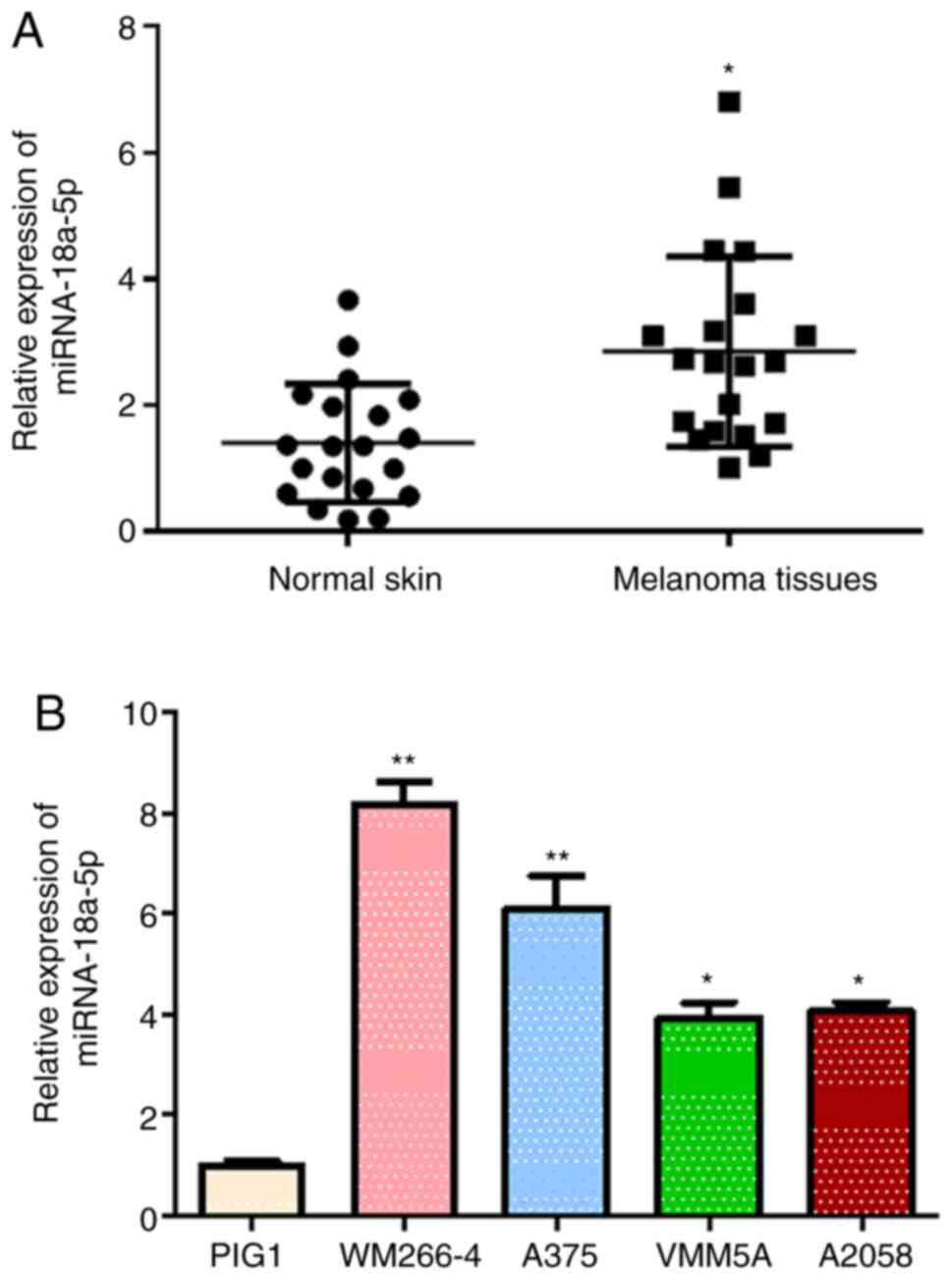
Elevated miR-18a-5p expression in
melanoma tissues and cells
The alterations of miR-18a-5p expression in melanoma
tissues and established cell lines were identified to investigate
the potential involvement of miR-18a-5p in melanoma pathogenesis.
miR-18a-5p expression levels in melanoma tissues collected from 20
patients were identified to be significantly higher compared with
that in the corresponding adjacent normal skin tissues (Fig. 1A). The miR-18a-5p expression levels
in four human melanoma cell lines WM266-4, A375, VMM5A and A2058
were observed to be significantly elevated compared with those in
the normal skin cell line PIG1 (Fig.
1B). miR-18a-5p expression levels were increased most
significantly in the WM266-4 and A375 cell lines compared with the
other two melanoma cell lines, so these cells were used as cellular
models for the following assays. Increased miR-18a-5p expression in
melanoma tissues and cell lines suggested the potential roles of
miR-18a-5p during melanoma pathogenesis.
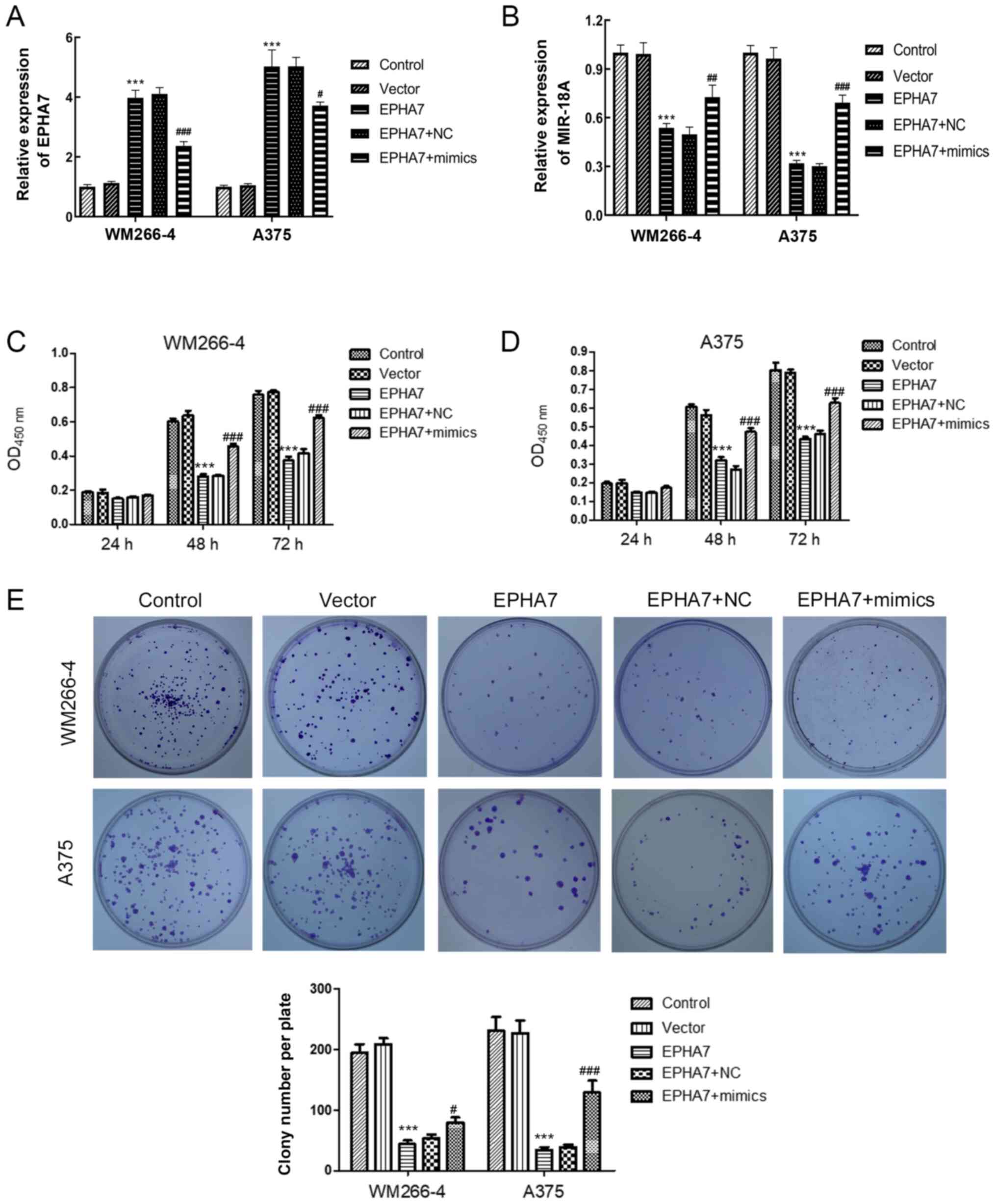
miR-18a-5p promotes proliferation and
induces apoptosis and autophagy in melanoma cells
The expression of miR-18a-5p in two melanoma cell
lines, WM266-4 and A375, was knocked down through transfection
using specific inhibitors targeting miR-18a-5p (Fig. 2A) to investigate the cellular
function of elevated miR-18a-5p expression in melanoma development.
The proliferation rates of WM266-4 and A375 cells were revealed to
be markedly decreased by miR-18a-5p inhibitors, compared with the
NC groups, as evidenced by the CCK-8 assay (Fig. 2B). The colony formation assay showed
a significant reduction in colony formation efficiency of WM266-4
and A375 cells induced by miR-18a-5p inhibitors (Fig. 2C). The percentages of apoptotic
WM266-4 and A375 cells were significantly increased through
transfection with miR-18a-5p inhibitors in the flow cytometry assay
(Fig. 2D). The Hoechst staining
assay also revealed a marked elevation of apoptotic WM266-4 and
A375 cells following miR-18a-5p inhibitor treatment compared with
the NC groups (Fig. 2E). The
results of western blot analysis revealed cleaved-caspase-3 and
cleaved-caspase-9, the two apoptosis marker proteins, to be
markedly upregulated in both WM266-4 and A375 cells following
miR-18a-5p knockdown, whereas the expression levels of
pro-caspase-3 and pro-caspase-9 were notably decreased (Fig. 2F). The LC3-II protein levels in
WM266-4 and A375 cells were notably elevated by transfection with
miR-18a-5p inhibitors, whereas autophagy target protein p62 levels
were markedly reduced (Fig. 2F).
The aforementioned findings indicated the roles miR-18a-5p served
in regulating apoptosis and autophagy during melanoma
development.
miR-18a-5p suppresses EPHA7 expression
by binding with its 3′-UTR region in melanoma cells
The expression of EPHA7 was further studied in
melanoma cells with alterations in miR-18a-5p expression to
investigate the correlation between miR-18a-5p and EPHA7 expression
during melanoma pathogenesis. The expression of miR-18a-5p was
significantly overexpressed following transfection with miR-18a-5p
mimics (Fig. 3A). EPHA7 expression
levels in WM266-4 and A375 cells were observed to be significantly
suppressed by transfection with miR-18a-5p mimics, but were
significantly elevated by transfection with miR-18a-5p inhibitors
compared with the NC groups (Fig.
3B). Western blotting results were consistent with RT-qPCR
(Fig. 3C). The expression of EPHA7
in clinical melanoma tissues was also significantly lower compared
with normal skin tissues (Fig. 3D).
The expression of EPHA7 exhibited a significantly negative
correlation with the miR-18a-5p expression in clinical melanoma
tissues and normal skin tissues (Fig.
3E). In addition, EPHA7 expression levels were observed to be
significantly lower in WM266-4, A375, VMM5A and A2058 cells
compared with PIG1 cells (Fig. 3F).
miR-18a-5p mimics were observed to cause a marked decrease in the
luciferase activity of WM266-4 and A375 cells expressing the WT
EPHA7 3′-UTR sequences, but not in WM266-4 and A375 cells
expressing the MUT EPHA7 3′-UTR sequences (Fig. 3G and H). These findings suggested
that miR-18a-5p may inhibit EPHA7 expression by directly binding
with EPHA7 3′-UTR sequences in melanoma cells.
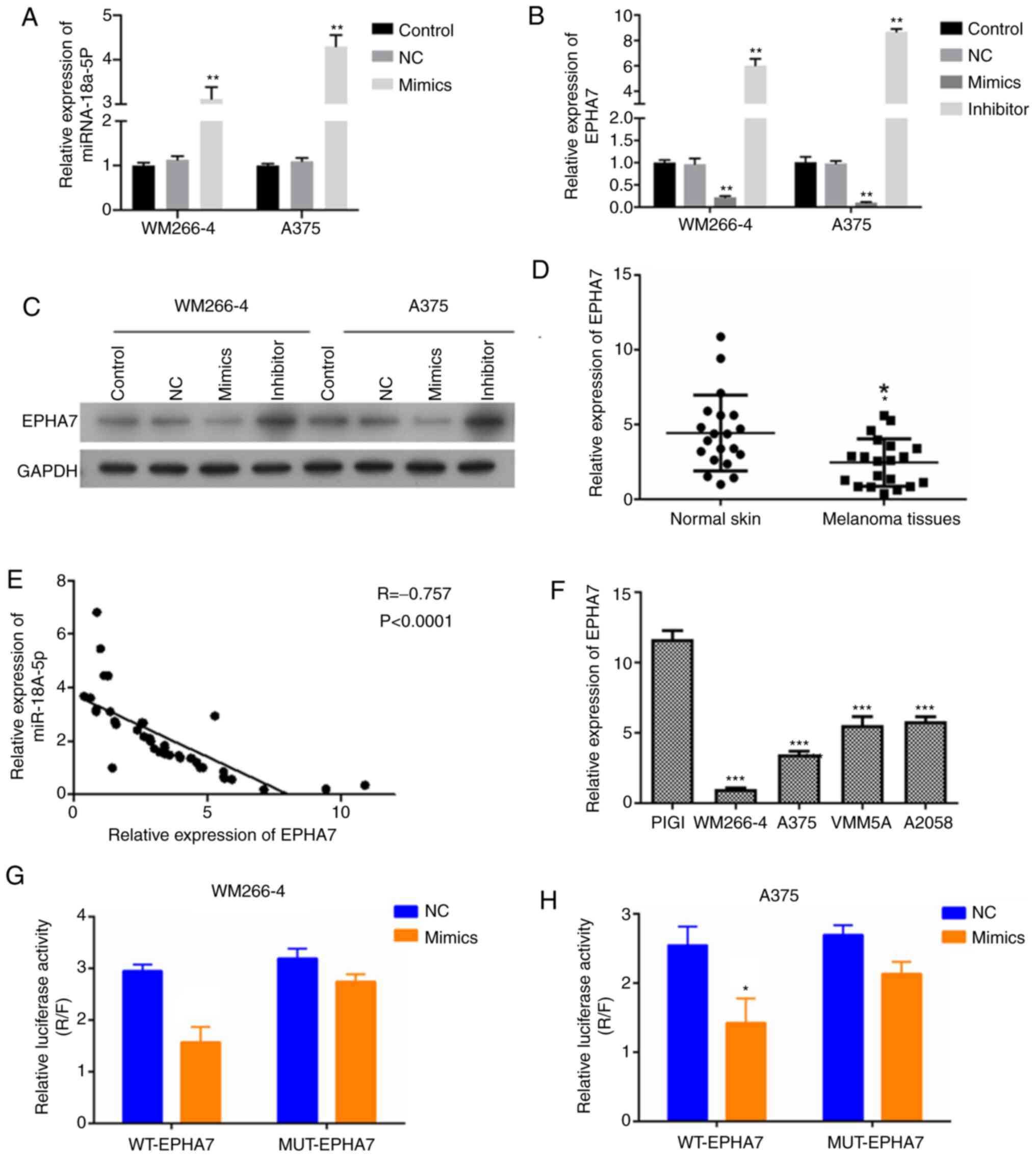 | Figure 3.miR-18a-5p suppresses EPHA7
expression by binding to its 3′-UTR region in melanoma cells. (A)
Relative miR-18a-5p expression levels in WM266-4 and A375 cells
transfected with miR-18a-5p mimics. Gene expression was measured by
RT-qPCR. (B) Relative EPHA7 expression levels in WM266-4 and A375
cells transfected with miR-18a-5p mimics or inhibitors. Gene
expression was measured by RT-qPCR. (C) EPHA7 protein expression in
WM266-4 and A375 cells following transfection with miR-18a-5p
mimics or inhibitors. Protein expression was analyzed by western
blotting, with GAPDH as the internal standard. (D) Relative EPHA7
expression in melanoma tissues and adjacent normal skin tissues
collected from 20 patients. EPHA7 expression was measured by
RT-qPCR. (E) Negative correlation between miR-18a-5p and EPHA7
expression in melanoma tissues and adjacent normal skin tissues.
(F) Expression of EPHA7 in the indicated cell lines was
examined by RT-qPCR. (G and H) Binding of miR-18a-5p with
EPHA7 3′-UTR sequences in WM266-4 and A375 cells was
confirmed by a Dual-Luciferase reporter assay. miR-18a-5p mimics
significantly repressed luciferase activity in cells expressing WT
EPHA7 3′-UTR sequences, but not in cells expressing the MUT
EPHA7 3′-UTR sequences. *P<0.05, **P<0.01,
***P<0.001 vs. control group. miR, microRNA; RT-qPCR, reverse
transcription-quantitative PCR; NC, negative control; EPHA7, ephrin
receptor A7; UTR, untranslated region; WT, wild-type; MUT,
mutant. |
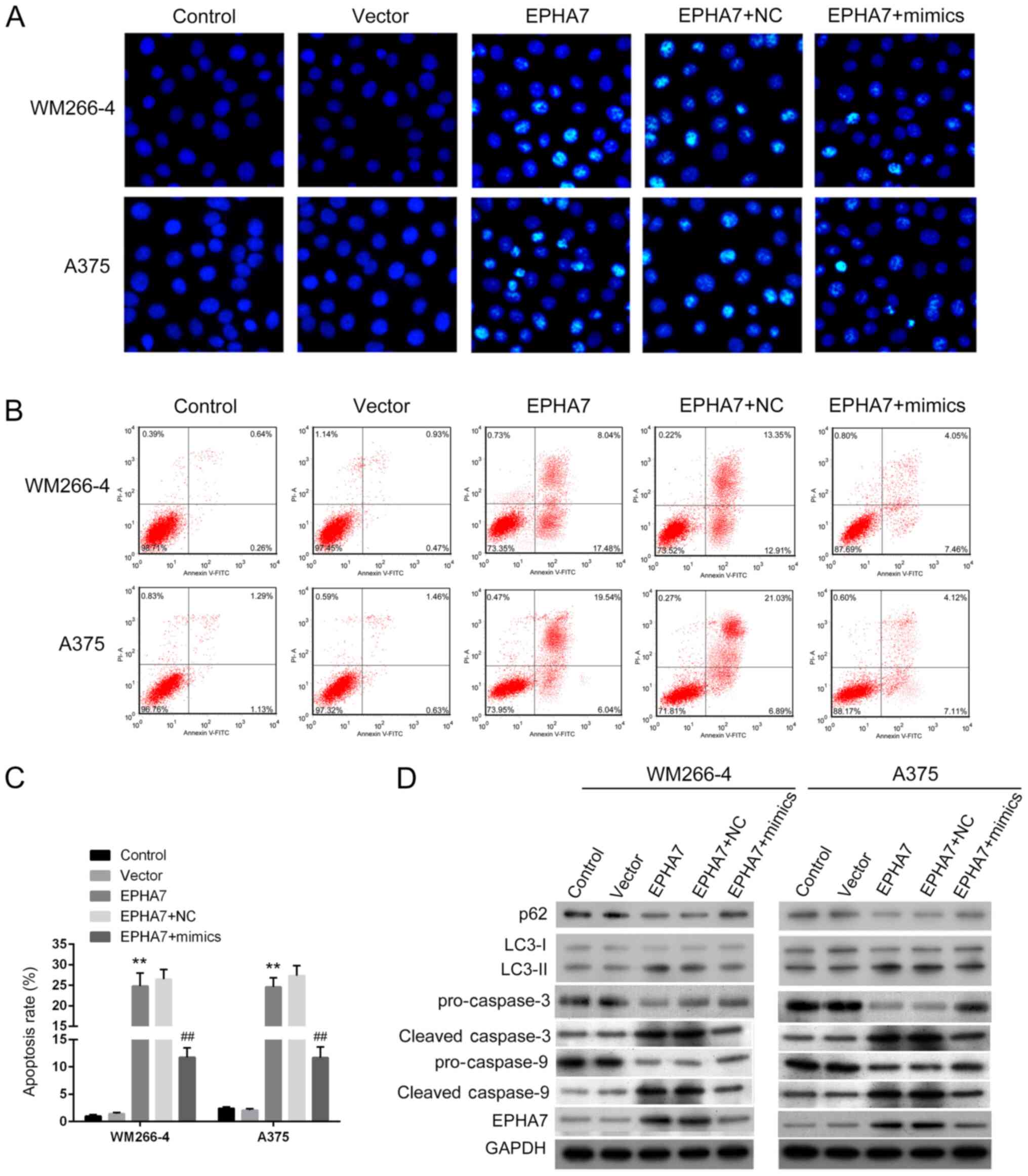
miR-18a-5p promotes melanoma cell
proliferation and inhibits apoptosis and autophagy by suppressing
EPHA7 expression
WM266-4 and A375 cells that overexpressed
EPHA7 were established and treated with miR-18a-5p mimics to
investigate the regulatory roles of EPHA7 in miR-18a-5p-promoted
melanoma cell functions. EPHA7 expression in WM266-4 and A375 cells
was observed to be significantly increased by transfection with
EPHA7-overexpressing vector in the present study, but the effect of
overexpression was partially inhibited by the addition of
miR-18a-5p mimics (Fig. 4A). The
level of miR-18a was significantly suppressed by transfection with
EPHA7-overexpressing vector and this could be reversed with the
addition of miR-18a-5p mimics (Fig.
4B). Overexpression of EPHA7 significantly decreased the
proliferation rates of WM266-4 and A375 cells compared with the NC
groups, as assessed by a CCK-8 assay (Fig. 4C). Nevertheless, the lowered cell
proliferation rates in WM266-4 and A375 cells due to EPHA7
overexpression were significantly reversed by transfection with
miR-18a-5p mimics (Fig. 4C and D).
The colony formation assay revealed that EPHA7 overexpression led
to significantly reduced numbers of WM266-4 and A375 colonies,
which was significantly recovered by transfection with miR-18a-5p
mimics (Fig. 4E). EPHA7
overexpression markedly increased apoptotic WM266-4 and A375 cells
compared with the untransfected or empty vector-transfected cells
(Fig. 5A). miR-18a-5p mimics
transfection notably reduced the number of apoptotic cells
(Fig. 5A). The apoptotic rates in
WM266-4 and A375 cells were identified to be significantly elevated
by EPHA7 overexpression (Fig. 5B and
C). Transfection with miR-18a-5p mimics significantly
eliminated the increase in apoptotic cells triggered by
overexpression of EPHA7 (Fig. 5B and
C). The expression levels of apoptosis marker proteins
cleaved-caspase-3 and cleaved-caspase-9 in WM266-4 and A375 cells,
in addition to autophagy-related protein LC3-II, were upregulated
by overexpression of EPHA7 and were inhibited by simultaneous
transfection with miR-18a-5p mimics (Fig. 5D). Targets that indicate excessive
autophagy degradation, p62 and LC3-I, and pro-caspase-3 and
pro-caspase-9 proteins, exhibited completely opposite changes
(Fig. 5D). These results indicated
that miR-18a-5p could induce melanoma cell proliferation and
suppress apoptosis and autophagy by inhibiting EPHA7
expression.
Discussion
Non-coding RNAs, including miRNA, long non-coding
RNA and circular RNA, have been identified as crucial epigenetic
regulators of various biological processes and pathogenic
conditions (23,24). miRNAs have also been associated with
melanoma in previous studies (16,17),
but the precise roles and molecular mechanisms of miRNAs in the
initiation and development of melanoma requires further study.
miR-18a-5p promotes proliferation and migration, and suppresses
apoptosis of lung cancer cells, and is also significantly increased
in malignant melanoma tissues (17,19);
however, its pathogenic roles and mechanisms in melanoma cells
remains to be elucidated. In the present study, significantly
increased miR-18a-5p expression levels were recorded in clinical
melanoma tissues and cell lines. By eliminating miR-18a-5p
expression in melanoma cells, miR-18a-5p was demonstrated to
promote melanoma cell proliferation and suppress apoptosis and
autophagy in melanoma cells. miR-18a-5p was also shown to directly
bind with the 3′-UTR region of the EPHA7 gene and suppress its
expression in melanoma cells, which mediated the effects of
miR-18a-5p expression on melanoma cell proliferation, apoptosis and
autophagy. These results revealed a novel miRNA-target network
underlying the pathogenesis of melanoma, which may be explored
further for non-coding RNA-based melanoma diagnosis and
treatment.
On the basis of previous studies, miR-18a-5p has
been demonstrated to serve as a significant epigenetic regulator in
various types of human cancer, including NSCLC (19), and breast (25,26)
and prostate cancers (27). The
regulatory functions of miR-18a-5p in melanoma cancer cell
proliferation and apoptosis were demonstrated in the present study
by transfection with miR-18a-5p inhibitors. miR-18a-5p knockdown
also led to a notable elevation of the two apoptosis marker
proteins caspase-3 and caspase-9, which serve as crucial components
of the mitochondria-cytochrome c-caspase cascade of
apoptosis processes (28). For the
first time, to the best of our knowledge, the present study linked
the cellular functions of miR-18a-5p to melanoma development and
progression, which further established the widespread roles of
miR-18a-5p in tumorigenesis and broadened the existing knowledge on
melanoma molecular pathogenic mechanisms in terms of non-coding
RNAs.
The pathogenic functions of miR-18a-5p in different
types of cancer may be mediated by regulating distinct target genes
(27). The suppressive effects of
miR-18a-5p on EPHA7 expression was further validated in two
melanoma cell lines in the present study. The Dual-Luciferase
reporter assay also confirmed the direct binding of miR-18a-5p with
the 3′-UTR region of the EPHA7 gene. The effects of EPHA7 on
melanoma cell proliferation and apoptosis processes were reversed
by transfection with miR-18a-5p mimics. These findings revealed
EPHA7 as a target gene of miR-18a-5p in melanoma pathogenesis.
Notably, the alteration of EPHA7 expression is also associated with
the development of other types of human cancer, including
glioblastoma multiforme, and colorectal, prostate and gastric
cancers (9,10,29–31).
The miR-18a-5p/EPHA7 axis may also mediate the pathogenesis of the
types of human cancer mentioned above as indicated by the
involvement of miR-18a-5p in melanoma; this requires further
investigation.
In addition, the miR-18a-5p/EPHA7 axis was
established to alter the expression of two autophagy-related
proteins LC3-I/II and p62 in melanoma cells. LC3II is one of the
most specific autophagy biomarkers induced by autophagy-associated
genes, including autophagy-related protein 3 (ATG3) and ATG7, and
is known to closely bind with autophagosome membranes (32). By contrast, p62 protein is a
commonly known degradation target of autophagy and the selective
p62 degradation by autophagy has been observed to mediate the
inhibitory roles of autophagy in tumorigenesis (33,34).
In the present study, LC3-II activation was demonstrated to be
elevated by miR-18a-5p inhibitors, but p62 protein expression was
suppressed by miR-18a-5p knockdown in melanoma cells. Similar
LC3-II activation and alterations in p62 protein expression were
also observed to be caused by EPHA7 overexpression. Molecular
evidence suggested that the miR-18a-5p/EPHA7 axis modulated
autophagy in melanoma cells, which may also be involved in
miR-18a-5p-regulated melanoma development and progression.
Autophagy has as established role in melanoma development and as a
common response to antitumor therapies (35,36).
The pathogenic roles of autophagy alteration by miR-18a-5p in
melanoma pathogenesis requires further investigations involving
cellular and animal models. A limitation of the present study
remains that the role of miR-18a-5p in melanoma needs to be
validated through a xenograft tumor model. Furthermore, the
therapeutic potential of miR-18a-5p inhibitors against melanoma
remains to be further determined in clinical practice.
To conclude, miR-18a-5p was demonstrated to be
highly expressed in melanoma tissues and cell lines, promoting
proliferation, and suppressing apoptosis and autophagy of melanoma
cells by directly targeting and inhibiting the expression of EPHA7.
These findings clarified the development and progression of
non-coding RNA-mediated melanoma, which may be explored further to
design novel diagnostic methods and therapies for patients with
melanoma.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RF conceived and designed the research, drafted and
revised the manuscript. YG performed the experiments. YG and WS
analyzed the data and prepared the figures. RF and YG edited and
revised the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Medical Ethics
Committee of the Guangzhou First People's Hospital (approval no.
K-2019-135-01) and all patients provided written consent prior to
surgery.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bastian BC: The molecular pathology of
melanoma: An integrated taxonomy of melanocytic neoplasia. Annu Rev
Pathol. 9:239–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wahid M, Jawed A, Mandal RK, Dar SA,
Akhter N, Somvanshi P, Khan F, Lohani M, Areeshi MY and Haque S:
Recent developments and obstacles in the treatment of melanoma with
BRAF and MEK inhibitors. Crit Rev Oncol Hematol. 125:84–88. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ankeny JS, Labadie B, Luke J, Hsueh E,
Messina J and Zager JS: Review of diagnostic, prognostic, and
predictive biomarkers in melanoma. Clin Exp Metastasis. 35:487–493.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Corrie PG, Marshall A, Dunn JA, Middleton
MR, Nathan PD, Gore M, Davidson N, Nicholson S, Kelly CG, Marples
M, et al: Adjuvant bevacizumab in patients with melanoma at high
risk of recurrence (AVAST-M): Preplanned interim results from a
multicentre, open-label, randomised controlled phase 3 study.
Lancet Oncol. 15:620–630. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ichihashi N and Kitajima Y: Chemotherapy
induces or increases expression of multidrug resistance-associated
protein in malignant melanoma cells. Br J Dermatol. 144:745–750.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li S, Wu Z, Ma P, Xu Y, Chen Y, Wang H, He
P, Kang Z, Yin L, Zhao Y, et al: Ligand-dependent EphA7 signaling
inhibits prostate tumor growth and progression. Cell Death Dis.
8:e31222017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Holland SJ, Gale NW, Gish GD, Roth RA,
Songyang Z, Cantley LC, Henkemeyer M, Yancopoulos GD and Pawson T:
Juxtamembrane tyrosine residues couple the Eph family receptor
EphB2/Nuk to specific SH2 domain proteins in neuronal cells. EMBO
J. 16:3877–3888. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang J, Kataoka H, Suzuki M, Sato N,
Nakamura R, Tao H, Maruyama K, Isogaki J, Kanaoka S, Ihara M, et
al: Downregulation of EphA7 by hypermethylation in colorectal
cancer. Oncogene. 24:5637–5647. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang LF, Fokas E, Juricko J, You A, Rose
F, Pagenstecher A, Engenhart-Cabillic R and An HX: Increased
expression of EphA7 correlates with adverse outcome in primary and
recurrent glioblastoma multiforme patients. BMC Cancer. 8:792008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xia J, Jia P, Hutchinson KE, Dahlman KB,
Johnson D, Sosman J, Pao W and Zhao Z: A meta-analysis of somatic
mutations from next generation sequencing of 241 melanomas: A road
map for the study of genes with potential clinical relevance. Mol
Cancer Ther. 13:1918–198. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin S and Gregory RI: MicroRNA biogenesis
pathways in cancer. Nat Rev Cancer. 15:321–333. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li Y, Qiu C, Tu J, Geng B, Yang J, Jiang T
and Cui Q: HMDD v2.0: A database for experimentally supported human
microRNA and disease associations. Nucleic Acids Res. 42((Database
Issue)): D1070–D1074. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Valastyan S, Reinhardt F, Benaich N,
Calogrias D, Szász AM, Wang ZC, Brock JE, Richardson AL and
Weinberg RA: Retraction notice to: A pleiotropically acting
microRNA, miR-31, inhibits breast cancer metastasis. Cell.
161:4172015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu M, Zhou K and Cao Y: MicroRNA-944
affects cell growth by targeting EPHA7 in non-small cell lung
cancer. Int J Mol Sci. 17:14932016. View Article : Google Scholar
|
|
16
|
Penna E, Orso F, Cimino D, Tenaglia E,
Lembo A, Quaglino E, Poliseno L, Haimovic A, Osella-Abate S, De
Pittà C, et al: microRNA-214 contributes to melanoma tumour
progression through suppression of TFAP2C. EMBO J. 30:1990–2007.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stark MS, Klein K, Weide B, Haydu LE,
Pflugfelder A, Tang YH, Palmer JM, Whiteman DC, Scolyer RA, Mann
GJ, et al: The prognostic and predictive value of melanoma-related
MicroRNAs using tissue and serum: A MicroRNA expression analysis.
EBioMedicine. 2:671–680. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen X, Wu L, Li D, Xu Y, Zhang L, Niu K,
Kong R, Gu J, Xu Z, Chen Z and Sun J: Radiosensitizing effects of
miR-18a-5p on lung cancer stem-like cells via downregulating both
ATM and HIF-1α. Cancer Med. 7:3834–3847. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liang C, Zhang X, Wang HM, Liu XM, Zhang
XJ, Zheng B, Qian GR and Ma ZL: MicroRNA-18a-5p functions as an
oncogene by directly targeting IRF2 in lung cancer. Cell Death Dis.
8:e27642017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Linck L, Liebig J, Völler D, Eichner N,
Lehmann G, Meister G and Bosserhoff A: MicroRNA-sequencing data
analyzing melanoma development and progression. Exp Mol Pathol.
105:371–379. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
World Medical Association: World medical
association declaration of Helsinki: Ethical principles for medical
research involving human subjects. JAMA. 310:2191–2194. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak JK and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dawson MA and Kouzarides T: Cancer
epigenetics: From mechanism to therapy. Cell. 150:12–27. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kita Y, Yonemori K, Osako Y, Baba K, Mori
S, Maemura K and Natsugoe S: Noncoding RNA and colorectal cancer:
Its epigenetic role. J Hum Genet. 62:41–47. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Calvano Filho CM, Calvano-Mendes DC,
Carvalho KC, Maciel GA, Ricci MD, Torres AP, Filassi JR and Baracat
EC: Triple-negative and luminal A breast tumors: Differential
expression of miR-18a-5p, miR-17-5p, and miR-20a-5p. Tumour Biol.
35:7733–7741. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang N, Zhang H, Liu Y, Su P, Zhang J,
Wang X, Sun M, Chen B, Zhao W, Wang L, et al: SREBP1, targeted by
miR-18a-5p, modulates epithelial-mesenchymal transition in breast
cancer via forming a co-repressor complex with Snail and HDAC1/2.
Cell Death Differ. 26:843–859. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang G, Han G, Zhang X, Yu Q, Li Z, Li Z
and Li J: Long non-coding RNA FENDRR reduces prostate cancer
malignancy by competitively binding miR-18a-5p with RUNX1.
Biomarkers. 23:435–445. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Burgess JT, Bolderson E, Adams MN, Baird
AM, Zhang SD, Gately KA, Umezawa K, O'Byrne KJ and Richard DJ:
Activation and cleavage of SASH1 by caspase-3 mediates an apoptotic
response. Cell Death Dis. 7:e24692016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Guan M, Xu C, Zhang F and Ye C: Aberrant
methylation of EphA7 in human prostate cancer and its relation to
clinicopathologic features. Int J Cancer. 124:88–94. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Oricchio E and Wendel HG: Mining the
cancer genome uncovers therapeutic activity of EphA7 against
lymphoma. Cell Cycle. 11:1076–1080. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang J, Li G, Ma H, Bao Y, Wang X, Zhou H,
Sheng Z, Sugimura H, Jin J and Zhou X: Differential expression of
EphA7 receptor tyrosine kinase in gastric carcinoma. Hum Pathol.
38:1649–1656. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li MY, Zhu XL, Zhao BX, Shi L, Wang W, Hu
W, Qin SL, Chen BH, Zhou PH, Qiu B, et al: Adrenomedullin
alleviates the pyroptosis of leydig cells by promoting autophagy
via the ROS-AMPK-mTOR axis. Cell Death Dis. 10:4892019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Komatsu M and Ichimura Y: Physiological
significance of selective degradation of p62 by autophagy. FEBS
Lett. 584:1374–1378. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mathew R, Karp CM, Beaudoin B, Vuong N,
Chen G, Chen HY, Bray K, Reddy A, Bhanot G, Gelinas C, et al:
Autophagy suppresses tumorigenesis through elimination of p62.
Cell. 137:1062–1075. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li Z, Jiang K, Zhu X, Lin G, Song F, Zhao
Y, Piao Y, Liu J, Cheng W, Bi X, et al: Encorafenib (LGX818), a
potent BRAF inhibitor, induces senescence accompanied by autophagy
in BRAFV600E melanoma cells. Cancer Lett. 370:332–344. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Meng XX, Yao M, Zhang XD, Xu HX and Dong
Q: ER stress-induced autophagy in melanoma. Clin Exp Pharmacol
Physiol. 42:811–816. 2015. View Article : Google Scholar : PubMed/NCBI
|