Introduction
Osteosarcoma (OS) is an aggressive malignancy that
primarily develops in the bones of adults and children (1). Worldwide, an estimated 4 million
people die of OS annually, with the disease peaking between the
ages of 15–19 years old (2). In
Europe and America, the 5-year survival rate for localized OS is
~65–70%, whereas the 5-year survival rate is <20% for metastatic
OS (3). Surgical resection is the
predominant method used for the treatment of OS (4). However, the incidence (50%) and
metastatic rates (70%) following surgery remain high, which
seriously affects the health and life of patients with OS (5). Consequently, research into the
potential mechanisms of OS and the identification of novel
therapeutic targets for OS has become a high priority.
Nasopharyngeal carcinoma-associated gene 6 (NGX6)
has been identified as a tumor metastasis suppressor gene, encoding
a class of membrane proteins (e.g. catenin-β1, transcription factor
4 and transmembrane protein 8B) primarily located in the nuclear
and cell membrane (6). Previous
studies have reported a variety of biological functions for NGX6,
including an ability to suppress angiogenesis, modulate the protein
expression levels of related genes, regulate cell cycle
progression, participate in cell signaling transduction pathways
(e.g., the epidermal growth factor receptor and Wnt/β-catenin
signaling pathways) and enhance the sensitivity of patients to
antitumor drugs (7–10). NGX6 was also identified to be
aberrantly expressed in numerous different types of human
malignancy, such as nasopharyngeal carcinoma (NPC) (11), and gastric (12), colon (12) and lung cancers (13). Wang et al (11) reported that NGX6 expression levels
were downregulated in NPC, and the overexpression of NGX6
suppressed NPC cell proliferation and invasion. Guo et al
(10) revealed that NGX6 expression
levels were downregulated in colorectal cancer cells, which
suppressed cell invasion and adhesion in colorectal cancer.
However, the biological role and regulatory mechanism of NGX6 in OS
remains poorly understood.
The Wnt/β-catenin signaling pathway is considered as
a β-catenin-dependent extracellular signaling pathway, which exerts
a crucial role in regulating numerous biological processes, such as
cell proliferation, apoptosis, migration, survival and
differentiation (14–17). The aberrant activation of the
Wnt/β-catenin signaling pathway has been closely associated with
the development of various types of human malignancy, such as
hepatocellular carcinoma (HCC), colorectal cancer (CRC) and OS
(15–17). For instance, silencing of the
sclerostin gene facilitated the proliferation, while repressing the
apoptosis, of OS cells by stimulating the Wnt/β-catenin signaling
pathway (18). In addition, the
knockdown of Sox2 inhibited the migration and invasion of OS cells
by blocking the Wnt/β-catenin signaling pathway (19). Moreover, a previous study
demonstrated that NGX6 suppresses CRC cell invasion and adhesion by
inhibiting the Wnt/β-catenin signaling pathway (10). Nevertheless, to the best of our
knowledge, there are few studies on the underlying regulatory
mechanism between NGX6 and the Wnt/β-catenin signaling pathway in
OS.
The present study aimed to analyze the NGX6
expression levels in OS tissues and cell lines. In addition, in
vitro experiments were performed to determine the effect of
NGX6 on the viability, apoptosis, migration and invasion of OS
cells. The association between NGX6 and the Wnt/β-catenin signaling
pathway in OS progression was also identified. The findings
illustrated the functional role of NGX6 in OS, providing a
potential novel therapeutic target for the treatment of OS.
Materials and methods
Clinical specimens
The use of patient tissues was approved by the
ethics committee of Qingdao West Coast Hospital, Affiliated
Hospital of Qingdao University (approval no. QYFYWZLL25779A) and
written informed consent was obtained from all patients. In total,
60 patients (27 males and 33 females; age range, 35–63 years old)
histologically diagnosed with OS were recruited in our hospital
between February 2017 and April 2019. The inclusion criteria were:
i) First-time diagnosis; and ii) no prior history of radiotherapy,
chemotherapy and other adjuvant therapy. The exclusion criteria
were: i) Presence of other malignant tumors; and ii) treatment for
OS before admission. OS tissues and adjacent normal tissues
(distance from tumor margin, 2 cm) were collected from these
patients prior to the administration of treatment.
Cell culture
The human OS cell lines (MG-63, Saos-2, U2OS and
HOS) and the human osteoblast cell line hFOB1.19 were purchased
from The Cell Bank of Type Culture Collection of the Chinese
Academy of Sciences. All cells were cultured in DMEM (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% FBS (HyClone;
Cytiva) and 1% penicillin-streptomycin, and maintained at 37°C with
5% CO2.
Cell transfection
U2OS and HOS cells (6×105 cells/well) in
the logarithmic growth phase were transfected with 20 nM pcDNA3.1
(pcDNA)-NGX6 or pcDNA-negative control (NC; both Sangon Biotech
Co., Ltd.) using Lipofectamine® 2000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). Cells were randomly
divided into the BLANK (untransfected cells), pcDNA-NC and
pcDNA3.1-NGX6 groups. Following transfection for 48 h at 37°C, the
cells were used for follow-up experiments.
To determine whether the regulatory effects of NGX6
on OS cells were associated with the Wnt/β-catenin signaling
pathway, U2OS and HOS cells transfected with pcDNA-NGX6 were
treated with BML284 (20 µM; Abcam), an activator of the
Wnt/β-catenin signaling pathway, for 0, 24, 48, 72 or 96 h at room
temperature. Cells were divided into the following groups: i)
pcDNA-NC; ii) pcDNA3.1-NGX6; and iii) pcDNA3.1-NGX6 + BML284.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from tissues or cells using
TRIzol® Plus RNA Isolation reagent (Invitrogen; Thermo
Fisher Scientific, Inc.). The concentration of total RNA was
measured using a NanoDrop 2000 spectrophotometer (Thermo Fisher
Scientific, Inc.). Total RNA (500 ng) was reverse transcribed into
cDNA at 42°C for 45 min using Moloney Murine Leukaemia Virus
Reverse Transcriptase (Invitrogen; Thermo Fisher Scientific, Inc.).
qPCR was subsequently performed using SYBR® Premix Ex
Taq™ (Takara Biotechnology Co., Ltd.) on a StepOnePlus™ Real-Time
PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.).
The following thermocycling conditions were used for the qPCR:
Initial denaturation at 95°C for 3 min; followed by 40 cycles at
95°C for 15 sec, annellation at 60°C for 30 sec, elongation at 72°C
for 1 min, and a final extension at 72°C for 5 min. The primer
sequences used for the qPCR are listed in Table I. The mRNA expression levels of
target genes were quantified using the 2−ΔΔCq method
(21). GAPDH or U6 was used as the
internal loading control.
 | Table I.Primer sequences used for reverse
transcription-quantitative PCR. |
Table I.
Primer sequences used for reverse
transcription-quantitative PCR.
| Gene | Primer sequence
(5′-3′) |
|---|
| Nasopharyngeal | F:
CAACAGCCTCAAGATCATCAGCA |
|
carcinoma-associated gene 6 | R:
GAGGAGGGGAGATTCAGTGTGGT |
| β-catenin | F:
TGAGGACAAGCCACAAGATTAC |
|
| R:
TCCACCAGAGTGAAAAGAACG |
| GAPDH | F:
GAGTCAACGGATTTGGTCGT |
|
| R:
TTGATTTTGGAGGGATCTC |
Western blotting
Total protein was extracted from tissues or OS cell
lines (U2OS and HOS) using RIPA lysis buffer (Beyotime Institute of
Biotechnology). Total protein was quantified using a BCA Protein
assay kit (Invitrogen; Thermo Fisher Scientific, Inc.) and 50 µg
protein/lane was separated via 10% SDS-PAGE. The separated proteins
were subsequently transferred onto a polyvinylidene fluoride
membrane and blocked with 5% skimmed milk for 2 h at 25°C. The
membranes were then incubated with the following primary antibodies
(Abcam) overnight at 4°C: Anti-GAPDH (1:1,000; cat. no. ab9485),
anti-β-catenin (1:1,000; cat. no. ab32572), anti-c-Jun (1:1,000;
cat. no. ab40766) and anti-c-Myc (1:1,000; cat. no. ab32072). After
washing three times with TBST (Tween-20; 0.05%) for 5 min, the
membranes were incubated with a horseradish peroxidase-conjugated
anti-rabbit IgG secondary antibody (1:2,000; cat. no. ab6721;
Abcam) for 2 h at 25°C. Protein bands were visualized using an
chemiluminescent substrate kit (Invitrogen; Thermo Fisher
Scientific, Inc.) and analyzed using Gel-Pro Analyzer software
(version 4.0; Media Cybernetics, Inc.). GAPDH was used as the
internal loading control.
MTT assay
The transfected U2OS and HOS cells (2×104
cells/well) were seeded into 96-well plates. Following incubation
for 24, 48, 72 or 96 h at 37°C, 20 µl MTT reagent (5 mg/ml;
Sigma-Aldrich; Merck KGaA) was added into each well. After 4 h of
incubation at 37°C, 150 µl DMSO was added into each well to
terminate the reaction. The absorbance was measured at 450 nm using
a microplate reader (Bio-Rad Laboratories, Inc.).
Flow cytometric analysis of
apoptosis
Early apoptosis in transfected U2OS and HOS cells
were analyzed using an Annexin V-FITC apoptosis detection kit
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. Briefly, cells were centrifuged at 200 × g
for 5 min at 4°C and resuspended in binding buffer. Cells were
subsequently stained with 5 µl Annexin V-FITC and 5 ml propidium
iodide for 15 min at 4°C in a dark room. Apoptosis was detected
using a FACScan flow cytometer (version 2.0; BD Biosciences) and
the data were analyzed using CellQuest software (version 5.1; BD
Biosciences).
Wound healing assay
U2OS and HOS cells were plated into six-well plates
containing DMEM at a density of 5×105 cells/well. Upon
reaching 90% confluence, the cell monolayer was scratched using a
sterile 100-µl pipette tip to form wounds. The cells were then
incubated in serum-free DMEM for 24 h at 37°C. Cells were observed
and photographed at 0 and 24 h using an inverted light microscope
(Olympus Corporation; magnification, ×200) and measured using
ImageJ software (version 1.46; National Institutes of Health). The
relative migration rate was calculated as follows: (original gap
distance-gap distance at 24 h)/original gap distance ×100. Relative
migration was normalized to the BLANK or pcDNA-NC group.
Transwell Matrigel assay
Cell invasion was assessed using Transwell chambers
(Corning, Inc.) pre-coated (at 37°C for 30 min) with
Matrigel® (BD Biosciences). Briefly, transfected U2OS
and HOS cells were resuspended in serum-free medium and 200 µl cell
suspension (1×105 cells) was placed in the upper
chamber. Subsequently, 600 µl medium containing 10% FBS was plated
into the lower chambers. Following 24 h of incubation at 37°C with
5% CO2, the invasive cells in the lower chamber were
fixed with 4% paraformaldehyde at room temperature for 20 min and
stained with 0.5% crystal violet at 37°C for 30 min. Cells in six
independent fields per well were imaged using a light microscope
(Olympus Corporation; magnification, ×200), and the number of
invading cells were counted.
Establishment of xenograft tumor model
mice
Animal experiments were approved by the ethics
committee of Qingdao West Coast Hospital (approval no.
QYFYWZLL25779) and were performed in accordance with the Guide for
the Care and Use of Laboratory Animals (version 1996) (20). Male specific pathogen free mice
(BALB/c; age, 4 weeks; weight, 20–25 g; n=30) were purchased from
the Medical College of Shanghai Jiaotong University (Shanghai,
China). The animals were housed in a sterile environment at a
controlled temperature of 20°C with 40% relative humidity, 12-h
light/dark cycles, and free access to food and water. A volume of
100 µl U2OS cells (0.1×108 cells/ml) at the logarithmic
growth phase from the different groups (pcDNA-NGX6, pcDNA-NC,
pcDNA-NGX6 + BML284) were resuspended in PBS and subcutaneously
injected into the posterior limb of mice (n=10/group) to create a
subcutaneous tumor-bearing model. The tumor volume was measured
with a Vernier caliper every week following injection. At the end
of the 4th week, mice were anesthetized with 50 mg/kg pentobarbital
sodium and sacrificed by cervical dislocation. The tumors were
removed and weighed. The experiment lasted for 4 weeks and no mice
died during this period.
Statistical analysis
Statistical analysis was performed using SPSS 22.0
software (IBM Corp.). All experiments were performed in triplicate
and data are presented as the mean ± SD. A paired Student's t-test
was used to determine the significant differences between two
groups (Fig. 1A), while a one-way
ANOVA test followed by Tukey's post hoc test was used to determine
the statistical differences between >2 groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
NGX6 expression levels are
downregulated in OS tissues and cell lines
RT-qPCR analysis revealed that NGX6 expression
levels were significantly downregulated in the OS tissues compared
with the normal tissues from patients (P<0.001; Fig. 1A). Consistently, NGX6 expression
levels were also significantly downregulated in OS cell lines
(MG-63, Saos-2, U2OS and HOS) compared with the human osteoblastic
cell line hFOB1.19, but especially in U2OS and HOS cells
(P<0.01; Fig. 1B). Therefore,
U2OS and HOS cells were selected for subsequent experiments. These
findings suggested that NGX6 expression levels may be downregulated
in OS tissues and cell lines.
Overexpression of NGX6 inhibits the
viability, while promoting the apoptosis of OS cells
To determine the effect of NGX6 on OS progression,
NGX6 was overexpressed by the transfection of pcDNA-NGX6 into U2OS
and HOS cells. RT-qPCR illustrated that the NGX6 expression levels
were significantly upregulated in the pcDNA3-NGX6 groups compared
with the BLANK groups in both cell lines (P<0.01; Fig. 2A). The results of the MTT assay
revealed that the optical density (OD)450 values of the pcDNA-NGX6
groups were significantly decreased at 48 h in both cell lines
compared with the BLANK groups (P<0.05; Fig. 2B). The levels of apoptosis of U2OS
and HOS cells in the pcDNA-NGX6 groups were significantly increased
compared with the BLANK groups (**P<0.01; Fig. 2C). Conversely, the transfection of
cells with pcDNA-NC did not influence the viability or apoptotic
rate of U2OS and HOS cells (Fig. 2B and
C). These results indicated that the overexpression of NGX6 may
impede the viability and promote the apoptosis of U2OS and HOS
cells.
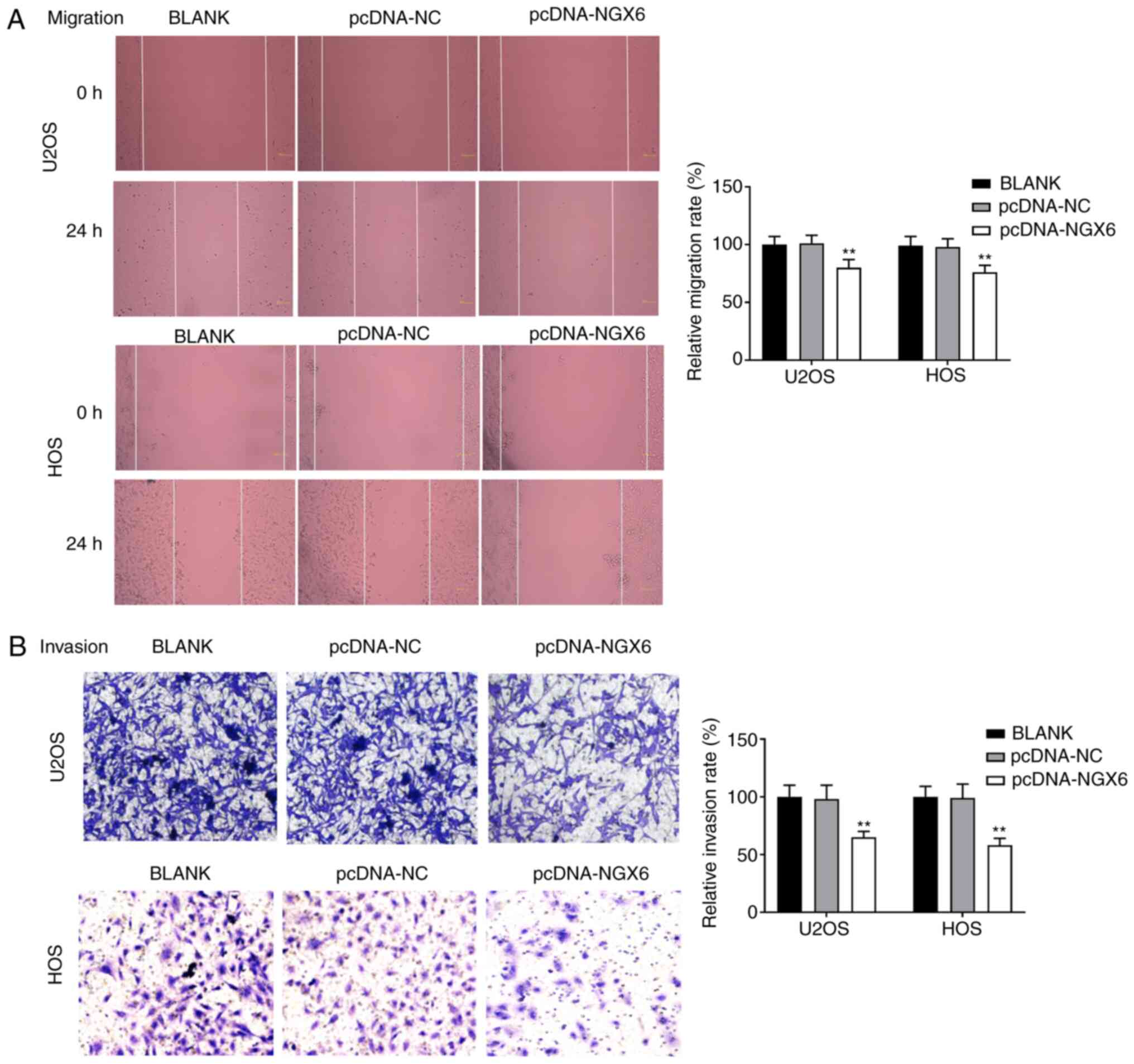
Overexpression of NGX6 suppresses the
migration and invasion of OS cells
The relative migration and invasion rates were
significantly decreased in the pcDNA-NGX6 groups compared with the
BLANK groups in both cell lines (P<0.01; Fig. 3A and B). The transfection of cells
with pcDNA-NC did not influence the migration and invasion rates of
U2OS and HOS cells. Taken together, these data indicated that the
overexpression of NGX6 may repress the migration and invasion of
U2OS and HOS cells.
NGX6 overexpression inhibits the
Wnt/β-catenin signaling pathway
To determine the potential mechanism of NGX6 in OS,
transfected U2OS and HOS cells were treated with BML284
(Wnt/β-catenin signaling pathway activator). In both cell lines,
the expression levels of β-catenin, c-Jun and c-Myc were all
significantly downregulated in the pcDNA-NGX6 groups compared with
the pcDNA-NC groups (P<0.01; Fig.
4). Conversely, BML284 treatment significantly reversed the
inhibitory effects of pcDNA-NGX6 on the expression levels of
β-catenin, c-Jun and c-Myc in U2OS and HOS cells
(##P<0.01; Fig. 4).
These results indicated that the transfection of pcDNA-NGX6 may
block the Wnt/β-catenin signaling pathway, while the intervention
with BML284 may reverse the inhibitory effect of pcDNA-NGX6 on the
Wnt/β-catenin signaling pathway.
NGX6 overexpression inhibits the
viability, migratory and invasive abilities, while facilitating the
apoptosis of OS cells by blocking the Wnt/β-catenin signaling
pathway
To explore the possible effect of the Wnt/β-catenin
signaling pathway on the occurrence and development of OS in
vitro, U2OS cells were treated with an activator of the
Wnt/β-catenin signaling pathway (BML284). The results indicated
that treatment with BML284 reversed the inhibitory effects of
pcDNA-NGX6 on the viability, migration and invasion, and the
promoting effect on the apoptosis of U2OS cells (P<0.05,
P<0.01; Fig. 5A-D).
Collectively, the results implied that treatment BML284 could
reverse the effect of NGX6 overexpression on the progression of OS
in vitro.
 | Figure 5.NGX6 overexpression inhibits the
viability, migration and invasion, while inducing the apoptosis of
OS cells by blocking the Wnt/β-catenin signaling pathway. (A) MTT
assay was used to analyze the viability of U2OS cells transfected
with pcDNA-NGX6 in the presence or absence of BML284 treatment (20
µM). (B) Levels of apoptosis of U2OS cells transfected with
pcDNA-NGX6 in the presence or absence of BML284 treatment (20 µM)
were analyzed using flow cytometry. (C) Migration rate of U2OS
cells transfected with pcDNA-NGX6 in the presence or absence of
BML284 treatment (20 µM) was analyzed using a wound healing assay
(magnification, ×200). (D) Invasion rate of U2OS cells transfected
with pcDNA-NGX6 in the presence or absence of BML284 treatment (20
µM) was analyzed using a Transwell Matrigel assay (magnification,
×200). *P<0.05, **P<0.01 vs. pcDNA-NC; #P<0.05,
##P<0.01 vs. pcDNA-NGX6. NGX6, nasopharyngeal
carcinoma-associated gene 6; NC, negative control; OD, optical
density; PI, propidium iodide. |
Overexpression of NGX6 inhibits the
growth of xenograft tumors in vivo by blocking the Wnt/β-catenin
signaling pathway
To verify the inhibitory effect of pcDNA-NGX6 on the
tumorigenesis of OS, xenograft tumor model mice were established. A
significant decrease was observed in both the tumor volume
(P<0.05; Fig. 6A) and tumor
weight (P<0.01; Fig. 6B) in the
pcDNA-NGX6 group compared with the pcDNA-NC group. However, the
addition of BML284 treatment partially rescued the inhibitory
effect of pcDNA-NGX6 on the tumor growth and weight of mice
(P<0.05, P<0.01). In addition, the expression levels of
β-catenin, c-Jun and c-Myc in the tumor tissues were significantly
downregulated in the pcDNA-NGX6 group compared with the pcDNA-NC
group (P<0.01; Fig. 6C), while
the addition of BML284 partially rescued this inhibitory effect of
pcDNA-NGX6 on the expression levels of β-catenin, c-Jun and c-Myc
in the tumor tissues of mice (P<0.05; Fig. 6C). The results indicated that
treatment with BML284 reversed the suppressive effect of NGX6
overexpression on the growth of xenograft tumors in
vivo.
Discussion
NGX6 expression levels were discovered to be
downregulated in colorectal (22),
gastric (11) and liver cancers
(23). Liu et al also
reported that NGX6 expression levels were downregulated in gastric
cancer tissues, and these low expression levels of NGX6 facilitated
the progression of gastric cancer (11). In addition, Zhang et al
(9) reported that the expression
levels of NGX6 were downregulated in colorectal cancer tissues, and
the downregulation of NGX6 exerted a promoting effect on the
occurrence and metastasis of colorectal cancer. The findings of the
present study were similar to those of previous studies; for
instance, the results revealed that NGX6 expression levels were
significantly downregulated in OS tissues and cell lines.
Peng et al (24) previously revealed that NGX6
inhibited the proliferation and migration of NPC cells. NGX6 was
discovered to suppress cell proliferation, invasion and metastasis,
regulate the cell cycle and inhibit tumor angiogenesis in colon
cancer (10). Similar to the
effects of NGX6 observed in other types of cancer, the results of
the present study demonstrated that the overexpression of NGX6
inhibited the cell viability, migration and invasion, while
promoting the apoptosis of U2OS and HOS cells. These findings
indicated that NGX6 may function as a tumor suppressor gene during
OS progression.
The Wnt/β-catenin signaling pathway serves a crucial
role in tumorigenesis and the abnormal activation of the
Wnt/β-catenin signaling pathway has been observed in various types
of human malignancies, such as HCC, CRC and OS (16,17,25,26).
Numerous genes have been identified to exert their tumor
suppressive roles in OS through blocking the Wnt/β-catenin
signaling pathway, including TraB domain containing 2B (27), bone morphogenetic protein 9
(28) and forkhead box protein O1
(29). Notably, NGX6 is involved in
the regulation of the Wnt/β-catenin signaling pathway in CRC
(25,30). Liu et al (31) demonstrated that NGX6 overexpression
downregulated the expression levels of the downstream target genes
of the Wnt/β-catenin signaling pathway, cyclin D1, c-Jun and c-Myc,
in colon cancer cells. Guo et al (10) illustrated that NGX6 suppressed the
translocation of β-catenin from the nucleus and cytoplasm to the
plasma membrane, thereby inhibiting the activity of transcription
factor 4 and downregulating the expression levels of the Wnt-target
genes, c-Myc, cyclin D1 and cyclooxygenase-2 in colon cancer cells.
In the present study, the overexpression of NGX6 significantly
downregulated the expression levels of β-catenin, c-Jun and c-Myc
in OS cells. These findings were consistent with previous studies,
which further suggested that the Wnt/β-catenin signaling pathway
may be blocked by NGX6 in OS. Thus, the blocked Wnt/β-catenin
signaling pathway may directly contribute to the antitumor effect
of NGX6 in colon cancer. Guo et al (30) also discovered that NGX6 inhibited
the proliferation, invasiveness and extracellular matrix adhesion,
in addition to promoting the apoptosis, of colon cancer cells
through suppressing the Wnt/β-catenin signaling pathway. Consistent
with these findings, the current study revealed that NGX6
suppressed the viability, migration and invasion, and promoted the
apoptosis of OS cells, through blocking the Wnt/β-catenin signaling
pathway. In addition, the migration of OS cells was inhibited by
the NGX6-mediated suppression of the Wnt/β-catenin signaling
pathway. The verification experiments further illustrated the
regulatory relationship between NGX6 and the Wnt/β-catenin
signaling pathway in OS; the BML284-induced activation of the
Wnt/β-catenin signaling pathway reversed the antitumor effects of
NGX6 on the viability, apoptosis, migration and invasion of OS
cells.
Furthermore, the present study also discovered that
NGX6 inhibited tumor growth in vivo through blocking the
Wnt/β-catenin signaling pathway. These findings indicated that NGX6
may inhibit the tumorigenesis of OS both in vitro and in
vivo through blocking the Wnt/β-catenin signaling pathway.
In conclusion, the findings of the present study
revealed that the expression levels of NGX6 were downregulated in
OS tissues and cell lines. The overexpression of NGX6 significantly
inhibited the viability, migration and invasion, and induced
apoptosis in OS cells in vitro. The growth of xenograft
tumors in vivo was also suppressed by NGX6 overexpression.
Additionally, the inhibitory effect of NGX6 on OS progression was
suggested to possibly occur through the suppression of the
Wnt/β-catenin signaling pathway. These data implied that NGX6 may
function as a potential therapeutic target for the treatment of
OS.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LL made substantial contributions to the conception
and design of the study. RW, ZZ and XW made substantial
contributions to the acquisition, analysis and interpretation of
data, as well as the drafting and revision of the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the ethics
committee of Qingdao West Coast Hospital (approval no.
QYFYWZLL25779A), and written informed consent was obtained from all
patients. The animal experiments were approved by the ethics
committee of Qingdao West Coast Hospital (approval no.
QYFYWZLL25779) and performed in accordance with the Guide for the
Care and Use of Laboratory Animals (version 1996).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Biermann JS, Chow W, Reed DR, Lucas D,
Adkins DR, Agulnik M, Benjamin RS, Brigman B, Budd GT, Curry WT, et
al: NCCN Guidelines Insights: Bone Cancer, Version 2.2017. J Natl
Compr Canc Netw. 15:155–167. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mirabello L, Troisi RJ and Savage SA:
Osteosarcoma incidence and survival rates from 1973 to 2004: Data
from the Surveillance, Epidemiology, and End results program.
Cancer. 115:1531–1543. 2010. View Article : Google Scholar
|
|
3
|
Siegel HJ and Pressey JG: Current concepts
on the surgical and medical management of osteosarcoma. Expert Rev
Anticancer Ther. 8:1257–1269. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mialou V, Philip T, Kalifa C, Perol D,
Gentet JC, Marec-Berard P, Pacquement H, Chastagner P, Defaschelles
AS and Hartmann O: Metastatic osteosarcoma at diagnosis: Prognostic
factors and long-term outcome-the French pediatric experience.
Cancer. 104:1100–1109. 2010. View Article : Google Scholar
|
|
5
|
Friebele JC, Peck J, Pan X, Abdel-Rasoul M
and Mayerson JL: Osteosarcoma: A meta-analysis and review of the
literature. Am J Orthop (Belle Mead NJ). 44:547–553.
2015.PubMed/NCBI
|
|
6
|
Ma J, Li J, Zhou J, Li XL, Tang K, Zhou M,
Yang JB, Yan Q, Shen SR, Hu GX and Li GY: Profiling genes
differentially expressed in NGX6 overexpressed nasopharyngeal
carcinoma cells by cDNA array. J Cancer Res Clin Oncol.
128:683–690. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
He S, Zhong Y, Shuai C, Gao D, Wei P, Li G
and Peng S: Tumor suppressor NGX6 inhibits the growth and
metastasis of multiple cancers. Tumour Biol. 37:5751–5760. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang L, Ma J, Li J, Li X, Zhang Q, Peng S,
Peng C, Zhou M, Xiong W, Yang J, et al: NGX6 gene inhibits cell
proliferation and plays a negative role in EGFR pathway in
nasopharyngeal carcinoma cells. 95:64–73. 2005.PubMed/NCBI
|
|
9
|
Zhang XM, Wang XY, Sheng SR, Wang JR and
Li J: Expression of tumor related genes NGX6, NAG-7, BRD7 in
gastric and colorectal cancer. World J Gastroenterol. 9:1729–1733.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guo Q, Shen S, Liao M, Lian P and Wang X:
NGX6 inhibits cell invasion and adhesion through suppression of
Wnt/beta-catenin signal pathway in colon cancer. Acta Biochim
Biophys Sin (Shanghai). 42:450–456. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang YT, Sun XY, Wang H, Huang TT, Wang Y
and Yang MX: Effects of NGX6 expression on proliferation and
invasion of nasopharyngeal carcinoma cells and survival of
patients. Eur Rev Med Pharmacol Sci. 21:5378–5385. 2017.PubMed/NCBI
|
|
12
|
Liu J, Zhu X, Xu X and Dai D: DNA promoter
and histone H3 methylation downregulate NGX6 in gastric cancer
cells. Med Onco. 31:8172014. View Article : Google Scholar
|
|
13
|
Lin ZF, Shen XY, Lu FZ, Ruan Z, Huang HL
and Zhen J: Reveals new lung adenocarcinoma cancer genes based on
gene expression. Eur Rev Med Pharmacol Sc. 16:1249–1256. 2012.
|
|
14
|
Nusse R and Clevers H: Wnt/β-catenin
signaling, disease, and emerging therapeutic modalities. Cell.
169:985–999. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin CH, Ji T, Chen CF and Hoang BH: Wnt
signaling in osteosarcoma. Adv Exp Med Biol. 804:33–45. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Anastas JN and Moon RT: WNT signalling
pathways as therapeutic targets in cancer. Nat Rev Cancer.
13:11–26. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Takebe N, Miele L, Harris PJ, Jeong W,
Bando H, Kahn M, Yang SX and Ivy SP: Targeting Notch, Hedgehog, and
Wnt pathways in cancer stem cells: Clinical update. Nat Rev Clin
Oncol. 12:445–464. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zou J, Zhang W and Li XL: Effects of SOST
gene silencing on proliferation, apoptosis, invasion, and migration
of human osteosarcoma cells through the Wnt/β-catenin signaling
pathway. Calcif Tissue Int. 100:551–564. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tang L, Wang D and Gu D: Knockdown of Sox2
inhibits OS cells invasion and migration via modulating
Wnt/beta-catenin signaling pathway. Pathol Oncol Res. 24:907–913.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Clark DJ, Gebhart FG, Gonder CJ, Keeling
ME and Kohn DF: Special Report: The 1996 Guide for the Care and Use
of Laboratory Animals. ILAR J. 38:41–48. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu M, Peng Y, Wang X, Guo Q, Shen S and
Li G: NGX6 gene mediated by promoter methylation as a potential
molecular marker in colorectal cancer. Bmc Cancer. 10:160. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xiao JD and Shen SR: Expression and
significance of NGX6 gene in human hepatocellular carcinoma. Zhong
Nan Da Xue Xue Bao Yi Xue Ban. 33:937–941. 2008.(In Chinese).
PubMed/NCBI
|
|
24
|
Peng SP, Li XL, Wang L, Ou-Yang J, Ma J,
Wang LL, Liu HY, Zhou M, Tang YL, Li WS, et al: The role of NGX6
and its deletion mutants in the proliferation, adhesion and
migration of nasopharyngeal carcinoma 5-8F cells. Oncology.
71:273–281. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Logan CY and Nusse R: The Wnt signaling
pathway in development and disease. Annu Rev Cell Dev Biol.
20:781–810. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ma Y, Zhu B, Liu X, Yu H, Yong L, Liu X,
Shao J and Liu Z: Inhibition of oleandrin on the proliferation show
and invasion of osteosarcoma cells in vitro by suppressing
Wnt/β-catenin signaling pathway. J Exp Clin Cancer Res. 34:1152015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li R, Liu J, Wu H, Liu L, Wang L and Zhang
S: TIKI2 suppresses growth of osteosarcoma by targeting
Wnt/β-catenin pathway. Mol Cell Biochem. 392:109–116. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lv Z, Wang C, Yuan T, Liu Y, Song T, Liu
Y, Chen C, Yang M, Tang Z, Shi Q and Weng Y: Bone morphogenetic
protein 9 regulates tumor growth of osteosarcoma cells through the
Wnt/β-catenin pathway. Oncol Rep. 31:989–994. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Guan H, Tan P, Xie L, Mi B, Fang Z, Li J,
Yue J, Liao H and Li F: FOXO1 inhibits osteosarcoma oncogenesis via
Wnt/β-catenin pathway suppression. Oncogenesis. 4:e1662015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Guo Q, Wu M, Lian P, Liao M, Xiao Z, Wang
X and Shen S: Synergistic effect of indomethacin and NGX6 on
proliferation and invasion by human colorectal cancer cells through
modulation of the Wnt/beta-catenin signaling pathway. Mol Cell
Biochem. 330:71–81. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu F, Sheng S, Wang X, Peng Y, Guo Q,
Xiao Z and Liu J: Role of NGX6 in Wnt/β-catenin signaling pathway
in colon cancer. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 36:235–242.
2011.(In Chinese). PubMed/NCBI
|