Introduction
Anthracyclines are chemotherapy drugs that inhibit
the activity of topoisomerase IIα, thereby negatively impacting DNA
synthesis in cancer cells; anthracyclines have been widely used in
the treatment of various solid and hematological malignancies
(1,2). It is well known, however, that
anthracyclines can inflict severe damage to the heart, and
cardiotoxicity represents one of the most important adverse effects
of this class of compounds (1–4).
Anthracycline-induced cardiotoxicity is cumulative, dose dependent,
and irreversible; therefore, these drugs increase the risk of heart
failure, in turn leading to increased mortality and decreased
quality of life in patients (1–4).
Although the precise mechanism of cardiotoxicity is not fully
understood, cardiac apoptosis (programmed cell death) mediated by
either of the following two mechanisms has been proposed: One is
the excessive production of reactive oxygen species, the other is
DNA damage by inhibition of topoisomerase IIβ in cardiomyocytes
(1,2,5).
Retrospective pooled analysis by Swain et al
(3) documented that rates of heart
failure caused by doxorubicin (DOX), a typical anthracycline drug,
were 5, 26 and 48% at cumulative doses of 400, 500 and 700
mg/m2, respectively, providing a rationale for limiting
the cumulative lifetime dose of DOX. Nevertheless, there are
individual differences in the maximal cumulative dose tolerated,
and anthracyclines can lead to the occurrence and development of
heart failure even at lower doses (5–7). In
addition, the risk of heart failure may be augmented by
pre-existing loading conditions such as hypertension and valvular
disease (8), and by combination
with other chemotherapy drugs such as molecularly targeted drugs
and immune checkpoint inhibitors (9). These facts indicate that
anthracyclines have no safe treatment dose.
Several strategies for reducing the risk of heart
failure in anthracycline-treated patients have been proposed,
including pharmacological therapy to prevent cardiotoxicity
(10). Although some antioxidants,
such as vitamin E and probucol, have been observed to provide
cardioprotective action in DOX-treated animal models (2,11),
there is (to date) no direct evidence supporting clinically
efficacy (12). Alternatively, it
has been demonstrated (in both animals and humans) that standard
drugs for heart failure, such as β-blockers and
angiotensin-converting enzyme (ACE) inhibitors/angiotensin II
receptor blockers (ARBs), are effective in attenuating DOX-induced
cardiac damage (2,10,13).
In fact, pharmacological therapy with these drugs is generally
accepted as a preventive strategy against cardiac dysfunction in
anthracycline-treated patients (10). It should be noted, however, that the
response rate to the pharmacological therapy progressively
decreases with increasing time delay between the end of
chemotherapy to the onset of cardiac dysfunction (i.e., with
delayed start of the pharmacological therapy) (13,14).
The delayed recognition of cardiotoxicity results in a poor
prognosis, presumably because of the accumulation of irreversible
cardiac dysfunction and a lack of clinical response to the
pharmacological therapy. Therefore, early detection of cardiac
abnormalities and early adequate therapy would be extremely
important in preventing the occurrence and development of heart
failure after anthracycline therapy (15).
If we could predict the risk of cardiotoxicity prior
to the administration of anthracycline, it would help to render
chemotherapy in cancer patients safer and more effective. Genetic
factors have been shown to determine individual susceptibility to
DOX-induced cardiotoxicity (DICT) (16,17).
Therefore, in the present study, we identified genes closely
associated with DICT (i.e., predictive biomarkers) in mice in
vivo, and then examined the roles of these genes in DOX-induced
apoptosis of H9c2 cells (rat cardiac myoblasts) in
vitro.
Materials and methods
Animals and chemicals
Four-week-old adult male C57BL/6J mice (22–24 g
each), were obtained from Japan SLC (Hamamatsu, Japan). The animals
were maintained on a 12-h/12-h light/dark cycle in a temperature-
and humidity-controlled room. Experiments were conducted in
accordance with the standards established by the Japanese
Pharmacological Society and were approved by the Tohoku Medical and
Pharmaceutical University of Institutional Animal Care and Use
Committee (Experimental Protocol no. 18013). Animals were allowed
free access to laboratory pellet chow (CE-2; CLEA Japan, Inc.) and
water throughout the experiments. DOX was purchased from Sandoz K.
K. All other reagents, unless stated, were of the highest grade
available and were supplied by either Sigma Chemical Co. or Wako
Pure Chemical Industries, Ltd.
DOX administration and collection of
samples
One week before the start of dosing, 50 µl of blood
was collected from a cut on the tail vein of each mouse, and total
RNA was extracted for reverse transcription-quantitative PCR
(RT-qPCR). Mice were injected intraperitoneally with 3.33 mg/kg DOX
every other day for 18 days, resulting in a cumulative DOX dose of
30 mg/kg. This cumulative dose of DOX in mice is estimated to be
comparable to approximately 100 mg/m2 in humans
(18), which is considerably lower
when compared to clinical cardiotoxic doses (3). However, the cumulative dose of DOX we
used is sufficient to cause damage to the murine heart, as
supported by many other studies (19–21);
thus, the susceptibility to DICT may be greater in mice than in
humans. The control mice were injected intraperitoneally with an
equivalent volume of saline. Animals were euthanized by
exsanguination via jugular puncture on the seventh day after the
last DOX administration under inhalation anesthesia with 2%
isoflurane; whole blood and heart samples were collected. A small
portion of whole blood was transferred into
ethylenediaminetetraacetic acid (EDTA)-coated tubes and used for
measurement of hematological parameters. The remaining blood was
immediately centrifuged at 1,000 × g for 10 min at 4°C to separate
serum for measurement of cardiac injury parameters. Heart samples
were immediately frozen and stored at −80°C until used for RNA
isolation. As an index of cardiac hypertrophy, the body
weight-normalized heart weight was calculated.
Measurements of hematological
parameters and serum cardiac injury parameters
As hematological parameters, white blood cells
(WBCs), red blood cells (RBCs) and platelets were counted, and
hemoglobin (Hb) levels and hematocrit (Hct) were assessed using a
hematology analyzer (EYM-230; Erma, Inc.). As indices of cardiac
injury, serum levels of lactate dehydrogenase (LDH), aspartate
aminotransferase (AST), creatine kinase MB isoenzyme (CK-MB), and
troponin T were determined; the first two were measured using a
colorimetric kit (Wako) as described in our previous reports
(22,23), and the last two were measured using
a diagnostic kit (Cloud-Clone Corp.) according to the supplier's
instructions.
Cell culture
Rat heart-derived embryonic myoblast H9c2 (2–1) cells
were obtained from DS Pharma Biomedical. Cells were maintained in
Dulbecco's modified Eagle's medium (DMEM) supplemented with 10%
fetal bovine serum, 100 U/ml penicillin G and 100 µg/ml
streptomycin at 37°C in a humidified 5% CO2−95% air
incubator under standard conditions. Staining with 0.2% Trypan blue
was performed to determine viable cell counts. To maintain
exponential growth, cells were seeded at a density of
5×104 cells/ml and were passaged every 3–4 days. For the
remaining assays cells were cultured in 2-ml aliquots in 35-mm
dishes.
RNA extraction and RT-qPCR
Total RNA was isolated from blood using a
NucleoSpin® RNA blood kit (MACHEREY-NAGEL GmbH & Co.
KG) according to the manufacturer's instructions. Total RNA was
purified from mouse heart and H9c2 cells by extraction using ISOGEN
reagent (Nippon Gene) according to the manufacturer's protocol. The
quantity and the purity of extracted RNA were determined on a
NanoDrop ND-1000 spectrophotometer. RNA samples with 260/280 ratios
higher than 1.8 were used for experiments. Total RNA (100 ng) was
converted into cDNA using the ReverTra Ace® qPCR RT Kit
(TOYOBO, Osaka, Japan). Aliquots of the resulting cDNA preparations
then were subjected to qPCR analysis using the KOD SYBR®
qPCR Mix (Toyobo). A CFX Connect™ Real-Time PCR system (Bio-Rad
Laboratories, Inc.) was used to determine mRNA expression levels of
the genes encoding connective tissue growth factor (Ctgf),
interleukin 6 (Il6), programmed cell death 1 gene
(Pdcd1, also called PD-1), superoxide dismutase-3
(Sod3), glutathione peroxidase-3 (Gpx3), and
metallothionein-1 (Mt1). The primer pairs used were obtained
from the Takara Perfect Real Time Primers collection (Takara Bio).
cDNA/Taq-polymerase was denatured at 98°C for 2 min, and then 40
amplification cycles (each cycle: At 98°C for 10 sec, at 60°C for
10 sec, and at 68°C for 30 sec) were performed. The primer
sequences and GenBank accession numbers are listed in Table I. Transcript levels were normalized
to those of the housekeeping gene Gapdh (encoding
glyceraldehyde-3-phosphate dehydrogenase) using the following
formulae: ΔCq=Cq target-Cq reference; and ΔΔCq=mean value of ΔCq
sample-ΔCq control. Finally, the 2−ΔΔCq method (24) was used to calculate the differences
in mRNA transcription levels. The results of all assays were
checked against melting curves to confirm the presence of single
PCR products. At least two independent experiments were conducted
and samples were assessed in (at least) triplicate in each
experiment.
 | Table I.Primers used for reverse
transcription-quantitative PCR (RT-qPCR) analysis. |
Table I.
Primers used for reverse
transcription-quantitative PCR (RT-qPCR) analysis.
| A, Mouse |
|---|
|
|---|
| Gene | GenBank accession
number | F primer sequence
(5′-3′) | R primer sequence
(5′-3′) |
|---|
| Gpx3 | NM_008161.3 |
TCAACGTAGCCAGCTACTGAGGTC |
CTGTTTGCCAAATTGGTTGGAA- |
| Il6 | NM_031168.1 |
CCACTTCACAAGTCGGAGGCTTA |
GCAAGTGCATCATCGTTGTTCATAC |
| Mt1 | NM_013602.3 |
TCTAAGCGTCACCACGACTTCA |
GTGCACTTGCAGTTCTTGCAG |
| Pdcd1 | NM_008798.2 |
TGGCAATCAGGGTGGCTTC |
GACTCAGGCGGTTCCAGTTCA |
| Sod3 | NM_011435.3 |
GTGTCCCAAGACAATCCCACAA |
GGGAGTACTCTCAAAGGTGCTCA |
| Ctgf | NM_010217.2 |
ACCCGAGTTACCAATGACAATACC |
CCGCAGAACTTAGCCCTGTATG |
| Gapdh | NM_008084.3 |
TGTGTCCGTCGTGGATCTGA |
TTGCTGTTGAAGTCGCAGGAG |
|
| B, Rat |
|
| Gene | GenBank
accession number | F primer
sequence (5′-3′) | R primer
sequence (5′-3′) |
|
| Il6 | NM_012589.2 |
ATTGTATGAACAGCGATGATGCAC |
CCAGGTAGAAACGGAACTCCAGA |
| Pdcd1 | NM_001106927.1 |
GCGCTTGCACTGTTGAGTGAG |
TGCCCAACAATAGGATTCAGGAG |
| Gapdh | NM_017008.4 |
GGCACAGTCAAGGCTGAGAATG |
ATGGTGGTGAAGACGCCAGTA |
Il6 and Pdcd1 knockdown
Small interfering RNA (siRNA)-Il6 (siIl6) and
siRNA-Pdcd1 (siPdcd1) were transfected into H9c2 cells using
the HyperFect transfection reagent (Qiagen) according to the
manufacturer's protocol. A non-targeting siRNA (Mock) was used as a
vehicle control to assess the non-sequence-specific effects of
transfected siRNAs. The siRNAs used were siIl6, a FlexiTube siRNA
(ID no. SI01525356, Qiagen) and siPdcd1, a Silencer®
Select Pre-designed siRNA Product (ID no. s154115, Thermo Fisher
Scientific, Inc.); the negative control siRNA was obtained as
AllStars Neg. Control siRNA (ID no. AM4611, Qiagen). Transfections
consisted of 4×104 cells combined with a given siRNA
(final concentration, 10 nM) in the presence of HyperFect reagent;
the mixtures then were incubated for 24 h before assessment of
Il6 or Pdcd1 expression.
IL-6 and PDCD1 immunofluorescence
Cells transfected with siRNAs were seeded into the
Lab-Tek® 8-well chambered cover glass system plates
(Thermo Fisher Scientific, Inc.) at 4×104 cells/ml and
incubated overnight under standard culture conditions. The
chambered slides were washed twice with phosphate-buffered saline
(PBS) adjusted to pH 7.4 and fixed in ice-cold 1:1 methanol:acetone
for 30 min. The slides were immersed for 10 min in 1% goat serum
and 0.25% Triton X-100 in PBS and then transferred to Blocking One
Histo (Nacalai Tesque) for 10 min. The slides then were washed with
PBS containing 0.1% Tween-20, incubated with primary antibody
anti-IL-6 rabbit polyclonal antibody (cat. no. NB600-1131, Novus
Biologicals) or anti-PDCD1 mouse monoclonal antibody (cat. no.
66220-1-Ig, Proteintech®) at 1:1,000 in PBS for 1 h at
room temperature, washed with PBS, and incubated with Alexa
Fluor-conjugated secondary antibodies (Thermo Fisher Scientific,
Inc.) for 1 h. After rinsing with PBS, a drop of UltraCruz™
Mounting Medium with DAPI (Santa Cruz Biotechnology, Inc.) was
added to each well. Cells were observed under a confocal
fluorescence microscope C-1 (Nikon) and cell components were
visualized by fluorescent intensity in blue (405 nm) for nuclear
DNA, green (488 nm) for PDCD1-positive cells, and red (594 nm) for
IL-6-positive cells.
Apoptosis assay
Annexin V-mediated detection of phosphatidylserine
(PS) was used to identify cells at early stages of apoptosis,
because the redistribution of PS from the internal membranes to the
external membrane surface occurs in early apoptosis (25). Several previous reports have
employed an annexin V assay to determine apoptosis in DOX-treated
cardiomyocytes (26,27). On the other hand, caspase-3/7 is
known to be a key downstream effector in the apoptosis pathway
(28). To test both aspects of cell
death, we assessed the DOX-induced apoptosis using the
RealTime-Glo™ Annexin V Apoptosis assay (Promega Corp.) and the
Caspase-Glo® 3/7 Assay (Promega Corp.). Briefly, the
H9c2 cells were transfected with siRNAs, distributed in a 96-well
plate at a density of 4×103 cells per well, and allowed
to adhere overnight. For the RealTime-Glo™ Annexin V Apoptosis
assay, the adhered cells were incubated with 1 µM DOX in the
presences of annexin V-luciferase reagents and time-dependent
increases in luminescence were monitored, reflecting the apoptotic
process. Similarly, apoptosis was evaluated after incubation for 24
h with the concentration of DOX at 0.01, 0.03, 0.1, 0.3 or 1 µM.
These concentrations were set based on the results of a previous
study (29), in which DOX at 0.1 µM
or higher was shown to induce apoptosis of cardiomyocyte cells. For
the Caspase-Glo® 3/7 Assay, the adhered cells were
incubated with DOX at 0.1, 0.3 or 1 µM for 4 or 8 h;
Caspase-Glo® 3/7 reagents then were added to each well
and the contents were gently mixed. The resulting luminescent
intensity was measured using a Varioskan™ LUX multimode microplate
reader (Thermo Fisher Scientific, Inc.).
Statistical analysis
Data are expressed as mean ± standard error of the
mean (SEM). Differences between two groups were compared using
one-way analysis of variance (ANOVA). Multiple comparisons were
performed by one-way ANOVA followed by Tukey post hoc test. P-value
of less than 0.05 was considered significant. Correlation analyses
were performed using the Spearman's rank correlation coefficient
method. Statistical analyses were performed using Ekuseru-Toukei
2012 software (Social Survey Research Information Co., Ltd.).
Results
DICT and DOX-induced
hematotoxicity
Toxic effects of DOX on the heart and in blood cells
were evaluated in mice treated with a cumulative dose of 30 mg/kg.
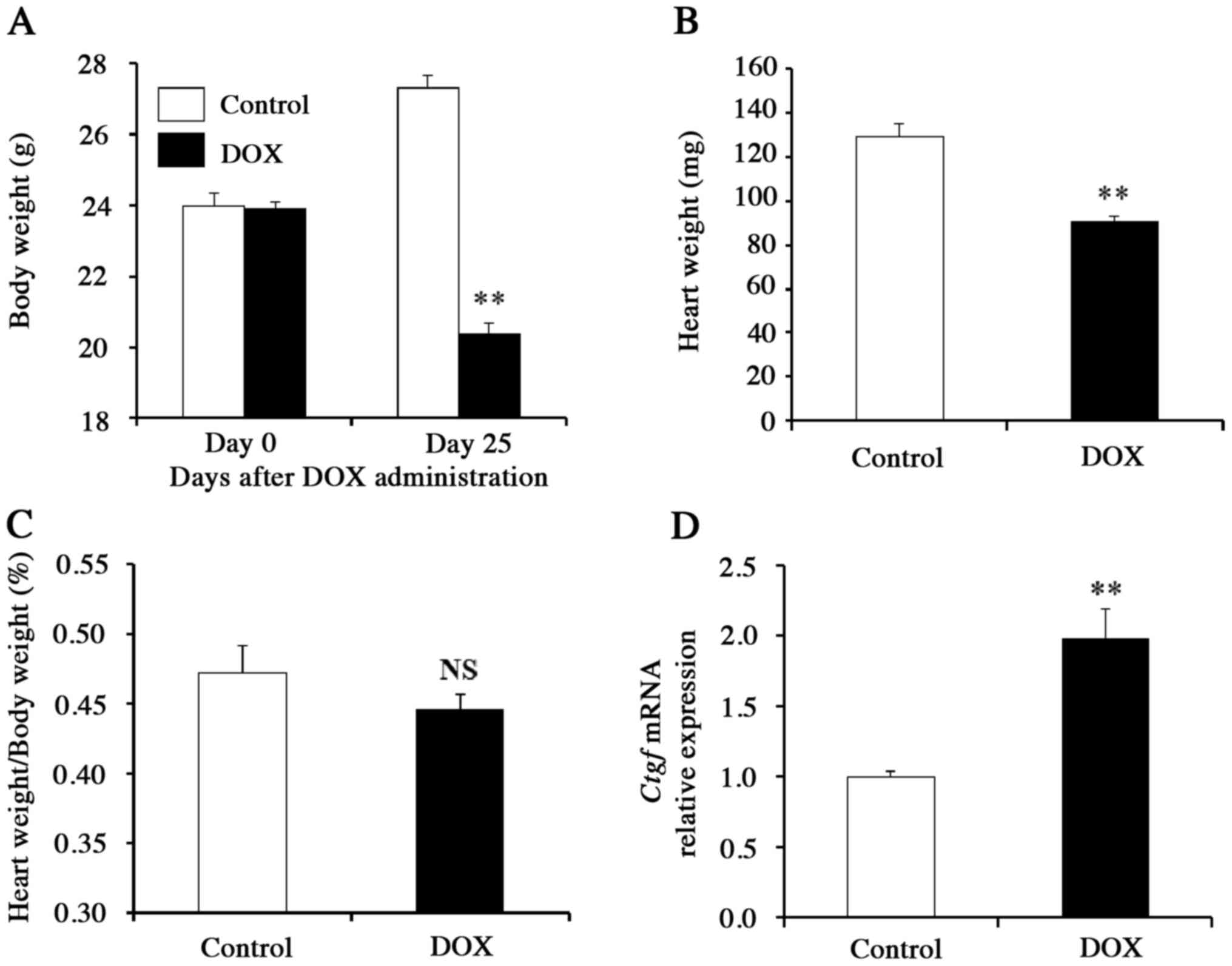
The body weight and heart weight of DOX-treated mice were almost
30% lower than those of the control mice (Fig. 1A and B), whereas the body
weight-normalized heart weight did not differ significantly between
these two groups (P>0.05; Fig.
1C), suggesting that cardiac hypertrophy is absent in the
DOX-treated mice. Gene expression analyses of pooled heart samples
revealed that the mRNA level of Ctgf (which encodes a
pro-fibrotic cytokine) was significantly increased in DOX-treated
mice compared to control animals (P<0.01; Fig. 1D). These findings suggested that
cardiac tissue is the primary target of DOX cardiotoxicity. To
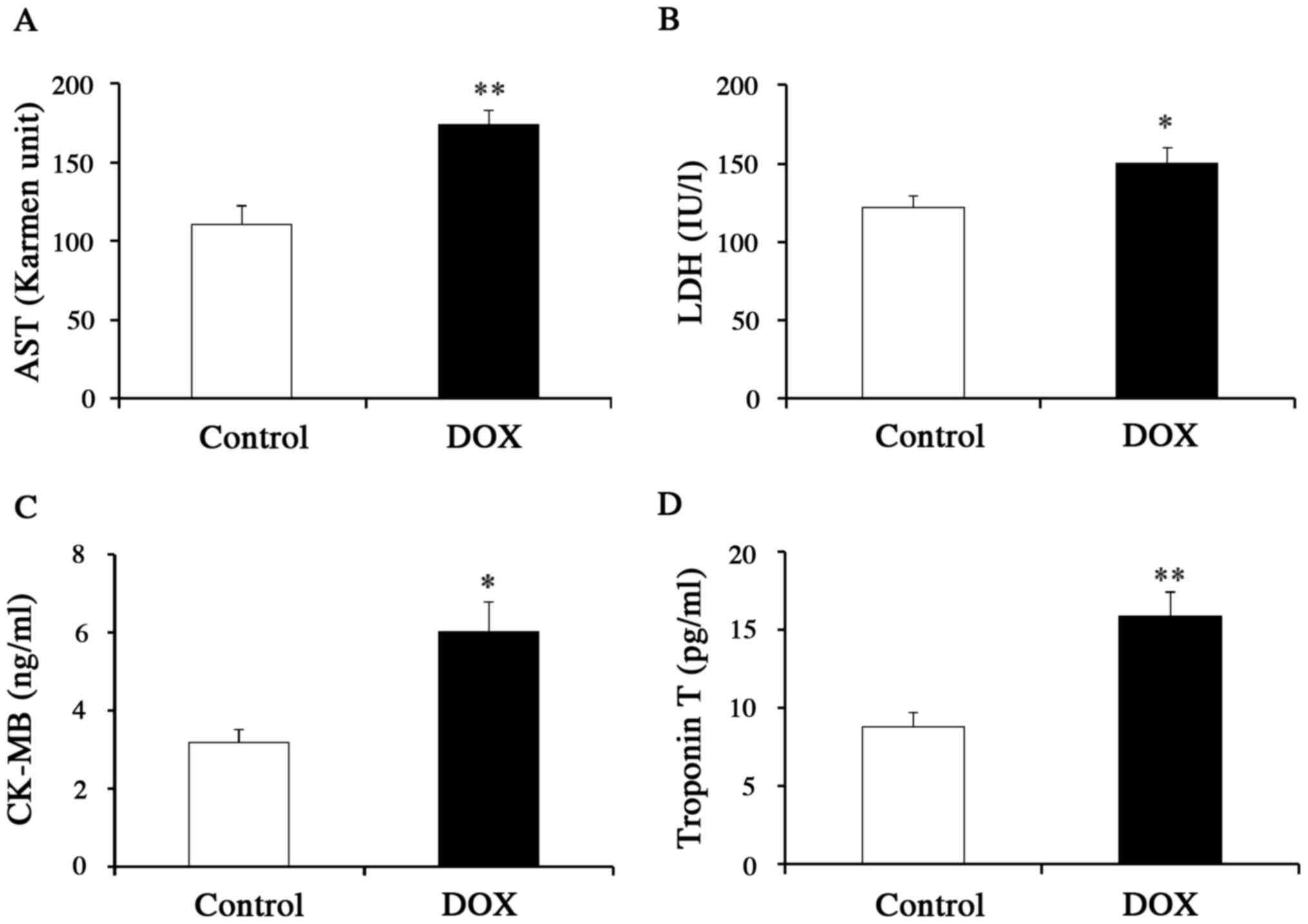
further clarify the effects of DOX on the heart, we measured the
serum levels of specific biomarkers for myocardial injury
(including CK-MB and troponin T) as well as the serum levels of
more general markers for inflammation (AST and LDH) (Fig. 2A-D). Mice that received DOX showed
higher levels of not only AST and LDH, but also CK-MB and troponin
T, as compared to control mice, supporting the use of the specific
biomarkers as indicators of DICT. We also measured the mRNA levels
of atrial natriuretic peptide (ANP) and brain natriuretic peptide
(BNP) in blood and heart, other biomarkers, at the first screening.
Unfortunately, there was poor reproducibility in the measurements,
so we did not adopt the data regarding natriuretic peptides.
Because DOX also can lead to hematotoxicity
(30), some hematological
parameters were measured in the small portion of whole blood
collected from mice of both groups. In the DOX-treated mice, WBC
values decreased to 45.8% of those in control mice, while platelet
counts increased to 115.7% of those in control mice. Nevertheless,
post-dose values of other parameters, such as RBC, Hb, and Hct, in
DOX-treated mice did not differ significantly from those values in
control mice (P>0.05) (data not shown), suggesting that DOX did
not cause anemia or hypoglobulinemia.
Correlation of DICT and mRNA
expression in blood
We next examined the correlation between the
severity of DICT and blood mRNA expression of genes prior to DOX
administration. Among 54 DOX-treated mice, twelve animals (22.2%)
had no significant change in serum levels of cardiac injury
parameters (P>0.05), while apparent cardiotoxicity (as evidenced
by increases in the levels of the parameters associated with
cardiotoxicity) was observed in 42 mice (77.8%), including ten mice
(18.5%) that were found dead. The death was considered due to
severe heart failure, because survival rate has conventionally been
used as an index of DICT (31–33).
Most of surviving mice that escaped from lethal DICT would develop
heart failure or cardiotoxicity. To facilitate correlation
analysis, susceptibility to DOX-induced heart damage was scored as
0 to 8 based on separate changes in parameters (AST, LDH, CK-MB,
and troponin T) in surviving mice as follows: 0, no change in
either parameter (no damage detected); 1, increase in either AST or
LDH=1 (such that increases in both yielded a value of 2); 3,
increase in either CK-MB or troponin T=3 (such that increases in
both yielded a value of 6); and 8, increases in all of four
parameters. For example, if three parameters except for troponin T
increased in a blood sample, its DICT score was determined as 5
(AST=1, LDH=1 and CK-MB=3). In addition, death was given a score of
10 as a maximum cardiac injury.
As a first screen for mRNA expression, we used
RT-qPCR to assess the blood levels of transcripts from 32 candidate
genes, including those encoding apoptosis-, autophagy- and
oxidative stress-related proteins, each of which might contribute
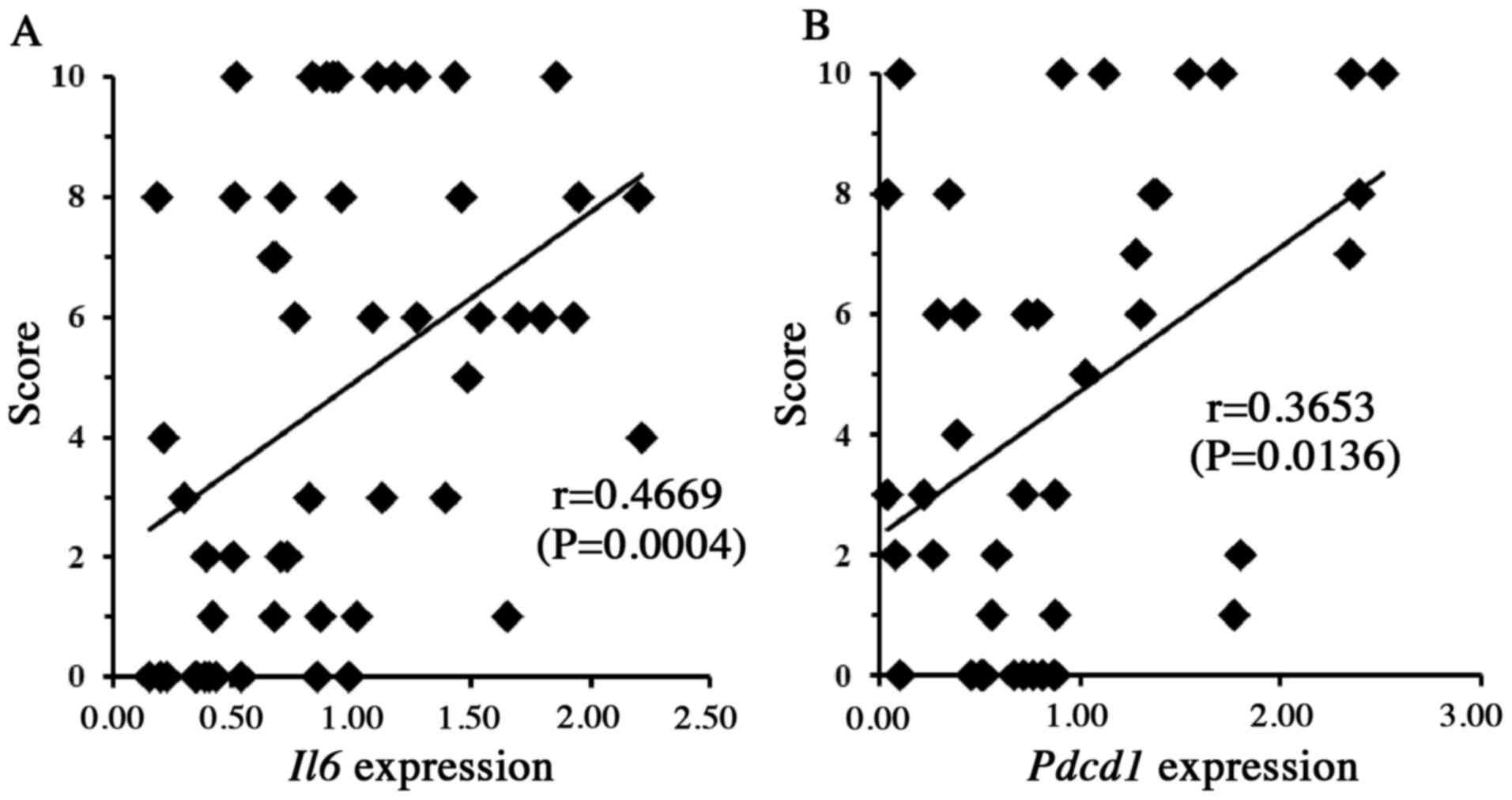
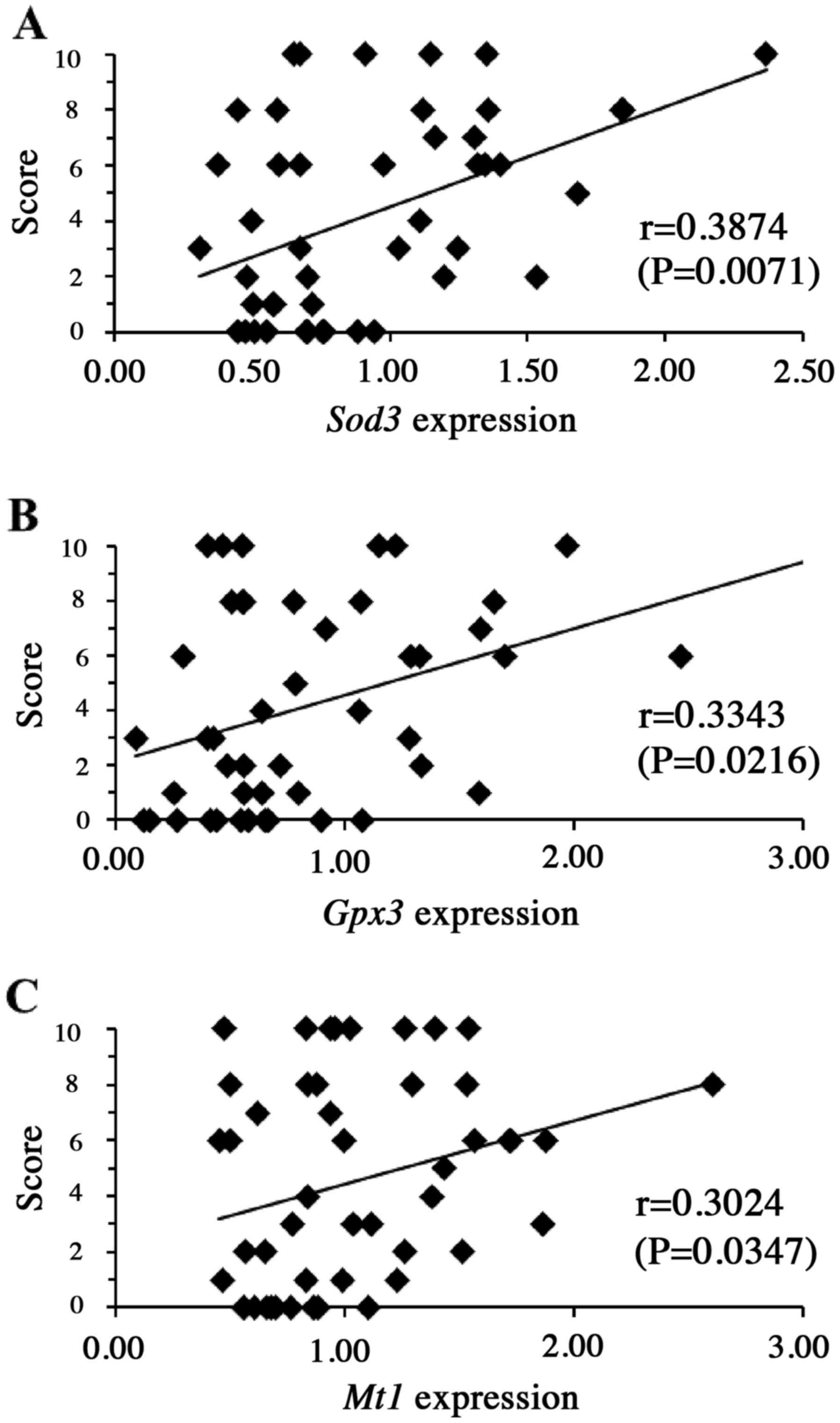
to the pathological mechanism(s) of DICT. The results revealed that
the susceptibility to DOX-induced heart damage (as described above)
was significantly and positively correlated with blood mRNA levels
of Il6 (P<0.01) and Pdcd1 (P<0.05) (Fig. 3), as well as with the blood mRNA
levels of the genes encoding Sod3 (P<0.01), Gpx3
(P<0.05) and Mt1 (P<0.05) (Fig. 4) prior to drug administration,
suggesting that the products of these five genes may have some role
in DICT. We chose to focus on the roles of Il6 and
Pdcd1, given that previous work has suggested that cell
signaling mediated by IL-6 and PDCD1 contributes to cytoprotection
in the heart and other tissues (34–39).
Therefore, in vitro experiments were performed to clarify
the roles of Il6 and Pdcd1 in DICT.
Effects of Il6 or Pdcd1 knockdown on
DOX-induced apoptosis in vitro
We next assessed the potential roles of Il6
and Pdcd1 on DOX-induced toxicity of H9c2 cells (embryonic
rat cardiac myoblasts), a line that is commonly used as a model for
studies of DICT. Because cell death by apoptosis is involved in the
primary mechanism of DICT (1,2,5), we
studied effects of knockdown of Il6 or Pdcd1 on
apoptotic response to DOX. The knockdown of endogenous Il6
and Pdcd1 was achieved by transfection with gene-specific
siRNAs (Fig. S1). Knockdown
efficacy was estimated by RT-qPCR; mRNA expression of Il6
and Pdcd1 were 0.32±0.03 and 0.23±0.05, respectively, when
expression following transfection with the control siRNA was
defined as 1.00 (Fig. S1A and B).
Mock cells (vehicle control cells) exhibited no significant change
in the accumulation of either mRNA (P>0.05). The knockdown of
Il6 and Pdcd1 was confirmed by assessing levels of
the two proteins by immunofluorescence analysis (Fig. S1C and D).
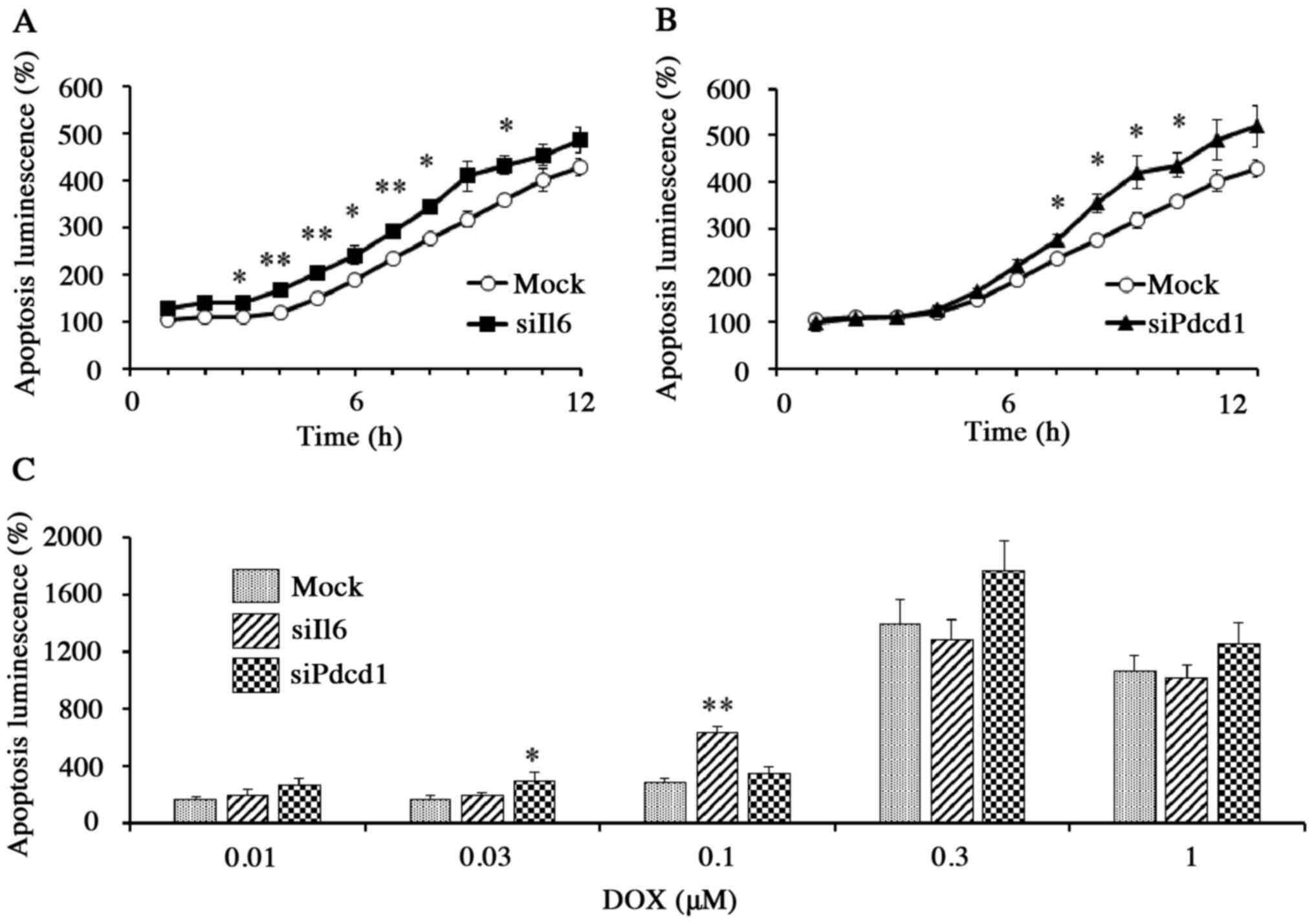
In mock cells exposed to DOX (1 µM), apoptosis (as
determined by a luminescence assay) increased with incubation time.
The DOX-induced apoptosis, however, was significantly increased in
Il6 knockdown cells (P<0.01) and in Pdcd1
knockdown cells (P<0.05) than in mock cells within the first 10
h of incubation (Fig. 5A and B).
Thus, DOX-induced apoptosis during the first 10 h of exposure was
significantly enhanced by either Il6 or Pdcd1
knockdown, although the effects were weakened or undetectable for
later time points (Fig. 5A-C). The
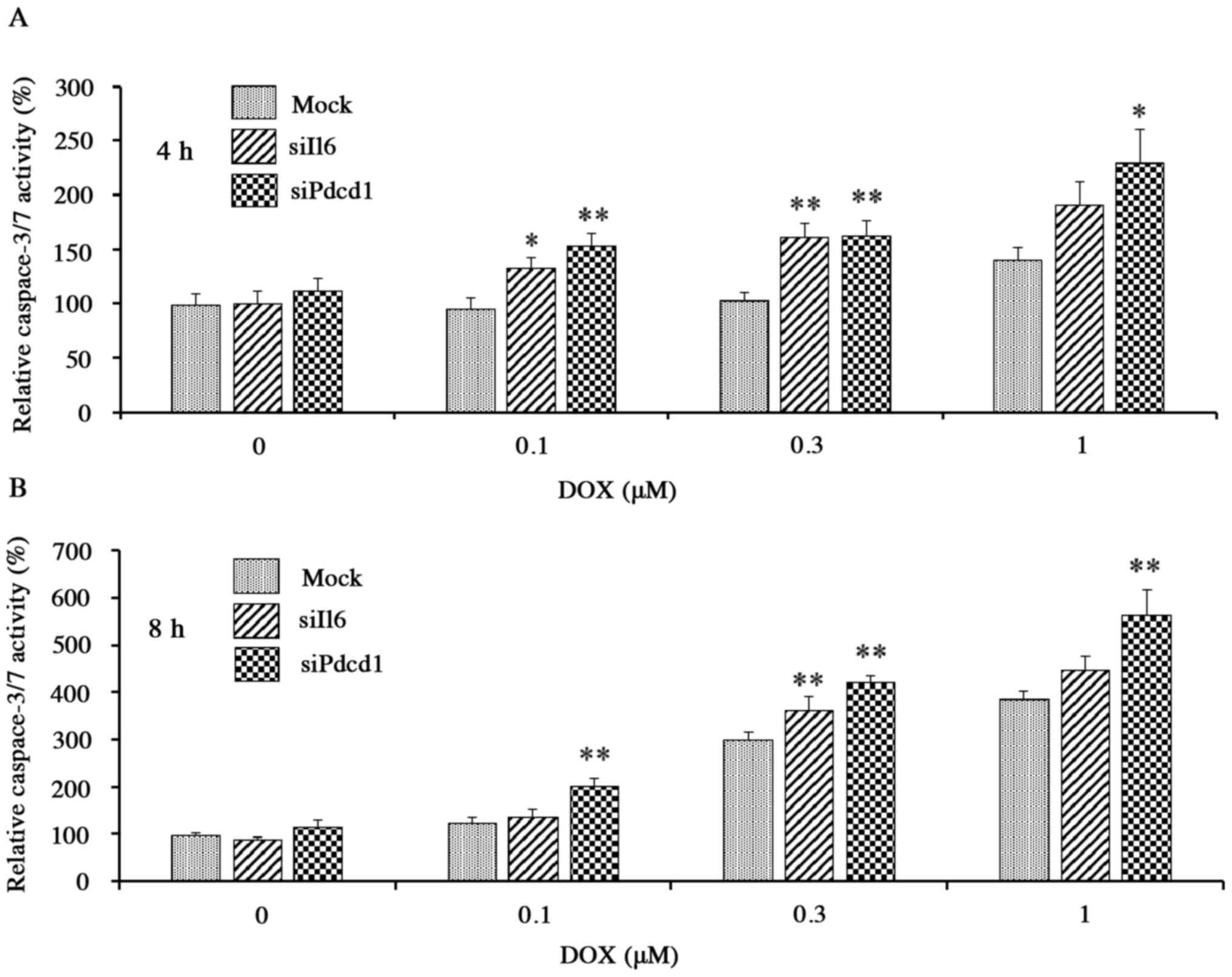
activity of caspase-3/7, a protease enzyme that plays an essential
role in apoptotic processes, also was increased in a DOX
concentration- and exposure time-dependent manner in mock cells
exposed to DOX (0.1–1 µM), confirming the ability of the
corresponding assay to detect DOX-induced apoptosis. The increase
in activity, however, was potentiated in each of the knockdown
cells compared to the mock cells (Fig.
6A and B); for example, after incubation for 4 h with 0.1 µM
DOX, activities in Il6 and Pdcd1 knockdown cells were
elevated by ~30 and ~60%, respectively, compared to mock cells
(Fig. 6A). Thus, by two separate
assays, DOX-induced apoptosis was enhanced by knockdown of
Il6 or Pdcd1, indicating that these genes have a
preventive role against DICT.
Discussion
DICT can result in acute or chronic adverse effects.
Both acute and chronic toxicity may lead to cardiac dysfunction or
cardiomyopathy, eventually leading to severe heart failure and
death (40,41). While acute cardiotoxicity following
treatment with a high dose of DOX is now rare (40), late-onset chronic cardiotoxicity
induced by the cumulative DOX dose remains common, occurring in up
to 65% of patients (42). In the
present study, we used a chronic cardiotoxicity model, in which
DICT was established in mouse by repeated administration of
DOX.
DOX-treated mice showed elevations (compared to
control mice) in serum levels of CK-MB and troponin T, specific
markers of myocardial cell injury, as well as in serum levels of
non-specific cytosolic enzymes, such as AST and LDH. Although we
did not assess the early cardiotoxicity markers after DOX
administration, the data on early markers may help to find new
information and knowledge about DICT. We need consider these points
in our next study. The mice also had elevated (compared to control
mice) cardiac tissue levels of the Ctgf transcript, which is
associated with fibrosis after heart tissue damage. These
biochemical changes indicate that DOX induces lethal injury in
myocardial cells. It should be noted, however, that no myocardial
injury was found in a subset (22.2%) of DOX-treated mice, although
the majority of mice displayed apparent DICT, including death.
Thus, individual differences are likely to exist in sensitivity of
the heart to DOX. Clinical findings also have indicated that
individuals vary greatly in their sensitivity to DOX (5–7); for
instance, some patients are highly tolerant to DOX at cumulative
doses exceeding 1,000 mg/m2, while others exhibit
cardiotoxicity at cumulative doses below 400 mg/m2
(5).
It is possible that differences among individuals
are attributable to genetic factors, which can be associated with
the pathogenesis of DICT. To assess this possibility, we analyzed
the correlation between mRNA expression of candidate genes in blood
before DOX administration and the severity of DICT (using a score
based on the changes in parameters known to be indicative of
cardiac injury). Among 32 candidate genes, we have identified the
five genes (Il6, Pdcd1, Sod3, Gpx3 and Mt1) that were
correlated with susceptibility to DICT. According to previous
reports (34–39), cell signaling mediated by IL-6 and
PDCD1 may be involved in protection from myocardial damage, such as
ischemic damage, and therefore we investigated the roles of
Il6 and Pdcd1 in DICT. As shown in Fig. 3, higher mRNA expression of
Il6 or Pdcd1 was positively correlated with the DICT
score, indicating that the expression levels of these genes might
serve as predictive markers for DICT. In addition, these results
led to the expectation that the expression of Il6 or
Pdcd1 (at the mRNA or protein level) may be factors
influencing the development of DICT. In the next experiment,
therefore, DOX toxicity was assessed in cells of the rat
cardiomyocyte line H9c2 in which Il6 or Pdcd1
expression had been subjected to knockdown via transfection with
gene-specific siRNAs (Fig. S1).
The cell toxicity of DOX was evaluated by monitoring apoptosis, a
process known to be one of the primary mechanisms of DICT (1,2,5).
Apoptosis was assessed by an annexin V assay, previously
demonstrated to show high sensitivity and specificity for cell
death (26), and by a caspase-3/7
assay known to be indicative of apoptotic processes (28). Contrary to our expectation, cells
subjected to Il6 and Pdcd1 knockdown exhibited
increased (rather than decreased) levels of DOX-induced apoptosis,
suggesting that both of these genes play a protective role against
the DOX-induced cardiomyocyte apoptosis, at least in this rat
cardiomyocyte line.
IL-6 has been shown to be a pro-inflammatory
cytokine with cardioprotective potential (35). Indeed, many reports have stated that
the expression of this cytokine in cardiomyocytes attenuates
myocardial ischemia-reperfusion damage (34,35,39).
However, consistent with our results for blood concentrations of
Il6 transcripts in mice, the circulating levels of IL-6 have
been shown to be elevated in patients with congestive heart failure
(43), and IL-6 is known to be
released from the border zone of myocardial infarcts (44). The increased circulating levels of
Il6 transcript and protein may reflect the release of these
gene products into blood as the result of a compensatory
mechanism(s) intended to reduce the vulnerability of heart tissue
to various stresses such as ischemia and chemical stimuli, leading
to cardioprotective action. In contrast, IL-6 has also been found
to act as a deleterious cytokine associated with oxidative stress
on the heart. The cytokine is indicated to induce apoptosis in
cardiomyocytes (45), in agreement
with our findings in terms of role of IL-6 on apoptosis.
Furthermore, involvement of IL-6 (and oxidative stress) in the
pathogenesis of cardiac heart failure (46) and atrial fibrillation (47) has been reported. The cardiac role of
IL-6 is complex and remains poorly understood (48).
PDCD1, an immune inhibitory receptor, has been shown
to inhibit lymphocyte activation and cytokine production (49). A previous study in mouse
demonstrated that neutralization of PDCD1 with an anti-PDCD1
monoclonal antibody enhances DOX-induced nephropathy, suggesting
that the PDCD1 pathway protects renal tissue from DOX-associated
toxicity (38). That finding would
be in agreement with our results suggesting the beneficial action
of PDCD1 in preventing DOX-induced cardiotoxicity, although the
underlying mechanism(s) of this effect remain unclear. Further
studies will be needed to determine the details of the pathways and
mechanisms whereby IL-6 and PDCD1 counteract DOX toxicity.
Extensive efforts have been made in various model
systems to understand the role of gene expression in the
mechanism(s) of DICT pathogenesis (21,50–55).
These studies assessed early and time-dependent molecular changes
that occur following DOX administration, but did not include
assessment of the levels of the tested molecules prior to DOX
administration. For instance, troponins, which are sensitive
tissue-specific markers of heart damage, are known to accumulate to
elevated levels in blood only after cardiac tissue damage has
occurred (56), but (to our
knowledge) the pre-exposure levels of troponins have not previously
been investigated in the context of DOX exposure. Invasive and
noninvasive clinical approaches are currently being tested for use
in predicting cardiotoxicity in DOX-treated cancer patients, but
there have been technical complications (57,58).
In that context, the results obtained in the present study may
contribute to the early prediction of DICT and to new therapeutic
strategies of cardioprotection. To our knowledge, the present
report is the first to demonstrate a role for Il6 and
Pdcd1 as predictive and protective factors in DICT. If
animals with diverse genetic backgrounds are used, further genes
associated with DICT may be found. Nevertheless, we used C57BL/6J
inbred mice to minimize the individual differences including age,
body weight, physical condition and genetic background, all of
which could significantly influence the DICT. Although it is
difficult to apply our findings to a human population in terms of
genetic diversity, we believe that Il6 and Pdcd1 may
also provide a beneficial role in cardioprotection in clinical
chemotherapy with DOX.
Pharmacological strategies for preventing DICT have
also been proposed in many studies; β-blockers, ACE inhibitors,
ARBs and statins can reduce DICT in animals and in humans (1,10,13).
It is noteworthy that dexrazoxane, which reduces oxidative stress
(by iron chelation) and inhibits topoisomerase IIβ, protects the
heart from anthracycline-induced toxicity (21,59,60).
In fact, dexrazoxane is licensed in many parts of the world for two
different indications; prevention of cardiotoxicity from
anthracycline-based chemotherapy, and prevention of tissue injuries
after extravasation of anthracycline (60). Based on information on predictive
gene expression in individual patients, a pharmacological approach
may be useful for further effective protection against DICT.
In conclusion, the pre-existing level of expression
of both Il6 and Pdcd1 in cardiomyocytes may play an
important role in protection against DOX-induced damage, such that
the expression of these genes in blood serves as a predictive
marker for DICT. These findings may provide useful information for
prevention of cardiotoxicity in cancer patients receiving DOX
therapy.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The current study was supported in part by a
Grant-in-Aid for Scientific Research (C) (grant no. KAKENHI
17K08458) from the Japan Society for the Promotion of Science, and
by a Matching Fund Subsidy for Private Universities from the
Ministry of Education, Culture, Sports, Science and Technology of
Japan.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SK performed the experimental work, data collection
and writing of the paper. AH participated in the study design,
interpreted the data and revised the paper. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Experiments were conducted in accordance with the
standards established by the Japanese Pharmacological Society and
were approved by the Tohoku Medical and Pharmaceutical University
of Institutional Animal Care and Use Committee (Experimental
Protocol no. 18013).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
DOX
|
doxorubicin
|
|
ACE
|
angiotensin-converting enzyme
|
|
ARBs
|
angiotensin II receptor blockers
|
|
PDCD1
|
programmed cell death 1
|
|
Sod3
|
superoxide dismutase-3
|
|
Gpx3
|
glutathione peroxidase-3
|
|
Mt1
|
metallothionein-1
|
|
DICT
|
DOX-induced cardiotoxicity
|
|
LDH
|
lactate dehydrogenase
|
|
AST
|
aspartate aminotransferase
|
|
CK-MB
|
creatine kinase MB isoenzyme
|
References
|
1
|
McGowan JV, Chung R, Maulik A, Piotrowska
I, Walker JM and Yellon DM: Anthracycline chemotherapy and
cardiotoxicity. Cardiovasc Drugs Ther. 31:63–75. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nebigil CG and Désaubry L: Updates in
anthracycline-mediated cardiotoxicity. Front Pharmacol. 9:12622018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Swain SM, Whaley FS and Ewer MS:
Congestive heart failure in patients treated with doxorubicin: A
retrospective analysis of three trials. Cancer. 97:2869–2879. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ewer MS and Ewer SM: Cardiotoxicity of
anticancer treatments: What the cardiologist needs to know. Nat Rev
Cardiol. 7:564–575. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jungsuwadee P: Doxorubicin-induced
cardiomyopathy: An update beyond oxidative stress and myocardial
cell death. Cardiovasc Reg Med. 3:e11272016.
|
|
6
|
Ohtani K, Fujino T, Ide T, Funakoshi K,
Sakamoto I, Hiasa KI, Higo T, Kamezaki K, Akashi K and Tsutsui H:
Recovery from left ventricular dysfunction was associated with the
early introduction of heart failure medical treatment in cancer
patients with anthracycline-induced cardiotoxicity. Clin Res
Cardiol. 108:600–611. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Raj S, Franco VI and Lipshultz SE:
Anthracycline-induced cardiotoxicity: A review of pathophysiology,
diagnosis, and treatment. Curr Treat Options Cardiovasc Med.
16:3152014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zamorano JL, Lancellotti P, Muñoz DR,
Aboyans V, Asteggiano R, Galderisi M, Habib G, Lenihan DJ, Lip GY,
Lyon AR, et al: 2016 ESC position paper on cancer treatments and
cardiovascular toxicity developed under the auspices of the ESC
committee for practice guidelines. Kardiol Pol. 74:1193–1233.
2016.(In Polish). View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Glass CK and Mitchell RN: Winning the
battle, but losing the war: Mechanisms and morphology of
cancer-therapy-associated cardiovascular toxicity. Cardiovasc
Pathol. 30:55–63. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Totzeck M, Mincu RI, Heusch G and Rassaf
T: Heart failure from cancer therapy: Can we prevent it? ESC Heart
Fail. 6:856–862. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hadi N, Yousif NG, Al-amran FG, Huntei NK,
Mohammad BI and Ali SJ: Vitamin E and telmisartan attenuates
doxorubicin induced cardiac injury in rat through down regulation
of inflammatory response. BMC Cardiovasc Disord. 12:632012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yamanaka S, Tatsumi T, Shiraishi J, Mano
A, Keira N, Matoba S, Asayama J, Fushiki S, Fliss H and Nakagawa M:
Amlodipine inhibits doxorubicin-induced apoptosis in neonatal rat
cardiac myocytes. J Am Coll Cardiol. 41:870–878. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cardinale D, Colombo A, Lamantia G,
Colombo N, Civelli M, De Giacomi G, Rubino M, Veglia F, Fiorentini
C and Cipolla CM: Anthracycline-induced cardiomyopathy: Clinical
relevance and response to pharmacologic therapy. J Am Coll Cardiol.
55:213–220. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Plana JC, Galderisi M, Barac A, Ewer MS,
Ky B, Scherrer-Crosbie M, Ganame J, Sebag IA, Agler DA, Badano LP,
et al: Expert consensus for multimodality imaging evaluation of
adult patients during and after cancer therapy: A report from the
American society of echocardiography and the european association
of cardiovascular imaging. J Am Soc Echocardiogr. 27:911–939. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cappetta D, Esposito G, Coppini R, Piegari
E, Russo R, Ciuffreda LP, Rivellino A, Santini L, Rafaniello C,
Scavone C, et al: Effects of ranolazine in a model of
doxorubicin-induced left ventricle diastolic dysfunction. Br J
Pharmacol. 174:3696–3712. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Henriksen PA: Anthracycline
cardiotoxicity: An update on mechanisms, monitoring and prevention.
Heart. 104:971–977. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Aminkeng F, Ross CJ, Rassekh SR, Hwang S,
Rieder MJ, Bhavsar AP, Smith A, Sanatani S, Gelmon KA, Bernstein D,
et al: Recommendations for genetic testing to reduce the incidence
of anthracycline-induced cardiotoxicity. Br J Clin Pharmacol.
82:683–695. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nair AB and Jacob S: A simple practice
guide for dose conversion between animals and human. J Basic Clin
Pharm. 7:27–31. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Neilan TG, Jassal DS, Scully MF, Chen G,
Deflandre C, McAllister H, Kay E, Austin SC, Halpern EF, Harmey JH
and Fitzgerald DJ: Iloprost attenuates doxorubicin-induced cardiac
injury in a murine model without compromising tumour suppression.
Eur Heart J. 27:1251–1256. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jenkins GR, Lee T, Moland CL, Vijay V,
Herman EH, Lewis SM, Davis KJ, Muskhelishvili L, Kerr S, Fucoe JC
and Desai VG: Sex-related differential susceptibility to
doxorubicin-induced cardiotoxicity in B6C3F1 mice.
Toxicol Appl Pharmacol. 310:159–174. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vijay V, Moland CL, Han T, Fuscoe JC, Lee
T, Herman EH, Jenkins GR, Lewis SM, Cummings CA, Gao Y, et al:
Early transcriptional changes in cardiac mitochondria during
chronic doxorubicin exposure and mitigation by dexrazoxane in mice.
Toxicol Appl Pharmacol. 295:68–84. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kanno S, Ishikawa M, Takayanagi M,
Takayanagi Y and Sasaki K: Potentiation of acetaminophen
hepatotoxicity and mortality by doxapram in mice. Biol Pharm Bull.
21:934–937. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kanno S, Tomizawa A, Hiura T, Osanai Y,
Kakuta M, Kitajima Y, Koiwai K, Ohtake T, Ujibe M and Ishikawa M:
Melatonin protects on toxicity by acetaminophen but not on
pharmacological effects in mice. Biol Pharm Bull. 29:472–476. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Blankenberg FG, Tait JF and Strauss HW:
Apoptotic cell death: Its implications for imaging in the next
millennium. Eur J Nucl Med. 27:359–367. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bennink RJ, van den Hoff MJ, van Hemert
FJ, de Bruin KM, Spijkerboer AL, Vanderheyden JL, Steinmetz N and
van Eck-Smit BL: Annexin V imaging of acute doxorubicin
cardiotoxicity (apoptosis) in rats. J Nucl Med. 45:842–848.
2004.PubMed/NCBI
|
|
27
|
Schwartz RG, Jain D and Storozynsky E:
Traditional and novel methods to assess and prevent
chemotherapy-related cardiac dysfunction noninvasively. J Nucl
Cardiol. 20:443–464. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Salvesen GS and Dixit VM: Caspases:
Intracellular signaling by proteolysis. Cell. 91:443–446. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
He H, Liu C, Wu Y, Zhang X, Fan J and Cao
Y: A multiscale physiologically-based pharmacokinetic model for
doxorubicin to explore its mechanisms of cytotoxicity and
cardiotoxicity in human physiological contexts. Pharm Res.
35:1742018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Eppstein DA, Kurahara CG, Bruno NA and
Terrell TG: Prevention of doxorubicin-induced hematotoxicity in
mice by interleukin 1. Cancer Res. 49:3955–3960. 1989.PubMed/NCBI
|
|
31
|
Li K, Sung RY, Huang WZ, Yang M, Pong NH,
Lee SM, Chan WY, Zhao H, To MY, Fok TF, et al: Thrombopoietin
protects against in vitro and in vivo cardiotoxicity induced by
doxorubicin. Circulation. 113:2211–2220. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu X, Chen Z, Chua CC, Ma YS, Youngberg
GA, Hamdy R and Chua BH: Melatonin as an effective protector
against doxorubicin-induced cardiotoxicity. Am J Physiol Heart Circ
Physiol. 283:H254–H263. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Montgomery MD, Chan T, Swigart PM, Myagmar
BE, Dash R and Simpson PC: An alpha-1A adrenergic receptor agonist
prevents acute doxorubicin cardiomyopathy in male mice. PLoS One.
12:e01684092017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dawn B, Xuan YT, Guo Y, Rezazadeh A, Stein
AB, Hunt G, Wu WJ, Tan W and Bolli R: IL-6 plays an obligatory role
in late preconditioning via JAK-STAT signaling and upregulation of
iNOS and COX-2. Cardiovasc Res. 64:61–71. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
McGinnis GR, Ballmann C, Peters B,
Nanayakkara G, Roberts M, Amin R and Quindry JC: Interleukin-6
mediates exercise preconditioning against myocardial ischemia
reperfusion injury. Am J Physiol Heart Circ Physiol.
308:H1423–H1433. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nishimura H, Okazaki T, Tanaka Y, Nakatani
K, Hara M, Matsumori A, Sasayama S, Mizoguchi A, Hiai H, Minato N
and Honjo T: Autoimmune dilated cardiomyopathy in PD-1
receptor-deficient mice. Science. 291:319–322. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Okazaki T, Tanaka Y, Nishio R, Mitsuiye T,
Mizoguchi A, Wang J, Ishida M, Hiai H, Matsumori A, Minato N and
Honjo T: Autoantibodies against cardiac troponin I are responsible
for dilated cardiomyopathy in PD-1-deficient mice. Nat Med.
9:1477–1483. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
38
|
Qin XH, Lee VW, Wang YP, Zheng GP, Wang Y,
Alexander SI and Harris DC: A protective role for programmed death
1 in progression of murine adriamycin nephropathy. Kidney Int.
70:1244–1250. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Smart N, Mojet MH, Latchman DS, Marber MS,
Duchen MR and Heads RJ: IL-6 induces PI 3-kinase and nitric
oxide-dependent protection and preserves mitochondrial function in
cardiomyocytes. Cardiovasc Res. 69:164–177. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sheng CC, Amiri-Kordestani L, Palmby T,
Force T, Hong CC, Wu JC, Croce K, Kim G and Moslehi J: 21st Century
cardio-oncology: Identifying cardiac safety signals in the era of
personalized medicine. JACC Basic Transl Sci. 1:386–398. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang YW, Shi J, Li YJ and Wei L:
Cardiomyocyte death in doxorubicin-induced cardiotoxicity. Arch
Immunol Ther Exp (Warsz). 57:435–445. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lipshultz SE, Colan SD, Gelber RD,
Perez-Atayde AR, Sallan SE and Sanders SP: Late cardiac effects of
doxorubicin therapy for acute lymphoblastic leukemia in childhood.
N Engl J Med. 324:808–815. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tsutamoto T, Hisanaga T, Wada A, Maeda K,
Ohnishi M, Fukai D, Mabuchi N, Sawaki M and Kinoshita M:
Interleukin-6 spillover in the peripheral circulation increases
with the severity of heart failure, and the high plasma level of
interleukin-6 is an important prognostic predictor in patients with
congestive heart failure. J Am Coll Cardiol. 31:391–398. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gwechenberger M, Mendoza LH, Youker KA,
Frangogiannis NG, Smith CW, Michael LH and Entman ML: Cardiac
myocytes produce interleukin-6 in culture and in viable border zone
of reperfused infarctions. Circulation. 99:546–551. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sun Y: Oxidative stress and cardiac
repair/remodeling following infarction. Am J Med Sci. 334:197–205.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Neri M, Fineschi V, Di Paolo M, Pomara C,
Riezzo I, Turillazzi E and Cerretani D: Cardiac oxidative stress
and inflammatory cytokines response after myocardial infarction.
Curr Vasc Pharmacol. 13:26–36. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li JY, He Y, Ke HH, Jin Y, Jiang ZY and
Zhong GQ: Plasma oxidative stress and inflammatory biomarkers are
associated with the sizes of the left atrium and pulmonary vein in
atrial fibrillation patients. Clin Cardiol. 40:89–94. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hartman MHT, Groot HE, Leach IM, Karper JC
and van der Harst P: Translational overview of cytokine inhibition
in acute myocardial infarction and chronic heart failure. Trends
Cardiovasc Med. 28:369–379. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Okazaki T, Maeda A, Nishimura H, Kurosaki
T and Honjo T: PD-1 immunoreceptor inhibits B cell
receptor-mediated signaling by recruiting src homology
2-domain-containing tyrosine phosphatase 2 to phosphotyrosine. Proc
Natl Acad Sci USA. 98:13866–13871. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Berthiaume JM and Wallace KB: Persistent
alterations to the gene expression profile of the heart subsequent
to chronic doxorubicin treatment. Cardiovasc Toxicol. 7:178–191.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Holmgren G, Synnergren J, Bogestål Y,
Améen C, Åkesson K, Holmgren S, Lindahl A and Sartipy P:
Identification of novel biomarkers for doxorubicin-induced toxicity
in human cardiomyocytes derived from pluripotent stem cells.
Toxicology. 328:102–111. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Thompson KL, Rosenzweig BA, Zhang J,
Knapton AD, Honchel R, Lipshultz SE, Retief J, Sistare FD and
Herman EH: Early alterations in heart gene expression profiles
associated with doxorubicin cardiotoxicity in rats. Cancer
Chemother Pharmacol. 66:303–314. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Todorova VK, Beggs ML, Delongchamp RR,
Dhakal I, Makhoul I, Wei JY and Klimberg VS: Transcriptome
profiling of peripheral blood cells identifies potential biomarkers
for doxorubicin cardiotoxicity in a rat model. PLoS One.
7:e483982012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yi X, Bekeredjian R, DeFilippis NJ,
Siddiquee Z, Fernandez E and Shohet RV: Transcriptional analysis of
doxorubicin-induced cardiotoxicity. Am J Physiol Heart Circ
Physiol. 290:H1098–H1102. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhao WJ, Wei SN, Zeng XJ, Xia YL, Du J and
Li HH: Gene expression profiling identifies the novel role of
immunoproteasome in doxorubicin-induced cardiotoxicity. Toxicology.
333:76–88. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Bertinchant JP, Polge A, Juan JM,
Oliva-Lauraire MC, Giuliani I, Marty-Double C, Burdy JY,
Fabbro-Peray P, Laprade M, Bali JP, et al: Evaluation of cardiac
troponin I and T levels as markers of myocardial damage in
doxorubicin-induced cardiomyopathy rats, and their relationship
with echocardiographic and histological findings. Clin Chim Acta.
329:39–51. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Shan K, Lincoff AM and Young JB:
Anthracycline-induced cardiotoxicity. Ann Intern Med. 125:47–58.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Gharib MI and Burnett AK:
Chemotherapy-induced cardiotoxicity: Current practice and prospects
of prophylaxis. Eur J Heart Fail. 4:235–242. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Ganatra S, Nohria A, Shah S, Groarke JD,
Sharma A, Venesy D, Patten R, Gunturu K, Zarwan C, Neilan TG, et
al: Upfront dexrazoxane for the reduction of anthracycline-induced
cardiotoxicity in adults with preexisting cardiomyopathy and
cancer: A consecutive case series. Cardiooncology.
5:12019.PubMed/NCBI
|
|
60
|
Langer SW: Dexrazoxane for the treatment
of chemotherapy-related side effects. Cancer Manag Res. 6:357–363.
2014. View Article : Google Scholar : PubMed/NCBI
|