Introduction
Burkitt lymphoma (BL) is a high-grade non-Hodgkin's
B cell lymphoma and the fastest growing type of cancer in humans
(1). The incidence of BL is ~1,500
cases per year in the United States (2). Currently, the treatment of BL
primarily consists of cytotoxic chemotherapy combined with
aggressive tumor lysis management, including administration of
allopurinol and adequate hydration (3). Therefore, numerous researchers are
actively searching for effective molecules for the treatment of BL
(4). An improved understanding of
the mechanisms involved in the development of BL is essential for
designing more individualized and effective therapeutic
strategies.
Long non-coding RNA (lncRNA) is a type of non-coding
RNA of >200 nucleotides in length (5). To date, it has been demonstrated that
lncRNAs serve a pivotal role in the developmental process of
various diseases, including cancer, cardiovascular diseases,
nervous system disorders, metabolic disorders and other diseases,
via both transcriptional and post-transcriptional regulation of
gene expression (6–10). Antisense non-coding RNA in the INK4
locus (ANRIL), also known as CDKN2B antisense RNA 1, is transcribed
as a 3,834 base pair lncRNA in the opposite direction from the
INK4b-ARF-INK4a gene cluster (11).
Common disease genome wide association studies have identified that
the ANRIL gene was a genetic susceptibility locus that was
associated with coronary disease, intracranial aneurysm and
numerous types of cancer (12). In
particular, the expression levels of ANRIL were revealed to be
upregulated in numerous types of cancer, including lung,
colorectal, bladder and cervical cancer, as well as hepatocellular
carcinoma (13–17), where they were involved in the
development of cancer through regulating the expression levels of
multiple downstream microRNAs or mRNAs (18,19).
However, to the best of our knowledge, very few studies have
reported the effects of ANRIL in BL.
TGF-β is a multifunctional biological activity
secreted cell factor, which participates in various cellular
biological processes via binding to the cell membrane receptor CD25
and activating downstream signaling pathways, including the SMAD
protein family (20). Kawabata
et al (21) reported that
TGF-β induced the apoptosis of B cell lymphoma Ramos cells through
the membrane spanning 4-domians A1/CD20 signaling pathway. Chen
et al (22) found that
downregulated ANRIL could inhibit the proliferation of human
esophageal squamous cell carcinoma through activating the TGF-β1
signaling pathway. In addition, another previous study discovered
that the ANRIL-induced cell invasion and metastasis in thyroid
cancer was mediated through the TGF-β/SMAD signaling pathway
(23).
In the present study, to explore the function of
ANRIL in the development of BL, the expression levels of ANRIL were
knocked down in BL cell lines using small interfering RNA (siRNA).
The results revealed that ANRIL silencing suppressed cell
proliferation and promoted cell apoptosis. Moreover, the knockdown
of ANRIL activated the TGF-β1 signaling pathway. Notably, the
protective effect of ANRIL silencing in BL could be inhibited by a
TGF-β receptor inhibitor. Thus, the present study provided a novel
target for the treatment of BL.
Materials and methods
Cell culture
The BL cell lines Daudi, CA46, Raji and Farage
(Shanghai Zhongqiao Xinzhou Biotechnology Co., Ltd.) were cultured
in RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (HyClone; GE Healthcare Life Sciences),
and maintained in a humidified incubator with 5% CO2 at
37°C.
Cell transfection
Daudi and CA46 cells were seeded (4×105)
into 6-well plates. Following culture for 24 h, 30 pmol siRNA
targeting ANRIL or negative control (NC) siRNA (JTS Scientific)
were transfected into Daudi and CA46 cells at 37°C for 48 h using
Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). The sequences of the NC siRNA and the siRNAs
targeting ANRIL were: NC siRNA, 5′-UUCUCCGAACGUGUCACGUTT-3′; ANRIL
siRNA-1, 5′-AAGCGAGGUCAUCUCAUUGCUCUAU-3′; and ANRIL siRNA-2,
5′-CGGACUAGGACUAUUUGCCACGACA-3′.
For rescue experiments, cells were pretreated with
10 µmol/l LY2109761 (Cayman Chemical Company) for 12 h prior to
transfection at 37°C. Subsequently, cells were transfected with
ANRIL siRNAs or NC siRNA as aforementioned, and then treated with
10 µmol/l LY2109761 for 48 h at 37°C with 5% CO2 for
rescue experiments.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from cells using the
RNAsimple Total RNA kit (Tiangen Biotech Co., Ltd.). Total RNA was
reverse transcribed into cDNA using oligo (dT)15, random primers
(GenScript), M-MLV reverse transcriptase (Tiangen Biotech Co.,
Ltd.), dNTPs, 5X buffer and RNase inhibitor (Tiangen Biotech Co.,
Ltd.) at 25°C for 10 min, 42°C for 50 min and 80°C for 10 min. qPCR
was subsequently performed to analyze the expression levels of
ANRIL using cDNA samples, primers (GenScript), SYBR Green (Beijing
Solarbio Science & Technology Co., Ltd.) and 2X Taq PCR
MasterMix (Tiangen Biotech Co., Ltd.). The following thermocycling
conditions were used for the qPCR: Initial denaturation at 94°C for
5 min; followed by 40 cycles at 94°C for 10 sec, 60°C for 20 sec
and 72°C for 30 sec; and final extension at 72°C for 150 sec. The
following primer pairs were used for the qPCR: ANRIL forward,
5′-CTCCAGACAGGGTCTCACTC-3′ and reverse, 5′-CTGTGTGTCTCCACACTAAG-3′;
and GAPDH forward, 5′-GACCTGACCTGCCGTCTAG-3′ and reverse,
5′-AGGAGTGGGTGTCGCTGT-3′. The 2−ΔΔCq method was used to
quantify the relative expression levels of ANRIL (24). GAPDH was used as the loading
control.
Cell Counting Kit 8 (CCK-8) assay
The proliferative ability of BL cells was analyzed
using CCK-8 reagent (Nanjing KeyGen Biotech. Co. Ltd.). Briefly,
3×103 cells per well were seeded in sterile 96-well
culture plates and transfected with ANRIL siRNAs or NC siRNA for 0,
24, 48, 72 or 96 h at 37°C as aforementioned. For rescue
experiments, cells were transfected with ANRIL siRNAs or NC siRNA
and treated with LY2109761 for 48 h as aforementioned. Following
incubation, 10 µl CCK-8 solution and 100 µl RPMI-1640 complete
medium were added to each well and incubated for 1 h at 37°C. The
optical density was measured at a wavelength of 450 nm using a
microplate reader.
Cell cycle distribution assay
The cell cycle distribution was analyzed using a
Cell Cycle and Apoptosis Analysis kit (Beyotime Institute of
Biotechnology). Cells were collected by centrifugation at 310 × g
for 5 min at room temperature, then fixed with cold 70% ethanol for
2 h at 4°C. Fixed cells were collected by centrifugation at 310 × g
for 5 min at 4°C and resuspended in 500 µl staining buffer.
Subsequently, cells were incubated with 25 µl propidium iodide (PI)
and 10 µl RNase A in the dark for 30 min at 37°C. The cell cycle
analysis was subsequently conducted using a NovoCyte flow cytometer
(ACEA Bioscience, Inc.; Agilent Technologies, Inc.). Cell cycle
distribution was analyzed using NovoExpress software (version
1.2.5; ACEA Bioscience, Inc.; Agilent Technologies, Inc.).
G1,S and G2 populations were quantified.
Flow cytometric analysis of
apoptosis
Apoptotic cells were detected using an Annexin
V-FITC Apoptosis Detection kit (Beyotime Institute of
Biotechnology). Briefly, cells were collected by centrifugation at
1,000 × g for 5 min at room temperature. Cells were stained with 5
µl Annexin V-FITC and 10 µl PI and incubated at room temperature in
the dark for 15 min. Subsequently, the apoptotic cells were
immediately detected using a NovoCyte flow cytometer (ACEA
Bioscience, Inc.; Agilent Technologies, Inc.) and analyzed using
NovoExpress software (version 1.2.5; ACEA Bioscience, Inc.; Agilent
Technologies, Inc.). The apoptotic rate of cells was calculated by
adding the percentage of early apoptotic (Annexin V-positive and
PI-negative) cells and late apoptotic (Annexin V-positive and
PI-positive) cells.
Immunofluorescence staining
Cells were seeded (4×105 cells/well) into
a sterile 6-well plate at 37°C for 24 h. Subsequently, cells were
transfected with ANRIL siRNA or NC siRNA as aforementiond. At 48 h
post-transfection, cells were fixed with 10% neutral formalin for
15–20 min at room temperature and then permeabilized with 0.1%
Triton X-100. Subsequently, the slides were blocked with undiluted
goat serum (Beijing Solarbio Science & Technology Co., Ltd.) at
room temperature for 15 min and incubated with anti-Ki67 primary
antibody (ProteinTech Group, Inc.; cat. no. 27309-1-AP; 1:50) at
4°C overnight. Following the primary antibody incubation, the
slides were incubated with a Cy3-conjugated goat anti-rabbit IgG
secondary antibody at room temperature for 1 h (Beyotime Institute
of Biotechnology; cat. no. A0516; 1:200). The nuclei were
counterstained with DAPI (Beyotime Institute of Biotechnology) at
room temperature for 5 min. Stained cells were visualized using a
fluorescent microscope (Olympus Corporation; magnification,
×400).
Hoechst staining
A total of 4×105 cells per well were
cultured in a sterile 6-well plate in an incubator at 37°C for 24 h
and then transfected with ANRIL siRNA or NC siRNA as
aforementioned. At 48 h post-transfection, cells were stained using
a Hoechst Staining kit (Beyotime Institute of Biotechnology),
according to the manufacturer's protocols. Stained cells were
visualized under a fluorescence microscope (magnification,
×400).
Western blotting
Total protein was extracted from cells using
high-efficiency RIPA lysis buffer containing 1 mmol/l PMSF (Beijing
Solarbio Science & Technology Co., Ltd.). Total protein was
quantified using a BCA Protein Assay kit (Beijing Solarbio Science
& Technology Co., Ltd.) and proteins (10 or 20 µg) were
separated via 10 or 15% SDS-PAGE. The separated proteins were
subsequently transferred onto PVDF membranes (EMD Millipore) and
blocked with 5% non-fat milk at room temperature for 1 h. The
membranes were then incubated with the following primary antibodies
overnight at 4°C: Anti-cyclin D1 (Cell Signaling Technology, Inc.;
cat. no. 2978; 1:500), anti-caspase-3 (ABclonal, Inc.; cat. no.
A19654; 1:500), anti-caspase-9 (ABclonal, Inc.; cat. no. A0019;
1:1,000), anti-cyclin-dependent kinase inhibitor 1A (p21;
ProteinTech Group, Inc.; cat. no. 10355-1-AP; 1:1,000), anti-E2F
transcription factor 1 (E2F1; ProteinTech Group, Inc.; cat. no.
12171-1-AP; 1:1,000), anti-Bcl-2 (ProteinTech Group, Inc.; cat. no.
12789-1-AP; 1:500), anti-Bax (ProteinTech Group, Inc.; cat. no.
50599-2-Ig; 1:500), anti-TGF-β1 (ProteinTech Group, Inc.; cat. no.
21898-1-AP; 1:500), anti-phosphorylated (p)-SMAD2/3 (Abcam; cat.
no. ab63399; 1:500), anti-SMAD2/3 (Affinity Biosciences; cat. no.
AF6367; 1:500), anti-p-SMAD1 (Cell Signaling Technology, Inc.; cat.
no. 9516; 1:1,000), anti-SMAD1 (ABclonal, Inc.; cat. no. A1101;
1:1,000), anti-sphingosine-1-phosphate receptor 2 (S1PR2;
ProteinTech Group, Inc.; cat. no. 21180-1-AP; 1:500) and anti-GAPDH
(ProteinTech Group, Inc.; cat. no. 60004-1-Ig; 1:10,000). Following
the primary antibody incubation, the membranes were incubated with
an HRP-conjugated goat anti-rabbit IgG antibody (Beijing Solarbio
Science & Technology Co., Ltd.; cat. no. SE134; 1:3,000) and
HRP-conjugated goat anti-mouse IgG secondary antibody (Beijing
Solarbio Science & Technology Co., Ltd.; cat. no. SE131;
1:3,000) at 37°C for 1 h. Protein bands were visualized using ECL
Plus reagent (Beijing Solarbio Science & Technology Co., Ltd.).
Densitometric analysis was performed using Gel-Pro-Analyzer
software (version 4.0; Media Cybernetics, Inc.).
Statistical analysis
Each experiment was performed in triplicate and data
are presented as the mean ± SD. Statistical analysis was performed
using GraphPad Prism software (version 8.0.3; GraphPad Software,
Inc.) and statistical differences between groups were analyzed
using one-way and two-way ANOVAs followed by a Tukey's multiple
comparisons test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression levels of lncRNA ANRIL in
BL cells
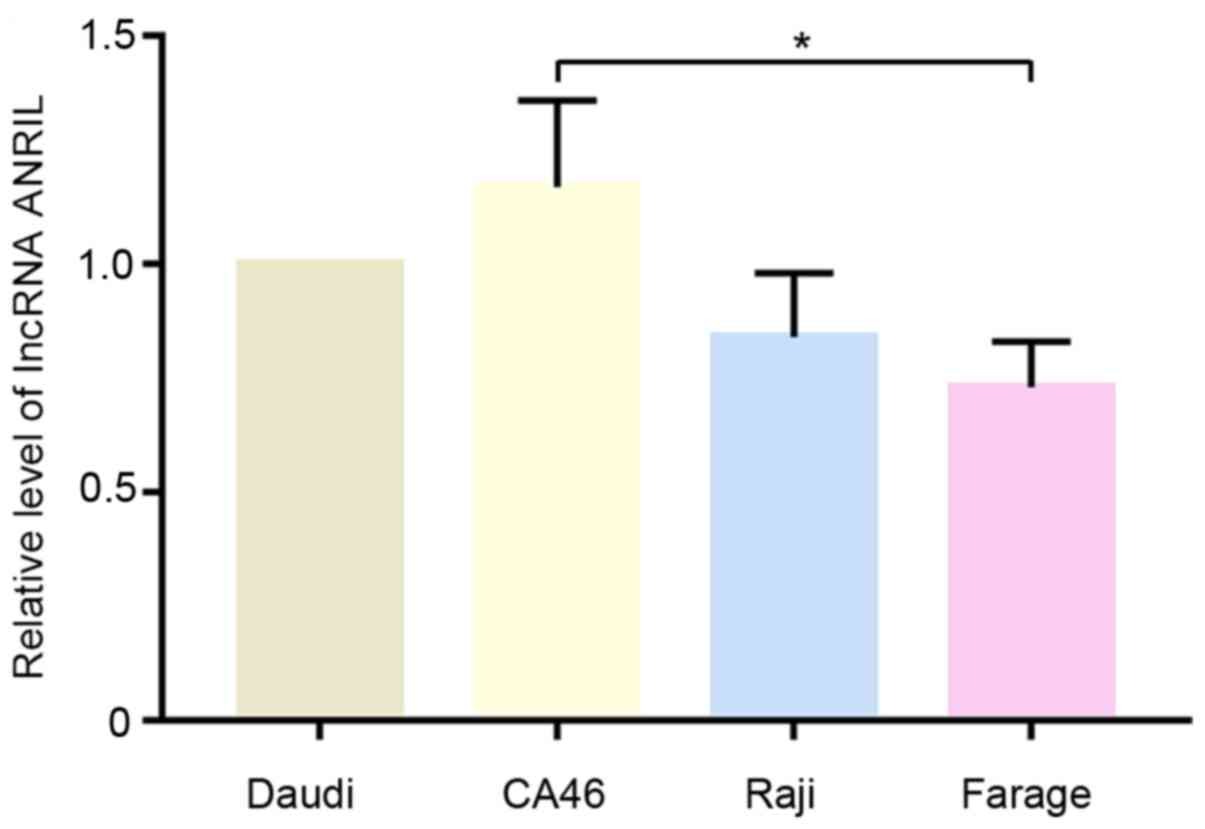
The relative expression levels of lncRNA ANRIL in
Daudi, CA46, Raji and Farage cells were analyzed using RT-qPCR. As
shown in Fig. 1, the expression
levels of ANRIL were notably higher in CA46 and Daudi cells
compared with Raji and Farage cells. Therefore, Daudi and CA46
cells were selected for subsequent experiments.
Knockdown of lncRNA ANRIL inhibits the
proliferation of BL cells
The effect of lncRNA ANRIL silencing on the
proliferation of BL cells was investigated. ANRIL expression levels
were significantly downregulated in Daudi (Fig. 2A) and CA46 (Fig. S1A) cells following the transfection
with ANRIL siRNA-1/2 compared with the NC siRNA group. The results
of the CCK-8 assay revealed that cell proliferation was
significantly suppressed in the ANRIL siRNA-1/2 groups compared
with the NC siRNA group in both cell lines (Figs. 2B and S1B). The genetic knockdown of ANRIL also
led to cell cycle arrest in the G1 phase in both cell
lines (Figs. 2C and S1C). Following the transfection for 48 h,
Ki67 expression levels (red fluorescence) were analyzed using
immunofluorescence staining (Figs.
2D and S1D). Ki67 expression
levels were markedly downregulated in the ANRIL siRNA-1/2 groups
compared with the NC siRNA group in both cell lines. Subsequently,
western blotting was used to analyze cyclin D1, p21 and E2F1
expression levels in Daudi and CA46 cells. As shown in Figs. 2E and S1E, the protein expression levels of
cyclin D1 and E2F1 in the ANRIL siRNA-1/2 groups were significantly
downregulated, while the protein expression levels of p21 were
significantly upregulated, compared with the NC siRNA group in both
cell lines.
Knockdown of lncRNA ANRIL promotes the
apoptosis of BL cells
The effect of lncRNA ANRIL silencing on the
apoptosis of BL cells was also investigated. Flow cytometry and
Hoechst staining were used to detect the levels of apoptotic cells.
As shown in Figs. 3A and S2A, the apoptotic rate of BL cells was
significantly increased in the ANRIL siRNA-1/2 groups compared with
the NC siRNA group in both cell lines. The transfection with ANRIL
siRNA-1 also markedly increased the number of Hoechst-positive
cells (Figs. 3B and S2B). In addition, the expression levels
of apoptosis-related proteins were analyzed using western blotting.
The results revealed that the knockdown of ANRIL significantly
downregulated Bcl-2 expression levels, while upregulating Bax,
cleaved-caspase-3/pro-caspase-3 and cleaved-caspase-9/pro-caspase-9
protein expression levels, in both cell lines compared with the NC
siRNA group (Figs. 3C and S2C).
Knockdown of lncRNA ANRIL activates
TGF-β1 signaling in BL cells
The mechanism of action of the lncRNA ANRIL in BL
cells was further investigated. The protein expression levels of
TGF-β1 signaling-related proteins, including TGF-β1, p-SMAD2/3,
p-SMAD1 and S1PR2, were analyzed using western blotting. The
results revealed that TGF-β1, p-SMAD2/3/SMAD2/3, p-SMAD1/SMAD1 and
S1PR2 expression levels were significantly upregulated in Daudi
(Fig. 4) and CA46 (Fig. S3) cells following the transfection
with ANRIL siRNA-1/2 compared with NC siRNA, indicating that ANRIL
may regulate the TGF-β1 signaling pathway in BL cells.
Inhibiting TGF-β1 signaling can
reverse the lncRNA ANRIL silencing-induced anti-proliferative and
pro-apoptotic effects on BL cells
As aforementioned, ANRIL siRNA-1 and siRNA-2
triggered a significant effect on BL cell proliferation, apoptosis
and TGF-β1 signaling, thus one of two siRNAs was selected for the
rescue experiments. To investigate whether the lncRNA ANRIL
affected the proliferation and apoptosis of BL cells through the
TGF-β1 signaling pathway, the TGF-β receptor inhibitor LY2109761
was used to treat cells prior to and after the transfection with
ANRIL siRNA-1. The results revealed that cell viability at 48 h was
significantly increased and cell apoptosis was inhibited in the
ANRIL siRNA+LY2109761 group compared with the ANRIL siRNA group in
both cell lines (Figs. 5A-C and
S4A-C). The western blotting
results revealed that LY2109761 partially reversed the ANRIL
knockdown-induced downregulation of cyclin D1 expression levels,
and ANRIL knockdown-induced upregulation of p21,
cleaved-caspase-3/pro-caspase-3 and cleaved-caspase-9/pro-caspase-9
expression levels in Daudi and CA46 cells (Figs. 5D and S4D). These findings indicated that the
lncRNA ANRIL knockdown-induced anti-proliferative and pro-apoptotic
effects on BL cells may be mediated via the TGF-β1 signaling
pathway.
Discussion
lncRNAs have been reported to be involved in the
angiogenesis, proliferation and metastasis of numerous types of
cancer. Due to the different origins of each type of cancer and the
microenvironments surrounding tumor cells, the mechanisms of each
lncRNA are different (25,26). The expression levels of lncRNA ANRIL
were discovered to be upregulated in a range of cancer types,
including breast (27), lung
(13), gastric (18) and esophageal squamous cancer
(22). However, to the best of our
knowledge, the expression levels and function of ANRIL in BL
remains unknown. The present study aimed to determine the effect of
the knockdown of ANRIL on the proliferation and apoptosis of BL
cells and the TGF-β1 signaling pathway.
Aberrant cell proliferation and apoptosis occurs in
the development of the majority of types of cancer (28). Four BL cell lines, Daudi, CA46, Raji
and Farage, were used in the present study. Due to higher
expression levels of lncRNA ANRIL in Daudi and CA46 cells, the cell
lines Daudi and CA46 were selected for further study. The present
results revealed that the genetic silencing of lncRNA ANRIL
inhibited cell proliferation and enhanced cell apoptosis, as
evidenced by decreased cell viability and downregulated Ki67
expression levels, and increased levels of apoptotic cells and
Hoechst-positive cells; these findings are consistent with those
reported in laryngeal squamous cell (29) and hepatocellular carcinoma (30). The cell cycle is a fundamental
process for cell life that serves an essential role in the accurate
regulation of the survival, reproduction, development and
inheritance of an organism. Cell cycle regulation is achieved
through cyclins (31). The present
study illustrated that the proportion of cells in the G1
phase was increased and the proportion of cells in the S phase was
reduced following the transfection with ANRIL siRNA. Furthermore,
the changes in the expression levels of important cell
cycle-related proteins in BL cells were also analyzed. It was
observed that the protein expression levels of cyclin D1 and E2F1
were downregulated, while p21 protein expression levels were
upregulated, following the transfection with ANRIL siRNA. Based on
the aforementioned findings, the knockdown of lncRNA ANRIL was
suggested to induce cell cycle arrest in the G1
phase.
The promotion of cell apoptosis is an effective
method to prevent the development of numerous types of cancer. The
Bcl-2 and caspase families are closely associated with cell
apoptosis in cancer (32). The
Bcl-2 and Bax proteins are two members of the Bcl-2 family that are
closely related to cell apoptosis. The Bcl-2 protein was discovered
to inhibit cell apoptosis by preventing the early stages of
cytoplasmic programmed cell death (33). The Bax protein forms a dimer with
the Bcl-2 protein to abolish the function of the Bcl-2 protein,
which subsequently regulates cell apoptosis (34). Caspase-3 and caspase-9 are key
components of the caspase family, which are of vital importance in
the occurrence and development of cancer (35,36).
Caspase-3 cleaves itself into an active state when caspase-9
signals are activated, which then cleaves DNA and leads to cell
apoptosis (37). Therefore, Bcl-2,
Bax, cleaved-caspase-3 and cleaved-caspase-9 are critical in
regulating apoptotic signaling pathways (38). The findings of the present study
discovered that the protein expression levels of Bcl-2 were
significantly downregulated, while Bax,
cleaved-caspase-3/pro-caspase-3 and cleaved-caspase-9/pro-caspase-9
expression levels were upregulated in lncRNA ANRIL knockdown cells,
indicating that ANRIL silencing may activate apoptotic
signaling.
TGF-β has been demonstrated to regulate
proliferation, differentiation, angiogenesis and other functions in
multiple types of cell, including cancer cells, epithelial cells
and fibrosblasts (39). TGF-β was
discovered to function as a tumor suppressor during the early
stages of tumor development (40).
Among the three TGF-β isoforms, TGF-β1 was found to be frequently
upregulated in hepatocellular carcinoma, esophageal squamous cell
carcinoma and thyroid cancer (41–43).
The downstream signaling of TGF-β is initiated through the binding
to its receptors, including type I TGF-β receptor and type II TGF-β
receptor (44). The activated TGF-β
receptor can initiate SMAD signaling by phosphorylation of SMAD2,
SMAD3 and SMAD1 (45,46). Zhao et al (43) reported that lncRNA ANRIL promoted
cell invasion and metastasis in thyroid cancer by inhibiting
TGF-β/SMAD signaling. In the present study, the genetic knockdown
of ANRIL significantly activated TGF-β signaling in BL cells, as
evidenced by upregulated TGF-β1, p-SMAD2/3/SMAD2/3, p-SMAD1/SMAD1
and S1PR2 expression levels. TGF-β signaling has been reported to
act as a proliferation inhibitor and apoptosis inducer in BL cell
lines (47,48). Hence, it was hypothesized that the
knockdown of ANRIL may exert an anti-proliferative and
pro-apoptotic effect in BL cells through the activation of the
TGF-β signaling. Subsequently, the effect of ANRIL inhibition on
the proliferation and apoptosis of BL cells was investigated in the
presence of the TGF-β receptor inhibitor LY2109761. It was observed
that cell proliferation was increased, while cell apoptosis was
decreased in the ANRIL siRNA+LY2109761 group compared with the
ANRIL siRNA group. Meanwhile, the expression levels of cyclin D1
were upregulated, while the expression levels of p21,
cleaved-caspase-3/pro-caspase-3 and cleaved-caspase-9/pro-caspase-9
were downregulated in the ANRIL siRNA+LY2109761 group compared with
the ANRIL siRNA group. These findings indicated that the TGF-β1
signaling pathway may serve an important role in the anti-BL effect
elicited by the genetic knockdown of ANRIL.
lncRNA ANRIL has been reported to affect multiple
signaling pathways, including the NF-κB, ATM-E2F1 and
AdipoR1/AMPK/SIRT1 signaling pathways (49–51).
The present study only investigated whether lncRNA ANRIL served an
important role in BL by modulating TGF-β signaling, which is a
limitation of the present study. In subsequent experiments, RNA-Seq
will be performed for cells from each group to investigate the
involvement of other significant signaling pathways.
In conclusion, the results of the present study
indicated that the genetic silencing of the lncRNA ANRIL may
inhibit cell proliferation and promote cell apoptosis in BL through
the activation of the TGF-β1 signaling pathway. These data provided
a potential novel strategy to treat BL.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant from the
Natural Science Foundation of Liaoning Province (grant no.
20170540350).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JJ and ZL performed the experiments and analyzed the
data; SM and WY designed the experiments and drafted the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Molyneux EM, Rochford R, Griffin B, Newton
R, Jackson G, Menon G, Harrison CJ, Israels T and Bailey S:
Burkitt's lymphoma. Lancet. 379:1234–1244. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sacks D, Baxter B, Campbell BCV, Carpenter
JS, Cognard C, Dippel D, Eesa M, Fischer U, Hausegger K, Hirsch JA,
et al: Multisociety Consensus Quality Improvement Revised Consensus
Statement for Endovascular Therapy of Acute Ischemic Stroke. Int J
Stroke. 13:612–632. 2018.PubMed/NCBI
|
|
3
|
Senbanjo IO: Tumor lysis and acute renal
failure in Burkitt's lymphoma: A review on pathophysiology and
management. Indian J Nephrol. 19:83–86. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bain BJ: Pseudoplatelets and apoptosis in
Burkitt lymphoma. Am J Hematol. 94:1168–1169. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Iyer MK, Niknafs YS, Malik R, Singhal U,
Sahu A, Hosono Y, Barrette TR, Prensner JR, Evans JR, Zhao S, et
al: The landscape of long noncoding RNAs in the human
transcriptome. Nat Genet. 47:199–208. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Maruyama R and Suzuki H: Long noncoding
RNA involvement in cancer. BMB Rep. 45:604–611. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Uchida S and Dimmeler S: Long noncoding
RNAs in cardiovascular diseases. Circ Res. 116:737–750. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qureshi IA, Mattick JS and Mehler MF: Long
non-coding RNAs in nervous system function and disease. Brain Res.
1338:20–35. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Losko M, Kotlinowski J and Jura J: Long
Noncoding RNAs in Metabolic Syndrome Related Disorders. Mediators
Inflamm. 2016:53652092016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dykes IM and Emanueli C: Transcriptional
and Post-transcriptional Gene Regulation by Long Non-coding RNA.
Genomics Proteomics Bioinformatics. 15:177–186. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shi S, Lu Y, Qin Y, Li W, Cheng H, Xu Y,
Xu J, Long J, Liu L, Liu C, et al: miR-1247 is correlated with
prognosis of pancreatic cancer and inhibits cell proliferation by
targeting neuropilins. Curr Mol Med. 14:316–327. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pasmant E, Sabbagh A, Vidaud M and Bièche
I: ANRIL, a long, noncoding RNA, is an unexpected major hotspot in
GWAS. FASEB J. 25:444–448. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nie FQ, Sun M, Yang JS, Xie M, Xu TP, Xia
R, Liu YW, Liu XH, Zhang EB, Lu KH, et al: Long noncoding RNA ANRIL
promotes non-small cell lung cancer cell proliferation and inhibits
apoptosis by silencing KLF2 and P21 expression. Mol Cancer Ther.
14:268–277. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang Z, Feng L, Liu P and Duan W: ANRIL
promotes chemoresistance via disturbing expression of ABCC1 by
regulating the expression of Let-7a in colorectal cancer. Biosci
Rep. 38:382018. View Article : Google Scholar
|
|
15
|
Zhu H, Li X, Song Y, Zhang P, Xiao Y and
Xing Y: Long non-coding RNA ANRIL is up-regulated in bladder cancer
and regulates bladder cancer cell proliferation and apoptosis
through the intrinsic pathway. Biochem Biophys Res Commun.
467:223–228. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang MD, Chen WM, Qi FZ, Xia R, Sun M, Xu
TP, Yin L, Zhang EB, De W and Shu YQ: Long non-coding RNA ANRIL is
upregulated in hepatocellular carcinoma and regulates cell
apoptosis by epigenetic silencing of KLF2. J Hematol Oncol.
8:502015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang JJ, Wang DD, Du CX and Wang Y: Long
Noncoding RNA ANRIL Promotes Cervical Cancer Development by Acting
as a Sponge of miR-186. Oncol Res. 26:345–352. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang EB, Kong R, Yin DD, You LH, Sun M,
Han L, Xu TP, Xia R, Yang JS, De W, et al: Long noncoding RNA ANRIL
indicates a poor prognosis of gastric cancer and promotes tumor
growth by epigenetically silencing of miR-99a/miR-449a. Oncotarget.
5:2276–2292. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chai L, Yuan Y, Chen C, Zhou J and Wu Y:
The role of long non-coding RNA ANRIL in the carcinogenesis of oral
cancer by targeting miR-125a. Biomed Pharmacother. 103:38–45. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Derynck R and Budi EH: Specificity,
versatility, and control of TGF-β family signaling. Sci Signal.
12:122019. View Article : Google Scholar
|
|
21
|
Kawabata KC, Ehata S, Komuro A, Takeuchi K
and Miyazono K: TGF-β-induced apoptosis of B-cell lymphoma Ramos
cells through reduction of MS4A1/CD20. Oncogene. 32:2096–2106.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen D, Zhang Z, Mao C, Zhou Y, Yu L, Yin
Y, Wu S, Mou X and Zhu Y: ANRIL inhibits p15(INK4b) through the
TGFβ1 signaling pathway in human esophageal squamous cell
carcinoma. Cell Immunol. 289:91–96. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guo J, Zhang K, Ji Y, Jiang X and Zuo S:
Effects of ethyl pyruvate on myocardial apoptosis and expression of
Bcl-2 and Bax proteins after ischemia-reperfusion in rats. Journal
of Huazhong University of Science and Technology. J Huazhong Univ
Sci Technol. 28:281–283. 2008. View Article : Google Scholar
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Δ Δ C(T)) Method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schmitz SU, Grote P and Herrmann BG:
Mechanisms of long noncoding RNA function in development and
disease. Cell Mol Life Sci. 73:2491–2509. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu X, Tudoran OM, Calin GA and Ivan M: The
Many Faces of Long Noncoding RNAs in Cancer. Antioxid Redox Signal.
29:922–935. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mehta-Mujoo PM, Cunliffe HE, Hung NA and
Slatter TL: Long Non-coding RNA ANRILin the Nucleus Associates With
Periostin Expression in Breast Cancer. Front Oncol. 9:8852019.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li R, He JL, Chen XM, Long CL, Yang DH,
Ding YB, Qi HB and Liu XQ: miR-200a is involved in proliferation
and apoptosis in the human endometrial adenocarcinoma cell line
HEC-1B by targeting the tumor suppressor PTEN. Mol Biol Rep.
41:1977–1984. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hao YR, Zhang DJ, Fu ZM, Guo YY and Guan
GF: Long non-coding RNA ANRIL promotes proliferation,
clonogenicity, invasion and migration of laryngeal squamous cell
carcinoma by regulating miR-181a/Snai2 axis. Regen Ther.
11:282–289. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang D, Bi C, Zhao Q, Ding X, Bian C,
Wang H, Wang T and Liu H: Knockdown long non-coding RNA ANRIL
inhibits proliferation, migration and invasion of HepG2 cells by
down-regulation of miR-191. BMC Cancer. 18:9192018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sherr CJ: Cancer cell cycles. Science.
274:1672–1677. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mei JM and Niu CS: Effects of CDNF on
6-OHDA-induced apoptosis in PC12 cells via modulation of Bcl-2/Bax
and caspase-3 activation. Neurol Sci. 35:1275–1280. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jiang H, Zhao PJ, Su D, Feng J and Ma SL:
Paris saponin I induces apoptosis via increasing the Bax/Bcl-2
ratio and caspase-3 expression in gefitinib-resistant non-small
cell lung cancer in vitro and in vivo. Mol Med Rep.
9:2265–2272. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Samarghandian S, Nezhad MA and Mohammadi
G: Role of caspases, Bax and Bcl-2 in chrysin-induced apoptosis in
the A549 human lung adenocarcinoma epithelial cells. Anticancer
Agents Med Chem. 14:901–909. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Galluzzi L, Kepp O and Kroemer G:
Caspase-3 and prostaglandins signal for tumor regrowth in cancer
therapy. Oncogene. 31:2805–2808. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim B, Srivastava SK and Kim SH: Caspase-9
as a therapeutic target for treating cancer. Expert Opin Ther
Targets. 19:113–127. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Takano S, Shiomoto S, Inoue KY, Ino K,
Shiku H and Matsue T: Electrochemical approach for the development
of a simple method for detecting cell apoptosis based on caspase-3
activity. Anal Chem. 86:4723–4728. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hastak K, Gupta S, Ahmad N, Agarwal MK,
Agarwal ML and Mukhtar H: Role of p53 and NF-kappaB in
epigallocatechin-3-gallate-induced apoptosis of LNCaP cells.
Oncogene. 22:4851–4859. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Massagué J: TGFbeta in Cancer. Cell.
134:215–230. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Huynh LK, Hipolito CJ and Ten Dijke P: A
Perspective on the Development of TGF-β Inhibitors for Cancer
Treatment. Biomolecules. 9:92019. View Article : Google Scholar
|
|
41
|
Abou-Shady M, Baer HU, Friess H, Berberat
P, Zimmermann A, Graber H, Gold LI, Korc M and Büchler MW:
Transforming growth factor betas and their signaling receptors in
human hepatocellular carcinoma. Am J Surg. 177:209–215. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gholamin M, Moaven O, Memar B, Farshchian
M, Naseh H, Malekzadeh R, Sotoudeh M, Rajabi-Mashhadi MT, Forghani
MN, Farrokhi F, et al: Overexpression and interactions of
interleukin-10, transforming growth factor beta, and vascular
endothelial growth factor in esophageal squamous cell carcinoma.
World J Surg. 33:1439–1445. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhao JJ, Hao S, Wang LL, Hu CY, Zhang S,
Guo LJ, Zhang G, Gao B, Jiang Y, Tian WG, et al: Long non-coding
RNA ANRIL promotes the invasion and metastasis of thyroid cancer
cells through TGF-β/Smad signaling pathway. Oncotarget.
7:57903–57918. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Vander Ark A, Cao J and Li X: TGF-β
receptors: In and beyond TGF-β signaling. Cell Signal. 52:112–120.
2018. View Article : Google Scholar
|
|
45
|
Derynck R, Akhurst RJ and Balmain A:
TGF-beta signaling in tumor suppression and cancer progression. Nat
Genet. 29:117–129. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Stelling A, Hashwah H, Bertram K, Manz MG,
Tzankov A and Müller A: The tumor suppressive TGF-β/SMAD1/S1PR2
signaling axis is recurrently inactivated in diffuse large B-cell
lymphoma. Blood. 131:2235–2246. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bakkebø M, Huse K, Hilden VI, Smeland EB
and Oksvold MP: TGF-β-induced growth inhibition in B-cell lymphoma
correlates with Smad1/5 signalling and constitutively active p38
MAPK. BMC Immunol. 11:572010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Spender LC, O'Brien DI, Simpson D, Dutt D,
Gregory CD, Allday MJ, Clark LJ and Inman GJ: TGF-beta induces
apoptosis in human B cells by transcriptional regulation of BIK and
BCL-XL. Cell Death Differ. 16:593–602. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhang B, Wang D, Ji TF, Shi L and Yu JL:
Overexpression of lncRNA ANRIL up-regulates VEGF expression and
promotes angiogenesis of diabetes mellitus combined with cerebral
infarction by activating NF-κB signaling pathway in a rat model.
Oncotarget. 8:17347–17359. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chen S, Zhang JQ, Chen JZ, Chen HX, Qiu
FN, Yan ML, Chen YL, Peng CH, Tian YF and Wang YD: The over
expression of long non-coding RNA ANRIL promotes
epithelial-mesenchymal transition by activating the ATM-E2F1
signaling pathway in pancreatic cancer: An in vivo and in vitro
study. Int J Biol Macromol. 102:718–728. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sun LY, Li XJ, Sun YM, Huang W, Fang K,
Han C, Chen ZH, Luo XQ, Chen YQ and Wang WT: lncRNA ANRIL regulates
AML development through modulating the glucose metabolism pathway
of AdipoR1/AMPK/SIRT1. Mol Cancer. 17:1272018. View Article : Google Scholar : PubMed/NCBI
|