Introduction
Cardiovascular disease is one of the leading causes
of human death (1). Acidosis,
electrolyte imbalance, hypoxia and ischemia can cause myocardial
cell damage, vascular remodeling, ventricular dysfunction and even
death. Cardiomyocyte apoptosis or programmed cell death is an
important pathological manifestation of ischemia-reperfusion injury
and is the primary cause of cardiac dysfunction (2). Therefore, an in-depth understanding of
the mechanism of cardiomyocyte apoptosis is the key to preventing
myocardial injury and treating heart disease. At present, multiple
apoptosis signaling cascades have been identified in
ischemia-reperfusion injury. Recently, non-coding ribonucleic acid
(RNA) microRNAs (miRNAs/miRs) and circular RNAs (circRNAs) have
been reported to be involved in cardiomyocyte apoptosis. For
example, miR-762 targets inhibition of NADH dehydrogenase subunit
2, which thereby inhibits cardiomyocyte apoptosis (3). Knockdown of mouse cardiomyocytes and
cardiac tissue circNCX1 promotes the targeted inhibition of cell
death-inducing p53-target protein 1 by miR-133a-3p, which inhibits
apoptosis and attenuates ischemia-reperfusion injury (4). However, the molecular mechanism of
cardiomyocyte apoptosis at the RNA expression level needs further
study. In addition, long non-coding RNAs (lncRNAs) have an
important role in a number of biological activities, such as
epigenetic regulation, cell cycle regulation and cell
differentiation regulation (5), but
their role in myocardial injury should be explored further.
lncRNAs are non-coding RNA molecules that are
>200 nucleotides in length. They have a mRNA-like structure.
After splicing, they have polyA tail and promoter structure. They
participate in various important regulatory processes, such as X
chromosome silencing, genomic imprinting and chromatin
modification, transcriptional activation, transcriptional
interference and intranuclear transport (6–8).
Furthermore, lncRNAs also have a role in the development of cancer,
Alzheimer's disease and neurological diseases (9,10).
lncRNAs act as a sponge to adsorb miRNAs and regulate target gene
expression. lncRNA AK038897 adsorbs miR-26a-5p via its role as a
competing endogenous RNA to promote the expression of
death-associated protein kinase 1 and aggravate cerebral
ischemia/reperfusion injury (11).
RNA-Seq data has revealed that the heart contains abundant lncRNAs.
The differential expression of lncRNAs in cardiac diseases suggests
that there may be a regulatory relationship between lncRNAs and
cardiovascular diseases. For example, lncCAREL is significantly
upregulated in neonatal rat cardiomyocytes that have lost the
ability to divide (12),
furthermore, knockdown of lncCPR can promote cardiomyocyte
proliferation (13). The lncRNA
2810403D21Rik/Mirf is known to promote ischemia-induced
cardiomyocyte apoptosis (14).
However, the functions and molecular mechanisms of cardiac lncRNAs
remain unclear. Therefore, the role of lncRNAs in heart disease,
especially ischemic heart disease, should be explored further. The
purpose of the present study was to investigate the role of lncRNA
HOX transcript antisense intergenic RNA (HOTAIR) in ischemic
myocardial injury and to explore its regulatory mechanism in
cardiomyocyte apoptosis.
Materials and methods
Cell culture
Rat H9c2 cells (American Type Culture Collection)
were cultured in Dulbecco's modified Eagle's medium (Corning Inc.)
containing 10% fetal bovine serum (cat. no. 10099141; Gibco; Thermo
Fisher Scientific, Inc.). The cells were incubated at 37°C in a
humidified atmosphere containing 5% CO2. To initiate
oxidative stress, H9c2 cells were exposed to
H2O2 (0–100 µM) for the indicated times at
37°C.
Experimental animals
All rat experiments conformed to the National
Institutes of Health Guidelines on the Use of Laboratory Animals
and was approved by The First People's Hospital of Tonglu
(Hangzhou, China). A total of 18 adult male Sprague-Dawley (SD)
rats at 8–10 weeks of age were purchased from Charles River
Laboratories, Inc., three were used to establish a myocardial
ischemia-reperfusion (MI/R) injury model. The MI/R model was
established as previously described (15). Briefly, SD rats were intubated and
artificially ventilated with a rodent ventilator under anesthesia
with 10% chloral hydrate (300 mg/kg, intraperitoneally). Symptoms
of peritonitis in the rats, such as abdominal muscle tension, were
checked. An electrocardiogram (ECG) was recorded following
subcutaneous placement of electrodes and connection to an
electrocardiograph. Coronary artery ligation was achieved with a
plastic snare fixed onto the left anterior descending (LAD)
coronary artery. A 6-0 silk suture was passed underneath the LAD
(2–3 mm inferior to the left auricle) and tied. Following 30 min of
ischemia, the plastic snare was removed and the myocardium was
reperfused for 180 min.
Microarray
Kangchen Biotech Co., Ltd., performed the microarray
in which six samples (three samples for the MI group and three for
the control group) were used for lncRNA microarray analysis by
Agilent Array (Agilent Technologies, Inc.). Sample preparation and
microarray hybridization were performed in accordance with the
manufacturer's standard protocols. Briefly, the total RNA from
myocardial tissues of rats was extracted using TRIzol®
(Invitrogen; Thermo Fisher Scientific, Inc.), and the purity and
integrity of RNA were detected. After hybridization, the different
fluorescence intensity of lncRNA was obtained by chip
hybridization, and the fluorescence intensity values were obtained
by image scanning. The differently expressed lncRNAs with P<0.05
and a fold-change value >2 were subsequently selected.
Cell Counting Kit-8 (CCK-8) assay
The H9c2 cells were seeded in 96-well plates at
1×104 cells per well. A CCK-8 (cat. no. HY-K0301;
MedChemExpress) was used to detect the viability of cells in
accordance with the manufacturer's instructions. The absorbance was
measured at 450 nm.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR).
Total RNA was isolated from the cultured cells using
TRIzol® reagent (Takara Biotechnology Co., Ltd.),
according to the supplier's instructions. The PrimeScript RT Master
Mix (Toyobo Life Science) was used to synthesize cDNA from the
extracted RNA at 37°C for 15 min, 50°C 5 min and 98°C 5 min. SYBR
Premix Ex Taq (Takara Biotechnology Co., Ltd.) was used to perform
the RT-qPCR, and GAPDH was the internal control. The primers used
were synthesized by Shangya Biotechnology. The primer sequence was
as follows: HOTAIR forward (Fw), 5′-CCTTATAAGCTCATCGGAGCA-3′ and
reverse (Rv), 5′-CATTTCTGGGTGGTTCCTTT-3′; rno-miR-130a-3p Fw,
5′-CGCCAGGGTTTTCCCAGTCACGACCAGTGCAATGTTAAAAGGGCAT-3′ and Rv,
5′-CGCGAGGAGAGAATTAATACGACTCAGTATACGCGATGCCCT-3′; mouse double
minute 4 (MDM4) Fw, 5′-CTCAGTGTCAACATCTGACAG-3′ and Rv,
5′-CATATGCTGCTCCTGCTGATC-3′; GAPDH Fw, 5′-GGAGCGAGATCCCTCCAAAAT-3′
and Rv, 5′-GGCTGTTGTCATACTTCTCATGG-3′; U6 Fw,
5′-GCGCGTCGTGAAGCGTTC-3′ and Rv, 5′-GTGCAGGGTCCGAGGT-3′. The
reaction conditions were as follows: Predenaturation at 95°C for 5
min, followed by 35 cycles of denaturation at 94°C for 45 sec,
annealing at 53–56°C for 45 sec and an extension at 72°C for 45
sec. PCR was carried out using the ABI PRISM® 7500
System (Applied Biosystems; Thermo Fisher Scientific, Inc.). The
expression of RNA relative to GAPDH was calculated using the
2−ΔΔCq method (13).
Construction of the plasmid and cell
transfection
The HOTAIR, MDM4-wild-type (WT), MDM4-mutant (MT)
and rno-miR-130a-3p sequences were designed and synthesized by
Shangya, which were further subcloned into pcDNA3.1 (Invitrogen;
Thermo Fisher Scientific, Inc.). The pcDNA3.1 vector was used as
control. Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to transfect or co-transfect the
plasmids (500 ng pcDNA3.1-vector, 500 ng HOTAIR, 600 ng MDM4-WT,
600 ng MDM4-MT and 500 ng rno-miR-130a-3p) into the H9c2 cells at
60% confluency in 6-well plates. After 48–72 h, the transfected
cells were used for the subsequent experiments.
Western blotting
The cells were washed with PBS and incubated on ice
in 1X RIPA buffer (Beyotime Institute of Biotechnology), containing
1X PhosSTOP protease inhibitor (Shanghai Yeasen Biotechnology Co.,
Ltd.) and 1X complete Protease Inhibitor Cocktail (Shanghai Yeasen
Biotechnology Co., Ltd.) for 30 min. The lysates were pre-cleared
by centrifugation at 12,000 × g for 10 min at 4°C, and the protein
was quantified using the Yeasen Protein Assay Kit (Shanghai Yeasen
Biotechnology Co., Ltd.). Then, protein lysate (20 µg) was resolved
via SDS-PAGE on 10% gel, and subsequently transferred to a PVDF
membrane (Bio-Rad Laboratories, Inc.). The blots were blocked using
5% skimmed milk for 1 h at room temperature, and then incubated
with the primary antibody overnight at 4°C. Then, the blots were
washed and incubated with secondary antibody for 2 h at room
temperature, followed by washing and visualization of the protein
bands using an ECL chemiluminescence kit (Hangzhou Fude Chemical
Co., Ltd.). GAPDH was used as the loading control. The primary
antibodies used were as follows: Bax (cat. no.2772; 1:1,000; CST
Biological Reagents Co., Ltd.), Bcl-2 (cat. no. 2764; 1:1,000; CST
Biological Reagents Co., Ltd.), MDM4 (cat. no. A300-287A; 1:1,000;
Bethyl Laboratories, Inc.) and GAPDH (cat. no. ab128915; 1:2,500;
Abcam). The secondary antibodies used were as follows: Anti-rabbit
(cat. no. 7074; 1:10,000; CST Biological Reagents Co., Ltd.) and
anti-mouse (cat. no. 6789; 1:10,000; Abcam).
Luciferase reporter gene assay
The H9c2 cells (5×104) were seeded in
96-well plates and incubated at 37°C for 24 h. A HOTAIR
3′-untranslated region (UTR)-Luc vector with WT or MT plasmids was
constructed at the miR-130a-3p binding site of the 3′UTR region of
lncRNA HOTAIR. A MDM4 3′-UTR-Luc vector with a WT or MT gene was
constructed at the miR-130a-3p binding site of the 3′UTR region of
the MDM4. The plasmids were co-transfected with the H9c2 cells
using Lipofectamine® 3000, harvested after 48 h, and the
luciferase activity was measured by a dual luciferase assay system
(Promega Corporation). Firefly luciferase activity was normalized
to Renilla luciferase activity.
Short hairpin (sh)RNA vectors
The sense and antisense oligonucleotides of the
shRNA HOTAIR (5′-AAAUCCAGAACCCUCUGACAUUUGC-3′) were synthesized and
cloned into the pENTR™/U6 vector (Invitrogen; Thermo Fisher
Scientific, Inc.). H9c2 cells (5×104) were transfected
with 1 µg shRNA using Lipofectamine® 3000 (Invitrogen;
Thermo Fisher Scientific, Inc.,), according to the manufacturer's
protocol. After 48 h, the transfected cells were used for
subsequent experiments.
Flow cytometry
Early and late apoptotic cells were detected using
the Annexin V-FITC/propidium iodide Apoptosis Detection Kit (BD
Pharmingen; BD Biosciences) after cell treatment. According to the
manufacturer's instructions, the stained cells were assayed by flow
cytometry (FACSCalibur™; BD Biosciences). The positive cells were
calculated and analyzed using FlowJo software (version 8; Tree
Star, Inc.).
RNA immunoprecipitation (RIP)
The RIP assay was performed using the Magna RIP™
RNA-Binding Protein Immunoprecipitation Kit (EMD Millipore),
according to the manufacturer's protocols. Briefly, cultured
chondrocytes were collected and resuspended in RIP lysis buffer
(Beijing Solarbio Science & Technology Co., Ltd.); then, the
cell extracts were incubated with RIP buffer containing magnetic
beads conjugated with human anti-Ago2 antibody (EMD Millipore) or
mouse immune globulin G (IgG) control (cat. no. ab172730; Abcam)
overnight at 4°C. The next day, the magnetic beads were incubated
with 50 µg/ml Proteinase K (cat. no. P2308; Sigma-Aldrich; Merck
KGaA) after washing three times. Total RNAs were isolated from the
extracts using the TRIzol® LS reagent (Thermo Fisher
Scientific, Inc.). Finally, the relative enrichment of HOTAIR and
miR-130a-3p were determined by RT-qPCR analysis.
miRNA regulatory network
StarBase (http://starbase.sysu.edu.cn/) and TargetScan
(http://www.targetscan.org/) databases
were used to explore target mRNAs.
Statistical analysis
Statistical analysis was performed using the
GraphPad Prism 7 (GraphPad Software, Inc.). The data are presented
as the mean ± standard deviation. Statistical comparisons were
performed using a paired t-test and one-way ANOVA. Following ANOVA,
Bonferroni's post hoc test was performed. P<0.05 was considered
to indicate a statistically significant difference.
Results
Downregulation of HOTAIR in the
ischemic myocardium mouse heart tissue and H2O2-treated H9c2
cells
Initially, the cardiac differential lncRNA in MI
rats was screened by lncRNA chip technology. It was found that
HOTAIR expression levels were downregulated in ischemic myocardium
rat heart tissue (Fig. 1A).
Excessive reactive oxygen species are produced under cardiac
pathological conditions, and H2O2 is often
used to mimic the induction of reactive oxygen species-induced
apoptosis in MI in vitro (16). To investigate the role of HOTAIR in
ROS-induced cardiomyocyte apoptosis, H9c2 cells were treated with
100 µM H2O2 at different time-points (0, 3, 6
and 12 h) and RT-qPCR was used to determine the expression levels
of HOTAIR. The present study found that HOTAIR expression
significantly decreased with prolonged H2O2
treatment time (Fig. 1B).
Similarly, H9c2 cells were treated with different concentrations of
H2O2, and the expression level of HOTAIR was
significantly reduced (Fig. 1C).
Further experiments found that HOTAIR was significantly
downregulated in mouse heart tissue with myocardial infarction
(Fig. 1D). These results suggested
that HOTAIR could be associated with reactive oxygen
species-induced cardiomyocyte injury.
 | Figure 1.Downregulation of HOTAIR in the
ischemic myocardium mouse heart tissue and
H2O2-treated H9c2 cells. (A) lncRNA
microarray was used to screen for differential lncRNAs in MI rats.
(B) H9c2 cells were treated with 100 µM H2O2
at different time-points (0, 3, 6 and 12 h), and HOTAIR expression
levels were detected by RT-qPCR. (C) After H9c2 cells were treated
with 0–100 µM H2O2 for 12 h, RT-qPCR was used
to detect the expression of HOTAIR. (D) After MI (0, 30, 60, 120
and 180 min) in rats, RT-qPCR was used to detect the expression of
HOTAIR in cardiac tissue. *P<0.05, **P<0.01 and ***P<0.001
vs. control group. MI, myocardial ischemia; lncRNA, long non-coding
RNA; RT-qPCR; reverse transcription-quantitative PCR; HOTAIR, HOX
transcript antisense intergenic RNA. |
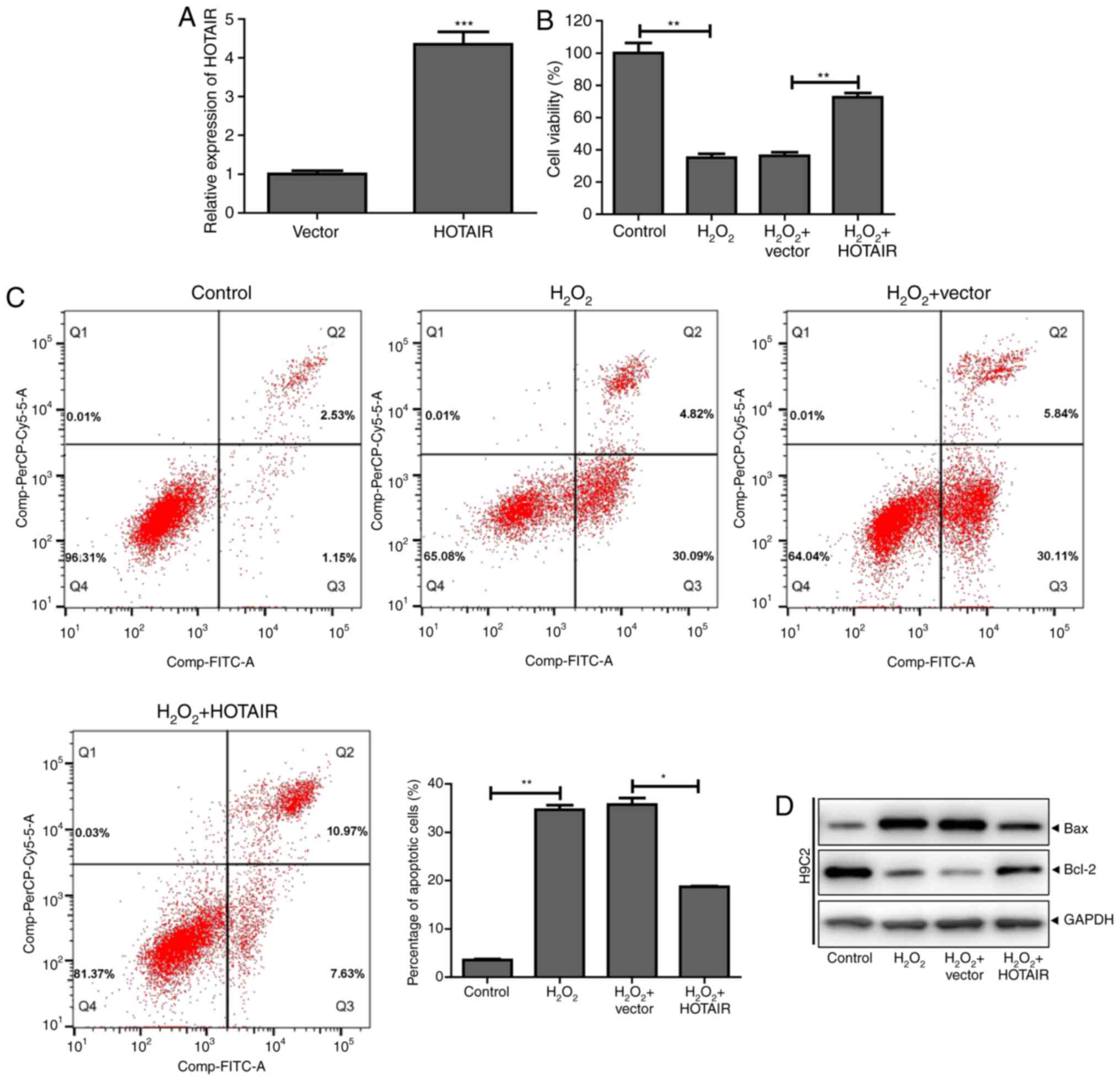
HOTAIR inhibits
H2O2-induced cardiomyocyte apoptosis
To further investigate the role of HOTAIR in
H2O2-induced cardiomyocyte apoptosis, a
HOTAIR overexpression plasmid was successfully constructed and
transfected into cells (Fig. 2A).
HOTAIR significantly increased H9c2 cell viability following
H2O2 treatment and reduced apoptosis
(Fig. 2B and C). Western blot
analysis showed that HOTAIR promoted the expression of the
anti-apoptotic protein Bcl-2 in H9c2 cells and inhibited the
expression of the proapoptotic protein Bax (Fig. 2D). These results indicated that
HOTAIR inhibited H2O2-induced cardiomyocyte
apoptosis.
HOTAIR downregulates miR-130a-3p
expression levels
The StarBase database was used to predict that
HOTAIR has a binding site with miR-130a-3p (Fig. 3A). To determine the regulatory
relationship between HOTAIR and miR-130a-3p, HOTAIR-WT luciferase
activity was found to be reduced in H9c2 cells co-transfected with
HOTAIR-WT and miR-130a-3p mimics by dual luciferase reporter assay
(Fig. 3B). The results of RIP
showed that HOTAIR-WT interacted directly with miR-130a-3p
(Fig. 3C). As shown in Fig. 3D and E, the expression of
miR-130a-3p was decreased following overexpression of HOTAIR in
H9c2 cells, whereas when the expression of HOTAIR was
downregulated, the expression of miR-130a-3p was increased. In
addition, miR-130a-3p was found to be significantly elevated in
ischemic myocardium mouse heart tissue and
H2O2-treated H9c2 cells (Fig. 3F and G). The results indicated that
HOTAIR can function as an miRNA sponge to adsorb miR-130a-3p.
 | Figure 3.HOTAIR downregulates miR-130a-3p
expression levels. (A) Bioinformatics analysis was used to predict
the binding site of HOTAIR to miR-130a-3p. (B) The luciferase
reporter gene detection system was used to detect luciferase
activity after co-transfection of HOTAIR-WT or -MT reporter
plasmids with scramble or miR-130a-3 mimics for 48 h, according to
the manufacturer's instructions. (C) H9c2 cells were collected,
lysed and incubated with magnetic beads that contained Ago2 or IgG
antibody, and HOTAIR and miR-130a-3p expression levels were
detected by RT-qPCR. (D) HOTAIR overexpression plasmid or control
plasmid was transfected into H9c2 cells, and miR-130a-3p expression
levels were detected by RT-qPCR. (E) shHOTAIR or shControl was
transfected into H9c2 cells, and miR-130a-3p expression levels were
detected by RT-qPCR. (F) H9c2 cells were treated with 100 µM
H2O2 at different time-points (0, 3, 6 and 12
h), and RT-qPCR was performed to detect miR-130a-3p expression. (G)
After MI (0, 30, 60, 120 and 180 min), the expression of
miR-130a-3p in cardiac tissue was determined using RT-qPCR.
*P<0.05, **P<0.01 and ***P<0.001 vs. control group. MI,
myocardial ischemia; RT-qPCR, reverse transcription-quantitative
PCR; Ago2, Argonaute 2; IgG, Immunoglobin G; miR, microRNA; HOTAIR,
HOX transcript antisense intergenic RNA; WT, wild-type; MT, mutant;
sh-, short hairpin RNA; NC, negative control. |
HOTAIR downregulates miR-130a-3p
expression and reduces H2O2-induced apoptosis
of H9c2 cells
In order to confirm that HOTAIR downregulates
miR-130a-3p expression and affects
H2O2-induced apoptosis of H9c2 cells,
miR-130a-3p mimics were synthesized to transfect H9c2 cells, which
was successful and miR-130a-3p expression significantly increased
(Fig. 4A). Then, H9c2 cells
transfected with the miR-130a-3p mimic were treated with
H2O2, which significantly decreased cell
viability and increased the proportion of cells undergoing
apoptosis. When the H9c2 cells were co-transfected with miR-130a-3p
mimics and HOTAIR overexpression vector, the cell viability
increased and the cell apoptosis decreased (Fig. 4B and C). Similarly, miR-130a-3p
mimics were found to inhibit the expression of Bcl-2 protein and
promote the expression of Bax protein, whereas H9c2 cells
co-transfected with HOTAIR overexpression vector and miR-130a-3p
mimics showed increased expression of Bcl-2 and decreased
expression of Bax (Fig. 4D). These
results indicated that HOTAIR could reduce the apoptosis of H9c2
cells induced by H2O2 by downregulating the
expression of miR-130a-3p.
miR-130a-3p targets the inhibition of
MDM4 and promotes H2O2-induced H9c2 cell
apoptosis
In order to elucidate the molecular mechanism by
which miR-130a-3p regulates apoptosis, TargetScan and RNA hybrids
were used to analyze potential target genes. MDM4 was revealed to
be a potential target gene of miR-130a-3p (Fig. 5A). MDM4 is known to be an important
inhibitor of apoptosis (15).
However, whether MDM4 participates in cardiomyocyte apoptosis needs
to be explored further. The expression of MDM4 protein was found to
be decreased in the heart tissue of rats with MI and in H9c2 cells
treated with H2O2 (Fig. 5B and C). Luciferase reporter assays
showed that miR-130a-3p mimics significantly reduced the activity
of MDM4-WT luciferase (Fig. 5D).
The MDM4 protein expression of H9c2 cells treated with miR-130a-3p
mimics decreased (Fig. 5E).
Whereas, when H9c2 cells were treated with miR-130a-3p inhibitor,
which successfully knocked down miR-130a-3p expression (Fig. 5F), MDM4 protein expression increased
(Fig. 5G). In addition,
overexpression of MDM4 in H9c2 cells (Fig. 5H) treated with
H2O2 resulted in increased cell viability,
decreased apoptosis, increased Bcl-2 protein expression and
decreased Bax protein expression. Transfection of miR-130a-3p
mimics into H9c2 cells that were overexpressing MDM4 resulted in
decreased cell viability, increased apoptosis, decreased Bcl-2
protein expression and increased Bax protein expression (Fig. 5I-K). The results showed that
miR-130a-3p could inhibit MDM4 to promote
H2O2-induced apoptosis of H9c2 cells.
 | Figure 5.miR-130a-3p targets the inhibition of
MDM4 and promotes H2O2-induced H9c2 cell
apoptosis. (A) TargetScan was used to predict the binding sites of
MDM4 and miR-130a-3p. (B) The expression of MDM4 in H9c2 cells
treated with 100 µm H2O2 at different
time-points (0, 3, 6 and 12 h) was detected by western blotting.
(C) After MI (0, 30, 60, 120 and 180 min), the expression of MDM4
was detected by western blotting. (D) The MDM4-WT or -MT reporter
plasmids were co-transfected with scramble or miR-130a-3p mimics,
respectively, in accordance with the manufacturer's instructions
for 48 h, and then the luciferase activity was detected by a
luciferase reporter gene detection system. Then, the H9c2 cells
were treated with scramble and (E) miR-130a-3p mimics for 48 h, and
the expression of MDM4 protein was detected by western blotting.
(F) H9c2 cells were successfully transfected with miR-130a-3p
inhibitor for 48 h, and (G) the expression of MDM4 protein was
detected by western blotting. (H) H9c2 cells were successfully
transfected with an MDM4 overexpression vector. (I) According to
the manufacturer's instructions, scramble or miR-130a-3p mimics
were transfected into H9c2 cells with MDM4 overexpression and
control plasmids. After treating with H2O2
for 12 h, a Cell Counting Kit-8 assay was used to detect the
changes in cell viability. (J) The apoptotic ratio was assessed by
flow cytometry. (K) Western blotting was performed to detect the
expression of MDM4, Bcl-2 and Bax protein. **P<0.01 and
***P<0.001 vs. NC-miRNA or as indicated. MDM4, mouse double
minute 4; miR/miRNA, microRNA; MI, myocardial ischemia; WT,
wild-type; NC, negative control; MT, mutant. |
HOTAIR inhibits
H2O2-induced H9c2 cell apoptosis via the
miR-130a-3p/MDM4 axis
The present study sought to determine whether HOTAIR
regulated H2O2-induced H9c2 cell apoptosis
via the miR-130a-3p/MDM4 axis. It was revealed that HOTAIR
increased MDM4-MT luciferase activity by inhibiting miR-130a-3p
(Fig. 6A). Subsequently, HOTAIR was
found to promote MDM4 expression, whereas depleting HOTAIR
expression inhibited MDM4 expression (Fig. 6B and C). Further assays indicated
that miR-130a-3p mimics inhibited HOTAIR-induced MDM4 expression,
whereas the miR-130a-3p inhibitor restored MDM4 reduction due to
HOTAIR knockdown (Fig. 6D and E).
MDM4 expression was successfully knocked down in H92c cells using
shMDM4 (Fig. 6F). Knockdown of MDM4
in H9c2 cells overexpressing HOTAIR, resulted in decreased cell
viability, increased apoptosis, decreased expression of Bcl-2
protein and increased expression of Bax protein (Fig. 6G-I). These results indicated that
HOTAIR inhibited H2O2-induced apoptosis of
H9c2 cells via the miR-130a-3p/MDM4 axis.
 | Figure 6.HOTAIR inhibits
H2O2-induced H9c2 cell apoptosis via the
miR-130a-3p/MDM4 axis. (A) The luciferase reporter gene detection
system was performed to detect luciferase activity after
co-transfection of MDM4-WT or -MT reporter plasmids with
miR-130a-3p mimics and HOTAIR for 48 h according to the
manufacturer's instructions. (B) HOTAIR overexpression plasmid or
control plasmid was transfected into H9c2 cells, and MDM4
expression was detected by western blotting. (C) shHOTAIR or
shControl was transfected into H9c2 cells, and MDM4 expression was
detected by western blotting. (D) Scramble or miR-130a-3p mimics
were transfected into H9c2 cells with HOTAIR and control plasmids
according to the manufacturer's instructions. H9c2 cardiomyocytes
were treated with H2O2 for 12 h, and MDM4
protein expression was detected by western blotting. (E) Scramble
or miR-130a-3p inhibitor was transfected into H9c2 cells with
shHOTAIR or shControl, according to the manufacturer's
instructions, H9c2 cardiomyocytes were treated with
H2O2 for 12 h, and MDM4 protein expression
was detected by western blotting. (F) After 48 h of transfection of
shMDM4 or shControl into H9C2 cells, western blotting was used to
detect the expression of MDM4 protein. (G) HOTAIR and its control
plasmid were co-transfected with shHOTAIR or shControl into H9c2
cells according to the manufacturer's instructions, H9c2
cardiomyocytes were treated with H2O2 for 12
h, and the cell viability was detected by a Cell Counting Kit-8
assay. (H) The apoptosis rate was determined by flow cytometry. (I)
The expression levels of MDM4, Bcl-2 and Bax protein were detected
by western blotting. **P<0.01 and ***P<0.001. MDM4, mouse
double minute 4; HOTAIR, HOX transcript antisense intergenic RNA;
WT, wild-type; miR, microRNA; sh-, short hairpin RNA; MT,
mutant. |
Discussion
Currently, patients worldwide suffer from MI
(17,18). However, MI/R injury is a difficult
problem for doctors. At present, the treatment of MI/R primarily
includes both non-pharmacological and pharmacological treatments
(18). To date, the most
encouraging measures are ischemic postconditioning, remote ischemic
preconditioning, atrial natriuretic peptide, adenosine,
cyclosporine and exenatide (18).
However, the overall therapeutic effect is not satisfactory, and
there are still varying degrees of microvascular dysfunction after
treatment (19,20). Therefore, it is necessary to study
new drugs and treatment methods to treat MI/R injury.
The present study found that the lncRNA, HOTAIR, was
significantly downregulated in ischemic myocardium mouse heart
tissues by lncRNA array. H9c2 cells were treated with
H2O2 to simulate reactive oxygen
species-induced cardiomyocytes. It was found that HOTAIR expression
levels gradually decreased with prolonged
H2O2 treatment time, and HOTAIR was lowly
expressed in the heart tissue of rats with myocardial infarction.
These results suggested that HOTAIR may be associated with reactive
oxygen species-induced cardiomyocyte injury. Overexpression of
HOTAIR in H9c2 cells enhanced cell viability and inhibited
apoptosis induced by H2O2.
lncRNAs are localized to the cytoplasm and function
as miRNA sponges to downregulate miRNA expression levels. Studies
have found that HOTAIR adsorbs miR-519d-3p to inhibit
hypoxia-induced cardiomyocyte injury (21). Li et al (22) found that the lncRNA H19 imprinted
maternally expressed transcript/miR-675 axis is involved in the
regulation of high glucose-induced apoptosis by targeting
voltage-dependent anion-selective channel protein 1. The present
study found a direct interaction between HOTAIR-WT and miR-130a-3p
by bioinformatics, dual luciferase reporter assay and RIP. The
results indicated that HOTAIR could adsorb miR-130a-3p. By
overexpressing HOTAIR and miR-130a-3p mimics in H9c2 cells, HOTAIR
downregulated miR-130a-3p expression levels to attenuate
H2O2-induced H9c2 cell apoptosis.
miR-130a-3p has been found to be associated with
apoptosis, for example Wang et al (23) reported that miR-130a-3p attenuates
activation and induces apoptosis of hepatic stellate cells and Chen
et al (24) demonstrated
that miR-130a-3p promotes apoptosis of nasopharyngeal carcinoma
cells. The present study found that MDM4 is a potential target gene
of miR-130a-3p through TargetScan analysis and using RNA hybrids.
The luciferase reporter assay found that transfection with
miR-130a-3p mimics significantly reduced MDM4-WT luciferase
activity. Transfection of miR-130a-3p mimics into H9c2 cells
overexpressing MDM4 resulted in decreased cell viability and
increased apoptosis, which indicated that miR-130a-3p targeted
inhibition of MDM4 promoted H2O2-induced H9c2
cell apoptosis.
Further studies in the present study showed that
miR-130a-3p mimics inhibited HOTAIR-induced MDM4 expression, and
that a miR-130a-3p inhibitor restored the reduction of MDM4
expression caused by HOTAIR depletion. In addition, knockdown of
MDM4 in H9c2 cells overexpressing HOTAIR was found to result in a
decrease in cell viability and increase in apoptosis. These results
indicated that HOTAIR inhibited H2O2-induced
apoptosis of H9c2 cells via the miR-130a-3p/MDM4 axis. He and Jiang
(25) previously found that
HOTAIR-induced apoptosis is mediated by sponging miR-130a-3p to
repress chondrocyte autophagy in knee osteoarthritis.
However, there are a few limitations of the present
study. Firstly, the conclusions of this study have not been
confirmed by conducting in vivo studies. Secondly, MDM4
could have other roles in ischemic cardiomyopathy, which needs to
be studied further. Finally, the specific role of lncRNA HOTAIR in
the cells has not been confirmed in this study. In summary, the
present study demonstrated that the lncRNA HOTAIR inhibited the
apoptosis of H9c2 cells induced by H2O2
through the miR-130a-3p/MDM4 axis. This study provides a novel
direction for prevention and treatment of ischemic
cardiomyopathy.
Acknowledgements
Not applicable.
Funding
This work was supported by grants from the Natural
Science Foundation of Zhejiang Province (grant. no.
LY18H270008)
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JF, WZ and PH and JW acquired the data, conducted
the formal analysis and utilized the software used in the study. JF
also developed the methodology, and wrote the original draft. WZ,
JW and PH also assisted with the visualization of the data and
study. JW conducted the initial funding acquisition, provided
resources and supervision, and helped to review and edit the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All rat experiments conform to the National
Institutes of Health Guidelines on the Use of Laboratory Animals
and were approved by The First People's Hospital of Tonglu
(approval no. 20190154; Hangzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Muñoz D, Uzoije P, Reynolds C, Miller R,
Walkley D, Pappalardo S, Tousey P, Munro H, Gonzales H, Song W, et
al: Polypill for cardiovascular disease prevention in an
underserved population. N Engl J Med. 381:1114–1123. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chouchani ET, Pell VR, James AM, Work LM,
Saeb-Parsy K, Frezza C, Krieg T and Murphy MP: A Unifying mechanism
for mitochondrial superoxide production during ischemia-reperfusion
injury. Cell Metab. 23:254–263. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yan K, An T, Zhai M, Huang Y, Wang Q, Wang
Y, Zhang R, Wang T, Liu J, Zhang Y, et al: Mitochondrial miR-762
regulates apoptosis and myocardial infarction by impairing ND2.
Cell Death Dis. 10:5002019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li M, Ding W, Tariq MA, Chang W, Zhang X,
Xu W, Hou L, Wang Y and Wang J: A circular transcript of ncx1 gene
mediates ischemic myocardial injury by targeting miR-133a-3p.
Theranostics. 8:5855–5869. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Engreitz JM, Ollikainen N and Guttman M:
Long non-coding RNAs: Spatial amplifiers that control nuclear
structure and gene expression. Nat Rev Mol Cell Biol. 17:756–770.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen CK, Blanco M, Jackson C, Aznauryan E,
Ollikainen N, Surka C, Chow A, Cerase A, McDonel P and Guttman M:
Xist recruits the X chromosome to the nuclear lamina to enable
chromosome-wide silencing. Science. 354:468–472. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Quinn JJ and Chang HY: Unique features of
long non-coding RNA biogenesis and function. Nat Rev Genet.
17:47–62. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sun M, Nie F, Wang Y, Zhang Z, Hou J, He
D, Xie M, Xu L, De W, Wang Z, et al: LncRNA HOXA11-AS promotes
proliferation and invasion of gastric cancer by scaffolding the
chromatin modification factors PRC2, LSD1, and DNMT1. Cancer Res.
76:6299–6310. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chi Y, Wang D, Wang J, Yu W and Yang J:
Long non-coding RNA in the pathogenesis of cancers. Cells.
8:10152019. View Article : Google Scholar
|
|
10
|
Adams BD, Parsons C, Walker L, Zhang WC
and Slack FJ: Targeting noncoding RNAs in disease. J Clin Invest.
127:761–771. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wei R, Zhang L, Hu W, Wu J and Zhang W:
Long non-coding RNA AK038897 aggravates cerebral
ischemia/reperfusion injury via acting as a ceRNA for miR-26a-5p to
target DAPK1. Exp Neurol. 314:100–110. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cai B, Ma W, Ding F, Zhang L, Huang Q,
Wang X, Hua B, Xu J, Li J, Bi C, et al: The long noncoding RNA
CAREL controls cardiac regeneration. J Am Coll Cardiol. 72:534–550.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ponnusamy M, Liu F, Zhang YH, Li RB, Zhai
M, Liu F, Zhou LY, Liu CY, Yan KW, Dong YH, et al: The long
non-coding RNA CPR regulates cardiomyocyte proliferation and
cardiac repair. Circulation. 139:2668–2684. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liang H, Su X, Wu Q, Shan H, Lv L, Yu T,
Zhao X, Sun J, Yang R, Zhang L, et al: LncRNA 2810403D21Rik/Mirf
promotes ischemic myocardial injury by regulating autophagy through
targeting Mir26a. Autophagy. 16:1077–1091. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yin Y, Guan Y, Duan J, Guo Wei, Zhu Y,
Quan W, Guo C, Zhou D, Wang Y, Xi M, et al: Cardioprotective effect
of Danshensu against myocardial ischemia/reperfusion injury and
inhibits apoptosis of H9c2 cardiomyocytes via Akt and ERK1/2
phosphorylation. Eur J Pharmacol. 699:219–226. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang JX, Zhang XJ, Li Q, Wang K, Wang Y,
Jiao JQ, Feng C, Teng S, Zhou LY, Gong Y, et al: MicroRNA-103/107
regulate programmed necrosis and myocardial ischemia/reperfusion
injury through targeting FADD. Circ Res. 117:352–363. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Khoo CM and Tai ES: Trends in the
incidence and mortality of coronary heart disease in asian pacific
region: the Singapore experience. J Atheroscler Thromb. 21 (Suppl
1):S2–S8. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dalen JE, Alpert JS, Goldberg RJ and
Weinstein RS: The epidemic of the 20(th) century: Coronary heart
disease. Am J Med. 127:807–812. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mauermann E, Puelacher C and Lurati Buse
G: Myocardial injury after noncardiac surgery: An underappreciated
problem and current challenges. Curr Opin Anaesthesiol. 29:403–412.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Heusch G and Gersh BJ: The pathophysiology
of acute myocardial infarction and strategies of protection beyond
reperfusion: A continual challenge. Eur Heart J. 38:774–784.
2017.PubMed/NCBI
|
|
21
|
Zhang D, Wang B, Ma M, Yu K, Zhang Q and
Zhang X: lncRNA HOTAIR Protects Myocardial Infarction Rat by
Sponging miR-519d-3p. J Cardiovasc Transl Res. 12((3)): 171–183.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li X, Wang H, Yao B, Xu W, Chen J and Zhou
X: lncRNA H19/miR-675 axis regulates cardiomyocyte apoptosis by
targeting VDAC1 in diabetic cardiomyopathy. Sci Rep. 6:363402016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang Y, Du J, Niu X, Fu N, Wang R, Zhang
Y, Zhao S, Sun D and Nan Y: MiR-130a-3p attenuates activation and
induces apoptosis of hepatic stellate cells in nonalcoholic
fibrosing steatohepatitis by directly targeting TGFBR1 and TGFBR2.
Cell Death Dis. 8:e27922017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen X, Yue B, Zhang C, Qi M, Qiu J, Wang
Y and Chen J: MiR-130a-3p inhibits the viability, proliferation,
invasion, and cell cycle, and promotes apoptosis of nasopharyngeal
carcinoma cells by suppressing BACH2 expression. Biosci Rep.
37:BSR201605762017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
He B and Jiang D: HOTAIR-induced apoptosis
is mediated by sponging miR-130a-3p to repress chondrocyte
autophagy in knee osteoarthritis. Cell Biol Int. 44:524–535. 2020.
View Article : Google Scholar : PubMed/NCBI
|