Introduction
Lung cancer is considered to be one of the most
frequent malignancies, with a high mortality rate, as there are 1.6
million tumor-related mortalities annually worldwide (1,2).
Non-small cell lung cancer (NSCLC) is the predominant type of lung
cancer and accounts for >80% of cases with a low 5-year survival
rate (3,4). Despite significant advances in the
diagnosis and treatment of lung cancer in recent decades, the
5-year survival rate still remains <20% (5). Poor prognosis in patients with NSCLC
is associated with the lack of early diagnostic biomarkers, as well
as high potentials of invasion and metastasis (6). Therefore, elucidating the detailed
molecular mechanism underlying the progression of NSCLC and
identifying novel biomarkers is urgently required for early
diagnosis, prevention and treatment.
Long non-coding (lnc) RNAs are functional
non-protein coding transcripts, with a length of >200
nucleotides (7). Based on emerging
research, lncRNAs exert a diverse set of functions in numerous
biological processes, such as cell proliferation, invasion,
differentiation, apoptosis and metastasis (8,9). In
addition, the expression profiles of lncRNA have been associated
with a number of diseases, including cancer, suggesting that they
may serve as potential mediators in carcinogenesis (10). PITPNA antisense RNA 1 (PITPNA-AS1)
is a newly reported lncRNA, which is located on chromosome 17p13.3
(11). It has been reported that
PITPNA-AS1 facilitates hepatocellular carcinoma progression via
regulation of the microRNA(miRNA/miR)-876-5p/WNT5A signaling
pathway (11). Moreover, emerging
evidence supports the hypothesis that PITPNA-AS1 accelerates
cervical cancer progression via regulating cellular proliferation
and apoptosis by targeting the miR-876-5p/c-MET axis (12). Therefore, PITPNA-AS1 may exert
oncogenic effects. These findings indicated that PITPNA-AS1 may
serve as a potential diagnostic and therapeutic target for
NSCLC.
The aims of the present study were to investigate
the role of lncRNA PITPNA-AS1 in NSCLC development and to identify
the underlying molecular mechanisms. These data may provide a novel
diagnostic and therapeutic target for the control of NSCLC
progression.
Materials and methods
The Cancer Genome Atlas (TCGA)
database analysis
Human RNA-sequencing data from NSCLC projects, which
included 1,038 patients with NSCLC and 322 normal tissues were
obtained from TCGA data in the UALCAN database (ualcan.path.uab.edu). A Mann-Whitney test was used by
UALCAN to determine the statistical differences in the PITPNA-AS1
expression levels between normal and tumor samples.
Cells and cell culture
A total of four human NSCLC cell lines (HCC827,
NCI-H1299, A549 and NCI-H1650) and one normal human bronchial
epithelioid cell line (BEAS-2B), were purchased from the Cell Bank
of the Chinese Academy of Sciences. The cell lines were cultured in
RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.) with 10%
FBS (Gibco; Thermo Fisher Scientific, Inc.), 100 µg/ml penicillin
and 0.1 µg/ml streptomycin at 37°C in a humidified incubator with
5% CO2.
Cell transfection
Cells were plated into 6-well plates
(1×106 cells per well), and transfection was performed
when cells in the logarithmic growth phase reached 80% confluence.
pIRES vectors containing (sh)RNAs targeting PITPNA-AS1
(shRNA-PITPNA-AS1-1 or shRNA-PITPNA-AS1-2; 1 µg) or a scrambled
shRNA [shRNA-negative control (NC); 1 µg] were synthesized by
Shanghai GenePharma Co., Ltd. miR-32-5p inhibitor
(5′-UGCAACUUAGUAAUGUGCAAUA-3′; 50 nM), miR-32-5p inhibitor NC
(inhibitor-NC; 5′-CAGUACUUUUGUGUAGUACAA-3′; 50 nM), miR-32-5p mimic
(forward, 5′-UAUUGCACAUUACUAAGUUGCA-3′ and reverse,
5′-CAACUUAGUAAUGUGCAAUAUU-3′; 50 nM) and miR-32-5p mimic NC (mimic
NC; 5′-UUCUCCGAACGUGUCACUGUU-3′; 50 nM) were designed and
synthesized by Shanghai GenePharma Co., Ltd. Transfection
experiments were performed using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. Cells were harvested 48 h following
transfection and the transfection efficiency was determined using
reverse transcription-quantitative PCR (RT-qPCR).
Cell Counting Kit-8 (CCK-8) assay
A549 cells were maintained in the exponential phase
and then seeded in 96-well plates (2×104 cells/well).
Cell proliferation was detected using a CCK-8 assay (Shanghai
YiSheng Biotechnology Co., Ltd.) according to the manufacturer's
instructions. At the indicated times of 24, 48 and 72 h, 10 µl
CCK-8 solution was added to each well and the cells were incubated
for an additional 4 h at 37°C. The optical density was measured at
450 nm using a microplate reader (Bio-Rad Laboratories, Inc.).
Colony formation assay
Cells were exposed to the indicated transfections
accordingly and were plated into 6-cm dishes (1,000 cells per
well), followed by incubation for 14 days at 37°C, until the
colonies could be observed under a fluorescence microscope
(magnification, ×10; Olympus Corporation). Subsequently, cells were
fixed with 4% paraformaldehyde for 10 min at room temperature and
stained with 0.2% crystal violet for 5 min at room temperature.
Immunofluorescence assay
Cells (5×104 cells/well) were plated on
coverslips in 24-well plates following transfection and were
cultured until 80% confluence was reached. Subsequently, cells were
fixed in 4% paraformaldehyde at room temperature for 10 min and
permeabilized with 0.05% Triton X-100. After blocking with 5%
normal goat serum (Boster Biological Technology) for 1 h at room
temperature, cells were incubated with a primary antibody against
Ki67 (cat. no. 12075S; 1:1,000; Cell Signaling Technology, Inc.)
overnight at 4°C. The cells were washed three times with PBS,
incubated with DyLight™ 488-conjugated secondary antibody (cat. no.
35553; 1:500; Invitrogen; Thermo Fisher Scientific, Inc.) for 2 h
at 37°C and stained with DAPI (Sigma-Aldrich; Merck KGaA) at room
temperature for 15 min. The stained cells were subsequently imaged
under a fluorescence microscope (magnification, ×200; Olympus
Corporation).
Transwell assay
The invasive ability of A549 cells was detected
using an 8-µm pore insert precoated with Matrigel (BD Biosciences)
overnight at 37°C. A total of 200 µl serum-free medium containing
cells was added to the upper chamber following 48 h of
transfection. RPMI-1640 containing 10% FBS was added to the lower
compartment as a chemoattractant. After 24 h of incubation, cells
invading the lower chamber were fixed with 4% formaldehyde for 20
min at 37°C and stained with 0.1% crystal violet for 30 min at
37°C. Images were captured using an inverted light microscope
(magnification, ×100; Olympus Corporation) and the number of
invasive cells were counted from five randomly selected fields.
Wound healing assay
For the wound healing assay, cells (3×105
cells/well) were incubated in a 6-well culture plate to achieve 80%
confluence. Subsequently, cells were incubated with serum-free
medium overnight at 37°C prior to the experiment. Then, the cell
monolayer was scratched using a sterilized 200-µl pipette tip. The
migrated cells were observed at 0 and 24 h following the creation
of the wound, using a phase contrast microscope (magnification,
×100; Olympus Corporation). Quantitative analysis of the wound
healing area was performed using ImageJ software (version 1.52r;
National Institutes of Health).
Luciferase activity reporter
assay
The TargetScan database 7.1 (http://www.targetscan.org/vert_71) was used to predict
the targets of PITPNA-AS1. The wild-type (WT) and mutant (MUT)
sequences of PITPNA-AS1 3′UTR were amplified by Shanghai GenePharma
Co., Ltd., cloned into a pGL3 luciferase vector (Promega
Corporation) and respectively named pGL3-PITPNA-AS1-WT and
pGL3-PITPNA-AS1-MUT. Cells were co-transfected with miR-32-5p mimic
(Shanghai GenePharma Co., Ltd.; 50 nM) or mimic-NC (Shanghai
GenePharma Co., Ltd.; 50 nM) and 50 ng pGL3-PITPNA-AS1-WT or
pGL3-PITPNA-AS1-MUT. Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) was used for transfection.
Following 48 h of transfection at 37°C, a Dual Luciferase Reporter
Assay kit (Promega Corporation) was used to evaluate the relative
luciferase signals, and firefly luciferase activity was normalized
to Renilla luciferase activity.
RT-qPCR
Total RNA was extracted from A549 cells using
TRIzol® (Thermo Fisher Scientific, Inc) following the
manufacturer's instructions. Subsequently, cDNA was synthesized at
42°C for 30 min using a reverse transcription kit (PrimeScript™ RT
Reagent kit; Takara Bio, Inc.). RT-qPCR was then performed with 2
µg cDNA, as the template using iTaq™ Universal SYBR®
Green Supermix (Bio-Rad Laboratories, Inc.) on an ABI 7500
instrument (Applied Biosystems; Thermo Fisher Scientific, Inc.).
The following thermocycling conditions were used: Initial
denaturation at 95°C for 10 min; followed by 40 cycles of
denaturation at 95°C for 15 sec and annealing at 60°C for 1 min;
and a final extension of 10 min at 72°C. The following primers
pairs were used: PITPNA-AS1 forward, 5′-GCAGGGTGGATAAAGAGGA-3′ and
reverse, 5′-CCTACTGACAGGATGTCCT-3′; GAPDH forward,
5′-ACAACTTTGGTATCGTGGAAGG-3′ and reverse,
5′-GCCATCACGCCACAGTTTC-3′; miR-32-5p forward,
5′-CGGTATTGCACATTACTAAGTTGCA-3′ and reverse,
5′-CTCGCTTCGGCAGCACA-3′; U6 forward, 5′-AGGGGCCATCCACAGTCTTC-3′ and
reverse, 5′-AACGCTTCACGAATTTGCGT-3′. GAPDH or U6 was used as the
internal reference gene. The 2−ΔΔCq method was used to
compare relative expression levels (13).
Western blot analysis
Protein samples were extracted from cells using RIPA
lysis buffer (Beyotime Institute of Biotechnology) for western blot
analysis. The concentration of the protein samples was determined
using the BCA protein assay kit (Beyotime Institute of
Biotechnology). The protein samples (40 µg/lane) were separated
using 10% SDS-PAGE and transferred onto PVDF membranes. After
blocking with 5% skimmed milk for 1.5 h at room temperature, the
blots were incubated with specific primary antibodies at 4°C
overnight. Subsequently the membranes were washed three times with
TBS-0.2% Tween-20, then incubated with a goat anti-rabbit
horseradish peroxidase-conjugated secondary antibody (1:3,000; cat.
no. 7074S; Cell Signaling Technology, Inc.) or horse anti-mouse
HRP-conjugated secondary antibody (1:3,000; cat. no. 7076S; Cell
Signaling Technology, Inc.) for 1.5 h at room temperature. The
immunoreactive protein bands on the membranes were visualized using
an enhanced chemiluminescence assay (EMD Millipore). The intensity
of the bands was semi-quantified using ImageJ software (version
1.52r; National Institutes of Health). The following primary
antibodies were used: Anti-Ki67 (cat. no. sc-23900; 1:1,000; Santa
Cruz Biotechnology, Inc.), anti-proliferating cell nuclear antigen
(PCNA; cat. no. 13110T; 1:1,000; Cell Signaling Technology, Inc.),
anti-MMP2 (cat. no. 40994S; 1:1,000; Cell Signaling Technology,
Inc.), anti-MMP9 (cat. no. 13667T; 1:1,000; Cell Signaling
Technology, Inc.), anti-E-cadherin (E-cad; cat. no. 3195T; 1:1,000;
Cell Signaling Technology, Inc.), anti-N-cadherin (N-cad; cat. no.
13116T; 1:1,000; Cell Signaling Technology, Inc.), anti-Vimentin
(Vim; cat. no. 5741T; 1:1,000; Cell Signaling Technology, Inc.) and
anti-GAPDH (cat. no. 5174T; 1:1,000; Cell Signaling Technology,
Inc.). GAPDH was used as an internal control.
Statistical analysis
All experiments were repeated independently in
triplicate. Data are presented as the mean ± standard deviation.
Statistical analysis was performed using GraphPad Prism version 6.0
(GraphPad Software, Inc.). Statistical comparisons between two
groups were performed using an unpaired Student's t-test. One-way
ANOVA followed by Tukey's post hoc test was conducted for
comparison among multiple groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
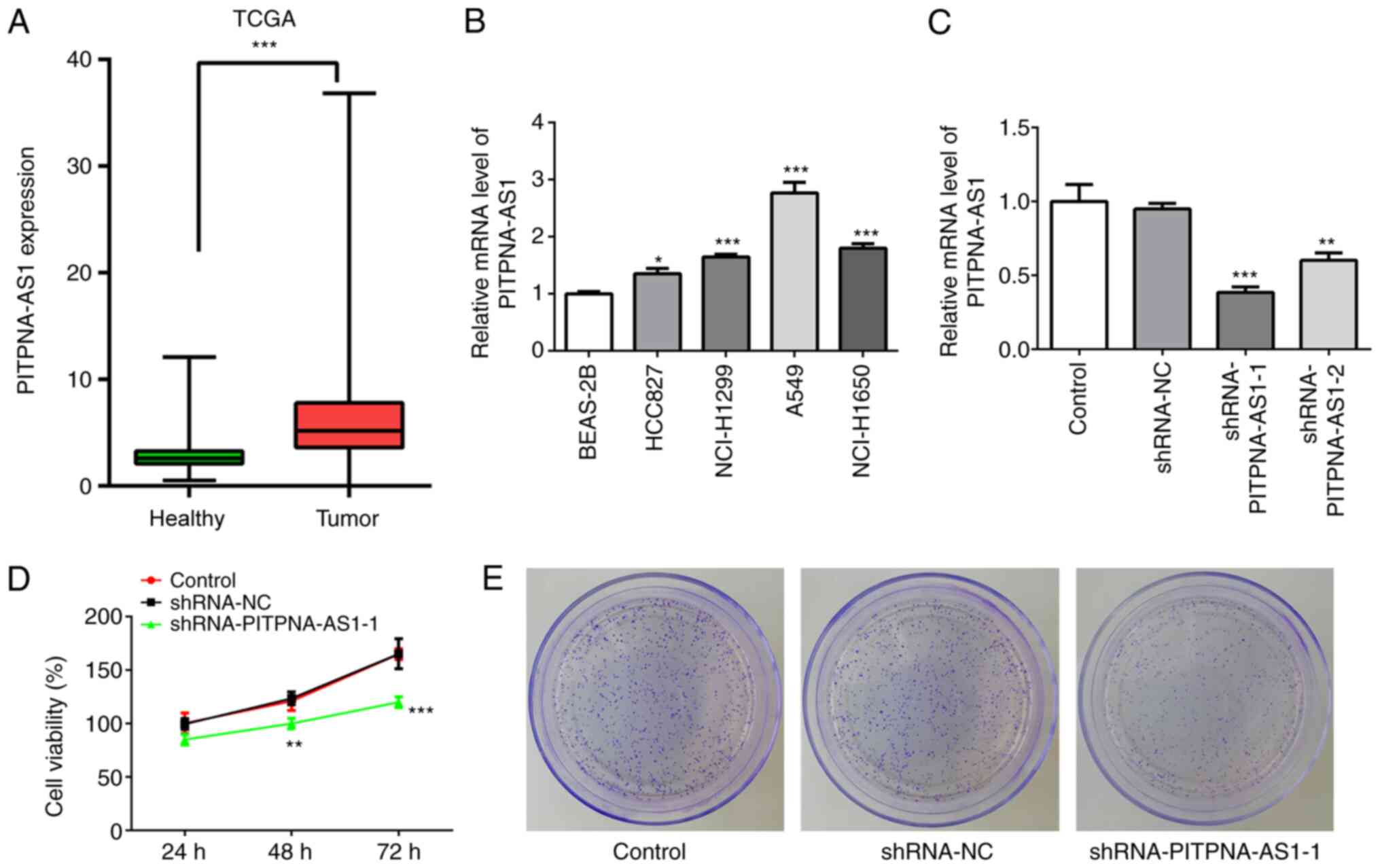
PITPNA-AS1 is highly expressed in
NSCLC cell lines
To investigate the role of PITPNA-AS1 in NSCLC, The
Cancer Genome Atlas (TCGA) database was used to analyze the
expression of PITPNA-AS1 in NSCLC tumor tissues (Tumor; n=1,038)
and adjacent non-tumor tissues (healthy; n=322). The mRNA
expression level of PITPNA-AS1 was increased in NSCLC tissues
compared with that in adjacent non-tumor tissue (Fig. 1A). Subsequently, the mRNA expression
level of PITPNA-AS1 was detected in several NSCLC cells using
RT-qPCR. The expression level of PITPNA-AS1 was increased in the
NSCLC cell lines, particularly in A549 cells, compared with that in
the BEAS-2B cells (Fig. 1B).
Therefore, the A549 cell line was selected for further
experimentation, as it exhibited the highest PITPNA-AS1 mRNA
expression level.
PITPNA-AS1 silencing inhibits the
proliferation of NSCLC cells
To evaluate the effect of PITPNA-AS1 on the
proliferation of NSCLC cells, the mRNA expression level of
PITPNA-AS1 was silenced via transfection with shRNA-PITPNA-AS1-1 or
shRNA-PITPNA-AS1-2. The results demonstrated that A549 cells
transfected with shRNA-PITPNA-AS1-1 displayed the lowest mRNA
expression levels of PITPNA-AS1 compared with the shRNA-NC
(Fig. 1C). Therefore,
shRNA-PITPNA-AS1-1 was used in the following experiments.
PITPNA-AS1 silencing significantly inhibited the
proliferation and colony formation of A549 cells compared with the
shRNA-NC group (Fig. 1D and E).
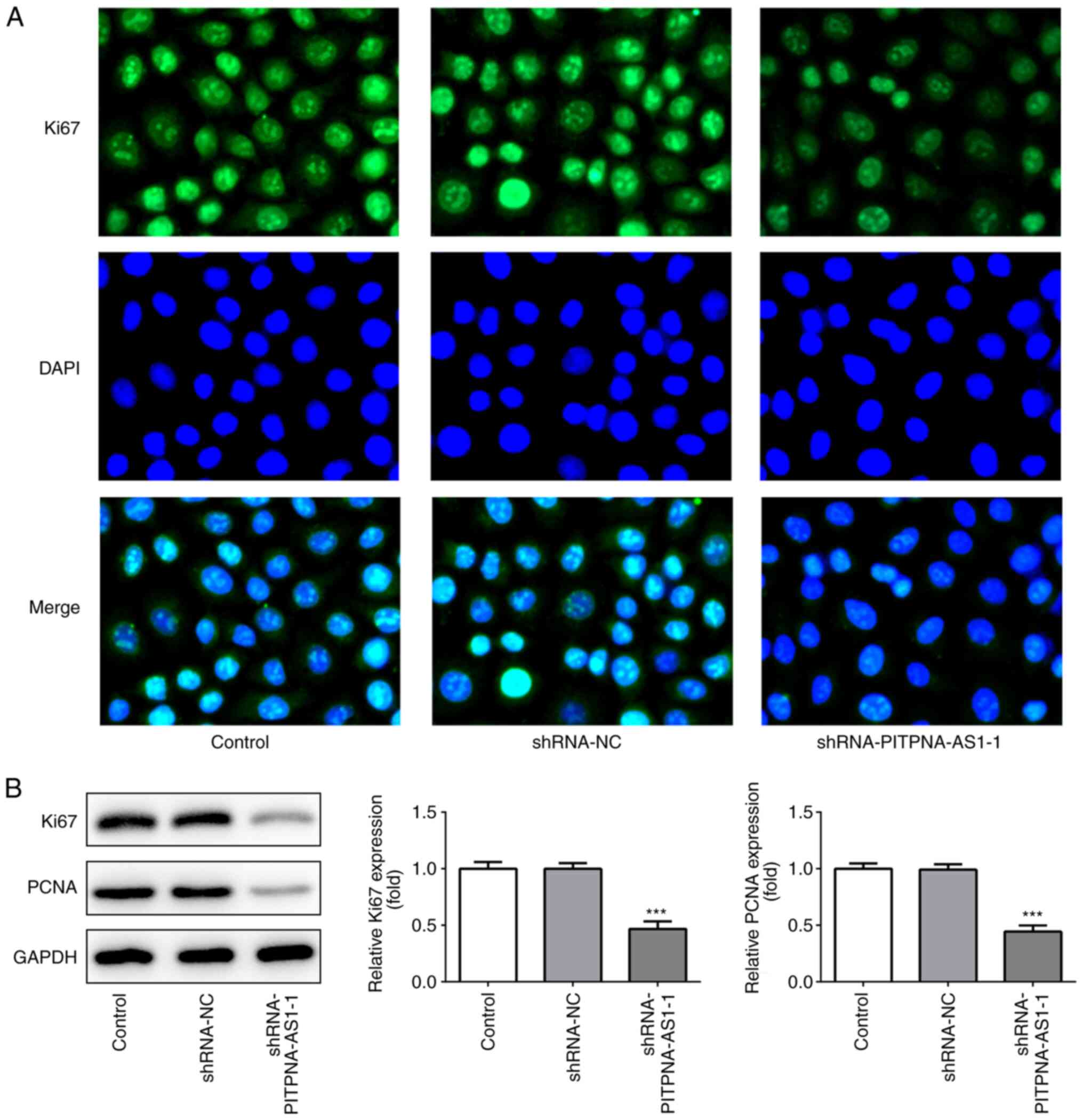
Subsequently, the expression levels of the proliferation-associated
proteins, Ki67 and PCNA, were detected using immunofluorescence
assay (Fig. 2A) and western blot
analysis (Fig. 2B).
PITPNA-AS1-knockdown significantly downregulated the expression
levels of Ki67 and PCNA. These results suggested that PITPNA-AS1
silencing suppressed the proliferation of A549 cells.
PITPNA-AS1 silencing reduces invasion,
migration and epithelial-mesenchymal transition (EMT) in NSCLC
cells
The effects of PITPNA-AS1 silencing on the invasion
and migration of A549 cells were detected using Transwell and wound
healing assays, respectively. The invasive ability of A549 cells
following transfection with shRNA-PITPNA-AS1-1 was significantly
alleviated compared with that in the shRNA-NC group (Fig. 3A and B). Moreover, the migratory
ability of A549 cells was suppressed (Fig. 3C and D), along with a significant
decrease in the expression levels of MMP2 and MMP9 (Fig. 3E).
The protein expression levels of EMT-related
proteins were determined using western blot analysis. Silencing of
PITPNA-AS1 significantly decreased the protein expression levels of
N-cadherin (N-cad) and vimentin (vim), which are important
mesenchymal marker genes (14),
while it enhanced the expression of E-cadherin (E-cad), which is a
crucial epithelial marker (Fig. 4)
(15). These results provided
evidence that PITPNA-AS1-silencing inhibited the invasion,
migration and EMT of NSCLC cells.
 | Figure 4.PITPNA-AS1 silencing suppresses EMT of
A549 cells. Western blot analysis was performed to examine the
expression levels of EMT-related proteins, including N-cad, E-cad
and Vim. **P<0.01, ***P<0.001 vs. shRNA-NC. NC, negative
control; sh, short inhibiting; PITPNA-AS1, PITPNA antisense RNA 1.
EMT, epithelial-to-mesenchymal transition; N-cad, N-cadherin;
E-cad, E-cadherin; Vim, Vimentin. |
miR-32-5p is a direct target of
PITPNA-AS1
The results from the TargetScan analysis identified
that miR-32-5p was one of the potential targets of PITPNA-AS1
(Fig. 5A). It was found that the
expression of miR-32-5p was decreased in NSCLC cells compared with
that in the BEAS-2B cells (Fig.
5B). The transfection efficiency of the miR-32-5p mimic was
assessed via RT-qPCR (Fig. 5C).
Subsequently, a luciferase activity reporter assay was utilized to
verify the potential association. Following transfection with
miR-32-5p mimic and PITPNA-AS1-WT, the luciferase activity was
significantly decreased compared with that in cells co-transfected
with mimic-NC and PITPNA-AS1-WT (Fig.
5D). However, co-transfection of miR-32-5p mimic and
PITPNA-AS1-MUT resulted in no change in the luciferase activity,
suggesting that miR-32-5p could bind to the PITPNA-AS1 3′UTR.
Moreover, significantly elevated miR-32-5p expression was
identified following transfection with shRNA-PITPNA-AS1-1 (Fig. 5E). Thus, these results suggested
that miR-32-5p was a direct target of PITPNA-AS1.
miR-32-5p inhibitor restores the
effects of PITPNA-AS1-silencing on the proliferation of NSCLC
cells
To investigate the regulatory association between
PITPNA-AS1 and miR-32-5p in NSCLC, the mRNA expression of miR-32-5p
was silenced using transfection with miR-211-5p inhibitor (Fig. 6A). Co-transfection with miR-32-5p
inhibitor and shRNA-PITPNA-AS1-1 increased the proliferative
ability of A549 cells compared with cells transfected with
shRNA-PITPNA-AS1-1 alone, which was accompanied by an increase in
Ki67 and PCNA expression levels (Fig.
6B and C). Collectively, it was indicated that PITPNA-AS1 could
regulate the proliferation of NSCLC cells by targeting
miR-32-5p.
 | Figure 6.miR-32-5p inhibitor restores the
effects of PITPNA-AS1-silencing on the proliferation, invasion and
migration of A549 cells. (A) Expression of miR-32-5p was evaluated
using RT-qPCR. ***P<0.001 vs. inhibitor-NC. The proliferative
ability of A549 cells was measured using (B) Cell Counting Kit-8
assay and (C) the expression levels of proliferation-related
proteins were tested using western blot analysis. (D) Transwell
assay was performed to determine invasion of A549 cells and the (E)
number of invaded cells was quantified. Magnification, ×100. (F)
Representative images and (G) relative quantification of cell
migration, as examined using a scratch assay. Magnification, ×100.
(H) Western blot analysis was executed for measuring the expression
of migration-associated proteins in A549 cells. **P<0.01,
***P<0.001 vs. shRNA-NC; ##P<0.01,
###P<0.001 vs. shRNA-PITPNA-AS1-1 + inhibitor-NC. NC,
negative control; sh, short inhibiting; PITPNA-AS1, PITPNA
antisense RNA 1; RT-qPCR, reverse transcription-quantitative
PCR. |
miR-32-5p inhibitor counteracts the
inhibitory effects of PITPNA-AS1-silencing on invasion, migration
and EMT in NSCLC cells
It was found that the miR-32-5p inhibitor reversed
the effect of PITPNA-AS1-silencing on the invasion (Fig. 6D and E) and migration (Fig. 6F and G) of A549 cells, which was
also coupled with an increase in the protein expression levels of
MMP2 and MMP9 (Fig. 6H).
Consistently, the expression levels of EMT-related proteins,
including N-cad, Vim and E-cad, in cells transfected with
shRNA-PITPNA-AS1-1 + miR-32-5p inhibitor demonstrated the opposite
trends compared with those in cells transfected with
shRNA-PITPNA-AS1-1 + inhibitor-NC (Fig.
7). Taken together, the results suggested that the miR-32-5p
inhibitor attenuated the effects of PITPNA-AS1-silencing on the
invasion, migration and EMT of NSCLC cells.
Discussion
Continuing advances in the development of
next-generation sequencing and transcriptomics suggest that the
investigation of lncRNAs in the regulation of cancer is a potential
research field (16). Worldwide
research has focused on identifying potential diagnostic and
prognostic markers, which may accurately predict survival outcomes.
Previous studies have reported that aberrant expression levels of
lncRNAs are associated with the development and progression of
different types of cancer, including NSCLC (17,18).
In the present study, significantly upregulated PITPNA-AS1
expression was observed in NSCLC tissues and cell lines, and the
TargetScan database predicted that miR-32-5p possesses potential
binding sites with PITPNA-AS1. Therefore, the role of PITPNA-AS1
and miR-32-5p in the progression of NSCLC was further
investigated.
PITPNA-AS1 is a newly reported lncRNA, which is
located on chromosome 17p13.3 (11). A previous study revealed that
PITPNA-AS1 could facilitate the progression of hepatocellular
carcinoma via the regulation of the miR-876-5p/WNT5A signaling
pathway (11). Analyzing data from
TCGA database, the present results demonstrated that the expression
of PITPNA-AS1 in NSCLC tissues was significantly increased compared
with that in the adjacent non-tumor tissues. In addition,
PITPNA-AS1 mRNA expression levels in NSCLC cell lines exhibited a
similar trend compared with that in NSCLC tissues, suggesting the
involvement of PITPNA-AS1 in NSCLC.
It is well-known that abnormal and uncontrolled
proliferation is a characteristic of cancer cells (19). Furthermore, invasion and migration
are considered as two dominant processes for tumor metastasis,
which leads to cancer-associated mortality (20). In this regard, interruption of the
aforementioned processes is an effective method for resistance of
cancer metastasis. The present study indicated that
PITPNA-AS1-silencing reduced the proliferation, invasion and
migration of A549 cells, suggesting the inhibitory effects of
PITPNA-AS1 silencing on NSCLC.
EMT, a crucial driver of tumor progression, has been
proposed as the classical tumor metastasis theory in previous years
(21). Cells undergoing EMT lose
the typical epithelial phenotype and are converted into a
mesenchymal phenotype, with an increased migratory ability and
invasiveness, which contributes to cancer metastasis (22). Aberrant expression of a large number
of genes is observed during the activation of EMT. For example, the
protein expression of E-cad, a crucial epithelial marker, is
decreased, while there is an increase in the expression levels of
N-cad and Vim, which are significant mesenchymal marker genes
(23,24). An increasing number of studies have
revealed that lncRNAs could modulate the invasion and metastasis of
NSCLC cells via the regulation of the EMT process. For example,
lncRNA FBXL19-AS1 accelerates the proliferation and metastasis of
NSCLC by mediating EMT (25), while
lncRNA double homeobox A pseudogene 8-downregulation suppresses
metastasis via inhibition of EMT in NSCLC (26). In the present study, increased E-cad
protein expression and decreased N-cad and vim expression levels
were found following PITPNA-AS1-knockdown, suggesting an inhibitory
effect of PITPNA-AS1 silencing on the EMT process.
An increasing number of studies have highlighted the
importance of lncRNAs, acting as competing endogenous RNAs in tumor
biology (27). Previous studies
have reported that lncRNAs could modulate gene expression by
sponging specific miRNAs, a class of endogenous conserved
non-coding RNAs, ~18–25 nucleotides in length, by indirectly
mediating gene expression at the post-transcriptional level
(28). In the present study, the
TargetScan database predicted that miR-32-5p was a potential target
of PITPNA-AS1, which was verified using a luciferase activity
reporter assay. It has been revealed that miR-32-5p inhibits the
proliferation and metastasis of cervical cancer, colorectal cancer
and clear cell renal cell carcinoma (29–31).
Furthermore, downregulated miR-32-5p expression is observed in
colorectal cancer cell lines, and SNHG14 can regulate
proliferation, metastasis and EMT in colorectal cancer progression
by targeting miR-32-5p (32). These
findings prompted further investigation of the role of PITPNA-AS1
and miR-32-5p in the tumorigenesis and progression of NSCLC. The
present study demonstrated that the effect of PITPNA-AS1-silencing
on cell proliferation, metastasis and EMT was rescued by a
miR-32-5p inhibitor. These observations indicated that PITPNA-AS1
could regulate the progression of NSCLC by targeting miR-32-5p.
In conclusion, to the best of our knowledge, the
present study demonstrated for the first time, that PITPNA-AS1 was
highly expressed in NSCLC tissues and cell lines. It was found that
PITPNA-AS1 regulated the proliferation, invasion, migration and EMT
of A549 cells, at least partially by targeting miR-32-5p,
suggesting the novelty of this study, which presents a new
promising target for the clinical diagnosis and therapeutic
interventions of NSCLC. However, the lack of studies verifying the
clinical value and prognostic value of PITPNA-AS1 and miR-32-5p in
clinical tissue samples, as well as the relationship of PITPNA-AS1
and miR-32-5p to the clinical prognosis data of patients are
limitations of the present research. Moreover, further experiments
are required to elucidate the specific signaling pathway or
specific signaling protein regulated by PITPNA-AS1 and miR-32-5p;
therefore, comprehensive analysis is required in the future.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
GC, ZZ and JL searched the literature, designed the experiments and performed the experiments. PZ, ZW, SG and HL analyzed and interpreted the data. JM and JS searched the literature, performed the experiments and wrote the manuscript. HL revised the manuscript. GC confirmed the authenticity of all the raw data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hao H, Zhou Z, Li S, Maquilan G, Folkert
MR, Iyengar P, Westover KD, Albuquerque K, Liu F, Choy H, et al:
Shell feature: A new radiomics descriptor for predicting distant
failure after radiotherapy in non-small cell lung cancer and cervix
cancer. Phys Med Biol. 63:0950072018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Reck M and Rabe KF: Precision Diagnosis
and Treatment for Advanced Non-Small-Cell Lung Cancer. N Engl J
Med. 377:849–861. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tian H, Zhou C, Yang J, Li J and Gong Z:
Long and short noncoding RNAs in lung cancer precision medicine:
Opportunities and challenges. Tumour Biol. 39:10104283176975782017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tian Y, Zhang N, Chen S, Ma Y and Liu Y:
The long non-coding RNA LSINCT5 promotes malignancy in non-small
cell lung cancer by stabilizing HMGA2. Cell Cycle. 17:1188–1198.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang H, Kanmangne D, Li R, Qian Z, Xia X,
Wang X and Wang T: miR 30a 3p suppresses the proliferation and
migration of lung adenocarcinoma cells by downregulating CNPY2.
Oncol Rep. 43:646–654. 2020.PubMed/NCBI
|
|
6
|
Tang XD, Zhang DD, Jia L, Ji W and Zhao
YS: lncRNA AFAP1-AS1 Promotes Migration and Invasion of Non-Small
Cell Lung Cancer via Up-Regulating IRF7 and the RIG-I-Like Receptor
Signaling Pathway. Cell Physiol Biochem. 50:179–195. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Qian H, Chen L, Huang J, Wang X, Ma S, Cui
F, Luo L, Ling L, Luo K and Zheng G: The lncRNA MIR4435-2HG
promotes lung cancer progression by activating β-catenin
signalling. J Mol Med (Berl). 96:753–764. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fatica A and Bozzoni I: Long non-coding
RNAs: New players in cell differentiation and development. Nat Rev
Genet. 15:7–21. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Santosh B, Varshney A and Yadava PK:
Non-coding RNAs: Biological functions and applications. Cell
Biochem Funct. 33:14–22. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kunej T, Obsteter J, Pogacar Z, Horvat S
and Calin GA: The decalog of long non-coding RNA involvement in
cancer diagnosis and monitoring. Crit Rev Clin Lab Sci. 51:344–357.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun J, Zhang Y, Li B, Dong Y, Sun C, Zhang
F, Jin L, Chen D and Wang W: PITPNA-AS1 abrogates the inhibition of
miR-876-5p on WNT5A to facilitate hepatocellular carcinoma
progression. Cell Death Dis. 10:8442019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guo Q, Li L, Bo Q, Chen L, Sun L and Shi
H: Long noncoding RNA PITPNA-AS1 promotes cervical cancer
progression through regulating the cell cycle and apoptosis by
targeting the miR-876-5p/c-MET axis. Biomed Pharmacother.
128:1100722020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lv W, Wang J and Zhang S: Effects of
cisatracurium on epithelial-to-mesenchymal transition in esophageal
squamous cell carcinoma. Oncol Lett. 18:5325–5331. 2019.PubMed/NCBI
|
|
15
|
Ding NH, Zhang L, Xiao Z, Rong ZX, Li Z,
He J, Chen L, Ou DM, Liao WH and Sun LQ: NEK4 kinase regulates EMT
to promote lung cancer metastasis. J Cell Mol Med. 22:5877–5887.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yeh IJ, Liu KT, Shen JH, Wu YH, Liu YH,
Yen MC and Kuo PL: Identification of the Potential Prognostic
Markers from the miRNA-lncRNA-mRNA Interactions for Metastatic
Renal Cancer via Next-Generation Sequencing and Bioinformatics.
Diagnostics (Basel). 10:2282020. View Article : Google Scholar
|
|
17
|
Huarte M: The emerging role of lncRNAs in
cancer. Nat Med. 21:1253–1261. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang L, Jin C, Yang G, Wang B, Hua P and
Zhang Y: lncRNA WTAPP1 promotes cancer cell invasion and migration
in NSCLC by downregulating lncRNA HAND2-AS1. BMC Pulm Med.
20:1532020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu W, Zhang H, Niu Y, Wu Y, Sun W, Li H,
Kong J, Ding K, Shen HM, Wu H, et al: Long non-coding RNA linc00673
regulated non-small cell lung cancer proliferation, migration,
invasion and epithelial mesenchymal transition by sponging
miR-150-5p. Mol Cancer. 16:1182017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen QF, Kong JL, Zou SC, Gao H, Wang F,
Qin SM and Wang W: lncRNA LINC00342 regulated cell growth and
metastasis in non-small cell lung cancer via targeting miR-203a-3p.
Eur Rev Med Pharmacol Sci. 23:7408–7418. 2019.PubMed/NCBI
|
|
21
|
Ombrato L and Malanchi I: The EMT
universe: Space between cancer cell dissemination and metastasis
initiation. Crit Rev Oncog. 19:349–361. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qian B, Wang X, Mao C, Jiang Y, Shi Y,
Chen L, Liu S, Wang B, Pan S, Tao Y, et al: Long non-coding RNA
linc01433 promotes migration and invasion in non-small cell lung
cancer. Thorac Cancer. 9:589–597. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen JH, Zhou LY, Xu S, Zheng YL, Wan YF
and Hu CP: Overexpression of lncRNA HOXA11-AS promotes cell
epithelial-mesenchymal transition by repressing miR-200b in
non-small cell lung cancer. Cancer Cell Int. 17:642017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gao YP, Li Y, Li HJ and Zhao B: lncRNA
NBR2 inhibits EMT progression by regulating Notch1 pathway in
NSCLC. Eur Rev Med Pharmacol Sci. 23:7950–7958. 2019.PubMed/NCBI
|
|
25
|
Yu DJ, Li YH and Zhong M: lncRNA
FBXL19-AS1 promotes proliferation and metastasis via regulating
epithelial-mesenchymal transition in non-small cell lung cancer.
Eur Rev Med Pharmacol Sci. 23:4800–4806. 2019.PubMed/NCBI
|
|
26
|
Ji X, Tao R, Sun LY, Xu XL and Ling W:
Down-regulation of long non-coding RNA DUXAP8 suppresses
proliferation, metastasis and EMT by modulating miR-498 through
TRIM44-mediated AKT/mTOR pathway in non-small-cell lung cancer. Eur
Rev Med Pharmacol Sci. 24:3152–3165. 2020.PubMed/NCBI
|
|
27
|
Pan JC, Meng XD and Gong ZH: The Role of
lncRNA as Competitive Endogenous RNA in Non-Small Cell Lung
Cancers. Prog. Biochem. Biophys. 45:1126–1135. 2018.
|
|
28
|
Jia YC, Wang JY, Liu YY, Li B, Guo H and
Zang AM: lncRNA MAFG-AS1 facilitates the migration and invasion of
NSCLC cell via sponging miR-339-5p from MMP15. Cell Biol Int.
43:384–393. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu YJ, Zhou HG, Chen LH, Qu DC, Wang CJ,
Xia ZY and Zheng JH: miR-32-5p regulates the proliferation and
metastasis of cervical cancer cells by targeting HOXB8. Eur Rev Med
Pharmacol Sci. 23:87–95. 2019.PubMed/NCBI
|
|
30
|
Liang H, Tang Y, Zhang H and Zhang C:
miR-32-5p regulates radiosensitization, migration and invasion of
colorectal cancer cells by targeting TOB1 gene. OncoTargets Ther.
12:9651–9661. 2019. View Article : Google Scholar
|
|
31
|
Wang M, Sun Y, Xu J, Lu J, Wang K, Yang
DR, Yang G, Li G and Chang C: Preclinical studies using miR-32-5p
to suppress clear cell renal cell carcinoma metastasis via altering
the miR-32-5p/TR4/HGF/Met signaling. Int J Cancer. 143:100–112.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ye T, Zhang N, Wu W, Yang B, Wang J, Huang
W and Tang D: SNHG14 promotes the tumorigenesis and metastasis of
colorectal cancer through miR-32-5p/SKIL axis. In Vitro Cell Dev
Biol Anim. 55:812–820. 2019. View Article : Google Scholar : PubMed/NCBI
|