Introduction
Neuropathic pain is the most frequently reported
form of chronic pain and seriously affects the quality of life of
patients (1). However, there are
few effective methods for treating neuropathic pain, hence the
requirement for pharmacological research into new analgesics
(2). Neuropathic pain arises from
primary lesions or dysfunctions of the peripheral and central
nervous systems, which often result in a long-lasting excitability
of spinal dorsal horn neurons (central sensitization) (3,4). In
general, hyperalgesia, allodynia and spontaneous pain are the
commonest symptoms of neuropathic pain (5). Chronic constriction injury (CCI) of
the sciatic nerve is a classic model used to study neuropathic pain
over a long period (6); it can
imitate the clinical neuropathic pain conditions resulting from
lumbar disk herniation or chronic entrapment of the peripheric
nerve (7). In recent years, ozone
has been widely used to relieve chronic pain in clinical practice
(8). For example, multicentre
clinical trials have indicated that ozone therapy can generate
valid effects and low morbidity rates when applied percutaneously
for the treatment of chronic low back pain (9–11).
Accumulating research has also indicated that ozone can treat
prolapse of the lumbar intervertebral disc or failed back surgery
syndrome effectively (12–15). Despite the wide use of ozone in the
treatment of clinical pain conditions, it is necessary to pay
attention to its potential toxicity due to its strong oxidizing
capacity (16). It has been
verified that ozone has useful antiseptic, antiviral and
disinfectant effects (17).
Previous studies have indicated that ozone may provide long-lasting
anti-inflammatory effects and reduce inflammation through the
immunomodulation and activation of cellular metabolism (18) and that it could also activate the
endogenous antioxidant system in endotoxic and septic shock models
(19). Although multiple studies
have been carried out in the past several years, the precise
analgesic mechanisms of ozone have yet to be elucidated (17–19).
Therefore, further research is warranted to illuminate the exact
biological mechanisms of ozone and to avoid potential detrimental
effects.
Kainate receptors are a type of ionotropic glutamate
receptor and serve important roles in mediating excitatory synapse
transmission in the central nervous system (20). These receptors have been implicated
in the pathogenesis of a number of neurological diseases, such as
stroke, Alzheimer's disease, epilepsy and neuropathic pain
(21). To date, five types of
kainate receptor subunits have been confirmed: Glutamate receptor
(GluR)5, GluR6, GluR7, KA1 and KA2 (22). Accumulating evidence has indicated
that GluR6 is expressed in the superficial dorsal horn and is
involved in nociception transmission (23). Our previous study demonstrated that
GluR6 is associated with visceral pain (24). However, few studies have
investigated the role of GluR6 in neuropathic pain and the
analgesic effect of ozone.
As a crucial nuclear transcription factor, the
NF-κB/p65 heterodimer has been demonstrated to be related to the
development and maintenance of neuropathic pain. This transcription
factor is expressed widely in the central nervous system (25,26).
It is natural to consider the question of whether
GluR6 is involved in neuropathic pain and the analgesic effect of
ozone. The present study investigated the role of GluR6 in
neuropathic pain and whether intrathecal injection of ozone could
alleviate pain through the GluR6-NF-κB/p65 signalling pathway in
the spinal cord of CCI rats.
Materials and methods
Animals
A total of 108 adult male Sprague-Dawley rats
(weight, 200–250 g; age, 8–10 weeks) from Experimental Animal
Centre, Shandong University, China) were used for the present
study. The rats were housed 4–5 per cage on a 12-h light/dark cycle
with ad libitum access to food and water and kept under
controlled environmental conditions (temperature, 23–25°C;
humidity, 60–70%). The rats were acclimated to the circumstances
for at least 5 days before the experiments. The experimental
surgeries were accomplished under anesthesia with sodium
pentobarbital (40 mg/kg, i.p.). All animal studies were approved by
the Ethics Committee of Clinical Medical College at Shandong
University (China). The experimental procedures were carried out in
accordance with the National Institute of Health Guide for the Care
and Use of Laboratory Animals (27), and efforts were made to minimize the
pain and discomfort of rats.
CCI of the sciatic nerve model
Neuropathic pain was induced by CCI of the sciatic
nerve, as described previously (28). In brief, rats were anaesthetized
with sodium pentobarbital (40 mg/kg, i.p.), then the left sciatic
nerve was bluntly dissected at mid-thigh level proximal to the
sciatic trifurcation without stretching the nerve structures and
the connective tissue was freed. Later, the sciatic nerve was
ligated loosely (4-0 chromic gut) with four ties (1 mm interval).
After slight shrinkage of the left posterior limb was observed, the
muscles and skin were sutured. Rats in the sham group were also
subjected to identical surgery but without nerve ligation.
Intrathecal (i.t.) implantation of
catheter
Rats were implanted with catheters intrathecally for
administration of drugs. In brief, rats were first anaesthetized
with sodium pentobarbital (40 mg/kg, i.p.). After blunt separation
of the occipital muscles, the cisternal membrane was exposed. A
polyethylene catheter (PE-10, 7.0–8.0 cm in length) was inserted
into the subarachnoid space through an incision in the cisterna
magna, and the cannula was advanced 7.0–7.5 cm caudally to the
level of lumbar enlargement in the spinal cord. After rats
recovered from anesthesia, 10 µl lidocaine was injected through the
catheter to confirm the correct catheterization site at the end of
the experiment. The catheter was verified as being correctly
implanted if paralysis and dragging of bilateral hind limbs
appeared within 30 sec after the injection. Rats with obvious motor
impairments were excluded from the experiment (29,30).
After recovering from the surgery for 5–7 day, the rats were used
for the following experiments.
GluR6 siRNAs and ozone injection
The GluR6 siRNAs (0.066 nmol dissolved in 10 µl
diluent; cat. no. sc-270102; Santa Cruz Biotechnology, Inc.) were
injected intrathecally using a microinjection syringe once every
day following CCI, over a period of 20 sec and followed by a flush
with 5 µl normal saline. The same dosages of negative GluR6 siRNAs
and vehicle were used as controls (30). Ozone at different concentrations
(10, 20, 30 µg/ml; 20 µl), generated by a medical ozone generator
(cat. no. CHY-31; Yuehua Co.), was injected intrathecally over 20
sec on day 7 after CCI through polyethylene catheters. The same
volume of oxygen was used as a control (31).
Assessment of neuropathic pain
behaviours
Mechanical allodynia was measured by the Von Frey
filaments test. In brief, after habituation for 30 min, the plantar
surface of each left hind paw of the rat was stimulated with a
sharp, cylindrical filament provided by an Electro Von Frey
(American Semmes-Weinstein Monofilaments Inc.), and the incidence
of foot withdrawal in response to mechanical stimulation was
recorded. The test was performed according to previously reported
procedures (32). The threshold of
mechanical withdrawal for each rat was obtained to assess
mechanical allodynia.
Thermal hyperalgesia was measured by the hot plate
test. In brief, rats were placed on a smooth glass floor of a
plastic cage. After habituation for 30 min, the plantar surface of
each left hind paw of the rat was stimulated with a heat source
provided by a Plantar Test Apparatus (Series 8 Model 390; IITC Life
Science Inc.) and the heat source was shut off when hind paw
movement occurred, or after 25 sec, to prevent tissue damage. The
intensity of the heat source (60A) was modulated to maintain the
paw withdrawal latency at 13±2 sec in normal rats. The thermal
stimuli were carried out 5 times to the same hind paw at 7-min
intervals, and the averaged seconds were obtained as the thermal
paw withdrawal latency to assess thermal hyperalgesia (20).
Sample preparation
The rats of each group were anesthetized with sodium
pentobarbital (40 mg/kg, i.p.) and decapitated immediately after
pain behaviour assessment. The spinal cord segments (L3-6) were
carefully isolated from each animal and snap frozen in liquid
nitrogen. The isolated spinal cord segments were homogenized with
ice-cold homogenization buffer that contained 50 mM
3-(N-morpholino) propanesulfonic acid (Sigma-Aldrich; Merck KGaA;
pH 7.4), 100 mM KCl, 1 mM Na3VO4
(Sigma-Aldrich; Merck KGaA), 20 mM sodium pyrophosphate, 1 mM EDTA,
1 mM p-nitrophenyl phosphate, 0.5 mM MgCl2, 1 mM
benzamidine, 1 mM phenylmethylsulfonyl fluoride, 0.2 mM
dithiothreitol, 320 mM sucrose, 1 mM EGTA, 50 mM NaF, 20 mM
β-phosphoglycerol, and 5 µg/ml pepstatin A, leupeptin and
aprotinin. The homogenates were then centrifuged at 4°C for 10 min
at 800 × g. The supernatants (the cytosol portion) were collected,
and the protein concentrations were confirmed with Lowry's method
(33). After that, the samples were
stored at −80°C and thawed only before use.
Reverse transcription-quantitative
(RT-q)PCR
RT-qPCR was used to measure GluR6 and NF-κB/p65
mRNAs. β-actin served as the internal control. The sequences of the
PCR primers were as follows: GluR6, forward,
5′-TTCCTGAATCCTCTCTCCCCT−3′ and reverse,
5′-ACCTCGCAATCACAAACAGTACA-3′; NF-κB/p65, forward,
5′-AGAGCAACGATTCCACCAA-3′ and reverse, 5′-GCAGTCTTTTCCCACCAGC-3′;
β-actin, forward, 5′-TACAACCTCCTTGCAGCTCC-3′ and reverse,
5′-GGATCTTCATGAGGTAGTCAGTC-3′.
First, the total RNAs were separated from
homogenized samples using TRIzol® reagent (Thermo Fisher
Scientific, Inc.). Then, the total RNAs were inversely transcribed
into the cDNAs using the MML-V reverse transcriptase kit
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. Then, the RT-qPCR master mix kit
(Fermentas; Thermo Fisher Scientific, Inc.) was used to detect
GluR6 and NF-κB/p65 mRNAs with the PCR system (FTC2000q PCR System;
Conrem). The following thermocycling conditions were used for the
qPCR: Initial denaturation for 15 min at 95°C, followed by 35
cycles of denaturation at 95°C for 15 sec, annealing at 55°C for 30
sec and elongation at 72°C for 30 sec. Finally, GluR6 and NF-κB/p65
mRNA levels were quantified with the relative quantification
2−∆∆Cq method (34).
Western blotting
Total protein was extracted from tissues on ice
using RIPA lysis buffer (Beyotime Institute of Biotechnology)
supplemented with protease and phosphatase inhibitors. Total
protein was quantified using a BCA protein assay kit, and 80 µg
protein per lane was separated by 12% SDS-PAGE and
electrotransferred onto a PVDF membrane (Amersham; Cytiva). After
blocking in TBS-0.05% Tween-20 (TBST) for 1.5 h at room temperature
and 3% BSA (Beijing Solarbio Science & Technology Co., Ltd.),
the membranes were incubated with the following primary antibodies
in TBST containing 3% BSA overnight at 4°C: Anti-GluR6 (1:1,000;
cat. no. EPR6307; Abcam), anti-NF-κB (1:1,000; cat. no. ab16502;
Abcam) and anti-β-actin (1:5,000; cat. no. ab8227; Abcam).
Following the primary antibody incubation, the membranes were
washed and incubated a goat anti-rabbit IgG secondary antibody
(1:20,000; cat. no. ab97051; Abcam) for 2 h in TBST. After being
visualized using nitro blue
tetrazolium/5-bromo-4-chloro-3-indolyl-phosphate color substrate
(Promega Corporation), the bands on the membranes were scanned and
analysed with an image analyzer and LabWorks software (version 4.5,
UVP). β-actin served as the internal control.
ELISA
ELISA was used to detect the protein levels of
TNF-α, IL-1β, and IL-6 in the spinal cord. Briefly, the spinal cord
tissues were pooled and homogenized in ice-cold PBS solution (pH
7.4, containing 1 mM phenylmethylsulfonyl fluoride, 1% Triton-X100,
1 g/ml leupeptin, and 10 g/ml aprotinin). Following centrifugation
at 10,000 × g at 4°C for 30 min, the supernatants were aliquoted
and stored at −80°C for future protein quantification. Cytokine
protein levels were analysed by rat TNF-α (cat. no. SRTA00), IL-1β
(cat. no. SRLB00) and IL-6 (cat. no. SR6000B) ELISA kits (R&D
Systems, Inc.) according to the manufacturer's protocols.
Statistical analyses
Data are expressed as the mean ± standard deviation.
SPSS 23.0 (IBM Corp.) was used to perform the statistical analyses
in the present study. Comparisons were performed by one-way
analysis of variance followed by Tukey's test. P<0.05 was
considered to indicate a statistically significant difference. For
Fig. 1, in combination with data
characteristics, the indicators of pain behavior scores, time, and
groups were standardized. The results demonstrated that the pain
behavior scores of CCI group were statistically significant
compared with sham group at d3, d7 and d14 time points
(P<0.01).
Results
Changes in pain behaviours and GluR6
expression in the spinal cord of rats following CCI at different
time points
In the present study, rats treated with CCI of the
sciatic nerve demonstrated obvious mechanical allodynia and thermal
hyperalgesia behaviours, and the pain behaviours were mostly
obvious on day 7 after CCI, which was in contrast to the sham group
(Fig. 1). To survey the change in
spinal GluR6 expression following CCI, the spinal cords of rats at
different time points were obtained and homogenized. GluR6 protein
expression was then measured by immunoblotting and GluR6 mRNA was
measured by RT-qPCR analysis. As shown in Fig. 2, the expression of GluR6 began to
increase on day 3 and reached its peak on day 7 after CCI.
Pre-intrathecal injection of GluR6
siRNAs attenuates CCI-induced pain behaviours and reduces spinal
GluR6 expression in rats on day 7 after CCI
To further ascertain the role of GluR6 in
CCI-induced neuropathic pain, we used GluR6 siRNAs to knock down
GluR6 expression through intrathecal injection once per day
following CCI. The alteration of GluR6 protein expression was
detected by immunoblotting, and GluR6 mRNA was measured by RT-qPCR
analysis. As shown in Figs. 3 and
4, pre-intrathecal injection of
GluR6 siRNAs significantly inhibited mechanical allodynia and
thermal hyperalgesia behaviours and decreased the expression of
spinal GluR6 in rats, which was in contrast to the CCI rats without
pretreatment with GluR6 siRNAs. Meanwhile, pre-intrathecal
injection of negative GluR6 siRNAs or vehicle had no effect on the
changes in pain behaviours or GluR6 expression in rats.
Pre-intrathecal injection of GluR6
siRNAs reduces spinal NF-κB/p65 expression in rats on day 7 after
CCI
The change in NF-κB/p65 protein expression in the
spinal cord of rats was measured by immunoblotting, and NF-κB/p65
mRNA was measured by RT-qPCR analysis. As shown in Fig. 5, the expression of NF-κB/p65 protein
and mRNA decreased significantly in the CCI group with pretreatment
with GluR6 siRNAs, which was in contrast to the CCI group without
pretreatment with GluR6 siRNAs.
Pre-intrathecal injection of GluR6
siRNAs reduces spinal IL-1β, IL-6 and TNF-α protein expression in
rats on day 7 after CCI
The changes in spinal IL-1β, IL-6 and TNF-α protein
expression in rats were measured by ELISA. As shown in Fig. 5, the expression of IL-1β, IL-6 and
TNF-α protein decreased significantly in the CCI group with
pretreatment of GluR6 siRNAs, which was in contrast to the CCI
group without pretreatment with GluR6 siRNAs.
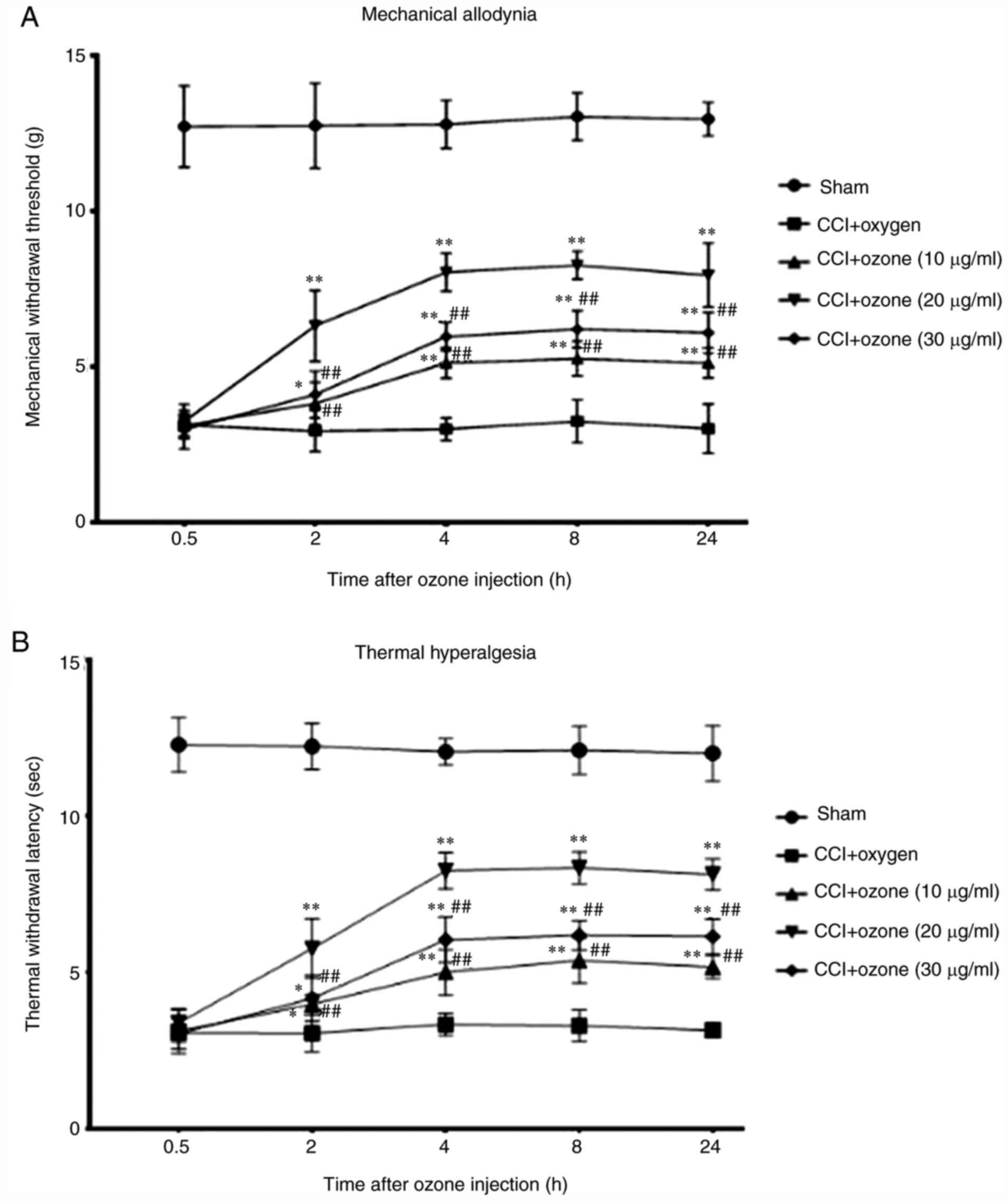
Intrathecal injection of ozone
attenuates CCI-induced neuropathic pain behaviours in rats
In the present study, ozone at different
concentrations was administered intrathecally on day 7 after CCI in
rats. Mechanical allodynia and thermal hyperalgesia of rats were
measured. As shown in Fig. 6,
intrathecal injection of ozone clearly attenuated mechanical
allodynia and thermal hyperalgesia induced by CCI in rats, in
contrast to the CCI group with intrathecal injection of oxygen, and
ozone at 20 µg/ml had the most evident effect.
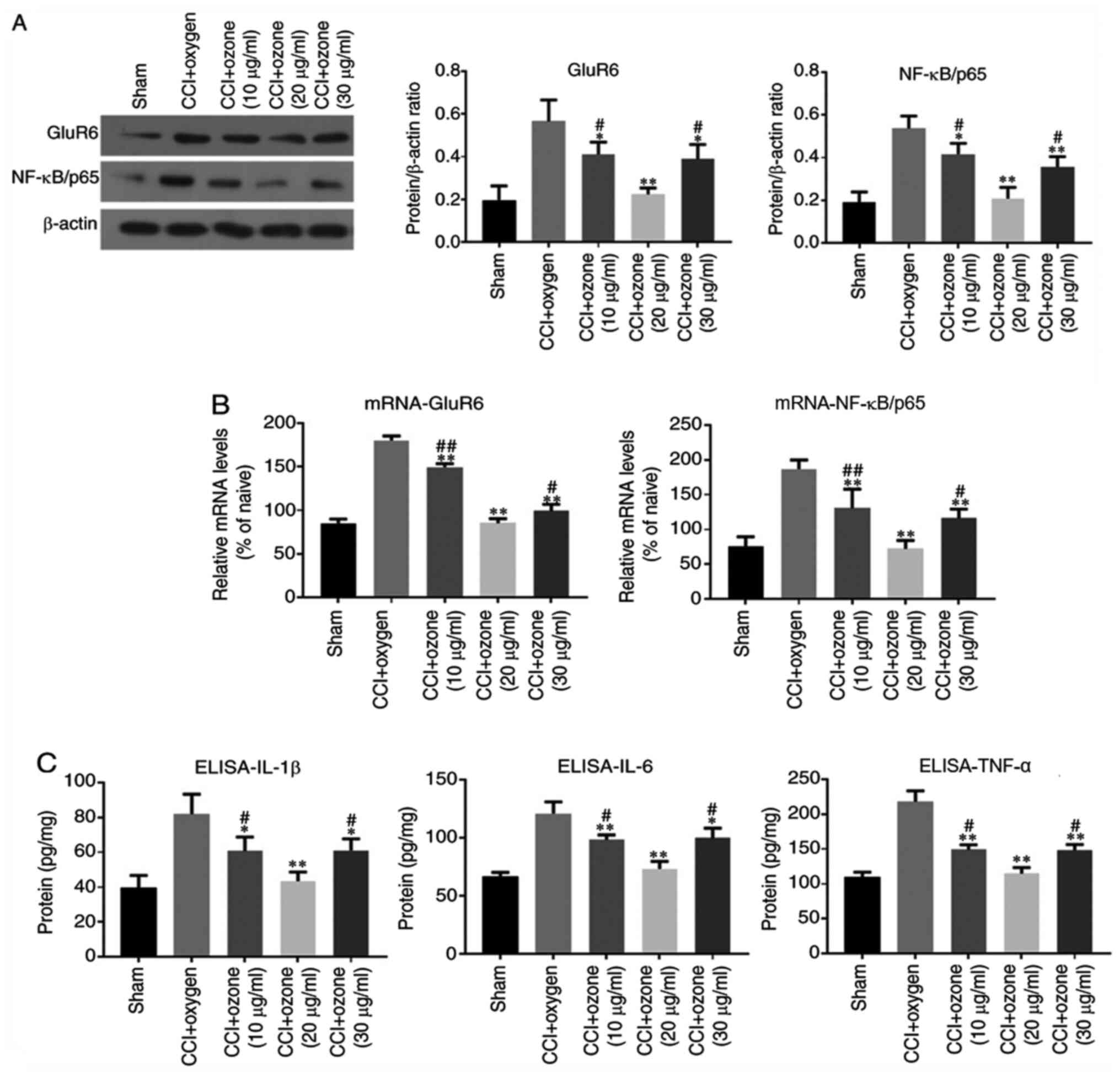
Intrathecal injection of ozone
decreases GluR6, NF-κB/p65, IL-1β, IL-6 and TNF-α expression in the
spinal cord of rats
To further investigate the mechanisms underlying the
pain-relieving effect of ozone, spinal GluR6 and NF-κB/p65
expression levels were measured by immunoblotting and RT-qPCR
assays. Spinal IL-1β, IL-6 and TNF-α protein expression was
measured by ELISA. As shown in Fig.
7, intrathecal injection of ozone clearly decreased GluR6,
NF-κB/p65, IL-1β, IL-6 and TNF-α expression in the spinal cord of
rats, which was in contrast to the CCI group with intrathecal
injection of oxygen, and ozone at 20 µg/ml had the most evident
effect.
Discussion
The present study demonstrated for the first time,
to the best of the authors' knowledge, that GluR6 participated in
the neuropathic pain induced by CCI and that intrathecal injection
of ozone suppressed the GluR6-NF-κB/p65 pathway to alleviate
neuropathic pain in the spinal cord of CCI rats. In recent years,
ozone has been widely used in clinical practice and is suggested to
be effective in treating a number of chronic pain diseases
(35,36). Nevertheless, in contrast to the
effective use of ozone in clinical practice, it is necessary to pay
close attention to its side effects due to its powerful oxidizing
capacity (37). It has been
reported in our previous study that ozone at 60 µg/ml can damage
astrocytes in vitro, while ozone of 20 µg/ml or 40 µg/ml has
no damaging effect (38). In
addition, ozone at a high-concentration (>40 µg/ml) can induce
neurotoxicity in spinal cord neurons due to ER Ca2+
release and CaMKII/MAPK signalling pathway activation, while ozone
at low concentration (<40 µg/ml) exhibits no neurotoxic effect
(39). Furthermore, ozone can
reduce apoptosis of nerve roots by blocking NF-κB signalling
pathway in a radiculoneuritis rat model (40). Wei et al (41) found that ozone can inhibit the
necrosis of the endometrial epithelial cells and reduce expression
of inflammatory factors, including IL-6 and TNF-α, in rats with
pelvic inflammatory disease. Re et al (42) reported that the analgesic effect of
ozone may involve two different mechanisms; a short-term mechanism
that may be associated with the direct oxidation of biomolecules,
and a long-term mechanism that may involve the activation of
antioxidant pathways. Although a number of studies have been
performed (38–42), the exact biological mechanisms
governing the efficacy of ozone remain to be elucidated.
In the present study, it was observed that rats with
CCI demonstrated obvious pain behaviours, which started on day 3,
reached their peak on day 7 and could persist for ≥14 days after
CCI. Meanwhile, intrathecal injection of low-concentration ozone
could alleviate CCI-induced neuropathic pain on day 7 after CCI and
ozone at 20 µg/ml had the most evident effect.
As one subunit of kainate receptors, GluR6 serves a
crucial role in neuronal cell death induced by cerebral
ischaemia/reperfusion, as demonstrated in previous studies
(33,43–45).
It has also been shown that GluR6 is expressed in the superficial
dorsal horn in adult rats and participates in nociceptive
transmission (23,46). However, thus far, the role of GluR6
in neuropathic pain has not been studied. Therefore, the present
study investigated whether GluR6 participated in the neuropathic
pain induced by CCI. It was also investigated whether intrathecal
injection of ozone could alleviate pain through the GluR6 pathway
in the spinal cord of CCI rats. In the present study, pain
behaviours and GluR6 expression were examined. It was demonstrated
that the expression of GluR6 in the spinal cord of rats increased
on day 3 and reached a peak on day 7 after CCI, which was
consistent with the alteration of pain behaviours. As the pain
behaviours of rats and the expression of GluR6 were significantly
changed on day 7 after CCI, the time point of day 7 was selected to
investigate the role of GluR6 in neuropathic pain. As demonstrated
in the present study, pre-intrathecal injection of GluR6 siRNAs not
only markedly inhibited the expression of GluR6 but also clearly
alleviated the pain behaviours induced by CCI, which demonstrated
that GluR6 might serve a crucial role in the neuropathic pain
induced by CCI.
The NF-κB/p65 heterodimer is a crucial transcription
factor in the central nervous system, which has been demonstrated
to be associated with the initiation and maintenance of
inflammation and neuropathic pain (26,31,47,48).
NF-κB/p65 is expressed in spinal dorsal horn neurons and has been
revealed to be activated by peripheral nerve injury or inflammation
(1,25,26,30,31).
NF-κB/p65 can interact with pro-inflammatory factors (IL-1β, IL-6
and TNF-α), which may amplify the neuroinflammatory responses and
give rise to central sensitization and hyperalgesia (25). A previous study by our group
demonstrated that suppression of spinal NF-κB/p65 expression could
clearly relieve mechanical allodynia and thermal hyperalgesia in
CCI rats (30). Furthermore, spinal
NF-κB/p65 activation in CCI rats has been reported to be inhibited
by MK-801, an NMDA receptor antagonist, which indicates that the
NMDA receptor may activate the NF-κB/p65 pathway (49). The present study investigated
whether GluR6 could activate the NF-κB/p65 pathway. In accordance
with the results above, the current study demonstrated that
peripheral nerve constriction injury could significantly activate
the expression of spinal NF-κB/p65, IL-1β, IL-6 and TNF-α in CCI
rats, and the alterations could be inhibited predominantly by
knockdown of GluR6 with intrathecal injection of GluR6 siRNAs,
which indicated that GluR6 could activate the spinal NF-κB/p65
pathway in rats with CCI.
Our previous study demonstrated that intrathecal
injection of low-concentration ozone could attenuate radiculitis in
rats with non-compressive lumbar disc herniation, probably through
the PDE2A-cGMP/cAMP-NF-κB/p65 pathway (31). The present study further
investigated whether intrathecal injection of low-concentration
ozone could alleviate neuropathic pain induced by CCI and
mechanism. It has been shown that oxygen has no analgesic effect on
the neuropathic pain induced by CCI (50), and in our preliminary experiment, it
was found that there was no statistical significance in pain
behaviors between CCI group and CCI + oxygen group (data not
shown), so the CCI + oxygen group was selected as the control
group. As shown in the present study, intrathecal treatment with
low-concentration ozone could alleviate mechanical allodynia and
thermal hyperalgesia and downregulate the expression of GluR6,
IL-1β, IL-6, TNF-α and NF-κB/p65 in the spinal cord of CCI rats. In
addition, ozone at 20 µg/ml had the most evident effect. Therefore,
it was hypothesized that the analgesic effects of ozone might be
mediated, at least in part, through the GluR6-NF-κB/p65 pathway.
However, further research is warranted to determine whether there
is an association between GluR6 and PDE2A or cGMP/cAMP.
In conclusion, the present study demonstrated that
GluR6 served a crucial role in the neuropathic pain induced by CCI,
which might serve as a potential new analgesic target in the
development of new therapies for neuropathic pain. Furthermore,
intrathecal injection of low-concentration ozone could attenuate
neuropathic pain and might downregulate the GluR6-NF-κB/p65 pathway
in CCI rats.
The present study had a number of limitations. The
lack of immunohistochemistry, the sample size for behavioral
assessment and the lack of determination of direct evidence that
GluR6 upregulation is associated with alleviation of neuropathic
pain induced by ozone are limitations of the study, which will be
addressed in the future.
Acknowledgements
The authors thank Professor Dongsheng Pei (Research
Center for Biochemistry and Molecular Biology, Xuzhou Medical
University) for his critical proposal of the study. The authors
also thank Dr Man Xu (Key Laboratory of Anesthesiology of Jiangsu
Province, Xuzhou Medical University) and Dr Xinxin Wan (Key
Laboratory of Anesthesiology of Jiangsu Province, Xuzhou Medical
University) for assisting in the experiment.
Funding
The present study was supported by grants from the
national natural Science Foundation of china (grant nos. 81271346,
81771199 and 81772443).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
WZ, LZ, TS and ZF designed the study. WZ and FW
performed the experiments and analyzed the data. WZ wrote the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Shandong Provincial Hospital is affiliated to
Shandong University. The experiments in the present study were
carried out in the laboratory at Shandong University. All animal
procedures were approved by the Ethics Committee of Clinical
Medical College at Shandong University and performed in compliance
with the guidelines set by the Ethics Committee for the Care and
Use of Laboratory Animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liu JC, Xue DF, Wang XQ, Ai DB and Qin PJ:
MiR-101 relates to chronic peripheral neuropathic pain through
targeting KPNB1 and regulating NF-κB signaling. Kaohsiung J Med
Sci. 35:139–145. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Baron R, Binder A and Wasner G:
Neuropathic pain: Diagnosis, pathophysiological mechanisms, and
treatment. Lancet Neurol. 9:807–819. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zimmermann M: Pathobiology of neuropathic
pain. Eur J Pharmacol. 429:23–37. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ma X, Wang H, Song T, Wang W and Zhang Z:
lncRNA MALAT1 contributes to neuropathic pain development through
regulating miR-129-5p/HMGB1 axis in a rat model of chronic
constriction injury. Int J Neurosci. 130:1215–1224. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Baron R: Mechanisms of disease:
Neuropathic pain-a clinical perspective. Nat Clin Pract Neurol.
2:95–106. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pu S, Li S, Xu Y, Wu J, Lv Y and Du D:
Role of receptor-interacting protein 1/receptor-interacting protein
3 in inflammation and necrosis following chronic constriction
injury of the sciatic nerve. Neuroreport. 29:1373–1378. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu L, Liu Y, Sun Y, Li H, Mi W and Jiang
Y: Analgesic effects of TLR4/NF-κB signaling pathway inhibition on
chronic neuropathic pain in rats following chronic constriction
injury of the sciatic nerve. Biomed Pharmacother. 107:526–533.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Al Habashneh R, Alsalman W and Khader Y:
Ozone as an adjunct to conventional nonsurgical therapy in chronic
periodontitis: A randomized controlled clinical trial. J
Periodontal Res. 50:37–43. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Magalhaes FN, Dotta L, Sasse A, Teixera MJ
and Fonoff ET: Ozone therapy as a treatment for low back pain
secondary to herniated disc: A systematic review and meta-analysis
of randomized controlled trials. Pain Physician. 15:E115–E129.
2012.PubMed/NCBI
|
|
10
|
Biazzo A, Corriero AS and Confalonieri N:
Intramuscular oxygen-ozone therapy in the treatment of low back
pain. Acta Biomed. 89:41–46. 2018.PubMed/NCBI
|
|
11
|
Costa T, Linhares D, Ribeiro da Silva M
and Neves N: Ozone therapy for low back pain, A systematic review.
Acta Reumatol Port. 43:172–181. 2018.PubMed/NCBI
|
|
12
|
Crockett MT, Moynagh M, Long N, Kilcoyne
A, Dicker P, Synnott K and Eustace SJ: Ozone-augmented percutaneous
discectomy: A novel treatment option for refractory discogenic
sciatica. Clin Radiol. 69:1280–1286. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ozcan S, Muz A, Yildiz Altun A and Onal
SA: Intradiscal ozone therapy for lumbar disc herniation. Cell Mol
Biol. 64:52–55. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Magalhães FN, Soares SC, Torres JM,
Ungaretti A, Cacciacarro MF, Teixeira MJ and Fonoff ET: Effects of
ozone applied by spinal endoscopy in patients with chronic pain
related to failed back surgery syndrome: A pilot study.
Neuropsychiatr Dis Treat. 9:1759–1766. 2013.PubMed/NCBI
|
|
15
|
Barbosa DC, Ângelos JSD, Macena GMJ,
Magalhães FNO and Fonoff ET: Effects of ozone on the pain and
disability in patients with failed back surgery syndrome. Rev Assoc
Med Bras. 63:355–360. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mauro RD, Cantarella G, Bernardini R, Rosa
MD, Barbagallo I, Distefano A, Longhitano L, Vicario N, Nicolosi D,
Lazzarino G, et al: The biochemical and pharmacological properties
of ozone: The smell of protection in acute and chronic diseases.
Int J Mol Sci. 20:6342019. View Article : Google Scholar
|
|
17
|
Fuccio C, Luongo C, Capodanno P, Giordano
C, Scafuro MA, Siniscalco D, Lettieri B, Rossi F, Maione S and
Berrino L: A single subcutaneous injection of ozone prevents
allodynia and decreases the over-expression of proinflammatory
caspases in the orbito-frontal cortex of neuropathic mice. Eur J
Pharmacol. 603:42–49. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Holz O, Tal-Singer R, Kanniess F, Simpson
KJ, Gibson A, Vessey RS, Janicki S, Magnussen H, Jörres RA and
Richter K: Validation of the human ozone challenge model as a tool
for assessing anti-inflammatory drugs in early development. J Clin
Pharmacol. 45:498–503. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zamora ZB, Borrego A, Lopez OY, Delgado R,
González R, Menéndez S, Hernández F and Schulz S: Effects of ozone
oxidative preconditioning on TNF-alpha release and
antioxidant-prooxidant intracellular balance in mice during
endotoxic shock. Mediators Inflamm. 2005:16–22. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lu Y, Sun YN, Wu X, Sun Q, Liu FY, Xing GG
and Wan Y: Role of
alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA)
receptor subunit GluR1 in spinal dorsal horn in inflammatory
nociception and neuropathic nociception in rat. Brain Res.
1200:19–26. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Marshall J, Blair LA and Singer JD:
BTB-Kelch proteins and ubiquitination of kainate receptors. Adv Exp
Med Biol. 717:115–125. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nasu-Nishimura Y, Jaffe H, Isaac JT and
Roche KW: Differential regulation of kainate receptor trafficking
by phosphorylation of distinct sites on GluR6. J Biol Chem.
285:2847–2856. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu LJ, Ko SW and Zhuo M: Kainate receptors
and pain: From dorsal root ganglion to the anterior cingulate
cortex. Curr Pharm Des. 13:1597–1605. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang WG, Zhang LC, Peng ZD and Zeng YM:
Intrathecal injection of GluR6 antisense oligodeoxynucleotides
alleviates acute inflammatory pain of rectum in rats. Neurosci
Bull. 25:319–323. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Luo JG, Zhao XL, Xu WC, Zhao XJ, Wang JN,
Lin XW, Sun T and Fu ZJ: Activation of spinal NF-κB/p65 contributes
to peripheral inflammation and hyperalgesia in rat adjuvant-induced
arthritis. Arthritis Rheumatol. 66:896–906. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ni H, Wang Y, An K, Liu Q, Xu L, Zhu C,
Deng H, He Q, Wang T, Xu M, et al: Crosstalk between NFκB-dependent
astrocytic CXCL1 and neuron CXCR2 plays a role in descending pain
facilitation. J Neuroinflammation. 16:12019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pei WM, Zou Y, Wang WT, Wei L, Zhao Y and
Li L: Tizanidine exerts anti-nociceptive effects in spared nerve
injury model of neuropathic pain through inhibition of TLR4/NF-κB
pathway. Int J Mol Med. 42:3209–3219. 2018.PubMed/NCBI
|
|
28
|
Sun W, Zhang L and Li R: Overexpression of
miR-206 ameliorates chronic constriction injury-induced neuropathic
pain in rats via the MEK/ERK pathway by targeting brain-derived
neurotrophic factor. Neurosci Lett. 646:68–74. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cao JL, He JH, Ding HL and Zeng YM:
Activation of the spinal ERK signaling pathway contributes
naloxone-precipitated withdrawal in morphine-dependent rats. Pain.
118:336–349. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sun T, Luo J, Jia M, Li H, Li K and Fu Z:
Small interfering RNA-mediated knockdown of NF-κBp65 attenuates
neuropathic pain following peripheral nerve injury in rats. Eur J
Pharmacol. 682:79–85. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang J, Wu M, Lin X, Li Y and Fu Z:
Low-concentration oxygen/ozone treatment attenuated radiculitis and
mechanical allodynia via PDE2A-cAMP/cGMP-NF-κB/p65 signaling in
chronic radiculitis rats. Pain Res Manag. 13:51928142018.
|
|
32
|
Liu S, Liu YP, Song WB and Song XJ:
EphrinB-EphB receptor signaling contributes to bone cancer pain via
Toll-like receptor and proinflammatory cytokines in rat spinal
cord. Pain. 154:2823–2835. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wei XE, Zhang FY, Wang K, Zhang QX and
Rong LQ: Fasudil hydrochloride protects neurons in rat hippocampal
CA1 region through inhibiting GluR6-MLK3-JNKs signal pathway. Cell
Biochem Biophys. 70:415–421. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Giurazza F, Guarnieri G, Murphy KJ and
Muto M: Intradiscal O2 O3: Rationale,
injection technique, short- and long-term outcomes for the
treatment of low back pain due to disc herniation. Can Assoc Radiol
J. 68:171–177. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Raeissadat SA, Tabibian E, Rayegani SM,
Dehgolan SR and Ghazani AB: An investigation into the efficacy of
intra-articular ozone (O2-O3) injection in
patients with knee osteoarthritis: A systematic review and
meta-analysis. J Pain Res. 11:2537–2550. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bocci V: Ozone as Janus: This
controversial gas can be either toxic or medically useful.
Mediators Inflamm. 13:3–11. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhou NB, Fu ZJ and Sun T: Effects of
different concentrations of oxygen-ozone on rats' astrocytes in
vitro. Neurosci Lett. 441:178–182. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li Y, Lin X, Zhao X, Xie J, Wang J, Sun T
and Fu Z: Ozone (O3) elicits neurotoxicity in spinal cord neurons
(SCNs) by inducing ER Ca(2+) release and activating the CaMKII/MAPK
signaling pathway. Toxicol Appl Pharmacol. 280:493–501. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wu MY, Xing CY, Wang JN, Li Y, Lin XW and
Fu ZJ: Therapeutic dosage of ozone inhibits autophagy and apoptosis
of nerve roots in a chemically induced radiculoneuritis rat model.
Eur Rev Med Pharmacol Sci. 22:1787–1797. 2018.PubMed/NCBI
|
|
41
|
Wei A, Feng H, Jia XM, Tang H, Liao YY and
Li BR: Ozone therapy ameliorates inflammation and endometrial
injury in rats with pelvic inflammatory disease. Biomed
Pharmacother. 107:1418–1425. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Re L, Sanchez GM and Mawsouf N: Clinical
evidence of ozone interaction with pain mediators. Saudi Med J.
31:1363–1367. 2010.PubMed/NCBI
|
|
43
|
Zhang J, Yan H, Wu YP, Li C and Zhang GY:
Activation of GluR6-containing kainate receptors induces
ubiquitin-dependent Bcl-2 degradation via denitrosylation in the
rat hippocampus after kainate treatment. J Biol Chem.
286:7669–7680. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nishimura YN, Jaffe H, Isaac JT and Roche
KW: Differential regulation of kainate receptor trafficking by
phosphorylation of distinct sites on GluR6. J Biol Chem.
285:2847–2856. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pei DS, Guan QH, Sun YF, Zhang QX, Xu TL
and Zhang GY: Neuroprotective effects of GuR6 antisense
oligodeoxynucleotides on transient brain ischemia/reperfusion
induced neuronal death in rat hippocampal CA1 region. J Neurosci
Res. 82:642–649. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lu CR, Willcockson HH, Phend KD, Lucifora
S, Darstein M, Valtschanoff JG and Rustioni A: Ionotropic glutamate
receptors are expressed in GABAergic terminals in the rat
superficial dorsal horn. J Comp Neurol. 486:169–178. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liu C, Zhang F, Liu H and Wei F: NF-κB
mediated CX3CL1 activation in the dorsal root ganglion contributes
to the maintenance of neuropathic pain induced in adult male
Sprague Dawley rats1. Acta Cir Bras. 33:619–628. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Li Y, Yang Y, Guo J, Guo X, Feng Z and
Zhao X: Spinal NF-κB upregulation contributes to hyperalgesia in a
rat model of advanced osteoarthritis. Mol Pain.
16:17448069209056912020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chou CW, Wong GT, Lim G, Wang S, Irwin MG
and Mao J: Spatiotemporal pattern of concurrent spinal and
supraspinal NF-κB expression after peripheral nerve injury. J Pain.
12:13–21. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lu L, Pan C, Chen L, Hu L, Wang C, Han Y,
Cheng Z, Yang Y and Liu W: AMPK activation by peri-sciatic nerve
administration of ozone attenuates CCI induced neuropathic pain in
rats. J Mol Cell Biol. 9:132–143. 2017. View Article : Google Scholar : PubMed/NCBI
|