Introduction
Ocular neovascularization is a type of
vision-impairment disorder characterized by aberrant or excessive
blood vessel formation (1).
Choroidal neovascularization (CNV) is a complication noted in the
advanced stage of wet-type age-related macular degeneration (AMD),
in the retinal neovascularization of diabetic retinopathy subjects,
in retinopathy of prematurity and in corneal neovascularization
(CrNV) (2). The etiology of the
occurrence of CrNV is diverse and includes inflammation, mechanical
and chemical injuries (3), as well
as hypoxia resulting from improper use of cornea contact lenses
(4). During the process of CrNV,
disturbed homeostasis of avascular conditions in ocular surfaces
may cause persistent and refractory keratitis, and subsequently
increase the risk of corneal graft rejection. In certain serious
cases, CrNV can lead to blindness (5). Although clinical treatment targeting
the vascular endothelial growth factor (VEGF)-A/VEGF receptor (R)2
signaling pathway has been proven to be an effective approach for
wet-type AMD and other ocular neovascular diseases, the side
effects and drug resistance, to some extent, partially limit the
application of anti-VEGF therapies (6). In addition, it has been demonstrated
that multiple other signaling pathways are involved in the
development of neovascularization (7). These signaling pathways may exert
their function by compensating for VEGF-A pro-angiogenic effects in
the process of neovascularization when VEGF-A/VEGFR2 signaling is
blocked. Therefore, these compensating effects may reduce the
sensitivity of anti-VEGF therapy in certain ocular
neovascularization cases (8).
Platelet-derived growth factor (PDGF)-BB is a
canonical mitogenic growth factor that mediates specific functions
in mural cells, such as pericytes or smooth muscle cells (SMCs)
(9). PDGF-BB is mainly produced by
the endothelial tip cells of the sprouting new vessels. PDGF-BB can
recruit pericytes to the sites of tip cells via binding to the
receptor of PDGF receptor (R)-β (10). The activation of PDGF-BB/PDGFR-β
signaling is initiated by the auto phosphorylation of PDGFR-β and
the cascade of intracellular signal transduction is then passed
through multiple pathways. These include the PI3K pathway, which is
important for chemotaxis and actin re-organization, and the ERK MAP
kinase pathway, which is indispensable in the process of cell
proliferation (11,12). Furthermore, the AKT and signal
transducer and activator of transcription 3 pathways are also
involved in PDGF-BB/PDGFR-β signal transduction (13). At present, the role of PDGF-BB in
promoting and stabilizing the maturation of newly formed vessels
has been identified. A previous study that utilized a laser-induced
CNV model reported that PDGFR-β positive cells were initially
located at the site of the Bruch's membrane and were subsequently
recruited to the CNV area (14),
suggesting that PDGF-BB may be involved in the recruitment of
PDGFR-β positive cells during CNV. It has also been reported that
residual or chemotactic cells other than endothelial cells (ECs)
contribute to the elevated expression levels of PDGF-BB (15,16).
By cooperating with VEGF-A and/or other cytokines, PDGF-BB recruits
ECs/endothelial progenitor cells (EPCs) to lesion sites in order to
form tube-like structures (17).
The increased proliferation and migration of
vascular ECs is considered an early stage of angiogenesis. A
previous study conducted on the overexpression of PDGFR-β in
spleen-derived EPCs revealed that the proliferation, migration and
tube formation of these EPCs were enhanced by stimulation with
recombinant PDGF-BB (18). This
finding suggested that the interaction between PDGF-BB and PDGFR-β
contributed to the induction of angiogenesis of EPCs at an early
stage. Accumulating evidence has revealed that infiltrated
macrophages exert an important role in the early stage of
angiogenesis (19). Macrophages can
be recruited by hypoxia or inflammatory cytokines, such as hypoxia
inducible factor-1 subunit α (HIF-1α), C-C motif chemokine receptor
(CCL)3, CCL4, tumor necrosis factor α, interferon γ and
interleukin-6 (20,21). The infiltrated macrophages then
secrete a series of pro-angiogenic cytokines, such as VEGF-A,
PDGF-BB and basic fibroblast growth factor (bFGF) (22). These cytokines exhibit chemotactic
effects and recruit ECs from pre-existing vessels (tip cells),
which in turn increases the proliferation of ECs (stalk cells) for
the formation of new vascular vessels (23). The role of PDGFs that are released
by macrophages has predominantly been studied in fibrotic diseases,
such as pulmonary, liver, kidney and cardiac fibrosis (24,25).
In these pathological conditions, the activated macrophages are the
main source of PDGFs, which promote the proliferation of
mesenchymal cells (MCs) (26). It
is commonly known that PDGFs are also released by ECs and are
involved in modulating the process of angiogenesis (27). It is also well-known that Cobalt
chloride (CoCl2) is a hypoxia-mimic agent that is
capable of stabilizing the expression level of HIF-1α by inhibiting
prolyl hydroxylases (PHD) (28).
CoCl2 can replace Fe2+ with Co2+
on PHD enzymes and block degradation of HIF-1α and hence facilitate
the establishment of hypoxia conditions in vitro (29). The protocol of inducing hypoxic
conditions by CoCl2 is frequently used in related in
vitro cellular assays (30–32).
Besides, there are also some other hypoxia-mimic agents that can
induce and stabilize HIF-1α expression, such as desferrioxamine and
nickel chloride (33). In the
present study, an alkali burn-induced corneal neovascular model was
used to investigate the role of PDGF-BB in CrNV in vivo and
CoCl2 was employed to induce hypoxic conditions in order
to assess the proliferation, migration and tube formation of
vascular ECs in vitro.
Materials and methods
Reagents and antibodies
Human retinal endothelial cells (HRECs) were
purchased from Shanghai Yaji Biological Technologies Inc. DMEM and
FBS were obtained from HyClone (Cytiva). Cobalt chloride
hexahydrate (CoCl2·6H2O) and
lipopolysaccharides (LPS) were provided by Sigma-Aldrich (Merck
KGaA). AG 1296 (cat. no. S8024) was obtained from Selleck
Chemicals. Matrigel Matrix was purchased from BD Biosciences. Cell
Counting Kit-8 (CCK-8) was obtained from Dojindo Molecular
Technologies, Inc. Rat anti-mouse CD31 antibody (cat. no. MEC13.3)
was purchased from BD Pharmingen (BD Biosciences). Alexa Fluor
488-conjugated secondary donkey anti-rat (H+L) antibody (cat. no.
A-21208) and Alexa Fluor 594-conjugated secondary goat anti-rabbit
(H+L) antibody (cat. no. R37117) were purchased from Invitrogen
(Thermo Fisher Scientific, Inc.). Goat anti-human PDGF-BB antibody
(cat. no. ab10845) was purchased from Abcam. Rat anti-mouse F4/80
antibody (cat. no. NB600-404) was from Novus Biologicals, LLC.
Rabbit anti-mouse PDGF-BB antibody (cat. no. abs135848) was
purchased from Absin Bioscience, Inc. The AxyPrep Multisource Total
RNA Miniprep Kit was purchased from Axygen (Corning, Inc.).
PrimeScript RT Master Mix and DRR041A SYBR Premix Ex Taq (Perfect
Real Time) were purchased from Takara Biotechnology Co., Ltd.
Primers were synthesized by Genewiz, Inc.
Induction of CrNV
All animal experiments followed the Guideline for
the Care and Use of Laboratory Animals of the Chinese Medical
Academy (34) and were approved by
the Soochow University Animal Care Committee (Suzhou, China), and
were performed in accordance with the ARVO Statement for the Use of
Animals in Ophthalmic and Vision Research (35). Specific pathogen-free 8-week-old
male C57B/6 (n=130; weighing 20–25 g) were obtained from Shanghai
SLAC Laboratory Animal Co., Ltd. The mice were anesthetized with an
intraperitoneal injection of 250 mg/kg of 1.8% Avertin
(Sigma-Aldrich; Merck KGaA). The left eye was treated topically
with 0.4% Benoxil [Santen Pharmaceutical (China) Co., Ltd.] and 2×2
mm filter paper saturated with 1N NaOH solution was placed on the
center of the cornea for 40 sec. Subsequently, the corneas were
immediately rinsed with balance solution for 15 sec. The central
epithelia of the corneas were scraped with iris restorer and the
layers were immediately treated with ofloxacin eye ointment [Santen
Pharmaceutical (China) Co., Ltd.]. The alkali injured mice were
divided into 2 groups, namely the vehicle group, which contained
mice treated with PBS, and the PDGF-BB antibody group, which
included mice treated with an antibody against PDGF-BB. This
antibody was dissolved in 2% hyaluronic acid at a concentration of
50 µg/ml. Either PBS or anti-PDGF-BB was applied topically onto the
alkali-injured corneas 3 times per day from day 1 to 7 following
alkali injury. Each experiment was repeated at least three times.
Mice were housed in the animal facility under specific
pathogen-free conditions at 23±3°C and 60±10% relative humidity and
in a 12/12 h day/night cycle with regular lab chow and water
available ad libitum. At the indicated time intervals (days 2, 4
and 7 after alkali injury), mice were sacrificed with an
intraperitoneal injection of sodium pentobarbitone (Virbac
Australia) at a dose of 300 mg/kg body weight. Mice whose breathing
and heartbeat had stopped were considered to be dead. The corneas
were removed from the experimental eyes for reverse
transcription-quantitative (RT-q)PCR. The corneas were placed
immediately into RNAlate (Qiagen GmbH) and stored at −86°C until
total RNA extraction. For another series of experiments to quantify
CrNV and the double-color immunofluorescence analysis, the mice
were sacrificed with an intraperitoneal injection of sodium
pentobarbitone at a dose of 300 mg/kg body weight at the indicated
time intervals (days 2, 4 or 14 after alkali injury), and the
corneas were immediately removed from the alkali-injured eyes.
Quantitation of CrNV
Briefly, the clear corneas from the aforementioned
alkali-injured eyes were removed rapidly along the limbus with a
microscissor. A total of three or four radial relaxing incisions of
each cornea were made. The excised corneas from the CrNV assay were
rinsed in PBS and fixed in 100% acetone for 20 min at room
temperature. Following washing and blocking with 2% BSA (Merck
KGaA) in PBS for 1 h at room temperature, the corneas were stained
overnight at 4°C with a rat anti-mouse CD31 antibody (1:100). On
day 2, CD31 was detected with an Alexa Fluor 488-conjugated
secondary donkey anti-rat antibody (1:50) that was incubated for 1
h at room temperature in the dark. The corneas were transferred to
slides, covered with fluorescent mounting medium (Dako; Agilent
Technologies, Inc.) and stored at 4°C in the dark. The stained
whole mounts were analyzed with a fluorescence microscope (MZ16;
Leica Microsystems GmbH). Each whole mount image was captured at
×25 magnification. The areas covered with neovascular tubes were
determined with ImageJ software (available at http://rsb.info.nih.gov/ij/index.html),
version 1.62 (National Institutes of Health). The mean vascularized
area of the control whole mounts was defined as 100%; vascularized
areas were then related to this value (vessel ratio).
RT-qPCR
Total RNA from the corneas and/or HRECs was isolated
using the AxyPrep miniprep kit and cDNA was synthesized with the
PrimeScript™ RT Master Mix (Takara Biotechnology Co., Ltd.)
according to the manufacturer's protocols. The mRNAs encoding
PDGF-BB, PDGFR-β, VEGF-A, matrix metallopeptidase
(MMP)−2, MMP-9, thrombospondin (TSP)−1,
TSP-2, a disintegrin and metalloproteinase with thrombospondin
motifs (ADAMTS)−1 and ADAMTS-2 were amplified
using the appropriate primers. The sequences of the PCR primer
pairs are listed in Table I.
DRR041A SYBR Premix Ex Taq (Perfect Real-Time) was used for qPCR.
The assay was performed using an iCycler iQ™ Multi-Color Real Time
PCR detection system (Bio-Rad Laboratories, Inc.). The PCR
thermocycling conditions were as follows: Initial denaturation at
95°C for 1 min, followed by 40 cycles of denaturation at 95°C for 5
sec and annealing at 60°C for 30 sec. Each sample was assayed in
triplicate for both the target and internal control
(β-actin) genes. Relative quantitative gene expression was
calculated using the 2−ΔΔCq method (36).
 | Table I.Sequences of the primers used for
reverse transcription-quantitative PCR. |
Table I.
Sequences of the primers used for
reverse transcription-quantitative PCR.
| Gene | Primer sequences
(5→3) | Product size
(bp) | Annealing
temperature (°C) | PCR cycles |
|---|
| VEGF-A | F:
CTGTCTAATGCCCTGGAGCC | 124 | 60 | 40 |
|
| R:
ACGCGAGTCTGTGTTTTTGC |
|
|
|
| PDGF-BB | F:
TTGGACCTGAACATGACCCG | 101 | 60 | 40 |
|
| R:
ATGGCCGGCTCAGCAATGGTC |
|
|
|
| PDGFR-β | F:
AGACACGGGAGAATACTTTTGC | 126 | 60 | 40 |
|
| R:
AGTTCCTCGGCATCATTAGGG |
|
|
|
| TSP-1 | F:
TGCTATCACAACGGAGTTCAGT | 108 | 60 | 40 |
|
| R:
GCAGGACACCTTTTTGCAGATG |
|
|
|
| TSP-2 | F:
GACACGCTGGATCTCACCTAC | 156 | 60 | 40 |
|
| R:
GAAGCTGTCTATGAGGTCGCA |
|
|
|
|
ADAMTS-1 | F:
TTCCACGGCAGTGGTCTAAAG | 100 | 60 | 40 |
|
| R:
CCACCAGGCTAACTGAATTACG |
|
|
|
|
ADAMTS-2 | F:
GTGCATGTGGTGTATCGCC | 191 | 60 | 40 |
|
| R:
AGGACCTCGATGTTGTAGTCA |
|
|
|
| MMP-2 | F:
GATACCCCTTTGACGGTAAGGA | 112 | 60 | 40 |
|
| R:
CCTTCTCCCAAGGTCCATAGC |
|
|
|
| MMP-9 | F:
GGGACGCAGACATCGTCATC | 139 | 60 | 40 |
|
| R:
TCGTCATCGTCGAAATGGGC |
|
|
|
| β-actin | F:
GCCGTCTTCCCCTCCATCGTG | 102 | 60 | 40 |
|
| R:
TCTCTTGCTCTGGGCCTCGTC |
|
|
|
Double-color immunofluorescence
analysis
For immunofluorescence analysis, the eyes were
harvested and rinsed twice with PBS and fixed in 4%
paraformaldehyde for 24 h at 4°C on day 4 following alkali injury.
The eyes were subsequently placed in 30% sucrose for 24 h and
frozen in Tissue-Tek® O.C.T.™ Compound (Sakura Finetek
Japan Co., Ltd.). The samples were cut on a Leica CM3050 S cryotome
(8-µm thick). All sections were blocked in 5% BSA (MP Biomedicals,
LLC) for 1 h at room temperature and subsequently incubated with
rat anti-mouse F4/80 (1:100) and rabbit anti-mouse PDGF-BB (1:50)
antibodies at 4°C overnight. Following rinsing with PBS, the
sections were incubated with Alexa Fluor 488-conjugated secondary
donkey anti-rat (H+L) antibody (1:100) and Alexa Fluor
594-conjugated secondary goat anti-rabbit (H+L) antibody (1:100)
antibodies for 1 h at room temperature in the dark. Finally, the
sections were washed with PBS, mounted on VECTASHIELD®
mounting medium (Vector Laboratories, Inc.) and incubated with DAPI
at room temperature for 10 min. Immunofluorescence was visualized
with a Nikon Eclipse Ti fluorescence microscope (Nikon
Corporation). The images were processed using graphics software
(Adobe Photoshop; version 7.0; Adobe Systems, Inc.).
Cell culture
HRECs (3–5×105) were cultured in DMEM
containing 4.5 mM glucose supplemented with 10% (v/v) FBS, 100 U/ml
penicillin and 10 µg/ml streptomycin. The cells were maintained in
a humidified incubator with 5% CO2 atmosphere at 37°C
and were used for all experiments at a passage number of 2 to
6.
Proliferation assay
To evaluate the effect of exogenous PDGF-BB on the
proliferation of HRECs under normal or hypoxic conditions, a CCK-8
assay was carried out. HRECs were seeded in a 96-well plate
(2×103 cells per well), and the hypoxic conditions were
induced using 200 µM of CoCl2 for 12 h. The cells were
then treated with recombinant human (rh)-PDGF-BB (Cell Signaling
Technology, Inc.) in the presence and/or absence of AG 1296, which
is a selective inhibitor of PDGFR-β. Following incubation for 24 h
at 37°C, the medium was replaced with fresh DMEM containing CCK-8
(10 µl per well). The cells were subsequently incubated for an
additional 2 h. The absorbance was measured at 450 nm using a
microplate reader (Thermo Fisher Scientific, Inc.).
Wound healing assay
The HRECs were cultured in DMEM supplemented with
10% FBS, pretreated with CoCl2 (200 µM) and were seeded
into 6-well plates at a density of 2×105 cells per well
for 24 h. A control, which was not treated with CoCl2,
was included. When HRECs reached 80% confluence, the cells in each
well were scratched perpendicularly using a 200-µl pipette tip at
the center of the wells. Briefly, the monolayer of cells was gently
and slowly scratched with a new 200-µl pipette tip across the
center of the well. While scratching across the surface of the
well, the long-axial of the tip should always be perpendicular to
the bottom of the well. The resulting gap distance is therefore
equal to the outer diameter of the end of the tip. A straight line
was scratched in one direction. Then, the other two straight lines
in parallel with the first line were scratched according to the
same protocol. A total of three straight lines were made per well.
After scratching, the well was gently washed twice with PBS to
remove the detached cells. Fresh serum-free medium (DMEM) was then
added into each well. The cells were then treated with PBS (for
control and vehicle group) or rh-PDGF-BB protein with or without AG
1296 at 37°C for 24 h. The cells were grown at 37°C for 48 h,
during which images were captured by an Olympus TMS inverted phase
contrast microscope (Olympus Corporation) at 0 and 24 h. The width
of the gap was evaluated quantitatively using ImageJ software. The
analysis of the distance of migration was performed relative to the
starting wound width (0 h time-point). The assessment of each
experimental group was repeated three times.
Tube formation assay
The in vitro cord formation of HRECs was
assessed using Corning Matrigel® Matrix (Coring, Inc.),
as described in a previous report with some modifications (37). Briefly, a 96-well plate was
incubated on ice and coated with 50 µl per well of fully thawed
Matrigel for 10 min. The samples were centrifuged at 300 × g for 10
min at 4°C to remove the air bubbles. The samples were subsequently
incubated at 37°C for 30 min in order to cause Matrigel
solidification. HRECs that were pretreated with CoCl2
(200 µM) were cultured in different medium with or without
rh-PDGF-BB and/or AG 1296. A control, which was not treated with
CoCl2, was included. The cells were seeded on the
solidified Matrigel immediately at a density of 1.5×104
cells per well. The plates were placed in a humidified atmosphere
of 5% CO2 and 95% air at 37°C for 12 h to allow the
formation of capillary-like structures. Angiogenesis is the
formation of capillary tubes and was assessed following 12 h of
cultivation. The tube-like capillary structures were examined under
an Olympus TMS inverted phase contrast microscope (Olympus
Corporation). The micrographs were captured using an Olympus
digital camera.
Statistical analysis
All data are expressed as the mean ± SEM. Data was
analyzed using a Student's t-test (two-tailed) between two groups
or by one-way ANOVA with Tukey's multiple comparison within
multiple groups using SPSS software version 18.0 (SPSS, Inc.).
P<0.05 was considered to indicate a statistically significant
difference. Each experiment was independently repeated at least 3
times.
Results
CrNV is attenuated via inhibition of
PDGF-BB in vivo
Initially the role of PDGF-BB in CrNV was explored
using a topical treatment of neutralizing anti-PDGF-BB antibody on
alkali burned corneas. The area of CrNV was recorded under a
slit-lamp microscope and under a fluorescence microscope following
whole mount immunofluorescent staining with an anti-CD31 antibody
and an Alexa Fluor 488-conjugated secondary antibody (Fig. 1A). The results indicated that the
area of CrNV was reduced in anti-PDGF-BB-treated groups in
comparison with the vehicle-treated group, 2 weeks following alkali
injury (68.37±3.85 vs. 82.85±4.66%; Fig. 1B). The density of the neovascular in
the two groups was also compared (Fig.
1C). The number of branching points and tip cells were also
reduced in the anti-PDGF-BB-treated group (131±9 vs. 164±12;
5.8±0.5 vs. 7.8±0.7; Fig. 1D). The
data suggested that the inhibition of PDGF-BB suppressed CrNV and
reduced the proliferation of tip cells.
Intra-corneal angiogenesis-related
gene expression in the CrNV model
To investigate the effects of PDGF-BB on CrNV, the
expression levels of the angiogenesis-related cytokines were
examined at the indicated time-points using RT-qPCR assays. Both
PDGF-BB and PDGFR-β were expressed at the early stage of CrNV (days
2, 4 and 7 following alkali injury). At these indicated
time-points, the results showed that the expression levels of
PDGF-BB and PDGFR-β (both in vehicle and anti-PDGF-BB
groups) were highest on day 4 after alkali injury compared with the
expression levels of PDGF-BB and PDGFR-β on days 2
and 7, and the expression levels of PDGF-BB and
PDGFR-β (both in vehicle and anti-PDGF-BB groups) were
downregulated on day 7 compared with expression levels of
PDGF-BB and PDGFR-β on day 4. The dynamic
intracorneal expression of PDGF-BB and PDGFR-β suggested the
possible involvement of PDGF-BB/PDGFR-β interactions in
alkali-induced CrNV. The intra-corneal expression levels of
VEGF-A, MMP-2 and MMP-9 were reduced, while the
expression levels of TSP-1, TSP-2, ADAMTS-1 and
ADAMTS-2 were increased in the anti-PDGF-BB-treated group
compared with those of the vehicle-treated group (Fig. 2). These data suggested that the
inhibition of PDGF-BB decreased the expression levels of the
pro-angiogenic factors VEGF-A, MMP-2 and MMP-9 and
increased the expression levels of the anti-angiogenic factors
TSP-1, TSP-2, ADAMTS-1 and ADAMTS-2.
 | Figure 2.Expression levels of pro-angiogenic
genes were downregulated and the expression levels of
anti-angiogenic genes were upregulated following inhibition of
PDGF-BB in the CrNV model. Total mRNA was extracted from corneas on
days 2, 4 and 7 following treatment with alkali. The expression
levels of the angiogenic genes were detected by reverse
transcription-quantitative PCR. The levels of PDGF-B, PDGFR-β,
VEGF-A, MMP-2 and MMP-9 were downregulated in the
anti-PDGF-BB group, whereas the expression levels of TSP-1,
TSP-2, ADAMTS-1 and ADAMTS-2 were upregulated. The data
are presented as mean ± SEM. *P<0.05, **P<0.01,
***P<0.001. PDGF, platelet-derived growth factor; PDGFR-β,
platelet-derived growth factor receptor; CrNV, corneal
neovascularization; VEGF, vascular endothelial growth factor; MMP,
matrix metalloproteinase; TSP, thrombospondin; ADAMTS, a
disintegrin and metalloproteinase with thrombospondin motifs. |
Intra-corneal infiltrated macrophages
produces PDGF-BB
Subsequently, the types of cells responsible for the
secretion of PDGF-BB were examined. It is commonly known that
infiltrated inflammatory cells, such as macrophages, play an
important role in the early stages of angiogenesis by secreting a
series of angiogenic cytokines (38,39).
Therefore, the number of intracorneal macrophages were detected and
it was determined whether PDGF-BB was expressed by macrophages
using immunohistochemical assays. The results indicated that
PDGF-BB was detectable in F4/80 positive cells on day 4 following
alkali injury (Fig. 3). This
finding suggested that intra-corneal macrophages may express
PDGF-BB and that they could be one of the sources of PDGF-BB
secretion.
 | Figure 3.PDGF-BB was partially derived from
infiltrated macrophages in the CrNV model. The eyes of the animals
from the induced CrNV model were enucleated on day 4, and the
expression levels of F4/80 and PDGF-BB were detected using
immunohistochemistry. The sections were fixed in 4%
paraformaldehyde and incubated with primary anti-mouse F4/80 and
anti-mouse PDGF-BB antibodies for staining. Alexa Fluor 488 (green)
and Alexa Fluor 594 (red) secondary antibodies were used to bind to
the anti-F4/80 and anti-PDGF-BB antibodies, respectively, whereas
the nuclei were counterstained with DAPI. On day 4, F4/80 positive
cells were detected in the stroma of cornea, and part of F4/80
positive cells were co-localized with PDGF-BB (white arrows).
Magnification, ×10 left panel, ×200 right panel. Representative
images from 3 independent experiments are shown. Scale bar, 50 µm.
Arrows indicate the double-positive cells. PDGF, platelet-derived
growth factor; CrNV, corneal neovascularization. |
Expression of PDGFR-β is upregulated
in HRECs under inflammatory or hypoxic conditions
The expression of PDGFR-β in HRECs under different
conditions was also examined. RT-qPCR indicated that PDGFR-β was
expressed in HRECs at a low level and that the expression levels of
PDGFR-β in HRECs increased in a concentration-dependent manner when
the cells were treated with LPS or CoCl2 to induce
inflammatory or hypoxic conditions (Fig. 4A).
PDGF-BB promotes proliferation,
migration and tube formation of HRECs
Subsequently, it was investigated whether
PDGF-BB/PDGFR-β signaling affects the proliferation of vascular
ECs. The results indicated that the proliferation of HRECs was
increased in the rh-PDGF-BB treated group, in which cells were
pretreated with CoCl2 compared with the PBS-treated
control and the rh-PDGF-BB-treated groups that were not exposed to
CoCl2 (1.675±0.75 vs. 1.103±0.11). This effect was
inhibited when the cells were treated with the PDGFR-β inhibitor,
AG 1296 (1.232±0.09; Fig. 4B).
To assess the effects of rh-PDGF-BB on the migration
of HRECs, the distance of migration was compared between vehicle
and experimental groups using a wound healing assay. The mobility
of HRECs that were pretreated with CoCl2 was increased
by treatment with rh-PDGF-BB (232±10.32 vs. 185±6.43 µm) compared
with that of PBS-treated cells following 24 h incubation from the
scratch (Fig. 5A). This positive
effect was inhibited by administration of AG 1296 (210±10.60 µm;
Fig. 5B).
To further evaluate the effects of the
PDGF-BB/PDGFR-β signaling pathway on the biological function of
HRECs in the formation of vascular cells, a tube formation assay
was performed using rh-PDGF-BB and AG 1296. The results indicated
that rh-PDGF-BB (20 ng/ml) increased tube formation of HRECs, while
this effect was abrogated when the cells were further treated with
AG 1296 (Fig. 6A). It was evident
by the formation of additional branch points (vehicle group was
117.3±5.94, rh-PDGF-BB group was 153.0±4.30 and rh-PDGF-BB+AG1296
group was 127.5±7.01; Fig. 6B), and
total tube length (vehicle group was 14,064±404.3 µm, rh-PDGF-BB
group was 18,604±738.7 µm and rh-PDGF-BB+AG1296 group was;
15,957±488.4 µm; Fig. 6C). The
results suggested that PDGF-BB could promote proliferation,
migration and tube formation of HRECs in vitro under hypoxic
conditions.
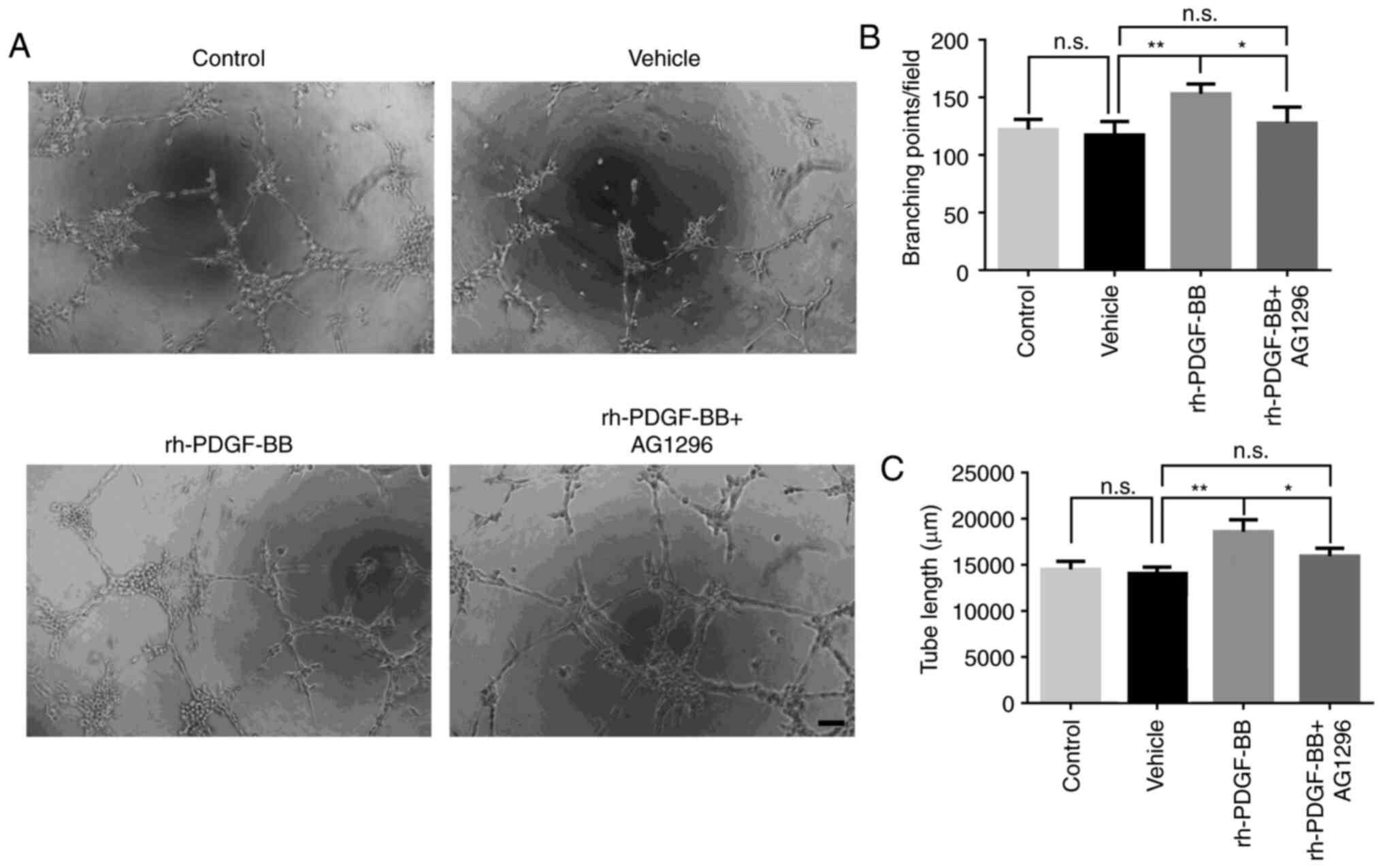 | Figure 6.Effects of rh-PDGF-BB on tube
formation of HRECs. (A) HRECs were pretreated with 200 mM
CoCl2 and were seeded on a Matrigel coated 96-well plate
at a density of 1×104 cells per well. Digital
microphotographs were obtained from 5 randomly selected high-power
fields following 6 h of incubation in different cultures (vehicle,
20 ng/ml, rh-PDGF-BB with or without 1 µM AG 1296). Magnification,
×100; scale bar, 200 µm. (B) Branch points and (C) tube lengths in
the rh-PDGF-BB group were increased compared with those of the
vehicle group (153.0±4.30 vs. 117.3±5.94, 18,604±738.7 vs.
14,064±404.3 µm), and this increase was inhibited with AG 1296
treatment (127.5±7.01, 15,957±488.4 µm). The data are presented as
mean ± SEM, *P<0.05, **P<0.01. n.s., no significance; PDGF,
platelet-derived growth factor; rh, recombinant human; HRECs, human
retinal endothelial cells; CoCl2, Cobalt chloride. |
Discussion
Corneal transparency is required for optimal vision.
The invasion of new vessels from the corneal limbus to the normal
avascular corneal stroma, causes the impairment of the avascular
state of the cornea, which can result in visual damage and even
lead to blindness (40).
Angiogenesis is a complex process involving interactions of
multiple ligand/receptor signaling pathways and cell types, as well
as the modulation of various cytokines (41). The process consists of two stages of
vessel bud formation resulting from migration and proliferation of
vascular ECs, and the maturation of vascular tubes (42). This process includes an event of
recruiting mural cells from pericytes and/or SMC (43). PDGF-BB is a well-characterized
trophic factor that is crucial for biological functions, such as
survival and proliferation of various cells including pericytes and
SMC (10). Furthermore, it has been
shown that PDGF-BB is produced by vascular ECs and contributes to
maintaining the maturity of newly formed vessels (27). Studies have demonstrated that during
fibrosis, MCs, such as pericytes and SMCs, are recruited and
further differentiated into fibroblasts by PDGF-BB, these cells
promote the formation of local fibrosis and lesion scars (26,44).
Furthermore, the expression of PDGFR-β, the receptor of PDGF-BB,
has been reported in EPCs, which are the precursor cells for
vascular ECs (45). A previous
study has also shown that the expression of PDGFR-β in EPCs
promotes the migration and proliferation of vascular ECs and
angiogenesis by interacting with PDGF-BB (46). Exogenous PDGF-BB was shown to
promote early migration of cells following wound healing in a rat
rotator cuff repair model (47).
Previous studies have indicated that PDGF-BB/PDGFR-β signaling is
very important in maintaining the maturity of vessels via the
recruitment of pericytes in the advanced stages of angiogenesis
(10,44,48).
However, the PDGF-BB/PDGFR-β signaling has not yet been well
characterized during CrNV. In the present study, the intra-corneal
expression of PDGFR-β and the role of PDGF-BB/PDGFR-β signaling in
CrNV following alkali injury were investigated.
The results indicated that PDGFR-β was dynamically
expressed in corneas in a murine CrNV model on days 2 and 7
following alkali injury, suggesting the possible involvement of
PDGF-BB/PDGFR-β signaling in the process of CrNV. Further analysis
revealed that CrNV was suppressed when the corneas were topically
treated with neutralizing anti-PDGF-BB antibody, suggesting that
blockage of PDGF-BB could inhibit neovascularization of the cornea.
The intra-corneal dynamic expression of angiogenesis-associated
cytokines, such as the pro-angiogenic factors VEGF-A, MMP-2
and MMP-9, and the anti- angiogenic factors TSP-1, TSP-2,
ADAMTS-1 and ADAMTS-2, was assessed between the vehicle
and the neutralizing anti-PDGF-BB antibody-treated groups. The data
indicated that the mRNA expression levels of VEGF-A, MMP-2
and MMP-9 were increased and that the expression levels of
TSP-1, TSP-2, ADAMTS-1 and ADAMTS-2 were reduced when
the biological function of PDGF-BB was blocked by the neutralizing
anti-PDGF-BB antibody, suggesting that PDGF-BB was involved in the
regulation of the expression of these cytokines. The results were
in agreement with a previous study by Park et al (49), which showed that the pro-angiogenic
effect of PDGF-BB was impaired by inhibition of PDGF-BB/PDGFR-β
signaling, resulting in suppressed expression of MMP-2 and MMP-9.
The data from the present study and previous reports supports the
hypothesis that PDGF-BB/PDGFR-β may have an important role in the
early stages of CrNV.
The expression levels of PDGFR-β in HRECs were
further investigated under different conditions, and the effects of
rh-PDGF-BB on proliferation, migration and tube formation of these
cells were explored in vitro. Although PDGF-BB/PDGFR-β
signaling was reported to exert a significant role in embryonic and
pathological vascular formation (50), the expression levels of PDGFR-β in
ECs were not determined. During the course of embryonic
development, PDGFR-β expression is ubiquitous and is noted in
several cell types, including ECs and pericytes. However, PDGFR-β
expression levels are downregulated postnatally (51). The upregulation of the expression
levels of PDGFR-β on EPCs is also reported under some pathological
conditions, such as inflammation, wound healing and tumorigenesis.
Upon activation of PDGF-BB/PDGFR-β signaling, the infiltration of
EPCs is facilitated and leads to differentiation from progenitor
cells into ECs. Moreover, the proliferation and mobility of ECs are
also enhanced (52). In addition to
the effect of PDGF-BB/PDGFR-β signaling on EPCs, this pathway
exerts proliferative and chemotactic effects on lymphatic ECs
(53). In the present study, it was
demonstrated that the expression levels of PDGFR-β on HRECs were
significantly increased in a dose dependent manner when HRECs were
stimulated with LPS or CoCl2, indicating that the
expression levels of PDGFR-β could be regulated under specific
conditions, such as inflammation or hypoxia, which are common
causes for postnatal pathological angiogenesis (43). Furthermore, the proliferation,
migration and tube formation of HRECs were also increased when the
cells were pretreated with CoCl2 and further treated
with rh-PDGF-BB. In contrast to rh-PDGF-BB, the aforementioned
biological functions of HRECs were suppressed when the cells were
pretreated with CoCl2 and further treated with AG 1296,
an inhibitor used for the PDGF-BB receptor. These in vitro
results suggested that the interactions between PDGF-BB and PDGFR-β
can promote angiogenesis in CoCl2-stimulated HRECs.
A previous study demonstrated that ECs can be
activated by inflammatory cells, such as macrophages, via the
secretion of pro-angiogenic factors (54). PDGF-BB is a pro-angiogenic factor
with the ability to activate ECs, and is also possibly secreted by
inflammatory cells in alkali-injured corneas (55). In the present study, the
intra-corneal expression levels of PDGF-BB were examined.
Considering that macrophages are an important source for the
secretion of pro-angiogenic factors, such as VEGF-A, bFGF and MMP-9
(39), the expression levels of
PDGF-BB in F4/80 positive macrophages were investigated using
immunohistochemical methods. The results indicated that PDGF-BB was
expressed in F4/80 positive macrophages in alkali-injured corneas,
suggesting that F4/80 positive macrophages can be considered an
important source for PDGF-BB. The secretion of this growth factor
activates ECs by binding with its receptor and therefore serving a
significant role in CrNV (56). To
the best of the authors' knowledge, it is still unclear whether
PDGF-BB is derived from macrophages. In the present study, the data
suggested that PDGF-BB could be secreted from infiltrated
macrophages in the CrNV model. This is a novel finding that may be
useful to further understand the underlying mechanism of macrophage
regulation during CrNV.
In the present study, the results demonstrated that
CrNV was suppressed in vivo via the inhibition of PDGF-BB at
the early stages of angiogenesis. The expression levels of the
angiogenic genes, namely VEGF-A, MMP-2 and MMP-9 were
enhanced, while the expression levels of TSP-1, TSP-2,
ADAMTS-1 and ADAMTS-2 were reduced following treatment
with the rh-PDGF-BB protein. In addition, exogenous PDGF-BB
promoted proliferation, migration and tube formation of
hypoxic-HRECs in vitro and provided evidence that PDGF-BB
may exert a critical role in experimental CrNV. However, there are
some limitations of the present study. First, the mechanism of
increased intra-corneal expression of PDGF-BB/PDGFR-β after alkali
injury is still unclear and needs to be further explored. Second,
it would be more useful to additionally apply PDGFR-β knockout mice
to examine the effects of PDGF-BB/PDGFR-β in alkali-burned CrNV by
comparing groups among vehicle (wild-type)-treated mice,
anti-PDGF-BB-treated mice and PDGFR-β knockout mice. Third, it
would be more suitable to choose a vascular EC line that originated
from corneal neovascular in this study when performing the relevant
in vitro experiments. However, considering that there are
still no vascular EC lines originating from CrNV worldwide, HRECs
had to be used to perform in vitro experiments.
In summary, the present study demonstrated that the
PDGF-BB/PDGFR-β signaling pathway exerts multiple activities,
including mitogenesis, angiogenesis and macrophage activation
(57). The data indicated that
endogenous PDGF-BB is partially derived from infiltrated
macrophages and that the PDGF-BB/PDGFR-β pathway played an
important role in the early stages of angiogenesis in the CrNV
model. The findings of the present study may aid the understanding
of the role of PDGF-BB in angiogenesis and could provide novel
options to target PDGF-BB in the treatment of angiogenesis.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation in China (grant nos. 81970830, 81671641,
81200727 and 31600736), Suzhou Municipal Natural Science Foundation
(grant no. SYS201745), Jiangsu Provincial Medical Youth Talent
(grant no. QNRC2016718), Jiangsu Provincial Medical Innovation Team
(grant no. CXTDA2017039) and the Soochow Scholar Project of Soochow
University (grant no. R5122001).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LC, GL confirm the authenticity of all the raw data.
LC, GL and HW designed the study, led the experiments, prepared
figures and wrote the manuscript. CR, LC, WZ and WL analyzed the
data and prepared the figures. PL and GL conceived, designed and
coordinated the study, and drafted the manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments followed the Guideline for
the Care and Use of Laboratory Animals of the Chinese Medical
Academy and were approved by the Soochow University Animal Care
Committee (approval number 202012A121, Suzhou, China), and were
performed in accordance with the ARVO Statement for the Use of
Animals in Ophthalmic and Vision Research.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Campochiaro PA: Ocular neovascularization.
J Mol Med (Berl). 91:311–321. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cheung LK and Eaton A: Age-related macular
degeneration. Pharmacotherapy. 33:838–855. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Clements JL and Dana R: Inflammatory
corneal neovascularization: Etiopathogenesis. Semin Ophthalmol.
26:235–245. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yeung KK, Yang HJ, Nguyen AL and Weissman
BA: Critical contact lens oxygen transmissibility and tearlens
oxygen tension to preclude corneal neovascularization. Eye Contact
Lens. 44 (Suppl 1):S291–S295. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Voiculescu OB, Voinea LM and Alexandrescu
C: Corneal neovascularization and biological therapy. J Med Life.
8:444–448. 2015.PubMed/NCBI
|
|
6
|
Rahbari NN, Kedrin D, Incio J, Liu H, Ho
WW, Nia HT, Edrich CM, Jung K, Daubriac J, Chen I, et al: Anti-VEGF
therapy induces ECM remodeling and mechanical barriers to therapy
in colorectal cancer liver metastases. Sci Transl Med.
8:360ra1352016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cabral T, Mello LGM, Lima LH, Polido J,
Regatieri CV, Belfort R Jr and Mahajan VB: Retinal and choroidal
angiogenesis: A review of new targets. Int J Retina Vitreous.
3:312017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao Y and Adjei AA: Targeting
angiogenesis in cancer therapy: Moving beyond vascular endothelial
growth factor. Oncologist. 20:660–673. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hellberg C, Ostman A and Heldin CH: PDGF
and vessel maturation. Recent Results Cancer Res. 180:103–114.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lindblom P, Gerhardt H, Liebner S,
Abramsson A, Enge M, Hellstrom M, Backstrom G, Fredriksson S,
Landegren U, Nystrom HC, et al: Endothelial PDGF-B retention is
required for proper investment of pericytes in the microvessel
wall. Genes Dev. 17:1835–1840. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dell S, Peters S, Müther P, Kociok N and
Joussen AM: The role of PDGF receptor inhibitors and PI3-kinase
signaling in the pathogenesis of corneal neovascularization. Invest
Ophthalmol Vis Sci. 47:1928–1937. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ricci C and Ferri N: Naturally occurring
PDGF receptor inhibitors with potential anti-atherosclerotic
properties. Vascul Pharmacol. 70:1–7. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Park DY, Lee J, Kim J, Kim K, Hong S, Han
S, Kubota Y, Augustin HG, Ding L, Kim JW, et al: Plastic roles of
pericytes in the blood-retinal barrier. Nat Commun. 8:152962017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Strittmatter K, Pomeroy H and Marneros AG:
Targeting platelet-derived growth factor receptor β(+) scaffold
formation inhibits choroidal neovascularization. Am J Pathol.
186:1890–1899. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ho CL, Hsu LF, Phyliky RL and Li CY:
Autocrine expression of platelet-derived growth factor B in B cell
chronic lymphocytic leukemia. Acta Haematol. 114:133–140. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Koehler NK, Roebbert M, Dehghani K,
Ballmaier M, Claus P, von Hoersten S, Shing M, Odin P, Strehlau J
and Heidenreich F: Up-regulation of platelet-derived growth factor
by peripheral-blood leukocytes during experimental allergic
encephalomyelitis. J Neurosci Res. 86:392–402. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Minardi S, Pandolfi L, Taraballi F, Wang
X, De Rosa E, Mills ZD, Liu X, Ferrari M and Tasciotti E: Enhancing
vascularization through the controlled release of platelet-derived
growth factor-BB. ACS Appl Mater Interfaces. 9:14566–14575. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang H, Yin Y, Li W, Zhao X, Yu Y, Zhu J,
Qin Z, Wang Q, Wang K, Lu W, et al: Over-expression of PDGFR-β
promotes PDGF-induced proliferation, migration, and angiogenesis of
EPCs through PI3K/Akt signaling pathway. PLoS One. 7:e305032012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu P, Li L, Liu G, van Rooijen N, Mukaida
N and Zhang X: Opposite roles of CCR2 and CX3CR1 macrophages in
alkali-induced corneal neovascularization. Cornea. 28:562–569.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rhee I: Diverse macrophages polarization
in tumor microenvironment. Arch Pharm Res. 39:1588–1596. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qian BZ and Pollard JW: Macrophage
diversity enhances tumor progression and metastasis. Cell.
141:39–51. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mayer A, Lee S, Jung F, Grütz G, Lendlein
A and Hiebl B: CD14+ CD163+ IL-10+
monocytes/macrophages: Pro-angiogenic and non pro-inflammatory
isolation, enrichment and long-term secretion profile. Clin
Hemorheol Microcirc. 46:217–223. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Marçola M and Rodrigues CE: Endothelial
progenitor cells in tumor angiogenesis: Another brick in the wall.
Stem Cells Int. 2015:8326492015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Noskovičová N, Petřek M, Eickelberg O and
Heinzelmann K; From Lung Development and Disease to Clinical
Studies, : Platelet-derived growth factor signaling in the lung.
From lung development and disease to clinical studies. Am J Respir
Cell Mol Biol. 52:263–284. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ostendorf T, Boor P, van Roeyen CR and
Floege J: Platelet-derived growth factors (PDGFs) in glomerular and
tubulointerstitial fibrosis. Kidney Int Suppl (2011). 4:65–69.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jaguin M, Fardel O and Lecureur V:
AhR-dependent secretion of PDGF-BB by human classically activated
macrophages exposed to DEP extracts stimulates lung fibroblast
proliferation. Toxicol Appl Pharmacol. 285:170–178. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kryza T, Achard C, Parent C, Marchand-Adam
S, Guillon-Munos A, Iochmann S, Korkmaz B, Respaud R, Courty Y and
Heuzé-Vourc'h N: Angiogenesis stimulated by human
kallikrein-related peptidase 12 acting via a platelet-derived
growth factor B-dependent paracrine pathway. FASEB J. 28:740–751.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang GL and Semenza GL: Desferrioxamine
induces erythropoietin gene expression and hypoxia-inducible factor
1 DNA-binding activity: Implications for models of hypoxia signal
transduction. Blood. 82:3610–3615. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yuan Y, Hilliard G, Ferguson T and
Millhorn DE: Cobalt inhibits the interaction between
hypoxia-inducible factor-alpha and von Hippel-Lindau protein by
direct binding to hypoxia-inducible factor-alpha. J Biol Chem.
278:15911–15916. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Taheem DK, Foyt DA, Loaiza S, Ferreira SA,
Ilic D, Auner HW, Grigoriadis AE, Jell G and Gentleman E:
Differential Regulation of Human Bone Marrow Mesenchymal Stromal
Cell Chondrogenesis by Hypoxia Inducible Factor-1α Hydroxylase
Inhibitors. Stem Cells. 36:1380–1392. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhu J, Tang Y, Wu Q, Ji YC, Feng ZF and
Kang FW: HIF-1α facilitates osteocyte-mediated osteoclastogenesis
by activating JAK2/STAT3 pathway in vitro. J Cell Physiol.
234:21182–21192. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ahani-Nahayati M, Solali S, Shams Asenjan
K, Movassaghpour Akbari AA, Talebi M, Zadi Heydarabad M,
Baharaghdam S and Farshdousti Hagh M: Promoter methylation status
of survival-related genes in MOLT-4 cells co-cultured with bone
marrow mesenchymal stem cells under hypoxic conditions. Cell J.
20:188–194. 2018.PubMed/NCBI
|
|
33
|
Lolmède K, Durand de Saint Front V,
Galitzky J, Lafontan M and Bouloumié A: Effects of hypoxia on the
expression of proangiogenic factors in differentiated 3T3-F442A
adipocytes. Int J Obes Relat Metab Disord. 27:1187–1195. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu G, Chen L, Cai Q, Wu H, Chen Z, Zhang
X and Lu P: Streptozotocin induced diabetic mice exhibit reduced
experimental choroidal neovascularization but not corneal
neovascularization. Mol Med Rep. 18:4388–4398. 2018.PubMed/NCBI
|
|
35
|
Le YZ, Ash JD, Al-Ubaidi MR, Chen Y, Ma JX
and Anderson RE: Targeted expression of Cre recombinase to cone
photoreceptors in transgenic mice. Mol Vis. 10:1011–1018.
2004.PubMed/NCBI
|
|
36
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Arnaoutova I and Kleinman HK: In vitro
angiogenesis: Endothelial cell tube formation on gelled basement
membrane extract. Nat Protoc. 5:628–635. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bock F, Maruyama K, Regenfuss B, Hos D,
Steven P, Heindl LM and Cursiefen C: Novel anti(lymph)angiogenic
treatment strategies for corneal and ocular surface diseases. Prog
Retin Eye Res. 34:89–124. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sunderkötter C, Goebeler M,
Schulze-Osthoff K, Bhardwaj R and Sorg C: Macrophage-derived
angiogenesis factors. Pharmacol Ther. 51:195–216. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nielsen SR and Schmid MC: Macrophages as
Key Drivers of cancer progression and metastasis. Mediators
Inflamm. 2017:96247602017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chung AS and Ferrara N: Developmental and
pathological angiogenesis. Annu Rev Cell Dev Biol. 27:563–584.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Carmeliet P: Angiogenesis in health and
disease. Nat Med. 9:653–660. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Carmeliet P: Angiogenesis in life, disease
and medicine. Nature. 438:932–936. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Rodriguez A, Friman T, Kowanetz M, van
Wieringen T, Gustafsson R and Sundberg C: Phenotypical differences
in connective tissue cells emerging from microvascular pericytes in
response to overexpression of PDGF-B and TGF-β1 in normal skin in
vivo. Am J Pathol. 182:2132–2146. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Capitão M and Soares R: Angiogenesis and
inflammation crosstalk in diabetic retinopathy. J Cell Biochem.
117:2443–2453. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sufen G, Xianghong Y, Yongxia C and Qian
P: bFGF and PDGF-BB have a synergistic effect on the proliferation,
migration and VEGF release of endothelial progenitor cells. Cell
Biol Int. 35:545–551. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kovacevic D, Gulotta LV, Ying L, Ehteshami
JR, Deng XH and Rodeo SA: rhPDGF-BB promotes early healing in a rat
rotator cuff repair model. Clin Orthop Relat Res. 473:1644–1654.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Bonnet CS and Walsh DA: Osteoarthritis,
angiogenesis and inflammation. Rheumatology (Oxford). 44:7–16.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Park ES, Lee KP, Jung SH, Lee DY, Won KJ,
Yun YP and Kim B: Compound K, an intestinal metabolite of
ginsenosides, inhibits PDGF-BB-induced VSMC proliferation and
migration through G1 arrest and attenuates neointimal hyperplasia
after arterial injury. Atherosclerosis. 228:53–60. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Maloney SC, Antecka E, Granner T,
Fernandes B, Lim LA, Orellana ME and Burnier MN Jr: Expression of
SIRT1 in choroidal neovascular membranes. Retina. 33:862–866. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Friedlaender GE, Lin S, Solchaga LA, Snel
LB and Lynch SE: The role of recombinant human platelet-derived
growth factor-BB (rhPDGF-BB) in orthopaedic bone repair and
regeneration. Curr Pharm Des. 19:3384–3390. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wyler von Ballmoos M, Yang Z, Völzmann J,
Baumgartner I, Kalka C and Di Santo S: Endothelial progenitor cells
induce a phenotype shift in differentiated endothelial cells
towards PDGF/PDGFRβ axis-mediated angiogenesis. PLoS One.
5:e141072010. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Miyazaki H, Yoshimatsu Y, Akatsu Y,
Mishima K, Fukayama M, Watabe T and Miyazono K: Expression of
platelet-derived growth factor receptor β is maintained by Prox1 in
lymphatic endothelial cells and is required for tumor
lymphangiogenesis. Cancer Sci. 105:1116–1123. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Riabov V, Gudima A, Wang N, Mickley A,
Orekhov A and Kzhyshkowska J: Role of tumor associated macrophages
in tumor angiogenesis and lymphangiogenesis. Front Physiol.
5:752014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Battegay EJ, Rupp J, Iruela-Arispe L, Sage
EH and Pech M: PDGF-BB modulates endothelial proliferation and
angiogenesis in vitro via PDGF beta-receptors. J Cell Biol.
125:917–928. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Chaoran Z, Zhirong L and Gezhi X:
Combination of vascular endothelial growth factor
receptor/platelet-derived growth factor receptor inhibition
markedly improves the antiangiogenic efficacy for advanced stage
mouse corneal neovascularization. Graefes Arch Clin Exp Ophthalmol.
249:1493–1501. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Shah P, Keppler L and Rutkowski J: A
review of platelet derived growth factor playing pivotal role in
bone regeneration. J Oral Implantol. 40:330–340. 2014. View Article : Google Scholar : PubMed/NCBI
|




















