Introduction
Following cardiopulmonary bypass (CPB), the
incidence rates of short-term cognitive abnormalities, memory and
learning ability decline and visual-motor response declines by
60–80% (1). The incidence of
postoperative cognitive dysfunction (POCD) is ~50–70% at 1 week
following open-heart surgery (2).
This incidence declines to 30–50% after 2 months; however, in 40%
of patients cognitive function is not restored to the preoperative
level after 5 years (3,4). Therefore, it is necessary to prevent
and treat POCD induced by CPB during cardiac surgery, which remains
a challenge and difficulty for clinicians.
The mechanism of POCD following CPB is complicated.
Persistent perfusion with blood flowing through a CPB machine with
a simulated human respiratory cycle, temperature decrease, damage
to important molecules in the blood, ischemia and reperfusion and
release of cytokines (IL-6 and TNF-α) and reactive oxygen species
(ROS) in large amounts caused by endotoxemia can directly trigger
systemic inflammatory response syndrome during CPB (5,6) and
oxidative stress (7), leading to
vital organ dysfunction and even permanent damage. Among these
factors, oxidative stress serves an important role in the
occurrence of POCD (8).
Additionally, changes in the central cholinergic system and levels
of Tau protein, calcium ions and γ-aminobutyric acid in the
hippocampus following CPB can promote the occurrence of POCD
(9).
In recent years, the protective effects mediated by
endogenous heme oxygenase 1 (HO-1) on cognitive functions have been
investigated (10). The antioxidant
enzyme HO-1 serves an important role in oxidative stress (11). HO-1 is overexpressed in a variety of
situations, including organ transplantation, acute kidney injury,
hypertension and atherosclerosis (12–16).
HO-1 has anti-inflammatory, anti-proliferative, anti-oxidative and
anti-apoptotic activities and remains a promising target for the
treatment of oxidative stress-related diseases (17). Nrf2, an anti-oxidative transcription
factor, binds to the antioxidant responsive element (ARE) to
initiate the expression of multiple antioxidant and
anti-inflammatory proteins as well as downstream detoxification
enzymes, which are key factors in regulating the transcription and
expression of HO-1 (18). Nrf2 can
be activated by protein kinases including PI3K/AKT (19). Activation of the AKT/mTOR pathway
reduces the occurrence of POCD in rats (20). A previous study demonstrated that
following PI3k/AKT pathway activation by phytoestrogens, the
downstream protein GSK-3β is inactivated, which subsequently
upregulates HO-1 expression, thereby inhibiting the neurotoxic
effects of amyloid β (Aβ)25-35 (21). Puerarin has been confirmed to
activate Nrf2 to upregulate HO-1 expression and protect primary
cultured hippocampal neurons in rats from
Aβ25-35-induced damage following GSK-3β inactivation
(22).
Opioids are currently the most widely used analgesic
drugs in clinical practice. Three types of classic opioid receptors
exist including µ-opioid receptors, δ-opioid receptors and κ-opioid
receptors (KORs). KORs are abundantly expressed in the prefrontal
cortex and other brain regions and can regulate mood and cognitive
functions. KORs have been demonstrated to alleviate brain damage
and improve functional recovery in animal models with both systemic
and regional cerebral ischemia (23). The KOR agonist U50488H was
administered in the hippocampus during nerve injury induced by
ischemia and the results demonstrate significant decline in
cognitive impairment (24).
However, its specific regulatory mechanism remains to be
elucidated. Charron et al (24) only reported that KOR agonists were
beneficial to the activation of hippocampal cholinergic neurons,
which thus counteracted the memory impairment caused by
scopolamine. However, whether KOR agonists induce transcription and
expression of HO-1 by regulating the PI3K/AKT/Nrf2 pathway in the
hippocampus of rats, inhibit oxidative stress following CPB and
improve cognitive functions following CPB are rarely examined. In
the present study, bloodless priming rat models of CPB were used
and the KOR agonist U50488H was administered to the rats to
investigate the protective effects of KORs on cognitive functions
following CPB. The HO-1 antagonist ZnPP-IX and the PI3K antagonist
LY294002 were then separately administered to observe the role of
the PI3K/AKT/Nrf2/HO-1 signaling pathway in the protective effects
on cognitive functions induced by KOR agonists following CPB.
Hence, the present study aimed to investigate possible protective
mechanisms and to provide an experimental basis for the clinical
application of KOR agonists.
Materials and methods
Experimental animals and grouping
Specific pathogen free Sprague-Dawley rats (n=50;
male; 400–480 g) were purchased from Liaoning Changsheng Biological
Co., Ltd. [SCXK (Liao): 2017–0001]. The present study was approved
by the China Medical University Laboratory Animal Welfare and
Ethics Committee (IACUC no. 2018048R). All experimental procedures
were performed in strict accordance with the guidelines for
management and protection of laboratory animals. The animals were
divided into the following groups: Sham operation (Sham group), CPB
(CPB group), KOR agonist (U50488H) + CPB (U50488H group), CPB +
U50488H + HO-1 antagonist (ZnPP-IX; ZnPP group) and CPB + U50488H +
PI3K antagonist (LY294002; LY294002 group) based on a random number
table, with 10 rats in each group. All rats were cultivated in
individual ventilated cages at 24±2°C and 40–70% humidity in a 12-h
light/dark cycle. Standard pelleted chow and drinking water were
available ad libitum.
Establishment of bloodless priming CPB
models with a beating heart
CPB rat models were established according to the
procedure reported by Sun et al (25). The rats were fasted for 6 h before
the operation but were allowed to drink freely. Rats were
anesthetized with 30 mg/kg 2% sodium pentobarbital (Sigma-Aldrich;
Merck KGaA) and underwent tracheal intubation (16G trocar) using a
light transmission method. Intraoperative maintenance was conducted
by intermittent administration of sodium pentobarbital and 1%
rocuronium (Zhejiang Xianju Pharmaceutical Co., Ltd.). Vital signs
including the heart rate, pulse oximetry and body temperature were
monitored. Catheter insertion (24G) was performed by puncturing the
left femoral artery and the pressure was measured. The needle (22G)
was inserted in the right femoral vein as a channel for fluid
supplementation. Another needle (22G) was indwelled in the caudal
artery for CPB perfusion. Catheter insertion (18G with a porous
tip) was performed via the right neck vein and the catheter was
placed at the level of the right atrium to be used as a drainage
end. Priming solution was prepared using 3 ml of succinylated
gelatin, 1 ml of lactated Ringer's solution, 1 ml of 20% mannitol,
250 IU/kg heparin and 0.5 ml of 5% sodium bicarbonate and 10 mg/kg
furosemide. Systemic heparinization (400 IU/kg) was injected into
the left femoral vein once the activated clotting time reached 480
sec. CPB was then started and mechanical ventilation was halted.
During CPB, the mean arterial pressure was maintained above 60
mmHg. Based on the blood gas report, drug application and
respiratory parameters were adjusted, the pH value was maintained
at 7.35–7.45, PaCO2 was 35–45 mmHg, hemoglobin was
>70 g/l and the hematocrit was maintained >25%. Subsequently,
10 min before the completion of CPB, the drainage and bypass rates
were gradually slowed. Mechanical ventilation was recovered
following completion of CPB. Catheters in various blood vessels
were removed in sequence and the anal temperature was maintained at
36.5–37.5°C. Fluid was supplemented appropriately, or vasoactive
drugs were applied if needed.
For rats in the Sham group, catheter insertion was
performed under anesthesia, but the CPB model was not established.
For CPB group rats, CPB models were established following catheter
insertion under anesthesia and bypass was maintained for 1 h. Rats
in the U50488H group were intravenously injected with U50488H (1.5
mg/kg, cat. no. 0495/25; Tocris Bioscience) before CPB surgery.
Rats in the ZnPP and LY294002 groups were first intravenously
injected with ZnPP-IX (5 mg/kg; Sigma-Aldrich; Merck KGaA) or
LY294002 (0.3 mg/kg; Sigma-Aldrich; Merck KGaA) and 30 min later
intravenously injected with U50488H (1.5 mg/kg) before CPB
surgery.
Neurological deficit score
According to the neurological scoring method of
Longa et al (26), rats were
scored for neurological deficits following the Morris water maze
test. The criteria were as follows: No symptoms of neurological
deficits (0 points); signs of flexion of the left upper limb after
lifting the tail, not fully extended (1 point); showing rotation to
the left and moving in circles (2 points); crawling to the left (3
points); involuntary movement and exhibiting disturbance of
consciousness (4 points).
Morris water maze test
To observe the changes in cognitive abnormalities
and memory and learning capability of CPB rats under U50488U
treatment, the Morris water maze test (Shanghai Xinsoft Information
Technology Co., Ltd.)was performed for rats in all groups on the
third day following CPB (27). The
water temperature was maintained at 22.0±10°C. A platform with an
area of ~38 cm2 was located 2–3 cm below the horizontal
plane. Black non-toxic stain was added to the water so that the rat
could not see the platform. The pool was evenly divided into four
quadrants (left top, right top, left bottom, right bottom) and the
platform was placed in the center of the left bottom quadrant.
Acquisition test (hidden platform training): Prior
to the operation, the rats were trained to find the platform twice
each day and once each night for 5 days. The rats were randomly
placed in the water in any of the four quadrants. The latency
period of finding the hidden platform was calculated from the time
the rat entered the water to the time that it climbed onto the
platform. Each training period was limited to 1 min. If the rat
could not find the hidden platform within 1 min, the latency was
recorded as 1 min. Then, the rat was placed on the platform to rest
for 1 min in order to help the rat locate the position of the
platform. The movement paths of the rats were imaged and recorded
by the system. In addition, the distance, duration, resting time,
number of times entering the water and rates were analyzed. This
experiment was used to assess the short-term memory and learning
ability of the animals.
Memory retention test (spatial exploration): The
platform was removed on day 6 and the rats were placed into the
water from the right top quadrant. The time spent swimming in each
quadrant, number of times crossing over the platform and swimming
distance within 1 min were recorded and analyzed by the system
automatically. The residence time, swimming distance and number of
times crossing over the original platform in the left bottom
quadrant (the quadrant where the platform was originally placed)
were recorded to evaluate memory storage as well as retrieval and
replication abilities of the rats.
Hematoxylin and eosin (H&E)
staining and terminal deoxynucleotidyl transferase dUTP nick end
labeling (TUNEL) assay
On the 7th day after the establishment of the rat
CPB model, the rats received an intraperitoneal injection of sodium
pentobarbital (200 mg/kg body weight) for sacrifice. The heartbeat
of the rats was assessed for ≥5 min to confirm mortality. The
hippocampal tissue was collected and post-fixed in perfusion
fixative for 2 h at room temperature, immersed in 20% sucrose
solution, then immersed in wax and embedded. The following day, the
hippocampus was coronally sectioned at 5-µm-thick sections for
H&E staining and TUNEL. A hematoxylin-eosin staining kit was
purchased from Boster Biological Technology (Wuhan, Hubei, China)
for H&E staining. A TUNEL assay kit (In situ Cell Death
Detection kit-POD; Sigma cat. no. 11684817910) was used for TUNEL
assay. The protocol was according to the manufacturer's protocol.
The H&E results and TUNEL-positive cells on each slice were
then observed using light microscopy (Olympus Corporation). Three
typical 200 × fields of view were randomly selected from each
section for quantification.
Immunofluorescence (IF)
After the wax-embedded hippocampal tissue sections
were deparaffinized and then rehydrated. For antigen retrieval, the
5-µm paraffin sections were autoclaved (121°C, 20 min) in a
solution of 0.1 M citric acid and 0.1 M sodium citrate. The
sections were blocked in the blocking solution and incubated at
37°C for 30 min. The serum was decanted and glial fibrillary acidic
protein (GFAP) antibodies (1:500; cat. no. ab7260; Abcam) and
Mito-Tracker Red CMXRos (Shanghai Biyuntian Biotechnology Co.,
Ltd.) were added and the sections were incubated overnight at 4°C.
After washed with PBS, the sections were incubated in Alexa Fluor
488-conjugated secondary antibodies (1:400) at 37°C for 30 min,
followed by 4′,6-diamidino-2-phenylindole (DAPI) to visualize cell
nuclei. Sections were imaged using a Zeiss Axio Observer
fluorescence microscope. Three typical 200 × fields of view were
randomly selected from each section for quantification.
ELISA
The S100 β (cat. no. SEA567Ra) and neuron specific
enolase (NSE; cat. no. SEA537Ra) expression levels in the rat
hippocampus, the serum expression levels of the inflammatory
factors IL-1β (cat. no. SEA563Ra) and IL-6 (cat. no. SEA079Ra) and
the expression levels of the oxidative stress indicators superoxide
dismutase (SOD; cat. no. SES134Ra), malondialdehyde (MDA, CEA597Ge)
and myeloperoxidase (MPO, cat. no. SEA601Ra) were detected by ELISA
kits. These ELISA kits were purchased from Wuhan USCN Business Co.,
Ltd. The protocol was performed according to the manufacturer's
instructions.
Western blot analysis
The hippocampal tissues were weighed, ground,
homogenized and centrifuged at 10,000 × g for 15 min at 4°C.
pre-cooled RIPA (cat. no. 89900; Thermo Fisher Scientific, Inc.)
lysate was added and was lysed on ice for 30 min. The supernatant
was extracted to detect the protein levels through BCA method (cat.
no. 23225; Thermo Fisher Scientific, Inc.). The proteins (30
µg/well) were loaded and separated by 12% sodium dodecyl sulfate
polyacrylamide gel electrophoresis. The proteins were transferred
to 12% polyvinylidene fluoride (PVDF) membrane for 1 h. The PVDF
membrane was washed with PBS-T (PBS plus 0.1% Tween-20). Then, 5%
skimmed milk blocking solution was added and the membrane was
placed in a shaker at 4°C overnight. Then, the membrane was washed
and primary antibodies to Bcl2 (1:1,000: cat. no. ab59348; Abcam),
Bax (1:1,000; cat. no. ab32503; Abcam), PI3K (1:1,000; cat. no.
ab191606; Abcam), AKT (1:1,000; cat. no. ab179463; Abcam),
phosphorylated (p)-AKT (1:1,000; cat. no. ab131443; Abcam), HO-1
(1:1,000; cat. no. ab13248; Abcam), Nrf2 (1:1,000; cat. no.
ab92946; Abcam), thioredoxin 1 (Trx1; 1:1,000; cat. no. ab185544;
Abcam), H3 (1:1,000; cat. no. ab1791; Abcam) or
glyceraldehyde-3-phosphate dehydrogenase (GAPDH; 1:1,000; cat. no.
ab9485; Abcam) were added and incubated for 1 h. The membrane was
washed three times consecutively. Then, the secondary antibody,
goat anti-rabbit IgG H&L (HRP; 1:10,000; cat. no. ab6721;
Abcam) antibody, was added and the membrane was placed on a shaker
for 1 h. The membrane was rinsed and developed by luminescence
(ECL; Amersham; Cytiva). A gel imaging system (Gel Doc™ XR; Bio-Rad
Laboratories, Inc.) was used for capturing images. Absorbance
values were analyzed using ImageJ (v1.8.0; National Institutes of
Health).
Statistical analysis
Statistical analysis was performed using SPSS 19.0
statistical software (IBM Corp.). Experimental data are expressed
as the mean ± standard deviation. One-way analysis of variance
(ANOVA) was performed followed with Tukey's post-hoc test for
comparison tests. A χ2 test was used to compare ratios.
A Kruskal-Wallis test with post-hoc Dunn's tests was performed in
Figs. 1A and D, 5A and S1D. Each experiment was repeated at
least three times. P<0.05 was considered to indicate a
statistically significant difference.
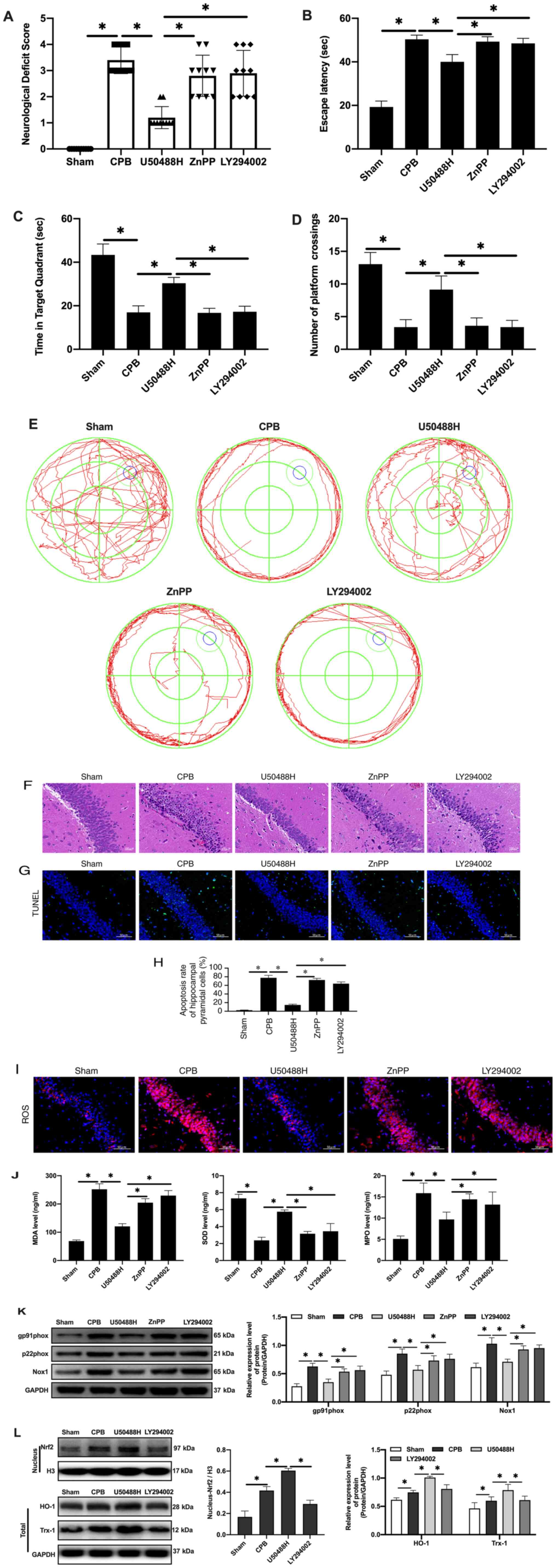 | Figure 5.KOR agonist activates Nrf2/HO-1 via
PI3K/AKT pathway to improve cognitive function in CPB rats. (A)
Neurological deficit score. (B) Time of escape latency. (C) Time in
target quadrant. (D) Number of platform crossings. (E) Water maze
track. (F) Hematoxylin and eosin staining scale bar, 100 µm). (G)
TUNEL staining (scale bar, 50 µm; n=10). (H) Apoptosis rate. (I)
Expression of ROS detected by immunofluorescence (scale bar, 50 µm;
n=10). (J) Oxidative stress factors detect by ELISA. (K) Expression
of gp91phox, p22phox and Nox1 proteins
detected by western blotting. (L) Expression of Nrf2/HO-1 signaling
pathway-related proteins detected by western blotting (n=10;
*P<0.05). KOR, κ-opioid receptor; Nrf2, nuclear factor erythroid
2-related factor 2; HO-1, heme oxygenase 1; CPB, cardiopulmonary
bypass; ROS, reactive oxygen species; Nrf2, nuclear factor
erythroid 2-related factor 2; HO-1, heme oxygenase 1; Nox1, NADPH
Oxidase 1; Trx1, thioredoxin 1. |
Results
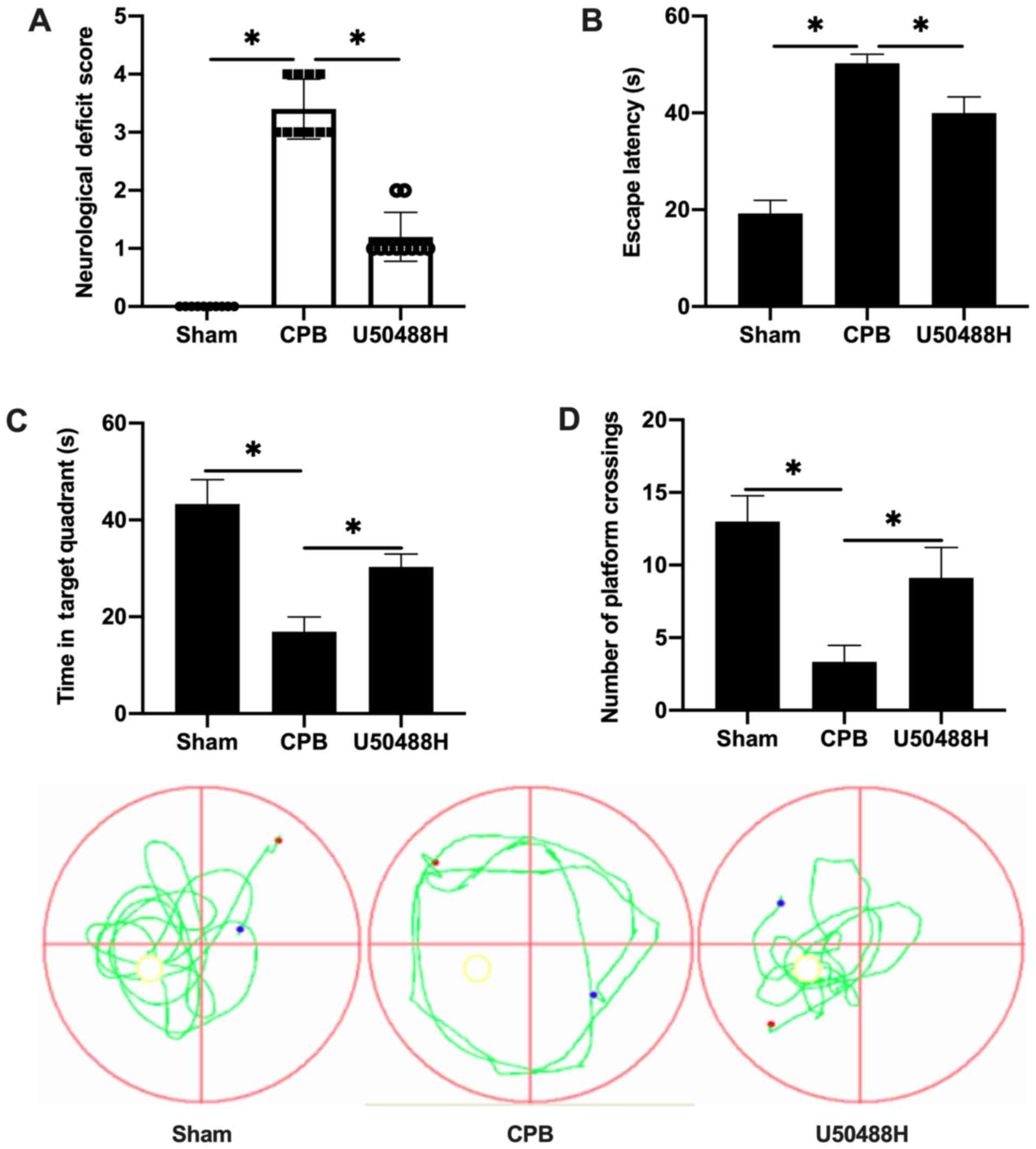
KOR agonist improves cognitive
function following CPB in rats
To observe the cognitive abnormalities and memory
and learning capability changes following CPB surgery, a rat CPB
model was successfully established and the Longa method used to
score neurological deficits. Treatment with U50488H improved the
performance of CPB rats and significantly decreased the
neurological deficit score (Fig.
1A). The Morris water maze navigation experiment results
demonstrated that the escape latency gradually decreased. The
escape latency in the CPB group was significantly longer than that
in the Sham group and U50488H could shorten the escape latency of
CPB rats (Fig. 1B). A spatial
exploration experiment was performed to assess the spatial memory
ability of rats. For rats in the CPB group, the retention time in
the target quadrant was significantly less compared with that in
the Sham group, however, rats in the U50488H group exhibited longer
target quadrant retention times compared with those in the CPB
group (Fig. 1C). In addition, the
number of crossing platform in U50488H group was significantly more
compared with that in CPB group (Fig.
1D). These results demonstrated that the KOR agonist
significantly improved the cognitive function of CPB rats.
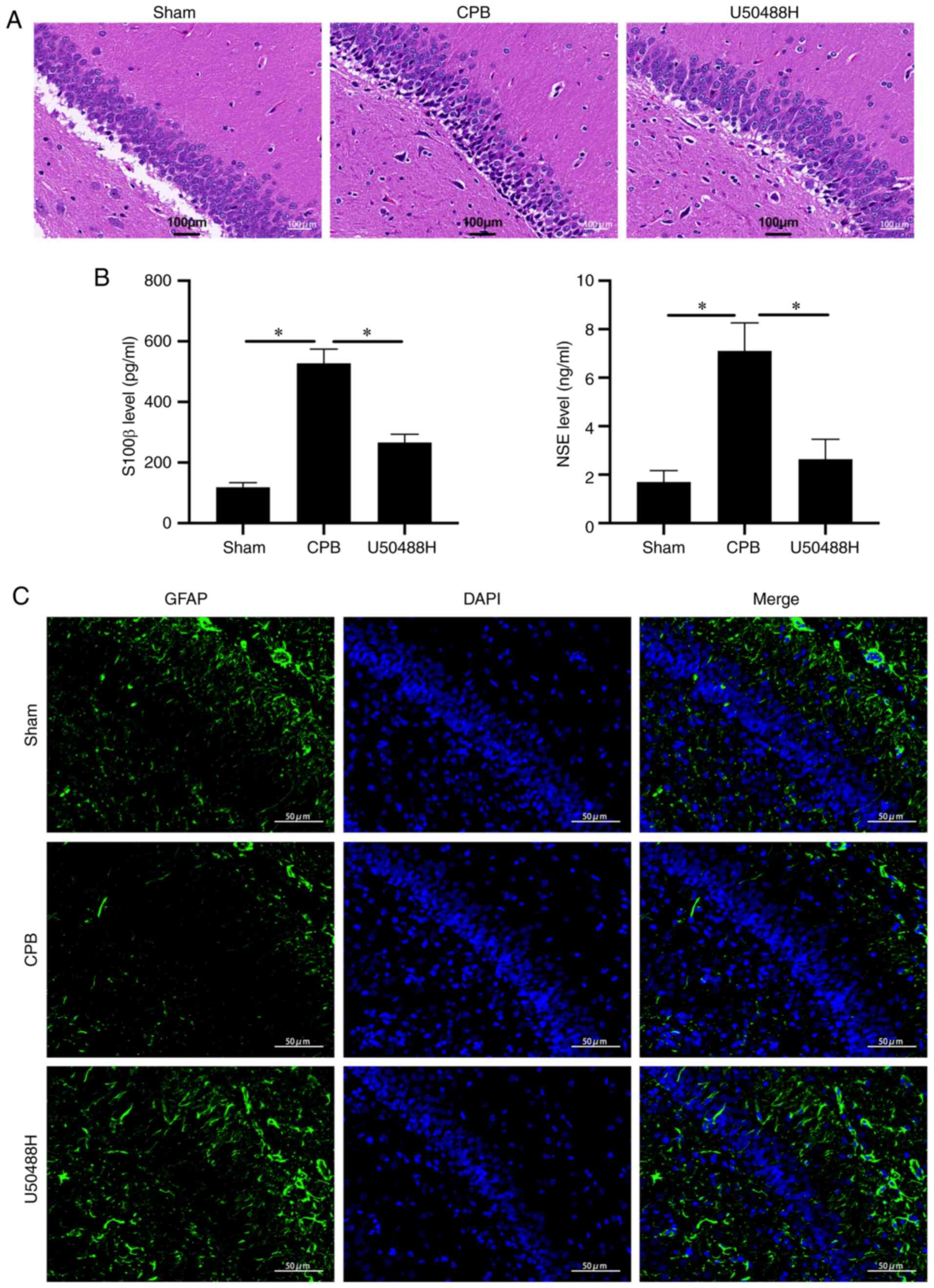
KOR agonist improves brain injury in
CPB rats
To observe the effect of U50448H on hippocampal
neuronal injury, H&E staining was performed and it demonstrated
that, in the CPB group, pyramidal cells in the hippocampal CA1 area
were significantly reduced, the cytoplasm was severely decreased,
nuclear volume was increased, nuclear vacuoles were enlarged and
the intercellular space was widened. Neuronal cell damage was
alleviated following U50488H treatment (Fig. 2A). When brain tissue is damaged,
S100 β and NSE proteins are continuously released into the plasma,
glial cells are damaged to varying degrees and blood-brain barrier
permeability increases (28). The
plasma levels of S100 β and NSE were significantly increased in CPB
model rats and could be significantly reduced by U50488 treatment
(Fig. 2B). In addition, under IF
detection, U50488H was found to increase GFAP content, suggesting
protection of hippocampal pyramidal cells (Fig. 2C). These results suggested that the
KOR agonist significantly improved hippocampal neuron damage in
rats subjected to CPB.
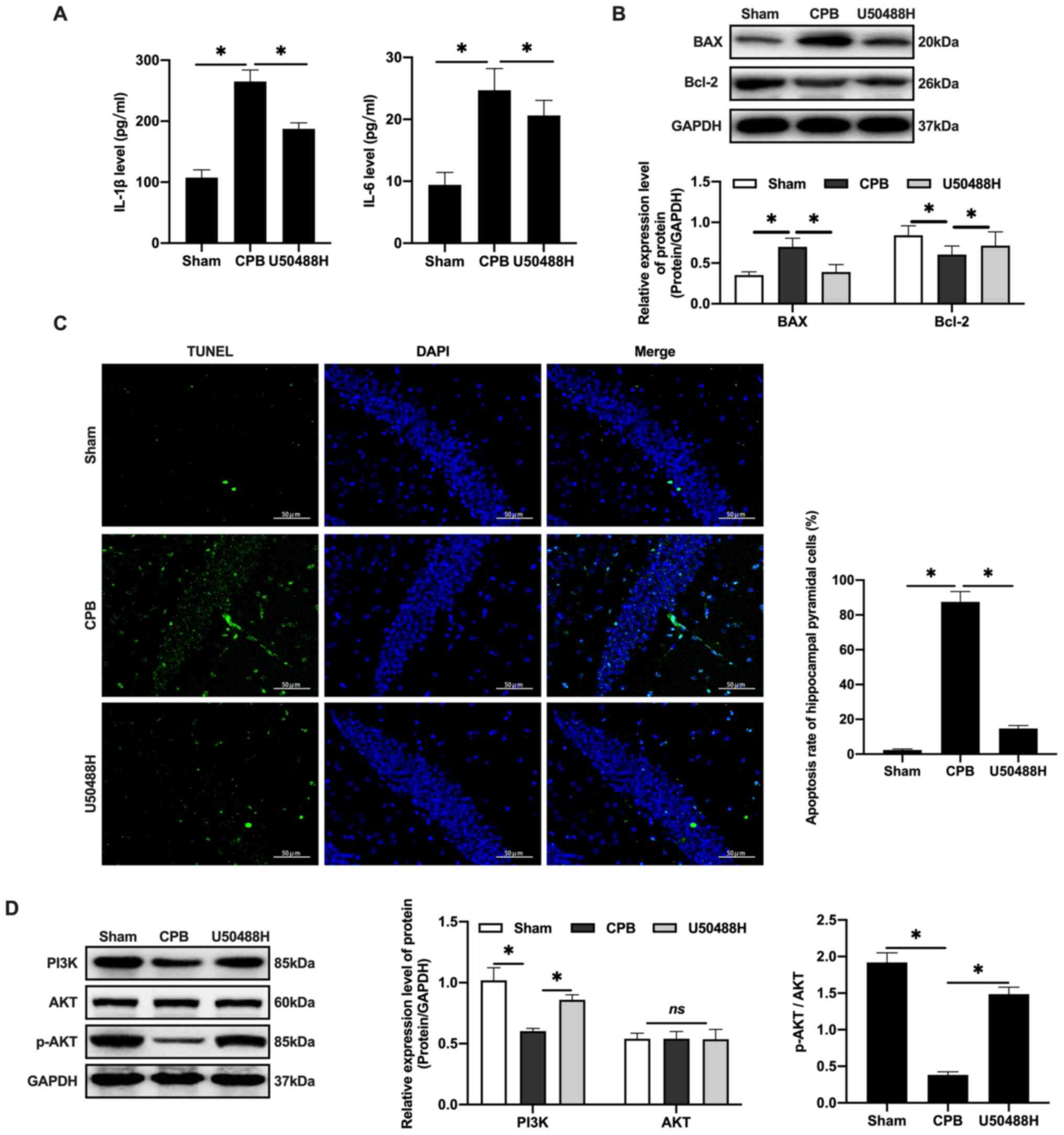
KOR agonist activates PI3K/AKT-related
proteins to inhibit neuronal apoptosis in CPB rats
CPB surgery can promote the release of inflammatory
factors, increase IL-1β and IL-6 plasma levels (Fig. 3A) and promote Bax and inhibit Bcl-2
in hippocampal neurons (Fig. 3B).
U50488H treatment was demonstrated to decrease the apoptosis rate
in rats (Fig. 3C). Previous studies
have demonstrated that activating the PI3K/AKT signaling pathway
can reduce the apoptosis rate of neurons caused by ischemia and
hypoxia and inhibit the expression of Bax, thereby exerting brain
protective effects (29–32). These results confirmed that U50488H
can activate PI3K to phosphorylate of AKT and the activated AKT
could regulate downstream proteins (Fig. 3D) and inhibit apoptosis, thus
protecting cerebral tissue.
KOR agonist activates Nrf2/HO-1 to
inhibit oxidative stress injury in CPB rats
Oxidative stress is the main cause of neuronal
apoptosis (33). Following CPB, it
is difficult for cerebral cells to produce ATP through aerobic
respiration (34,35). Therefore, the MDA and MPO levels
increase and the SOD content decreases in plasma (Fig. 4A). Under U50448H treatment the MDA
and MPO levels decreased, the SOD content increased and ROS
expression in cerebral tissues was significantly reduced compared
with the CPB group (Fig. 4B). In
addition, the expression of the NADPH oxidase subunit components
gp91phox, p22phox and NADPH Oxidase 1 was
upregulated in the CBP group (Fig.
4C) and the expression of these components was also reversed by
U50448H treatment (Fig. 4C).
Nrf2/HO-1 is the main regulatory pathway of oxidative stress. CPB
surgery could induce oxidative stress in the whole rat body and
activate and increase Nrf2 content in the nucleus (Fig. 4D). KOR agonists can promote entrance
of Nrf2 into the nucleus, which then combines with the ARE to
initiate transcription of proteins downstream of ARE, including
HO-1 and Trx-1, thus inhibiting injury (Fig. 4D).
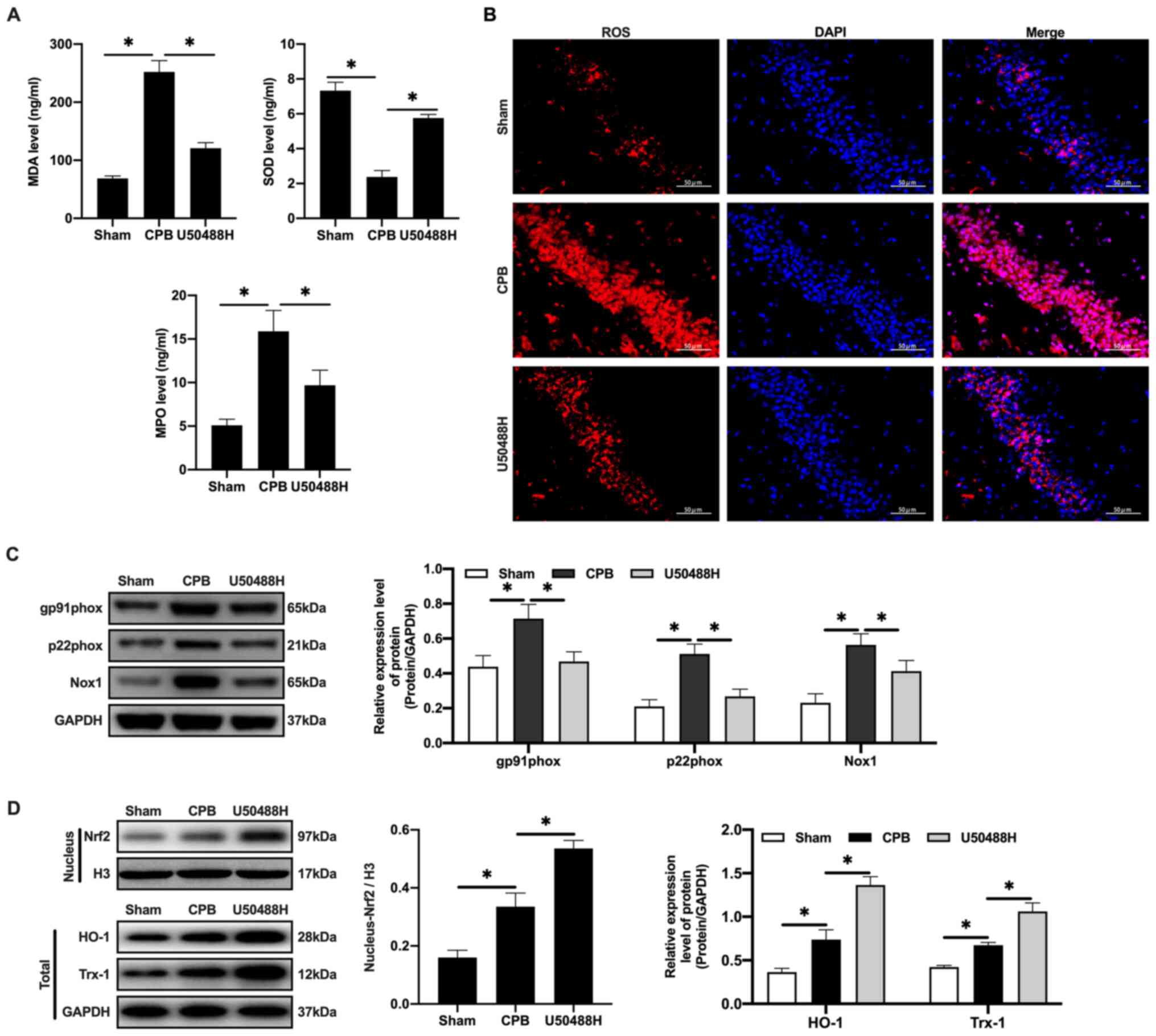 | Figure 4.KOR agonist activates Nrf2/HO-1 to
inhibit oxidative stress injury in CPB rats. (A) Oxidative stress
factors detected by ELISA. (B) The expression of ROS detected by
immunofluorescence (scale bar, 50 µm). (C) The expression of
Nrf2/HO-1 signaling pathway-related proteins detected by western
blotting. (D) Expression levels of gp91phox,
p22phox and Nox1 were detected by western blotting.
n=10; *P<0.05. KOR, κ-opioid receptor; CPB, cardiopulmonary
bypass; Nrf2, nuclear factor erythroid 2-related factor 2; HO-1,
heme oxygenase 1; MDA, malondialdehyde; SOD, superoxide dismutase;
MPO, myeloperoxidase; ROS, reactive oxygen species; Nox1, NADPH
Oxidase 1; Trx1, thioredoxin 1. |
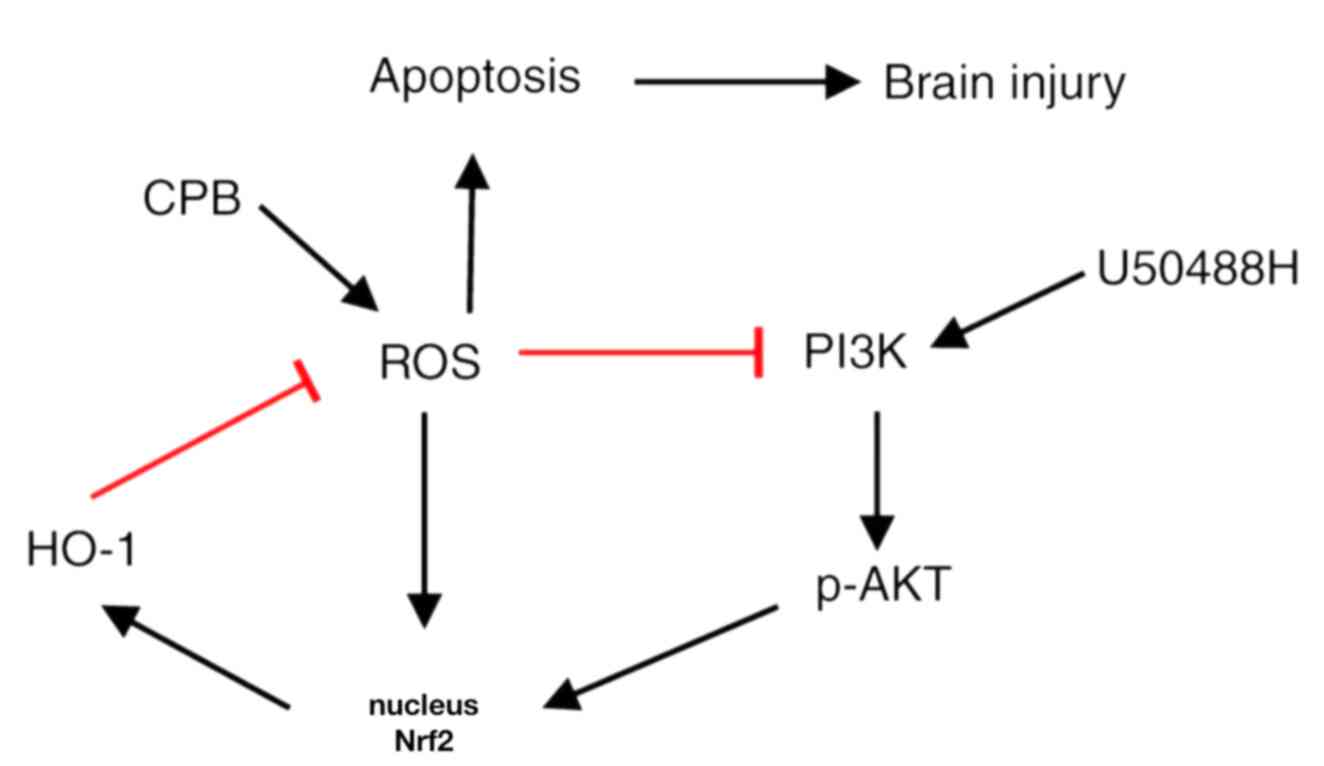
KOR agonist activated Nrf2/HO-1 via
the PI3K/AKT pathway to improve cognitive function in CPB rats
Nrf2 has been reported to be regulated by the
PI3K/AKT signaling pathway (36).
To explore the regulatory mechanism of PI3K/AKT/Nrf2/HO-1 in CPB,
rats were treated with PI3K and HO-1 inhibitors to observe the
protective effects of the KOR agonist on brain injury in CPB rats.
The results demonstrated that compared with the U50488H group, rats
treated with PI3K or HO-1 inhibitors demonstrated significantly
increased neurological deficit scores (Fig. 5A), extended escape latency in the
navigation experiment in the Morris water maze assay (Fig. 5B-E), fewer pyramidal cells and
increased damage in the hippocampal CA1 area (Fig. 5F), an increased apoptosis rate of
neuronal cells (Fig. 5G-H),
promotion of oxidative stress injury (Fig. 5I-J) and increased oxidative stress
injury related protein expression (Fig.
5K). Notably, when PI3K inhibitors were added, entrance of Nrf2
into the nucleus was inhibited and HO-1 and Trx-1 expression levels
were reduced (Fig. 5L). Therefore,
KOR agonists can improve cognitive function and reduce brain damage
in CPB rats through activation of Nrf2/HO-1 and regulation of the
PI3K/AKT pathway.
Discussion
POCD is a common complication following cardiac
surgery and may lead to an extended stay in an intensive care unit
or hospital, increased perioperative complications and mortality
and a decrease in the ability of the patient to lead an independent
life (2). At 1 week following
cardiac surgery, the incidence of POCD is as high as 50–70 and ~40%
of patients still have cognitive dysfunction at five years
following surgery (37,38). CPB surgery can reduce postoperative
neurocognitive ability because of hypoperfusion or low mean
arterial pressure, hemodynamic instability, cerebral thrombosis,
systemic inflammatory responses, anemia, hyperglycemia and
extracorporeal circulation trauma (39). In the present study, a CPB rat model
was successfully established and treated with a KOR agonist and
then the cognitive function, inflammatory response, oxidative
stress injury and apoptosis were observed. The results demonstrated
that a KOR agonist could improve cognitive function and reduce
brain damage in CPB rats, which is related to the activation of the
PI3K/AKT signaling pathway, and activate Nrf2/HO-1, thus inhibiting
oxidative stress injury.
KORs are distributed in the central nervous system
tissue and mRNAs of KORs can be detected in the hippocampal dentate
gyrus, hypothalamus, certain thalamic nuclei, cerebral cortex and
spinal cord (40). KORs have been
demonstrated to regulate emotional and cognitive functions, reduce
brain tissue damage and improve functional recovery in animal
models of cerebral ischemia (5).
Previous studies have also demonstrated that KOR expression is
reduced in the brain of Alzheimer's disease patients (41,42).
Activation of KORs in the brain of Alzheimer's rats can improve
cognitive impairment and protect neuronal cells by inhibiting the
formation of Aβ and its neurotoxicity (43). Charron et al (24) demonstrated that U50488H, a KOR
agonist, can specifically activate hippocampal cholinergic neurons
by activating KORs on hippocampal nerve cells, which can
significantly reduce hippocampal nerve damage caused by ischemia.
In the present study, U50488H (1.5 mg/kg) was injected into the
lateral ventricle before CPB surgery. It was found that U50488H
could reduce the neurological deficit score of rats, shorten the
escape latency, increase the number of crossings of the original
platform and extend the swimming distance and time in the target
quadrant in the Morris water maze and reduce hippocampal injury.
These results suggested that U50488H can improve cognitive function
and reduce brain injury in CPB rats.
The cerebral tissue damage induced by CPB is caused
by the difficulty for brain cells to produce ATP through aerobic
respiration (34,35). Glial cells activated following CPB
can secrete a variety of chemokines and inflammatory mediators,
leading to a large increase in the level of cell adhesion molecules
and disrupting the permeability of vascular endothelial cells,
causing cell dysfunction and eventually leading to apoptosis
(24). Following the activation of
KOR receptors in stroke rats, injury inhibits glutamic acid release
and NO production at the presynaptic membrane (43). Activation of KOR receptors also
reduces neurotoxicity, improves the survival rate of damaged
neurons, reduces neuronal apoptosis and the incidence of cerebral
infarction and can reverse the memory impairment caused by CPB
(44). Using the CPB rat model, the
present study confirmed that oxidative stress served an important
role in brain injury following CPB. CPB stimulated the oxidative
stress response of neuronal cells, which generated high
concentrations of ROS, affecting nerve cells, adversely affecting
metabolism and increasing the rate of neuronal cell apoptosis.
U50488H treatment could inhibit oxidative stress damage, reduce the
ROS content and increase the SOD concentration, thereby effectively
removing excess oxygen free radicals to avoid oxidative stress.
Harmful external environmental changes including
hypoxia can induce the expression of HO-1, which can protect cells
from a variety of harmful extracellular stimuli, including hypoxia
(24). Additionally, HO-1 is
regulated by the transcription factor Nrf2 (36). Under normal physiological
conditions, Nrf2 in combination with the cytoplasmic linker protein
Kelch-like ECH-associated protein 1 localizes to the cytoplasm and
is maintained at a low level (6).
When oxidative stress occurs, Nrf2 is not degraded by the ubiquitin
proteasome and is thus maintained at a high level (5). The PI3K/AKT/Nrf2/HO-1 pathway is an
important pathway in protection against epilepsy and
seizure-induced brain injury under Dynorphin treatment through
activation of κ-opioid receptor (36). The present study demonstrated that
U50488H could activate Nrf2, promote its nuclear translocation,
increase HO-1 expression and then protect cells from oxidative
stress damage. However, when the HO-1 inhibitor ZnPP-IX was
administered, the protective effect of U50488H on CPB rats was
blocked, suggesting that HO-1 mediates U50488H activation of KORs
in the hippocampus of CPB rats, which may be an important mechanism
of neural protection. To further explore the regulatory mechanism
of U50488H, the expression of the upstream protein Nrf2 was
detected. As a result of Nrf2 expression, the PI3K/AKT signaling
pathway was promoted and activated AKT participated in the
anti-apoptosis and oxidative stress processes under U50488H
treatment. In addition, PI3K inhibitor administration could inhibit
Nrf2 and HO-1 expression. Therefore, the present study demonstrated
that KOR agonists could activate Nrf2/HO-1 through the PI3K/AKT
pathway to improve cognitive function and reduce brain damage in
CPB rats (Fig. 6).
Acknowledgements
Not applicable.
Funding
This study was supported by the Key Scientific
Research Project of Liaoning Province (grant no.
2020JH2/10300051).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JF, LL and PQ performed the animal experiment,
prepared the CPB rat model and performed Neurological deficit score
and Morris water maze tests. LL and PQ also performed the H&E
staining, TUNEL assay and immunofluorescence. YD and YS contributed
to acquisition of funding support. JF designed the study. YD and YS
conceived and designed the study, acquired data, interpreted the
results and drafted the manuscript. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the China Medical
University Laboratory Animal Welfare and Ethics Committee (IACUC
no. 2018048R).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Martin J and Cheng DC: Neurologic
complications after cardiac surgery: Stroke, Delirium,
Postoperative Cognitive Dysfunction, and Peripheral Neuropathy.
Evidence-Based Practice in Perioperative Cardiac Anesthesia and
Surgery. Springer; pp. 619–636. 2020
|
|
2
|
Keith JR, Puente AE, Malcolmson KL, Tartt
S, Coleman AE and Marks HF Jr: Assessing postoperative cognitive
change after cardiopulmonary bypass surgery. Neuropsychology.
16:411–421. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kumpaitiene B, Svagzdiene M, Sirvinskas E,
Adomaitiene V, Petkus V, Zakelis R, Krakauskaite S, Chomskis R,
Ragauskas A and Benetis R: Cerebrovascular autoregulation
impairments during cardiac surgery with cardiopulmonary bypass are
related to postoperative cognitive deterioration: Prospective
observational study. Minerva Anestesiol. 85:594–603. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen K, Sun Y, Dong W, Zhang T, Zhou N, Yu
W, Diao Y, Guo S and Tian Y: Activated A7nachr improves
postoperative cognitive dysfunction and intestinal injury induced
by cardiopulmonary bypass in rats: Inhibition of the
proinflammatory response through the Th17 immune response. Cell
Physiol Biochem. 46:1175–1188. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kobayashi M, Li L, Iwamoto N,
Nakajima-Takagi Y, Kaneko H, Nakayama Y, Eguchi M, Wada Y, Kumagai
Y and Yamamoto M: The antioxidant defense system Keap1-Nrf2
comprises a multiple sensing mechanism for responding to a wide
range of chemical compounds. Mol Cell Biol. 29:493–502. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Takaya K, Suzuki T, Motohashi H, Onodera
K, Satomi S, Kensler TW and Yamamoto M: Validation of the multiple
sensor mechanism of the Keap1-Nrf2 system. Free Radic Biol Med.
53:817–827. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen HH, Chen YT, Huang YW, Tsai HJ and
Kuo CC: 4-Ketopinoresinol, a novel naturally occurring ARE
activator, induces the Nrf2/HO-1 axis and protects against
oxidative stress-induced cell injury via activation of PI3K/AKT
signaling. Free Radic Biol Med. 52:1054–1066. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Netto MB, de Oliveira Junior AN, Goldim M,
Mathias K, Fileti ME, da Rosa N, Laurentino AO, de Farias BX, Costa
AB, Rezin GT, et al: Oxidative stress and mitochondrial dysfunction
contributes to postoperative cognitive dysfunction in elderly rats.
Brain Behav Immun. 73:661–669. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Narasimhan M, Patel D, Vedpathak D,
Rathinam M, Henderson G and Mahimainathan L: Identification of
novel microRNAs in post-transcriptional control of Nrf2 expression
and redox homeostasis in neuronal, SH-SY5Y cells. PLoS One.
7:e511112012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hong-Qiang H, Mang-Qiao S, Fen X,
Shan-Shan L, Hui-Juan C, Wu-Gang H, Wen-Jun Y and Zheng-Wu P: Sirt1
mediates improvement of isoflurane-induced memory impairment
following hyperbaric oxygen preconditioning in middle-aged mice.
Physiol Behav. 195:1–8. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gozzelino R, Jeney V and Soares MP:
Mechanisms of cell protection by heme oxygenase-1. Annu Rev
Pharmacol Toxicol. 50:323–354. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yu M, Wang J, Fang Q, Liu P, Chen S, Zhe
N, Lin X, Zhang Y, Zhao J and Zhou Z: High expression of heme
oxygenase-1 in target organs may attenuate acute graft-versus-host
disease through regulation of immune balance of TH17/Treg. Transpl
Immunol. 37:10–17. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu B, Song HL, Yang Y, Yin ML, Zhang BY,
Cao Y, Dong C and Shen ZY: Improvement of liver transplantation
outcome by heme oxygenase-1-transduced bone marrow mesenchymal stem
cells in rats. Stem Cells Int. 2016:92350732016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Billings FT IV, Yu C, Byrne JG, Petracek
MR and Pretorius M: Heme oxygenase-1 and acute kidney injury
following cardiac surgery. Cardiorenal Med. 4:12–21. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lever JM, Boddu R, George JF and Agarwal
A: Heme oxygenase-1 in kidney health and disease. Antioxid Redox
Signal. 25:165–183. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chang M, Xue J, Sharma V and Habtezion A:
Protective role of hemeoxygenase-1 in gastrointestinal diseases.
Cell Mol Life Sci. 72:1161–1173. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hu L, Chen W, Tian F, Yuan C, Wang H and
Yue H: Neuroprotective role of fucoxanthin against cerebral
ischemic/reperfusion injury through activation of Nrf2/HO-1
signaling. Biomed Pharmacother. 106:1484–1489. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Balogun E, Hoque M, Gong P, Killeen E,
Green CJ, Foresti R, Alam J and Motterlini R: Curcumin activates
the haem oxygenase-1 gene via regulation of Nrf2 and the
antioxidant-responsive element. Biochem J. 371:887–895. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fang J, Wang H, Zhou J, Dai W, Zhu Y, Zhou
Y, Wang X and Zhou M: Baicalin provides neuroprotection in
traumatic brain injury mice model through Akt/Nrf2 pathway. Drug
Des Devel Ther. 12:2497–2508. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang P, Cao J, Liu N, Ma L, Zhou X, Zhang
H and Wang Y: Protective effects of edaravone in adult rats with
surgery and lipopolysaccharide administration-induced cognitive
function impairment. PLoS One. 11:e01537082016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Meng X, Wang M, Sun G, Ye J, Zhou Y, Dong
X, Wang T, Lu S and Sun X: Attenuation of Aβ25-35-induced parallel
autophagic and apoptotic cell death by gypenoside XVII through the
estrogen receptor-dependent activation of Nrf2/ARE pathways.
Toxicol Appl Pharmacol. 279:63–75. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zou Y, Hong B, Fan L, Zhou L, Liu Y, Wu Q,
Zhang X and Dong M: Protective effect of puerarin against
beta-amyloid-induced oxidative stress in neuronal cultures from rat
hippocampus: Involvement of the GSK-3β/Nrf2 signaling pathway. Free
Radic Res. 47:55–63. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chunhua C, Chunhua X, Megumi S and Renyu
L: Kappa opioid receptor agonist and brain ischemia. Transl
Perioper Pain Med. 1:27–34. 2014.PubMed/NCBI
|
|
24
|
Charron C, Messier C and Plamondon H:
Neuroprotection and functional recovery conferred by administration
of kappa- and delta 1-opioid agonists in a rat model of global
ischemia. Physiol Behav. 93:502–511. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sun Y, Song D, Wang M, Chen K and Zhang T:
α7 nicotinic acetylcholine receptor agonist attenuates the cerebral
injury in a rat model of cardiopulmonary bypass by activating the
Akt/GSK3β pathway. Mol Med Rep. 16:7979–7986. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mackensen GB, Sato Y, Nellgård B, Pineda
J, Newman MF, Warner DS and Grocott HP: Cardiopulmonary bypass
induces neurologic and neurocognitive dysfunction in the rat.
Anesthesiology. 95:1485–1491. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Johnsson P, Lundqvist C, Lindgren A,
Ferencz I, Alling C and Ståhl E: Cerebral complications after
cardiac surgery assessed by S-100 and NSE levels in blood. J
Cardiothorac Vasc Anesth. 9:694–699. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen K, Li G, Geng F, Zhang Z, Li J, Yang
M, Dong L and Gao F: Berberine reduces ischemia/reperfusion-induced
myocardial apoptosis via activating AMPK and PI3K-Akt signaling in
diabetic rats. Apoptosis. 19:946–957. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li X, Zhang J, Zhu X, Wang P, Wang X and
Li D: Progesterone reduces inflammation and apoptosis in neonatal
rats with hypoxic ischemic brain damage through the PI3K/Akt
pathway. Int J Clin Exp Med. 8:81972015.PubMed/NCBI
|
|
31
|
Pachori AS, Smith A, McDonald P, Zhang L,
Dzau VJ and Melo LG: Heme-oxygenase-1-induced protection against
hypoxia/reoxygenation is dependent on biliverdin reductase and its
interaction with PI3K/Akt pathway. J Mol Cell Cardiol. 43:580–592.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang L, Qu Y, Tang J, Chen D, Fu X, Mao M
and Mu D: PI3K/Akt signaling pathway is required for
neuroprotection of thalidomide on hypoxic-ischemic cortical neurons
in vitro. Brain Res. 1357:157–165. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Trushina E and McMurray C: Oxidative
stress and mitochondrial dysfunction in neurodegenerative diseases.
Neuroscience. 145:1233–1248. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Schurr A: Lactate, glucose and energy
metabolism in the ischemic brain (Review). Int J Mol Med.
10:131–136. 2002.PubMed/NCBI
|
|
35
|
Arrica M and Bissonnette B: Therapeutic
hypothermia. Semin Cardiothorac Vasc Anesth. 11:6–15. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dai H, Wang P, Mao H, Mao X, Tan S and
Chen Z: Dynorphin activation of kappa opioid receptor protects
against epilepsy and seizure-induced brain injury via
PI3K/Akt/Nrf2/HO-1 pathway. Cell Cycle. 18:226–237. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kennedy DO and Haskell CF: Cerebral blood
flow and behavioural effects of caffeine in habitual and
non-habitual consumers of caffeine: A near infrared spectroscopy
study. Biol Psychol. 86:298–306. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Neema PK, Dharan BS, Singha SK, Sethuraman
M and Rathod RC: Entropy score, patent ductus arteriosus (PDA), and
cardiopulmonary bypass (CPB): Ligation of PDA on CPB can compromise
cerebral blood flow. Ann Card Anaesth. 14:203–205. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gottesman RP, Grega MA, McKhann GM and
Selnes OA: Neurological complications of cardiac surgery: Stroke,
encephalopathy, and cognitive decline. Brain disorders in critical
illness: Mechanisms, diagnosis, and treatment. Camb Univ Press; pp.
410–418. 2011
|
|
40
|
Simonin F, Gavériaux-Ruff C, Befort K,
Matthes H, Lannes B, Micheletti G, Mattéi MG, Charron G, Bloch B
and Kieffer B: kappa-opioid receptor in humans: cDNA and genomic
cloning, chromosomal assignment, functional expression,
pharmacology, and expression pattern in the central nervous system.
Proc Natl Acad Sci USA. 92:7006–7010. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Silvia RC, Slizgi GR, Ludens JH and Tang
AH: Protection from ischemia-induced cerebral edema in the rat by
U-50488H, a kappa opioid receptor agonist. Brain Res. 403:52–57.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cai Z and Ratka A: Opioid system and
Alzheimer's disease. Neuromolecular Med. 14:91–111. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Rácz B and Halasy K: Kappa opioid receptor
is expressed by somatostatin- and neuropeptide Y-containing
interneurons in the rat hippocampus. Brain Res. 931:50–55. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li X, Sun Y, Jin Q, Song D and Diao Y:
Kappa opioid receptor agonists improve postoperative cognitive
dysfunction in rats via the JAK2/STAT3 signaling pathway. Int J Mol
Med. 44:1866–1876. 2019.PubMed/NCBI
|