Introduction
Mycoplasma pneumoniae pneumonia (MPP) is a
type of pneumonia induced by M. pneumoniae infection
(1). MPP frequently occurs in
children and infants. The clinical presentation is low-grade fever,
cough and asthma-like symptoms (2).
Despite the advancement in medical science, the incidence of severe
or fatal MPP continues to rise (3).
Severe MPP is characterized by pulmonary fibrosis, obstructive
bronchiolitis and copious pleural effusion (PE), and may be
life-threatening (4). Macrolide
antibiotics are the first-line therapy for MPP (5). However, macrolide resistance develops
frequently in children with MPP, with a rate of 87.2% reported in
one previous study (6). Alternative
and effective therapeutic strategies in children with MPP are
required.
Long non-coding RNAs (lncRNAs) are essential for the
regulation of respiratory diseases, including idiopathic pulmonary
fibrosis (IPF) (7), acute lung
injury (ALI) (8), and pneumonia
(9). The growth arrest-specific 5
(GAS5) lncRNA is crucial in numerous inflammatory diseases. GAS5
upregulation alleviates renal fibrosis and inflammatory reactions
in rats with diabetic nephropathy (10). GAS5-silencing decreases the
viability and aggravates the inflammatory injury of
lipopolysaccharide (LPS)-induced chondrocytes (11). Notably, GAS5 is poorly expressed in
ALI, and its overexpression attenuates inflammation in ALI mice
(12). However, the precise role of
GAS5 in the progression of MPP remains unclear.
As biological molecules, microRNAs (miRNAs)
participate in the progression of pneumonia. miR-217 contributes
toward lung injury and inflammation in interstitial pneumonia
(13). miR-21 is upregulated in and
promotes the development of ventilator-associated pneumonia
(14). miR-155 was reported to
induce impaired bacterial clearance and increase mortality in
patients suffering from pneumonia that developed following
influenza (15). Importantly,
miR-222-3p expression is enhanced in children with MPP (16). miR-222-3p is a target of GAS5 in
papillary thyroid carcinoma (17).
However, the specific regulatory association between GAS5 and
miR-222-3p in MPP remains to be elucidated.
The regulatory functions of miRNAs involve the
targeting of mRNAs through complementary sequences (18). Tissue inhibitor of metalloproteinase
3 (TIMP3), a member of the TIMP family, participates in various
inflammatory diseases, including liver ischemia/reperfusion injury
(19), osteoarthritis (20) and IPF (21). Notably, miR-222-3p may directly
target TIMP3 in osteosarcoma (22).
However, the potential regulatory mechanism of GAS5 associated with
the miR-222-3p/TIMP3 axis in MPP remains unknown.
The present study investigated the expression of
GAS5, miR-222-3p and TIMP3 in patients with MPP. Lipid-associated
membrane proteins (LAMPs) were induced in THP-1 cells to model MPP.
The associations among GAS5, miR-222-3p and TIMP3 were confirmed.
Subsequently, whether GAS5 controlled the viability and
inflammation of LAMP-induced THP-1 cells by regulating the
miR-222-3p/TIMP3 axis was investigated. The findings indicated a
potential novel therapeutic target for MPP.
Materials and methods
Patients and samples
A total of 25 children with MPP from the Pediatric
Intensive Care Unit of the Liaocheng Second People's Hospital
(Linqing, China) were enrolled between July 2017 and October 2018.
Exclusion criteria included premature delivery, immunodeficiency,
recurrent pneumonia and recent use of immunomodulators and
immunosuppressive agents. There were 16 children with PE and nine
children without PE. Additionally, 25 healthy children comprised
the control group. The healthy children had no history of MPP,
immune system diseases, or other acute and chronic infectious
diseases. Peripheral blood samples were collected from children in
the MPP and control groups. A portion of the peripheral blood
samples were used for the measurement of inflammatory cytokines and
another portion was used to extract peripheral blood mononuclear
cells (PBMCs). Next, the PBMCs were isolated by Ficoll-Plaque
density gradient centrifugation (GE Healthcare) from all blood
samples at 1,000 × g for 30 min at 20°C. The PBMC layer was
extracted and washed by adding three volumes of PBS, centrifuged at
250 × g for 10 min at 20°C. The PBMCs were maintained in RPMI-1640
medium (Invitrogen; Thermo Fisher Scientific, Inc.), containing 10%
fetal bovine serum (FBS; Invitrogen; Thermo Fisher Scientific,
Inc.) at 37°C in an atmosphere of 5% CO2. Logarithmic
growth phase cells were used for further assays. The present study
was conducted in accordance with the Declaration of Helsinki and
was approved by the Ethics Committee of the Liaocheng Second
People's Hospital (approval no. 2017–013). Written informed consent
was obtained from each child and their guardian.
Extraction of LAMPs from MP
MP M129 [29342; American Type Culture Collection
(ATCC)] was used as the standard MP strain. The MP was cultured in
PPLO broth medium (BioLife) at 37°C for 5–7 days and harvested when
the red pH indicator turned orange. Following centrifugation at
10,000 × g for 20 min at 4°C, MP pellets were resuspended in 10 ml
Tris-buffered saline (TBS; 50 mM Tris pH 8.0, 0.15 M NaCl),
containing 1 mM EDTA (TBSE). MP was then lysed for 1 h at 4°C by
adding 2% (v/v) Triton X-114 into the suspension. To isolate LAMPs,
the lysate was incubated for 10 min at 37°C to allow phase
separation. The upper aqueous phase was removed and replaced with
the same volume of TBSE. The phase separation procedure was
repeated twice. The final phase was resuspended in TBSE to the
original volume, and 2.5 volumes of ethanol were added to
precipitate LAMPs at −20°C overnight. After the supernatant was
discarded, the isolated LAMPs were resuspended in PBS, treated with
low temperature ultrasound and stored at −70°C. The concentration
of LAMPs was determined using an enhanced BCA Protein assay kit
(Beyotime Institute of Biotechnology).
Cell culture and treatment
THP-1 cells (1×105 cells/ml), obtained
from ATCC, were cultured in RPMI-1640 medium containing 10% FBS
(Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C in an
atmosphere of 5% CO2. THP-1 cells were harvested and
centrifuged after reaching exponential growth. The resulting cell
pellet was suspended in serum-free RPMI-1640 medium,
1×106 cells were distributed across a 24-well plate. The
cells were co-cultured with different levels of LAMPs (2, 4 and 6
µg/ml) for 16 h. Cells in the control group were treated with PBS.
THP-1 cells co-cultured with 6 µg/ml LAMPs were considered as
LAMP-induced THP-1 cells and were examined in subsequent
experiments.
Cell transfection
Short hairpin (sh)-GAS5, sh-negative control (NC)
and sh-TIMP3 were synthesized by Shanghai GenePharma Co., Ltd., and
then inserted into the pGLVU6/Puro vector (Shanghai GenePharma Co.,
Ltd.). The pcDNA3.1-NC (pcDNA3.1), pcDNA3.1-GAS5 (oe-GAS5),
miR-222-3p mimics (sense, 5′-AGCUACAUCUGGCUACUGGGU-3′ and
antisense, 5′-CCAGUAGCCAGAUGUAGCUUU-3′), NC mimics (sense,
5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense,
5′-ACGUGACACGUUCGGAGAATT-3′), miR-222-3p inhibitor
(5′-ACCCAGUAGCCAGAUGUAGCU-3′), and NC inhibitor
(5′-CAGUACUUUUGUGUAGUACAA-3′) were synthesized by Shanghai
GenePharma Co., Ltd. LAMP-induced THP-1 cells (1×105
cells/well) were seeded onto a 24-well plate and cultured until
growth was 80% confluent. The lipid complex was pre-prepared by
mixing 25 µl Lipofectamine® 3000 (Invitrogen; Thermo
Fisher Scientific, Inc.) diluted in serum-free RPMI-1640 medium and
25 µl specific nucleic acids
(sh-GAS5/sh-TIMP3/sh-NC/oe-GAS/pcDNA3.1 and/or miR-222-3p mimics or
mimics NC/miR-222-3p inhibitor/inhibitor NC; shRNA, 200 ng; miRNA,
50 nM) diluted in serum-free medium for 10 min. Cells were then
incubated with 50 µl lipid complex for 48 h at 37°C. After 48 h
transfection, the transfected cells were used for subsequent
assays.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from cells using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). The cDNA
samples were obtained through reverse transcription (37°C for 15
min; 85°C for 5 sec) using the PrimeScript RT Reagent kit (Takara
Bio, Inc.). miScript SYBR Green PCR kit (Qiagen, Inc.) was used to
conduct a qPCR analysis. RT-qPCR was performed using a 7500
Real-time PCR system (Applied Biosystems; Thermo Fisher Scientific,
Inc.) under the following conditions: 95°C for 3 min and 40 cycles
of 95°C for 15 sec and 60°C for 30 sec, and a final extension step
at 72°C for 10 min. Relative expression was calculated using the
2−ΔΔCq method (23).
GAPDH, U6 and β-actin were used for the normalization of GAS5,
miR-222-3p and TIMP3, respectively. The primer sequences are
presented in Table I.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Name of primer | Sequence
(5′-3′) |
|---|
| GAS5-F |
CTTCTGGGCTCAAGTGATCCT |
| GAS5-R |
TTGTGCCATGAGACTCCATCAG |
| GAPDH-F |
CGACTTATACATGGCCTTA |
| GAPDH-R |
TTCCGATCACTGTTGGAAT |
| miR-222-3p-F |
AGCTACATCTGGCTACTGGGT |
| miR-222-3p-R |
GCGAGCACAGAATTAATACGAC |
| U6-F |
CTCGCTTCGGCAGCACA |
| U6-R |
AACGCTTCACGAATTTGCGT |
| TIMP3-F |
ACCGAGGCTTCACCAAGATG |
| TIMP3-R |
CATCATAGACGCGACCTGTCA |
| β-actin-F |
TGGAATCCTGTGGCATCCATGAAAC |
| β-actin-R |
ACGCAGCTCAGTAACAGTCCG |
Western blot analysis
A Nuclear and Cytoplasmic Protein Extraction kit
(Beyotime Institute of Biotechnology) was used to extract nuclear
and cytoplasmic proteins. PBMCs were lysed using RIPA lysis buffer
(Beyotime Institute of Biotechnology) on ice to extract the total
protein. Protein concentration was evaluated by bicinchoninic acid
assay (Beyotime Institute of Biotechnology). Equal amounts (30 µg)
of protein samples were separated by 10% SDS-PAGE. The separated
proteins were transferred to polyvinylidene fluoride membranes,
blocked with 5% skimmed milk for 1 h at 37°C, and incubated at 4°C
overnight with primary antibodies against anti-TIMP3 (1:1,000; cat.
no. SAB4502973; Sigma-Aldrich; Merck KGaA) or anti-Tubulin (1:200;
cat. no. T3526; Sigma-Aldrich; Merck KGaA). The membranes were then
incubated with a horseradish peroxidase-labeled goat anti-rabbit
IgG (1:5,000; cat. no. 12-348; Sigma-Aldrich; Merck KGaA) secondary
antibody for 1 h at 25°C. Finally, the bands were visualized using
an enhanced chemiluminescence kit (Invitrogen; Thermo Fisher
Scientific, Inc.). Protein bands were semi-quantified by
densitometric analysis using ImageJ software (version 1.51,
National Institutes of Health).
Viability assay
The viability of LAMP-induced THP-1 cells was
measured using an MTT cell proliferation assay kit (Sigma-Aldrich;
Merck KGaA). In brief, the LAMP-induced THP-1 cells with different
transfections were seeded onto 96-well plates (2×103
cells/well) and cultured with 5% CO2 at 37°C. When the
cell reached 80% confluence, they were incubated with 20 µg MTT
reagents for 2 h at 37°C in a humidified culture chamber supplied
with 5% CO2. Following incubation, supernatants were
removed and 150 µl DMSO was added to dissolve the formazan
crystals. The optical density values were measured at 450 nm using
a plate reader and were used to evaluate cell viability.
ELISA
Peripheral blood samples from each group were
transferred to a serum separating tube and centrifuged at 1,000 × g
at 4°C for 10 min. The serum was harvested. Additionally,
LAMP-induced THP-1 cells from each group were centrifuged at 1,000
× g at 4°C for 10 min. Each supernatant was collected. The levels
of interleukin (IL)-6 and interferon (IFN)-γ in serum were measured
using a Human IL-6 ELISA kit (cat. no. ab178013; Abcam) and Human
IFN-γ ELISA kit (cat. no. ab46025; Abcam). The levels of IL-1β,
IL-6, tumor necrosis factor-α (TNF-α) and heme oxygenase-1 (HO-1)
in LAMP-induced THP-1 cells were assessed using Human IL-1β ELISA
kit (cat. no. ab214025; Abcam), Human IL-6 ELISA kit (cat. no.
ab178013; Abcam), Human TNF-α ELISA kit (cat. no. ab181421; Abcam)
and Human HO-1 ELISA kit (cat. no. ab207621; Abcam),
respectively.
Dual-luciferase reporter assay
The potential binding sites of GAS5 and miR-222-3p
or miR-222-3p and TIMP3 were predicted by starBase (http://starbase.sysu.edu.cn/starbase2/)
or TargetScan (http://www.targetscan.org/vert_72/). Wild-type (WT)
fragments of the 3′-UTR of GAS5/TIMP3 with putative binding sites
of miR-222-3p were purchased from Shanghai GenePharma Co., Ltd. and
cloned into a psiCHECK-2 Dual-Luciferase miRNA Target Expression
Vector (Promega Corporation). GAS5/TIMP3-3′-UTR-Mut reporter
containing mutant miR-222-3p binding sites was used and generated
using a Quikchange Multi Site-directed Mutagenesis kit (Stratagene;
Agilent Technologies, Inc.). Subsequently, the recombinant vectors
were co-transfected with miR-NC or miR-222-3p mimics into THP-1
cells. Luciferase activity was evaluated 48 h post-transfection by
Dual Luciferase Reporter assay system (Promega Corporation), and
firefly luciferase activity was normalized to that of
Renilla luciferase.
Statistical analyses
All statistical analyses were performed using
GraphPad Prism 7.0 (GraphPad Software, Inc.). Data are presented as
the mean ± standard deviation. The differences between two groups
or among multiple groups were assessed using Student's t-test or
one-way analysis of variance followed by Tukey's post-hoc test. The
significance of the correlations was determined by Pearson's
correlation analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
Decreased GAS5 expression in PBMCs of
patients with MPP
The demographic and clinical characteristics of the
children with MPP and control groups are presented in Table II. Age, sex and white blood cell
counts were not significantly different between the MPP and control
groups. The levels of neutrophils and C-reactive protein in
children with MPP were significantly higher than those of the
control group (P<0.001). RT-qPCR was performed to confirm
whether GAS5 was differentially expressed in the PBMCs of patients
with MPP. GAS5 expression was significantly decreased in PBMCs of
the MPP group compared with the control group (P<0.001; Fig. 1A). Additionally, GAS5 expression was
significantly decreased in MPP cases with PE compared with those
without PE (P<0.001; Fig. 1B).
The level of GAS5 expressed by THP-1 cells significantly decreased
in a dose-dependent manner following induction with LAMPs
(P<0.001; Fig. 1C). Ten
peripheral blood samples were randomly selected from control and
MMP groups, respectively. Next, the levels of IL-6 and IFN-γ in
serum were measured by ELISA. The results demonstrated that the
levels of IL-6 and IFN-γ in serum in the MMP group were higher than
those in the control group (P<0.001; Fig. 1D).
 | Figure 1.GAS5 expression is decreased in the
PBMCs of patients with MPP. (A) The expression of GAS5 in PBMCs of
patients with MPP and the control group was detected by RT-qPCR.
***P<0.001 vs. Control. (B) The expression of GAS5 in PBMCs of
patients with MPP with and without PE was assessed by RT-qPCR.
***P<0.001 vs. MPP without PE. (C) The expression of GAS5 in
different concentrations of LAMPs (2, 4 and 6 µg/ml) treated THP-1
cells was measured by RT-qPCR. ***P<0.001 vs. Control;
#P<0.05, ###P<0.001 vs. 2 µg/ml;
&&&P<0.001 vs. 4 µg/ml. (D) The levels of
IL-6 and IFN-γ in serum in the control and MMP groups were measured
by ELISA. ***P<0.001 vs. Control. PBMCs, peripheral blood
mononuclear cells; MPP, Mycoplasma pneumoniae pneumonia;
RT-qPCR, reverse transcription-quantitative PCR; PE, pleural
effusion; LAMPs, lipid-associated membrane proteins; IL,
interleukin; IFN, interferon; GAS5, growth arrest-specific 5. |
 | Table II.Demographic and clinical
characteristics of children with MPP and controls. |
Table II.
Demographic and clinical
characteristics of children with MPP and controls.
| Parameter | MPP (n=25) | Control (n=25) | P-value |
|---|
| Age, mean ± SD,
years | 6.5±2.8 | 6.7±2.8 |
0.786 |
| Sex, male/female,
n | 13/12 | 14/11 |
0.365 |
| White blood cells,
mean ± SD, ×109/l | 8.2±3.7 | 7.6±1.9 |
0.126 |
| Neutrophils mean ±
SD, ×109/l | 7.9±2.6 | 1.9±0.7 | <0.001 |
| C-reactive protein,
25th-75th percentile, mg/l | 17.1
(9.8–43.5) | 0.16
(0.09–0.7) | <0.001 |
| Lactate
dehydrogenase, mean ± SD, U/l | 456.4±150.5 | – | – |
| Lymphocytes
subgroups, mean ± SD, % |
|
|
|
|
CD3+ | 67.9±8.6 | – | – |
|
CD3+CD4+ | 36.7±7.1 | – | – |
|
CD3+CD8+ | 27.6±6.9 | – | – |
|
CD3−CD19+ | 18.5±5.1 | – | – |
| CD3−CD
(16+ 56+) | 12.8±6.5 | – | – |
|
CD19+CD23+ | 9.1±4.4 | – | – |
GAS5 enhances the viability and
inhibits the inflammation of LAMP-induced THP-1 cells
To investigate the effect of GAS5 on MPP in
vitro, LAMP-induced THP-1 cells were used as the MPP model at
the cellular level. As demonstrated in Fig. 2A, GAS5 expression was significantly
inhibited or enhanced by the transfection of sh-GAS5 or oe-GAS5
into LAMP-induced THP-1 cells (P<0.001). The MTT assay revealed
that GAS5-silencing significantly decreased the viability
(P<0.01), while GAS5-overexpression significantly increased the
viability of LAMP-induced THP-1 cells (P<0.001; Fig. 2B). The levels of IL-1β, IL-6 and
TNF-α in LAMP-induced THP-1 cells were significantly increased by
GAS5 silencing and decreased when GAS5 was overexpressed
(P<0.001; Fig. 2C-E).
Transfection of sh-GAS5 or oe-GAS5 significantly downregulated or
upregulated the level of the anti-inflammatory cytokine HO-1 in
LAMP-induced THP-1 cells (P<0.001; Fig. 2F).
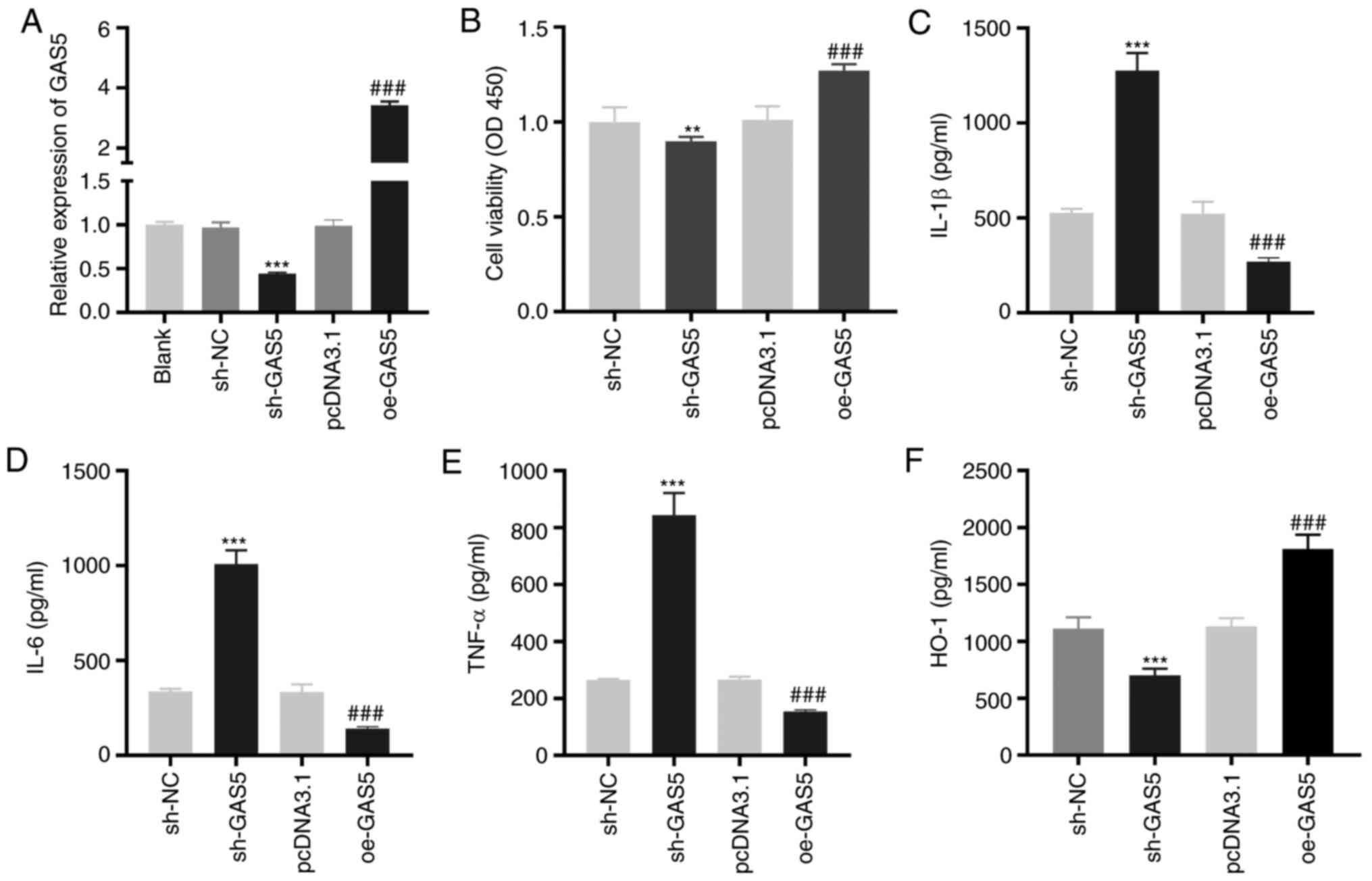 | Figure 2.GAS5 increases the viability and
inhibits the inflammation of LAMP-induced THP-1 cells. (A) The
transfection efficiency of sh-NC, sh-GAS5, pcDNA3.1 and oe-GAS5 in
LAMP-induced THP-1 cells was investigated by reverse
transcription-quantitative PCR. ***P<0.001 vs. sh-NC;
###P<0.001 vs. pcDNA3.1. (B) The viability of
LAMP-induced THP-1 cells was detected by MTT assay. **P<0.01 vs.
sh-NC; ###P<0.001 vs. pcDNA3.1. (C-F) ELISA was
performed to confirm the level of IL-1β, IL-6, TNF-α and HO-1 in
LAMP-induced THP-1 cells. ***P<0.001 vs. sh-NC;
###P<0.001 vs. pcDNA3.1. LAMPs, lipid-associated
membrane proteins; sh, short-hairpin RNA; NC, negative control; IL,
interleukin; TNF-α, tumor necrosis factor-α; HO-1, heme
oxygenase-1; GAS5, growth arrest-specific 5. |
miR-222-3p as a target of GAS5
Using the starBase online software, it was found
that GAS5 contained the target site of miR-222-3p (Fig. 3A). A dual-luciferase reporter assay
confirmed that miR-222-3p mimics reintroduction clearly decreased
the luciferase activity in THP-1 cells transfected with GAS5 WT
compared with the activity observed in miR-NC (P<0.001; Fig. 3B). RT-qPCR revealed that miR-222-3p
expression was significantly enhanced in PBMCs of patients with MPP
(P<0.001; Fig. 3C). As
demonstrated in Fig. 3D, a negative
correlation between GAS5 and miR-222-3p expression was observed in
PBMCs of all participants (R2=0.556, P<0.0001).
miR-222-3p was highly expressed in patients with MPP with PE
(P<0.001; Fig. 3E). Notably,
downregulation or upregulation of GAS5 significantly increased or
decreased, respectively, miR-222-3p expression in LAMP-induced
THP-1 cells (P<0.001; Fig.
3F).
 | Figure 3.miR-222-3p serves as a target of
GAS5. (A) The putative binding site of miR-222-3p in GAS5 was
predicted by starBase. (B) Relative luciferase activity in
LAMP-induced THP-1 cells was measured by dual-luciferase reporter
assay. ***P<0.001 vs. miR-NC. (C) RT-qPCR was performed to
measure the expression of miR-222-3p in PBMCs of patients with MPP
and the control group. ***P<0.001 vs. Control. (D) The
correlation between the expression of GAS5 and miR-222-3p was
analyzed in the PBMCs of all participants. (E) The expression of
miR-222-3p in PBMCs of MPP patients with and without PE was
detected by RT-qPCR. ***P<0.001 vs. MPP without PE. (F) The
effect of GAS5 upregulation and downregulation on the miR-222-3p
expression in LAMP-induced THP-1 cells was evaluated by RT-qPCR.
***P<0.001 vs. sh-NC; ###P<0.001 vs. pcDNA3.1.
LAMP, lipid-associated membrane protein; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; PBMCs,
peripheral blood mononuclear cells; MPP, Mycoplasma
pneumoniae pneumonia; PE, pleural effusion; sh, short hairpin
RNA; NC, negative control; GAS5, growth arrest-specific 5; WT,
wild-type; mut, mutant. |
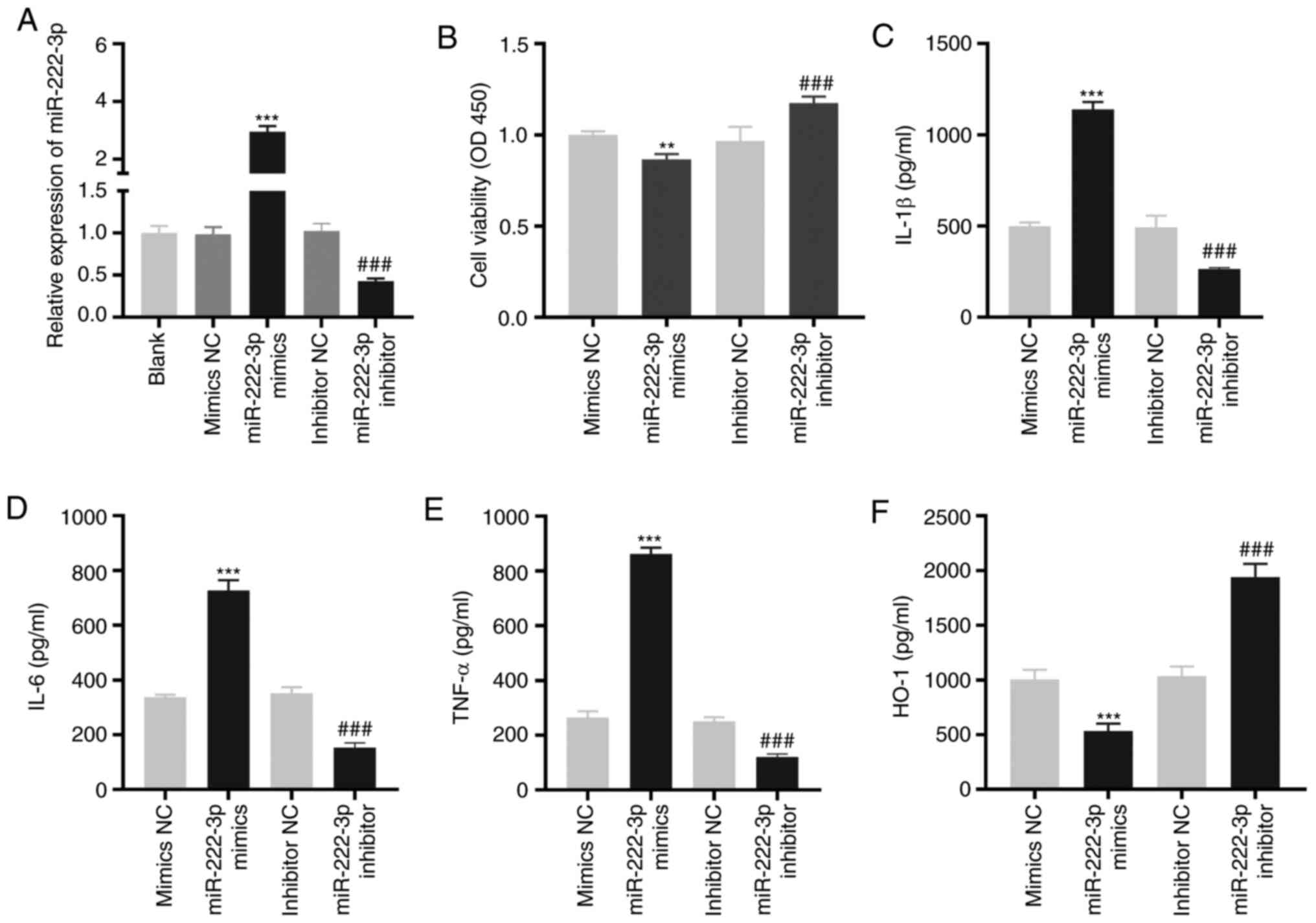
miR-222-3p decreases the viability and
increases the inflammation of LAMP-induced THP-1 cells
As demonstrated in Fig.
4A, miR-222-3p was enhanced or inhibited by the transfection of
miR-222-3p mimics or miR-222-3p inhibitors, respectively, into
LAMP-induced THP-1 cells (P<0.001). The overexpression of
miR-222-3p significantly decreased viability (P<0.01), while its
inhibition significantly increased the viability of LAMP-induced
THP-1 cells (P<0.001; Fig. 4B).
miR-222-3p-overexpression or inhibition increased or decreased,
respectively, the levels of IL-1β, IL-6 and TNF-α in LAMP-induced
THP-1 cells (P<0.001; Fig.
4C-E). Furthermore, the level of HO-1 in LAMP-induced THP-1
cells was significantly decreased or increased by the upregulation
or downregulation, respectively, of miR-222-3p (P<0.001;
Fig. 4F).
Targeting of TIMP3 by miR-222-3p
TargetScan was used to predict the binding site for
miR-222-3p on the 3′-UTR of TIMP3 (Fig.
5A). miR-222-3p mimics notably decreased the luciferase
activity of the WT TIMP3 reporter vector in THP-1 cells
(P<0.001; Fig. 5B).
Additionally, RT-qPCR demonstrated that TIMP3 expression was
significantly downregulated in PBMCs of patients with MPP
(P<0.001; Fig. 5C). As
illustrated in Fig. 5D, the
expression of TIMP3 was negatively correlated with miR-222-3p
expression in PBMCs of all participants (R2=0.787,
P<0.0001). Notably, TIMP3 expression was significantly
suppressed in patients with MPP with PE (P<0.001; Fig. 5E). Furthermore, western blot
analysis revealed that transfection of miR-222-3p mimics or
miR-222-3p inhibitor may suppress or promote, respectively, the
protein expression of TIMP3 in LAMP-induced THP-1 cells
(P<0.001; Fig. 5F).
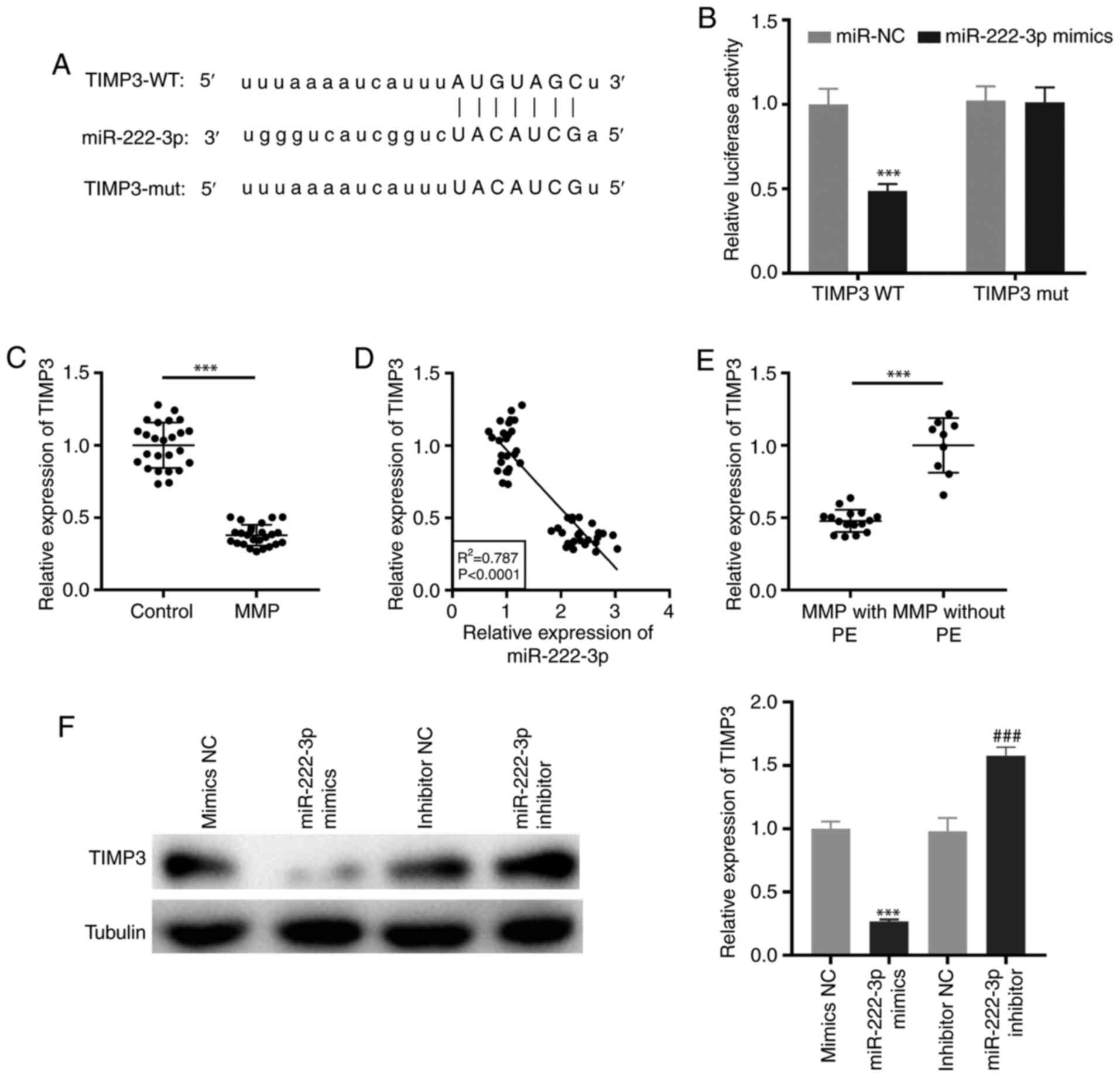 | Figure 5.TIMP3 is targeted by miR-222-3p. (A)
The binding site for miR-222-3p on the 3′-UTR of TIMP3 was
predicted by TargetScan. (B) Dual-luciferase reporter assay was
performed to measure the relative luciferase activity in
LAMP-induced THP-1 cells. ***P<0.001 vs. miR-NC. (C) The TIMP3
expression in PBMCs of patients with MPP and the control group was
detected by RT-qPCR. ***P<0.001 vs. Control. (D) The correlation
between the expression of TIMP3 and miR-222-3p was analyzed in the
PBMCs of all participants. (E) The expression of TIMP3 in PBMCs of
patients with MPP with and without PE was measured by RT-qPCR.
***P<0.001 vs. MPP without PE. (F) The protein expression of
TIMP3 in LAMP-induced THP-1 cells was measured by western blot
analysis. ***P<0.001 vs. NC mimics; ###P<0.001 vs.
NC inhibitors. TIMP3, tissue inhibitor of metalloproteinases-3;
3′-UTR, 3′-untranslated region; LAMP, lipid-associated membrane
protein; NC, negative control; PBMCs, peripheral blood mononuclear
cells; MPP, Mycoplasma pneumoniae pneumonia; RT-qPCR,
reverse transcription-quantitative PCR; PE, pleural effusion; WT,
wild-type; mut, mutant. |
GAS5 increases the viability and
attenuates the inflammation of LAMP-induced THP-1 cells by
regulating the miR-222-3p/TIMP3 axis
As demonstrated in Fig.
6A, the expression of TIMP3 was significantly inhibited by
transfecting with sh-TIMP3 in LAMP-induced THP-1 cells
(P<0.001). To investigate whether GAS5 regulated the
miR-222-3p/TIMP3 axis in MPP, feedback experiments were performed
in LAMP-induced THP-1 cells. GAS5-overexpression notably increased
TIMP3 protein expression, and miR-222-3p-overexpression or
TIMP3-knockdown rescued the increased TIMP3 protein expression
caused by GAS5-overexpression in LAMP-induced THP-1 cells
(P<0.001; Fig. 6B).
GAS5-overexpression increased cell viability (P<0.001). The
upregulation of miR-222-3p or downregulation of TIMP3 reversed the
promotion effect of GAS5-overexpression on the viability of
LAMP-induced THP-1 cells (P<0.05; Fig. 6C). GAS5-overexpression significantly
decreased the levels of IL-1β, IL-6 and TNF-α, and increased the
level of HO-1 in LAMP-induced THP-1 cells (P<0.001; Fig. 6D-G). Notably,
miR-222-3p-overexpression or TIMP-knockdown mitigated the
inhibitory effect of GAS5-overexpression on the levels of the
pro-inflammatory cytokines and weakened the promotion effect of
GAS5-overexpression on the level of anti-inflammatory cytokines in
LAMP-induced THP-1 cells (P<0.001).
 | Figure 6.GAS5 increases the viability and
attenuates the inflammation of LAMP-induced THP-1 cells via
regulating the miR-222-3p/TIMP3 axis. (A) The transfection
efficiency of sh-NC and sh-TIMP3 in LAMP-induced THP-1 cells was
measured by reverse transcription-quantitative PCR. ***P<0.001
vs. sh-NC. (B) The promoting effect of GAS5-overexpression on TIMP3
protein expression in LAMP-induced THP-1 cells was mitigated by
miR-222-3p-overexpression or TIMP3-knockdown. ***P<0.001 vs.
pcDNA3.1; ###P<0.001 vs. oe-GAS5. (C) Overexpression
of miR-222-3p or inhibition of TIMP3 reversed the enhancing effect
of GAS5-overexpression on the viability of LAMP-induced THP-1
cells. ***P<0.001 vs. pcDNA3.1; #P<0.05,
##P<0.01 vs. oe-GAS5. (D-G) miR-222-3p-overexpression
or TIMP3 inhibition not only reversed the decrease in IL-1β, IL-6
and TNF-α, but also weakened the promoting effect on the level of
HO-1 caused by GAS5-overexpression in LAMP-induced THP-1 cells.
***P<0.001 vs. pcDNA3.1; ###P<0.001 vs. oe-GAS5.
LAMP, lipid-associated membrane protein; TIMP3, tissue inhibitor of
metalloproteinases-3; sh, short-hairpin RNA; NC, negative control;
IL, interleukin; TNF-α, tumor necrosis factor-α; HO-1, heme
oxygenase-1; GAS5, growth arrest-specific 5. |
Discussion
Downregulation of lncRNAs is associated with the
pathogenesis of respiratory diseases. GAS5 expression is
downregulated in diverse respiratory diseases, including acute
respiratory distress syndrome (24), non-small cell lung cancer (25) and ALI (12). In the present study, GAS5 expression
was markedly decreased in PBMCs of patients with MPP and in
LAMP-induced THP-1 cells. Furthermore, the results of the present
study demonstrated that GAS5 may be associated with the development
of MPP. LncRNAs serve as critical factors to modulate cell
viability in certain inflammatory diseases. LncRNA CASC9 may
mitigate the effect of LPS on the viability of human small airway
epithelial cells (26). LncRNA TUG1
suppressed MRC-5 cell viability in a pneumonia model (27). Notably, GAS5 increases the viability
of endothelial progenitor cells, attenuating the development of
atherosclerosis (28). In the
present study, GAS5-overexpression increased the viability of
LAMP-induced THP-1 cells, suggesting that GAS5 may increase cell
viability in MPP. Certain lncRNAs are involved in the regulation of
inflammation in lung diseases. In sepsis-induced ALI, lncRNA TUG1
ameliorates inflammation by inhibiting miR-34b-5p (29). MALAT1-silencing increases IL-6 and
TNF-α levels in ALI rats (30).
Notably, GAS5 has an anti-inflammatory role in numerous diseases.
GAS5 regulates miR-429 to increase DUSP1 expression, attenuating
the inflammation of alveolar epithelial cells in ALI (12). GAS5 inhibits endoplasmic reticulum
stress-induced inflammation by targeting SERCA2b in diabetic
retinopathy (31). In the present
study, GAS5-overexpression suppressed the levels of IL-1β, IL-6 and
TNF-α, and enhanced the HO-1 level in LAMP-induced THP-1 cells.
These results indicated that GAS5 may attenuate inflammation in
MPP. By contrast, GAS5 has a pro-inflammatory role in certain
diseases. For instance, GAS5 accelerates microglial inflammatory
responses in Parkinson's disease (32). Silencing of GAS5 decreases the
levels of TNF-α and IL-6 in the supernatant of osteoarthritic
chondrocytes (33). GAS5 may exert
different regulatory effects on inflammation in different diseases.
The regulatory role of GAS5 in inflammation is controversial and
further research on the detailed regulatory mechanism of GAS5 on
the inflammation of MPP is required.
Previous studies have demonstrated that GAS5 may
interact with miRNAs to modulate the development of inflammatory
diseases (34,35). For instance, GAS5 attenuates
inflammation in systemic lupus erythematosus by suppressing
miR-92a-3p expression (36). GAS5
decreases TNF-α and IL-6 levels in diabetic nephropathy by
inhibiting miR-452-5p expression (37). In the present study, miR-222-3p was
determined to be a target of GAS5. miR-222-3p expression was
upregulated in patients with MMP compared with the children in the
control group. Notably, miR-222-3p expression was negatively
correlated with GAS5 expression in patients with MPP. We
hypothesized that GAS5 may influence MPP by regulating miR-222-3p.
miR-222 is upregulated and promotes the pathogenesis of
inflammatory diseases. For instance, miR-222 accelerates
endothelial cell inflammation and dysfunction in atherosclerosis
(38). miR-222 is highly expressed
and induces the inflammatory responses of microglia cells in
intracerebral hemorrhage (39).
Notably, miR-222-silencing attenuates staphylococcal enterotoxin
B-induced inflammation in ALI (40). In the present study, miR-222-3p
decreased the viability and accelerated the inflammation of
LAMP-induced THP-1 cells. Furthermore, miR-222-3p markedly reversed
the effects of GAS5-overexpression on LAMP-induced THP-1 cells.
These results suggested that GAS5 may alleviate MPP by inhibiting
miR-222-3p expression in vitro.
TIMP3 expression is usually downregulated in diverse
inflammatory diseases, including heart failure (41), interstitial lung disease (42) and cardiac ischemia/reperfusion
injury (43). Similarly, TIMP3
expression was clearly decreased in MPP and LAMP-induced THP-1
cells in the present study. TIMP3 generally exerts its role in
certain diseases by interacting with miRNAs. For instance, miR-712
facilitates endothelial inflammation and atherosclerosis by
inhibiting TIMP3 expression (44).
miR-34b-5p-silencing hampers bleomycin-induced pulmonary fibrosis
by increasing TIMP3 expression (21). Notably, miR-222 inhibition enhances
TIMP3 expression to alleviate the development of pulmonary artery
hypertension (45). In the present
study, TIMP3 was a target of miR-222-3p. The expression of TIMP3
was lower in patients with MMP than in the children in control
group. Notably, there was a negative correlation between the
expression of TIMP3 and miR-222-3p in patients with MPP. We
hypothesized that miR-222-3p may be involved in MPP development by
modulating TIMP3. Considering the interaction between GAS5 and
miR-222-3p, we hypothesized that silencing of GAS5 may downregulate
TIMP3 expression by increasing miR-222-3p expression in MMP. The
feedback data demonstrated that TIMP3-knockdown attenuated the
enhancing effect on cell viability and weakened the
anti-inflammatory effect caused by GAS5-overexpression in
LAMP-induced THP-1 cells. These findings suggested that GAS5 may
exert its protective effect by regulating the miR-222-3p/TIMP3 axis
in MPP.
There were two limitations to the present study. To
begin with, the detailed regulatory mechanism of GAS5 on the
inflammation of MPP is not fully understood. Furthermore, the
effect of GAS5 on MPP was investigated at the cellular level.
Establishment of an MPP animal model is required to confirm the
role of GAS5 in vivo.
In conclusion, the expression of GAS5 was
downregulated in patients with MPP and LAMP-induced THP-1 cells.
GAS5-overexpression increased the viability and attenuated the
inflammation of LAMP-induced THP-1 cells by regulating the
miR-222-3p/TIMP3 axis. These results indicated that GAS5 may be a
promising therapeutic target for MPP.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
LY and XL were responsible for conceptualization and
design of the study, and performed the methods and software
analysis, as well as having roles in the supervision of the entire
study. XZ was responsible for data acquisition, original draft
preparation and project management. XZ and XL were responsible for
reviewing and editing the manuscript. LY, XZ and XL confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by The Ethics
Committee of The Second People's Hospital of Liaocheng (Linqing,
China) and written informed consent was obtained from each child
and their guardian.
Patient consent for publication
Written informed consent for publication was
obtained from each child and their guardian.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Saraya T: The history of Mycoplasma
pneumoniae pneumonia. Front Microbiol. 7:3642016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Søndergaard M, Friis MB, Hansen DS and
Jørgensen IM: Clinical manifestations in infants and children with
Mycoplasma pneumoniae infection. PLoS One. 13:e01952882018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Izumikawa K, Izumikawa K, Takazono T,
Kosai K, Morinaga Y, Nakamura S, Kurihara S, Imamura Y, Miyazaki T,
Tsukamoto M, et al: Clinical features, risk factors and treatment
of fulminant Mycoplasma pneumoniae pneumonia: A review of
the Japanese literature. J Infect Chemother. 20:181–185. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Izumikawa K: Clinical features of severe
or fatal Mycoplasma pneumoniae pneumonia. Front Microbiol.
7:8002016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee H, Yun KW, Lee HJ and Choi EH:
Antimicrobial therapy of macrolide-resistant Mycoplasma
pneumoniae pneumonia in children. Expert Rev Anti Infect Ther.
16:23–34. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee E, Cho HJ, Hong SJ, Lee J, Sung H and
Yu J: Prevalence and clinical manifestations of macrolide resistant
Mycoplasma pneumoniae pneumonia in Korean children. Korean J
Pediatr. 60:151–157. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Du Y, Hao X and Liu X: Low expression of
long noncoding RNA CDKN2B-AS1 in patients with idiopathic pulmonary
fibrosis predicts lung cancer by regulating the p53-signaling
pathway. Oncol Lett. 15:4912–4918. 2018.PubMed/NCBI
|
|
8
|
Dai L, Zhang G, Cheng Z, Wang X, Jia L,
Jing X, Wang H, Zhang R, Liu M, Jiang T, et al: Knockdown of LncRNA
MALAT1 contributes to the suppression of inflammatory responses by
up-regulating miR-146a in LPS-induced acute lung injury. Connect
Tissue Res. 59:581–592. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang S, Feng C, Chen L, Huang Z, Zhou X,
Li B, Wang LL, Chen W, Lv FQ and Li TS: Identification of potential
key long non-coding RNAs and target genes associated with pneumonia
using long non-coding RNA sequencing (lncRNA-Seq): A preliminary
study. Med Sci Monit. 22:3394–3408. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang L, Zhao S and Zhu Y: Long noncoding
RNA growth arrest-specific transcript 5 alleviates renal fibrosis
in diabetic nephropathy by downregulating matrix metalloproteinase
9 through recruitment of enhancer of zeste homolog 2. FASEB J.
34:2703–2714. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li F, Sun J, Huang S, Su G and Pi G:
LncRNA GAS5 overexpression reverses LPS-induced inflammatory injury
and apoptosis through up-regulating KLF2 expression in ATDC5
chondrocytes. Cell Physiol Biochem. 45:1241–1251. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li J and Liu S: LncRNA GAS5 suppresses
inflammatory responses and apoptosis of alveolar epithelial cells
by targeting miR-429/DUSP1. Exp Mol Pathol. 113:1043572020.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pan J, Ye Z, Zhang N, Lou T and Cao Z:
MicroRNA-217 regulates interstitial pneumonia via IL-6. Biotechnol
Biotechnol Equip. 32:1541–1547. 2018. View Article : Google Scholar
|
|
14
|
Wang Z, Zheng Y, Fang Z and Zhang Y: The
role of miR-21 and its predicted target gene, PTEN, in the
development of ventilator associated pneumonia. Biomed Res.
28:3967–3973. 2017.
|
|
15
|
Podsiad A, Standiford TJ, Ballinger MN,
Eakin R, Park P, Kunkel SL, Moore BB and Bhan U: MicroRNA-155
regulates host immune response to postviral bacterial pneumonia via
IL-23/IL-17 pathway. Am J Physiol Lung Cell Mol Physiol.
310:L465–L475. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chu C, Lei X, Li Y, Luo Y, Ding Y, Zhou W
and Ji W: High expression of miR-222-3p in children with
Mycoplasma pneumoniae pneumonia. Ital J Pediatr. 45:1632019.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang X, Ye Y and Zhao S: LncRNA Gas5 acts
as a ceRNA to regulate PTEN expression by sponging miR-222-3p in
papillary thyroid carcinoma. Oncotarget. 9:3519–3530. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Muniategui A, Nogalescadenas R, Vazquez M,
Aranguren XL, Agirre X, Luttun A, Prosper F, Pascual-Montano A and
Rubio A: Quantification of miRNA-mRNA interactions. PLoS One.
7:e307662012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fujii T, Duarte S, Lee E, Ke B, Busuttil
RW and Coito AJ: Tissue inhibitor of metalloproteinase 3 deficiency
disrupts the hepatocyte E-cadherin/β-catenin complex and induces
cell death in liver ischemia/reperfusion injury. Liver Transpl.
26:113–126. 2020. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nakamura H, Vo P, Kanakis I, Liu K and
Bou-Gharios G: Aggrecanase-selective tissue inhibitor of
metalloproteinase-3 (TIMP3) protects articular cartilage in a
surgical mouse model of osteoarthritis. Sci Rep. 10:92882020.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hu RP, Lu YY and Zhang XJ: MiR-34b-5p
knockdown attenuates bleomycin-induced pulmonary fibrosis by
targeting tissue inhibitor of metalloproteinase 3 (TIMP3). Eur Rev
Med Pharmacol Sci. 23:2273–2279. 2019.PubMed/NCBI
|
|
22
|
Guo J, Liu Q, Li Z, Guo H, Bai C and Wang
F: miR-222-3p promotes osteosarcoma cell migration and invasion
through targeting TIMP3. Onco Targets Ther. 11:8643–8653. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li HB, Zi PP, Shi HJ, Gao M and Sun RQ:
Role of signaling pathway of long non-coding RNA growth
arrest-specific transcript 5/microRNA-200c-3p/angiotensin
converting enzyme 2 in the apoptosis of human lung epithelial cell
A549 in acute respiratory distress syndrome. Zhonghua Yi Xue Za
Zhi. 98:3354–3359. 2018.(In Chinese). PubMed/NCBI
|
|
25
|
Wu Y, Lyu H, Liu H, Shi X, Song Y and Liu
B: Downregulation of the long noncoding RNA GAS5-AS1 contributes to
tumor metastasis in non-small cell lung cancer. Sci Rep.
6:310932016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang HR, Guo XY, Liu XY and Song X:
Down-regulation of lncRNA CASC9 aggravates sepsis-induced acute
lung injury by regulating miR-195-5p/PDK4 axis. Inflamm Res.
69:559–568. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Meng J, Chen Y and Zhang C: Protective
impacts of long noncoding RNA taurine-upregulated 1 against
lipopolysaccharide-evoked injury in MRC-5 cells through inhibition
of microRNA-127. J Cell Biochem. 120:14928–14935. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yao J, Shi Z, Ma X, Xu D and Ming G:
lncRNA GAS5/miR-223/NAMPT axis modulates the cell proliferation and
senescence of endothelial progenitor cells through PI3K/AKT
signaling. J Cell Biochem. 120:14518–14530. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qiu N, Xu X and He Y: LncRNA TUG1
alleviates sepsis-induced acute lung injury by targeting
miR-34b-5p/GAB1. BMC Pulm Med. 20:492020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li H, Shi H, Ma N, Zi P, Liu Q and Sun R:
BML-111 alleviates acute lung injury through regulating the
expression of lncRNA MALAT1. Arch Biochem Biophys. 649:15–21. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jiang L, Wang C and Shen X: LncRNA GAS5
suppresses ER stress-induced apoptosis and inflammation by
regulating SERCA2b in HG-treated retinal epithelial cell. Mol Med
Rep. 22:1072–1080. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xu W, Zhang L, Geng Y, Liu Y and Zhang N:
Long noncoding RNA GAS5 promotes microglial inflammatory response
in Parkinson's disease by regulating NLRP3 pathway through sponging
miR-223-3p. Int Immunopharmacol. 85:1066142020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ji Q, Qiao X, Liu Y, Wang D and Yan J:
Silencing of long-chain non-coding RNA GAS5 in osteoarthritic
chondrocytes is mediated by targeting the miR-34a/Bcl-2 axis. Mol
Med Rep. 21:1310–1319. 2020.PubMed/NCBI
|
|
34
|
Li G, Du P, Qiang X, Jin D, Liu H, Li B
and Guo J: Low-expressed GAS5 injure myocardial cells and
progression of chronic heart failure via regulation of miR-223-3P.
Exp Mol Pathol. 117:1045292020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shangguan Y, Han J and Su H: GAS5
knockdown ameliorates apoptosis and inflammatory response by
modulating miR-26b-5p/Smad1 axis in cerebral ischaemia/reperfusion
injury. Behav Brain Res. 379:1123702020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu Q, Deng Y, Li C, Xie H, Liu Q, Ming S,
Wu D and Luo F: LncRNA GAS5 suppresses CD4+ T cell
activation by upregulating E4BP4 via inhibiting miR-92a-3p in
systemic lupus erythematosus. Immunol Lett. 227:41–47. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xie C, Wu W, Tang A, Luo N and Tan Y:
lncRNA GAS5/miR-452-5p reduces oxidative stress and pyroptosis of
high-glucose-stimulated renal tubular cells. Diabetes Metab Syndr
Obes. 12:2609–2617. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xue Y, Wei Z, Ding H, Wang Q, Zhou Z,
Zheng S, Zhang Y, Hou D, Liu Y, Zen K, et al: MicroRNA-19b/221/222
induces endothelial cell dysfunction via suppression of PGC-1α in
the progression of atherosclerosis. Atherosclerosis. 241:671–681.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bai YY and Niu JZ: miR-222 regulates brain
injury and inflammation following intracerebral hemorrhage by
targeting ITGB8. Mol Med Rep. 21:1145–1153. 2020.PubMed/NCBI
|
|
40
|
Chen L, Chen J, Xie G and Zhu L: MiR-222
inhibition alleviates Staphylococcal Enterotoxin B-induced
inflammatory acute lung injury by targeting Foxo3. J Biosci.
45:652020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kassiri Z, Oudit GY, Sanchez O, Dawood F,
Mohammed FF, Nuttall RK, Edwards DR, Liu PP, Backx PH and Khokha R:
Combination of tumor necrosis factor-alpha ablation and matrix
metalloproteinase inhibition prevents heart failure after pressure
overload in tissue inhibitor of metalloproteinase-3 knock-out mice.
Circ Res. 97:380–390. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nagar JK, Patel PP, Mohapatra JN, Sharma
MM, Pandya GM, Umar MM, Chatterjee AA, Deshpande SS, Jain MR and
Soni HM: Differential effects of dexamethasone and rosiglitazone in
a sephadex-induced model of lung inflammation in rats: Possible
role of tissue inhibitor of metalloproteinase-3. Indian J
Pharmacol. 47:153–159. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu H, Jing X, Dong A, Bai B and Wang H:
Overexpression of TIMP3 protects against cardiac
ischemia/reperfusion injury by inhibiting myocardial apoptosis
through ROS/Mapks pathway. Cell Physiol Biochem. 44:1011–1023.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Son DJ, Kumar S, Takabe W, Kim CW, Ni CW,
Alberts-Grill N, Jang IH, Kim S, Kim W, Won Kang S, et al: The
atypical mechanosensitive microRNA-712 derived from pre-ribosomal
RNA induces endothelial inflammation and atherosclerosis. Nat
Commun. 4:30002013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Xu Y, Bei Y, Shen S, Zhang J, Lu Y, Xiao J
and Li X: MicroRNA-222 promotes the proliferation of pulmonary
arterial smooth muscle cells by targeting P27 and TIMP3. Cell
Physiol Biochem. 43:282–292. 2017. View Article : Google Scholar : PubMed/NCBI
|