Introduction
Epithelial ovarian cancer (EOC) accounts for ~90% of
ovarian cancer cases, which is the most common cause of
gynaecological cancer-related death, causing >150,000 deaths
annually worldwide (1,2). Due to the asymptomatic nature of EOC
at early stages and the lack of an effective screening program for
assessing disease risk, patients are generally diagnosed at an
advanced stage (International Federation of Gynecology and
Obstetrics stage III and IV), which is associated with the
dissemination of cancer cells from the ovary to the peritoneal
cavity (1,3,4).
Surgical resection and platinum-based chemotherapy are the standard
treatment strategies for patients with EOC (4). However, the majority of patients
develop chemoresistance and recurrence, which results in a poor
5-year relative survival rate (5).
To improve the prognosis in patients with EOC, identifying the
molecular pathogenies underlying EOC is important for the
development of novel and effective drug targets.
Circular RNA (circ/circRNA)-ABCB10, a novel circRNA,
exerts oncological effect in several types of cancer, such as
breast and non-small-cell lung cancer (6–8). As
for EOC, our previous study revealed that circ-ABCB10 correlates
with deteriorated clinical features and poor prognosis in patients
with EOC. Moreover, the previous study indicated that circ-ABCB10
facilitates cell proliferation, inhibits cell apoptosis and
negatively regulates microRNA (miR)-1271, miR-1252 and miR-203 in
EOC cells (9). Meanwhile, miR-1271
suppresses cell proliferation and invasion by downregulating
calpain small subunit 1 (Capn4), and Capn4 enhances EOC cell
proliferation and migration by targeting the Wnt/β-catenin
signaling pathway (10,11). Based on the aforementioned studies,
it was hypothesized that circ-ABCB10 might competitively interact
with miR-1271 to promote EOC progression by activating Capn4 and
its downstream Wnt/β-catenin signaling pathway. Therefore, the
present study aimed to investigate the interaction between
circ-ABCB10 and miR-1271 in regulating cell proliferation,
apoptosis, invasion and the Capn4/Wnt/β-catenin signaling pathway
in EOC.
Materials and methods
Cell culture
Human EOC cell lines (OVCAR3, UWB1.289, SKOV3 and
CAOV3) were purchased from American Type Culture Collection and a
human normal ovarian epithelial cell line (IOSE80) was purchased
from BioVector NTCC, Inc. Cells were cultured as previously
described (9). OVCAR3, UWB1.289,
SKOV3 and IOSE80 cell lines were cultured in 90% RPMI-1640 medium
(Gibco; Thermo Fisher Scientific, Inc.) and 10% FBS (Gibco; Thermo
Fisher Scientific, Inc.); CAOV3 cell line was cultured in 90% DMEM
(Gibco; Thermo Fisher Scientific, Inc.) and 10% FBS (Gibco; Thermo
Fisher Scientific, Inc.); all cell lines were cultured under 95%
air and 5% CO2 at 37°C. Following culture, the relative
expression levels of circ-ABCB10 and miR-1271 in cells were
determined via reverse transcription-quantitative PCR
(RT-qPCR).
Cell transfection
Control overexpression plasmids (100 nM),
circ-ABCB10 overexpression plasmids (100 nM), control shRNA
plasmids (100 nM) and circ-ABCB10 shRNA plasmids (100 nM) were
constructed by Shanghai GenePharma Co., Ltd. using pEX-2 and pGPU6.
Control mimic (100 nM; 5′-AACACCGAACGAGACAGGATT-3′), miR-1271 mimic
(100 nM; 5′-CUUGGCACCUAGCAAGCACUCA-3′), control inhibitor (100 nM)
(5′-AAGAACAACACAAAAGAACAG-3′) and miR-1271 inhibitor (100 nM;
5′-UGAGUGCUUGCUAGGUGCCAAG-3′) were also synthesized by Shanghai
GenePharma Co., Ltd. SKOV3 (5×105 cells/well) and
UWB1.289 cells (5×105 cells/well) were transfected using
HilyMax (Dojindo Molecular Technologies, Inc.). The following four
groups were generated using SKOV3 cells: i) NC (−), cells
co-transfected with control shRNA plasmid and control inhibitor;
ii) circ (−), cells co-transfected with circ-ABCB10 shRNA plasmid
and control inhibitor; iii) miR (−), cells co-transfected with
miR-1271 inhibitor and control shRNA plasmid; and iv) circ (−) +
miR (−), cells co-transfected with circ-ABCB10 shRNA plasmid and
miR-1271 inhibitor. The following four groups were generated using
UWB1.289 cells: i) NC (+), cells co-transfected with control
overexpression plasmid and control mimic; ii) circ (+), cells
co-transfected with circ-ABCB10 overexpression plasmid and control
mimic; iii) miR (+), cells co-transfected with miR-1271 mimic and
control overexpression plasmid; and iv) circ (+) + miR (+), cells
co-transfected with circ-ABCB10 overexpression plasmid and miR-1271
mimic. At 24 h post-transfection, the relative expression levels of
circ-ABCB10 and miR-1271 were detected via RT-qPCR.
Cell proliferation, apoptosis and
invasion
Cell proliferation was assessed at 0, 24, 48 and 72
h post-transfection using a Cell Counting Kit-8 assay
(Sigma-Aldrich; Merck KGaA) according to the manufacturer's
protocol. At 48 h post-transfection, cell apoptosis was assessed
using an Annexin V-FITC Apoptosis Detection kit (Sigma-Aldrich;
Merck KGaA), according to the manufacturer's protocol. Apoptotic
cells (early and late apoptosis) were detected via flow cytometry
using CytoFLEX system (Beckman Coulter, Inc.), and FlowJo software
7.6 (FlowJo LLC) was used for data analysis. In addition, at 48 h
post-transfection, cell invasion was evaluated by performing a
Transwell assay. Each Transwell insert (size, 8 µm; Corning, Inc.)
was pre-coated with Matrigel (at 37°C for 1 h), then, SKOV3
(3×104 cells) and UWB1.289 cells (3×104
cells) suspended in serum-free culture medium (Gibco; Thermo Fisher
Scientific, Inc.) were added into the upper chamber (Corning).
Culture medium containing 10% FBS (Gibco; Thermo Fisher Scientific,
Inc.) was added into the lower chamber. After 24 h of incubation,
cells that invaded the Matrigel coated filters were fixed with
methanol (Sigma-Aldrich; Merck KGaA), stained with crystal violet
(at 37°C for 15 min; Sigma-Aldrich; Merck KGaA) and counted with a
BX 41 inverted microscope under ×200 magnification (Olympus
Corporation).
RT-qPCR
Total RNA was isolated from IOSE80, OVCAR3,
UWB1.289, SKOV3 and CAOV3 cells using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). Linear RNA was
digested using RNase R enzyme (Epicentre; Illumina, Inc.) for the
detection of circ-ABCB10, but linear RNA digestion was not
conducted for the detection of miR-1271, Capn4, Wnt1, β-catenin,
GAPDH and U6. Total RNA was reverse transcribed into cDNA using the
PrimeScript RT reagent kit (Takara Bio, Inc.). The synthetization
of cDNA was performed at 37°C for 15 min and 85°C for 5 sec.
Subsequently, qPCR was performed using the QuantiNova SYBR Green
PCR kit (Qiagen GmbH) at 95°C for 30 sec, followed by 40 cycles of
amplification (95°C for 5 sec and 60°C for 19 sec). The following
primers were used for qPCR: circ-ABCB10 forward,
5′-GTGCTGATGACCCTTCCTCTG-3′ and reverse,
5′-GGGAATCCTGAGTGACTTTGGT-3′; GAPDH forward,
5′-GAGTCCACTGGCGTCTTCAC-3′ and reverse,
5′-ATCTTGAGGCTGTTGTCATACTTCT-3′; miR-1271 forward,
5′-ACACTCCAGCTGGGCTTGGCACCTAGCAAG-3′ and reverse,
5′-TGTCGTGGAGTCGGCAATTC-3′; U6 forward,
5′-CGCTTCGGCAGCACATATACTA-3′ and reverse,
5′-ATGGAACGCTTCACGAATTTGC-3′; Capn4 forward,
5′-ACCCACTCCGTAACCTC-3′ and reverse, 5′-GGGTAGCAACCGTGAA-3′; Wnt1
forward, 5′-TACCTCCAGTCACACTCCCC-3′ and reverse,
5′-CCATGGCAGGAGAATAGGAA-3′; and β-catenin forward,
5′-GCCATTACAACTCTCCACAACCT-3′ and reverse,
5′-GACAGATAGCACCTTCAGCACTC-3′. circRNA and mRNA expression levels
were normalized to the internal reference gene GAPDH. miRNA
expression levels were normalized to the internal reference gene
U6. Expression levels were quantified using the 2−∆∆Cq
method (12).
Western blotting
Western blotting was performed as previously
described (9). The following
antibodies were used: Capn4 (1:1,000; cat. no. ab92356; Abcam),
Wnt1 (1:500; cat. no. ab15251; Abcam), β-catenin (1:400; cat. no.
ab68183; Abcam), GAPDH (1:10,000; cat. no. ab128915; Abcam) and
HRP-conjugated goat anti-rabbit IgG H&L (1:10,000; cat. no.
ab6721; Abcam).
Luciferase activity assay
The wild-type (WT) and mutant (MUT) circ-ABCB10
recombinant plasmids were constructed using the psiCHECK luciferase
vector (Hanbio Biotechnology Co., Ltd.). 293T cells
(5×105 cells/well) (American Type Culture Collection)
were co-transfected with WT-circ-ABCB10 (100 nM) or MUT-circ-ABCB10
(100 nM) and miR-1271 mimic or control mimic (100 nM) using HilyMax
(Dojindo Molecular Technologies, Inc.). The following four groups
were generated: i) WT + miR-1271; ii) WT + control; iii) MUT +
miR-1271; and iv) MUT + control. Luciferase activity was measured
using the luciferase activity detecting kit (Biothrive) with the
Dual-Luciferase Reporter Assay system (Promega Corporation) 48 h
after transfection, and the Firefly luciferase activity was
normalized to the Renilla luciferase activity.
Statistical analysis
Data are presented as the mean ± standard deviation
of three experimental repeats. Comparisons between two groups were
analyzed using the unpaired Student's t-test. Comparisons among
multiple groups were analyzed using one-way ANOVA followed by
Dunnett's or Tukey's post hoc test. P<0.05 was considered to
indicate a statistically significant difference. Statistical
analyses were performed by SPSS 22.0 Software (IBM Corp.), and
graphs were plotted by GraphPad Prism 7.02 Software (GraphPad
Software, Inc.).
Results
Comparison of circ-ABCB10 and miR-1271
expression levels between EOC and normal ovarian epithelial
cells
Compared with IOSE80 cells, circ-ABCB10 expression
was significantly increased in OVCAR3, SKOV3 and CAOV3 cells, but
was not significantly altered in UWB1.289 cells (Fig. 1A). miR-1271 expression was
significantly decreased in OVCAR3, UWB1.289, SKOV3 and CAOV3 cells
compared with IOSE80 cells (Fig.
1B). Therefore, SKOV3 and UWB1.289 cells were selected for
subsequent experiments.
circ-ABCB10 and miR-1271 expression
levels following transfection
In SKOV3 cells, circ-ABCB10 expression levels were
significantly decreased in the circ (−) group compared with the NC
(−) group, but were similar between the miR (−) group and the NC
(−) group, as well as between the circ (−) + miR (−) and circ (−)
groups (Fig. 2A). miR-1271
expression levels were significantly decreased in the miR (−) group
compared with the NC (−) group, and in the circ (−) + miR (−) group
compared with the circ (−) group. By contrast, miR-1271 expression
levels were significantly increased in the circ (−) group compared
with the NC (−) group (Fig. 2B). In
UWB1.289 cells, circ-ABCB10 expression levels were significantly
increased in the circ (+) group compared with NC (+) group, but
similar between the miR (+) group and the NC (+) group, as well as
between the circ (+) + miR (+) group and the circ (+) group
(Fig. 2C). Furthermore, miR-1271
relative expression levels were significantly elevated in the miR
(+) group compared with the NC (+) group, and in the circ (+) + miR
(+) group compared with the circ (+) group. By contrast, miR-1271
expression was significantly decreased in the circ (+) group
compared with the NC (+) group (Fig.
2D). Collectively, the results indicated that circ-ABCB10
negatively regulated miR-1271, whereas miR-1271 did not regulate
circ-ABCB10 in EOC.
 | Figure 2.Interaction between circ-ABCB10 and
miR-1271. (A) circ-ABCB10 and (B) miR-1271 expression levels in NC
(−), miR (−), circ (−) and circ (−) + miR (−) SKOV3 cells. (C)
circ-ABCB10 and (D) miR-1271 expression levels in NC (+), miR (+),
circ (+) and circ (+) + miR (+) UWB1.289 cells. **P<0.01,
***P<0.001. circ-ABCB10, circular RNA ABCB10; miR-1271, microRNA
1271; NC, negative control; NS, not significant; shRNA, short
hairpin RNA; OR, overexpression. |
circ-ABCB10 promotes cell
proliferation by targeting miR-1271
In SKOV3 cells, cell proliferation was significantly
increased in the miR (−) group compared with NC (−) group at 48 and
72 h. Similarly, cell proliferation was significantly increased in
the circ (−) + miR (−) group compared with circ (−) group at 48 and
72 h, but significantly decreased in the circ (−) group compared
with the NC (−) group at 48 and 72 h (Fig. 3A). In UWB1.289 cells, cell
proliferation was significantly reduced in the miR (+) group
compared with the NC (+) group at 72 h, and in the circ (+) + miR
(+) group compared with the circ (+) group at 48 and 72 h. By
contrast, cell proliferation was significantly enhanced in the circ
(+) group compared with the NC (+) group at 48 and 72 h (Fig. 3B). Collectively, the results
indicated that miR-1271 reversed the proproliferative effect of
circ-ABCB10 in EOC.
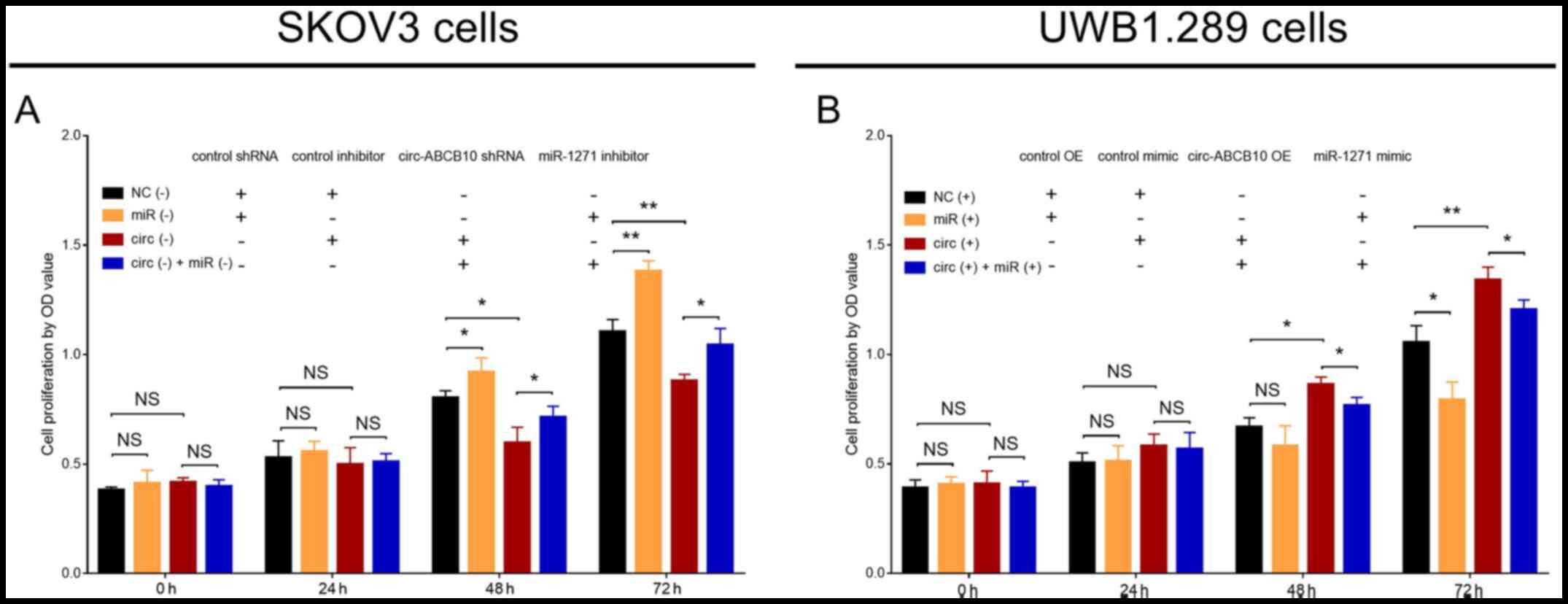 | Figure 3.Effect of circ-ABCB10 and miR-1271 on
cell proliferation. Cell proliferation at 0, 24, 48 and 72 h in (A)
NC (−), miR (−), circ (−) and circ (−) + miR (−) SKOV3 cells, and
(B) NC (+), miR (+), circ (+) and circ (+) + miR (+) UWB1.289
cells. *P<0.05, **P<0.01. circ-ABCB10, circular RNA ABCB10;
miR-1271, microRNA 1271; NC, negative control; OD, optical density;
shRNA, short hairpin RNA; OE, overexpression; NS, not
significant. |
circ-ABCB10 inhibits cell apoptosis by
targeting miR-1271
In SKOV3 cells, cell apoptosis was significantly
decreased in the miR (−) group compared with the NC (−) group, and
in the circ (−) + miR (−) group compared with the circ (−) group.
By contrast, cell apoptosis was significantly increased in the circ
(−) group compared with the NC (−) group (Fig. 4A and B). In UWB1.289 cells, cell
apoptosis was significantly increased in the miR (+) group compared
with the NC (+) group, and in the circ (+) + miR (+) group compared
with the circ (+) group. Moreover, cell apoptosis was significantly
reduced in the circ (+) group compared with the NC (+) group
(Fig. 4C and D). Collectively, the
results suggested that miR-1271 attenuated the antiapoptotic effect
of circ-ABCB10 in EOC.
 | Figure 4.Effect of circ-ABCB10 and miR-1271 on
cell apoptosis. (A) Cell apoptosis in NC (−), miR (−), circ (−) and
circ (−) + miR (−) SKOV3 cells. (B) Representative flow cytometry
plots in SKOV3 cells. (C) Cell apoptosis in NC (+), miR (+), circ
(+) and circ (+) + miR (+) UWB1.289 cells. (D) Representative flow
cytometry plots in UWB1.289 cells. *P<0.05, **P<0.01,
***P<0.001. circ-ABCB10, circular RNA ABCB10; miR-1271, microRNA
1271; NC, negative control; shRNA, short hairpin RNA; OE,
overexpression; PI, propidium iodide; AV, Annexin V. |
circ-ABCB10 promotes cell invasion by
targeting miR-1271
In SKOV3 cells, cell invasion was significantly
increased in the miR (−) group compared with the NC (−) group, and
in the circ (−) + miR (−) group compared with the circ (−) group.
By contrast, cell invasion was significantly decreased in the circ
(−) group compared with the NC (−) group (Fig. 5A and B). In UWB1.289 cells, cell
invasion was significantly decreased in the miR (+) group compared
with the NC (+) group, and in the circ (+) + miR (+) group compared
with the circ (+) group, but significantly increased in the circ
(+) group compared with the NC (+) group (Fig. 5C and D). Collectively, the results
indicated that miR-1271 reversed the proinvasive effect of
circ-ABCB10 in EOC.
 | Figure 5.Effect of circ-ABCB10 and miR-1271 on
cell invasion. (A) Cell invasion in NC (−), miR (−), circ (−) and
circ (−) + miR (−) SKOV3 cells. (B) Representative images of the
Transwell assay in SKOV3 cells under ×200 magnification. (C) Cell
invasion in NC (+), miR (+), circ (+) and circ (+) + miR (+)
UWB1.289 cells. (D) Representative images of the Transwell assay in
UBW1.289 cells under ×200 magnification. *P<0.05, **P<0.01.
circ-ABCB10, circular RNA ABCB10; miR-1271, microRNA 1271; NC,
negative control; shRNA, short hairpin RNA; OE, overexpression. |
circ-ABCB10 promotes the
Capn4/Wnt/β-catenin signaling pathway by targeting miR-1271
In SKOV3 cells, Capn4, Wnt and β-catenin expression
levels were significantly increased in the miR (−) group compared
with the NC (−) group, and in the circ (−) + miR (−) group compared
with the circ (−) group, but significantly decreased in the circ
(−) group compared with the NC (−) group (Figs. 6A-D and S1). In UWB1.289 cells, Capn4, Wnt and
β-catenin expression levels were significantly decreased in the miR
(+) group compared with the NC (+) group, and in the circ (+) + miR
(+) group compared with the circ (+) group. By contrast, Capn4, Wnt
and β-catenin expression levels were significantly increased in the
circ (+) group compared with the NC (+) group (Figs. 6E-H and S1). Collectively, the results suggested
that miR-1271 reversed the positive regulation of circ-ABCB10 on
the Capn4/Wnt/β-catenin signaling pathway in EOC.
 | Figure 6.Effect of circ-ABCB10 and miR-1271 on
the Capn4/Wnt/β-catenin signaling pathway. mRNA expression levels
of (A) Capn4, (B) Wnt and (C) β-catenin in SKOV3 cells. (D) Protein
expression levels of Capn4, Wnt and β-catenin in SKOV3 cells. mRNA
expression levels of (E) Capn4, (F) Wnt and (G) β-catenin in
UBW1.289 cells. (H) Protein expression levels of Capn4, Wnt and
β-catenin in UBW1.289 cells. *P<0.05, **P<0.01,
***P<0.001. circ-ABCB10, circular RNA ABCB10; miR-1271, microRNA
1271; Capn4, calpain small subunit 1; NC, negative control; shRNA,
short hairpin RNA; OE, overexpression. |
Direct interaction between circ-ABCB10
and miR-1271
To explore the interaction between circ-ABCB10 and
miR-1271, miR-1271 and circ-ABCB10 binding sequences were designed
(Fig. 7A). Subsequently, luciferase
activity was evaluated, which revealed that luciferase activity was
significantly reduced in wild-type/miR-1271 (+) cells compared with
wild-type/control (+) cells. However, luciferase activity was not
significantly different between mutant/miR-1271 (+) cells and
mutant/control (+) cells (Fig. 7B).
The results indicated that circ-ABCB10 directly bound to miR-1271
in EOC.
Discussion
EOC is a highly heterogeneous malignancy that is
characterized by different precursor lesions, tissues of origin,
molecular properties and clinical outcomes (2,4).
Despite advances in molecular predictors (for example, cancer
antigen 125 and human epididymal protein 4), surgical debulking and
chemotherapy for EOC in the past three decades, the clinical
outcomes remain undesirable with increased chemoresistance and
recurrence in patients with EOC (2,13). To
develop a novel and effective treatment strategy, the molecular
pathogenesis underlying EOC requires further investigation.
With the application of high-throughput
technologies, the underlying mechanisms and applications of
circRNAs as therapeutic targets are widely explored in various
diseases (14). circRNA, a
covalently closed non-coding RNA, is generated by non-random
back-splice events, is resistant to RNA degradation and has limited
protein coding ability (15).
circRNAs either function as oncogenic stimuli or tumor suppressors
in cancer development and progression by sponging miRNAs to
regulate the activities of downstream target genes or protein
expression (6,15). circ-ABCB10 is involved in the
tumorigenesis of various types of cancer (6–8). In
breast cancer, circ-ABCB10 knockdown inhibits breast cancer cell
proliferation but facilitates apoptosis by interacting with
miR-1271 (7). In non-small cell
lung cancer, circ-ABCB10 expression is upregulated in NSCLC cell
lines, which enhances cell proliferation and migration via sponging
miR-1252 to upregulate forkhead box 2 (FOXR2) synthase expression
(8). As for EOC, our previous study
uncovered that circ-ABCB10 expression is elevated in EOC cell lines
compared with normal ovarian epithelial cells, and it increases
cell proliferation, but decreases apoptosis and the expression of
miR-1271, miR-1252 and miR-203 (9).
miR-1271 serves as a tumor suppressive miRNA, which inhibits cell
proliferation and invasion via modulating downstream target genes
in various types of cancer (10,16,17).
In EOC, miR-1271 inhibits cell viability, invasion and epithelial
mesenchymal transition by directly binding to the 3′-untranslated
region of zinc finger E-box binding homeobox 1 mRNA in EOC
(17). In colorectal cancer,
miR-1271 promotes cell proliferation and invasion by suppressing
Capn4 (10). Capn4 facilitates cell
proliferation and migration in EOC by activating the Wnt/β-catenin
signaling pathway (11). Therefore,
it was hypothesized that circ-ABCB10 might promote the progression
of EOC via targeting miR-1271, Capn4 and the Wnt/β-catenin
signaling pathway.
In the present study, the results indicated that
circ-ABCB10 expression was upregulated, whereas miR-1271 expression
was downregulated in EOC cells compared with normal ovarian cells.
To investigate the role of the interaction between circ-ABCB10 and
miR-1271 in cell proliferation, invasion and apoptosis, rescue
experiments were performed. circ-ABCB10 negatively regulated
miR-1271, whereas miR-1271 did not regulate circ-ABCB10 in EOC.
Furthermore, circ-ABCB10 enhanced cell proliferation and invasion,
but inhibited cell apoptosis in EOC. By contrast, miR-1271
suppressed cell proliferation and invasion, and facilitated cell
apoptosis in EOC. miR-1271 reversed the proproliferative,
proinvasive and antiapoptotic effects of circ-ABCB10 in EOC.
Collectively, the results suggested that circ-ABCB10 facilitated
cell proliferation and invasion, but suppressed cell apoptosis by
negatively regulating miR-1271 in EOC. A few potential explanations
for the results of the present study include: i) circ-ABCB10 might
affect the function of its host gene ABCB10, which facilitates EOC
tumor progression; and ii) circ-ABCB10 inhibited miR-1271
expression, which downregulated its downstream proteins (e.g.
Capn4, FOXR2, regulator of G-protein signaling 17 and spindlin 1),
resulting in enhanced tumor growth, metastasis and invasion of EOC
(8,10,18,19).
The results also indicated that circ-ABCB10 increased Capn4
expression by repressing miR-1271.
Additionally, this study suggested that circ-ABCB10
promoted the Capn4/Wnt/β-catenin signaling pathway, whereas
miR-1271 repressed the Capn4/Wnt/β-catenin signaling pathway.
Moreover, miR-1271 reversed the positive regulation of circ-ABCB10
on the Capn4/Wnt/β-catenin signaling pathway in EOC. Collectively,
the results indicated that circ-ABCB10 facilitated the
Capn4/Wnt/β-catenin signaling pathway by downregulating miR-1271 in
EOC. To further confirm the sponging effect of circ-ABCB10 on
miR-1271, a luciferase activity assay was performed, which
indicated that circ-ABCB10 directly bound to miR-1271 in EOC.
Herein, several explanations were proposed: i) circ-ABCB10
upregulated the Capn4/Wnt/β-catenin signaling pathway and
subsequently induced mutations of the β-catenin gene (CTNNB1),
which promoted tumor cell proliferation, migration and invasion via
matrix metalloproteinase-2 (20);
and ii) circ-ABCB10 promoted Wnt/β-catenin pathway signaling and
amplified the activation of target genes (such as c-Myc and
cyclin-D1), which facilitated the malignant progression of EOC
(21).
In conclusion, this study indicated that circ-ABCB10
promoted cell proliferation and invasion, but suppressed apoptosis
by regulating the miR-1271-mediated Capn4/Wnt/β-catenin signaling
pathway in EOC, highlighting the involvement of the
circ-ABCB10/miR-1271/Capn4/Wnt/β-catenin signaling pathway in the
progression of EOC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XX conceived and designed the present study. XL and
YC performed the experiments and analyzed the data. XY interpreted
the data and wrote the manuscript. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
EOC
|
epithelial ovarian cancer
|
|
circ-ABCB10
|
circular RNA ABCB10
|
|
circRNA
|
circular RNA
|
|
FOXR2
|
forkhead box 2
|
References
|
1
|
Gloss BS and Samimi G: Epigenetic
biomarkers in epithelial ovarian cancer. Cancer Lett. 342:257–263.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Krzystyniak J, Ceppi L, Dizon DS and
Birrer MJ: Epithelial ovarian cancer: The molecular genetics of
epithelial ovarian cancer. Ann Oncol. 27 (Suppl 1):i4–i10. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Epidemiology Working Group Steering
Committee, Ovarian Cancer Association Consortium Members of the EWG
SC, ; Doherty JA, Jensen A, Kelemen LE, Pearce CL, Poole E,
Schildkraut JM, Terry KL, Tworoger SS, et al: Current gaps in
ovarian cancer epidemiology: The need for new population-based
research. J Natl Cancer Inst. 109:djx1442017.
|
|
4
|
Lheureux S, Gourley C, Vergote I and Oza
AM: Epithelial ovarian cancer. Lancet. 393:1240–1253. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Khairallah AS, Genestie C, Auguste A and
Leary A: Impact of neoadjuvant chemotherapy on the immune
microenvironment in advanced epithelial ovarian cancer: Prognostic
and therapeutic implications. Int J Cancer. 143:8–15. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang Y, Zhang Y, Jia L, Liu C and Xu F:
Circular RNA ABCB10 promotes tumor progression and correlates with
pejorative prognosis in clear cell renal cell carcinoma. Int J Biol
Markers. 34:176–183. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liang HF, Zhang XZ, Liu BG, Jia GT and Li
WL: Circular RNA circ-ABCB10 promotes breast cancer proliferation
and progression through sponging miR-1271. Am J Cancer Res.
7:1566–1576. 2017.PubMed/NCBI
|
|
8
|
Tian X, Zhang L, Jiao Y, Chen J, Shan Y
and Yang W: CircABCB10 promotes nonsmall cell lung cancer cell
proliferation and migration by regulating the miR-1252/FOXR2 axis.
J Cell Biochem. 120:3765–3772. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen Y, Ye X, Xia X and Lin X: Circular
RNA ABCB10 correlates with advanced clinicopathological features
and unfavorable survival, and promotes cell proliferation while
reduces cell apoptosis in epithelial ovarian cancer. Cancer
Biomark. 26:151–161. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li J, Xu J, Yan X, Jin K, Li W and Zhang
R: Suppression of Capn4 by microRNA-1271 impedes the proliferation
and invasion of colorectal cancer cells. Biomed Pharmacother.
99:162–168. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang X, Sun J, Xia D, Can X, Liu L, Zhang
J, Xu H, Du N, Liu W, Shen F, et al: Capn4 enhances osteopontin
expression through activation of the Wnt/β-catenin pathway to
promote epithelial ovarian carcinoma metastasis. Cell Physiol
Biochem. 42:185–197. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dochez V, Caillon H, Vaucel E, Dimet J,
Winer N and Ducarme G: Biomarkers and algorithms for diagnosis of
ovarian cancer: CA125, HE4, RMI and ROMA, a review. J Ovarian Res.
12:282019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao ZJ and Shen J: Circular RNA
participates in the carcinogenesis and the malignant behavior of
cancer. RNA Biol. 14:514–521. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen B and Huang S: Circular RNA: An
emerging non-coding RNA as a regulator and biomarker in cancer.
Cancer Lett. 418:41–50. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu X, Ma L, Rao Q, Mao Y, Xin Y, Xu H, Li
C and Wang X: miR-1271 inhibits ovarian cancer growth by targeting
cyclin G1. Med Sci Monit. 21:3152–3158. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jiao Y, Zhu G, Yu J, Mao Y, Xin Y, Xu H,
Li C and Wang X: miR-1271 inhibits growth, invasion and
epithelial-mesenchymal transition by targeting ZEB1 in ovarian
cancer cells. Onco Targets Ther. 12:6973–6980. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chi Y, Jin Q, Liu X, Xu L, He X, Shen Y,
Zhou Q, Zhang J and Jin M: miR-203 inhibits cell proliferation,
invasion, and migration of non-small-cell lung cancer by
downregulating RGS17. Cancer Sci. 108:2366–2372. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Du HY and Liu B: miR-1271 as a tumor
suppressor in breast cancer proliferation and progression via
targeting SPIN1. Eur Rev Med Pharmacol Sci. 22:2697–2706.
2018.PubMed/NCBI
|
|
20
|
Bodnar L, Stanczak A, Cierniak S, Smoter
M, Cichowicz M, Kozlowski W, Szczylik C, Wieczorek M and
Lamparska-Przybysz M: Wnt/β-catenin pathway as a potential
prognostic and predictive marker in patients with advanced ovarian
cancer. J Ovarian Res. 7:162014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Arend RC, Londoño-Joshi AI, Straughn JM Jr
and Buchsbaum DJ: The Wnt/β-catenin pathway in ovarian cancer: A
review. Gynecol Oncol. 131:772–779. 2013. View Article : Google Scholar : PubMed/NCBI
|





















