Introduction
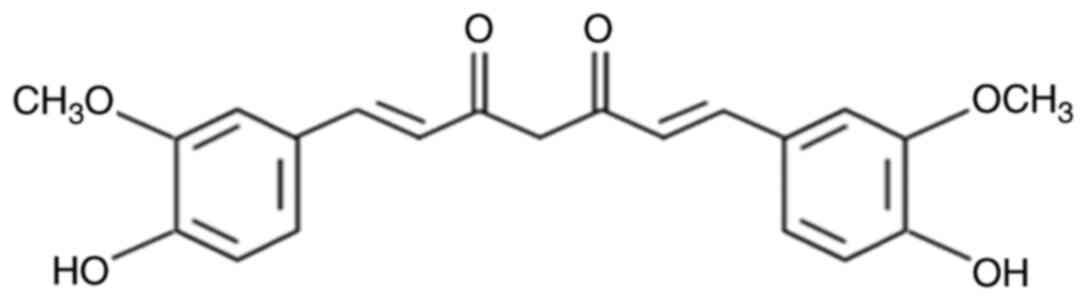
Curcumin (Fig. 1) is
an active component found in turmeric, a rhizomatous herbaceous
perennial plant of the ginger family (1). Curcumin is widely used as a culinary
spice and food colorant (2) and is
widely known to possess numerous beneficial health effects
(3). Recent studies indicate that
curcumin has a wide range of biological activities, including
anti-inflammatory, antioxidant, anticancer and antimicrobial
activities (4–6).
Intestinal hypermotility and hypomotility disorders
are associated with inflammatory bowel disease (IBD) and other
types of chronic gut inflammations, such as chronic enteritis and
allergic colitis (7). For instance,
infectious diarrhea and gastric stress ulcerations are
characterized by hypermotility, whilst intestinal
pseudo-obstruction, reflux esophagitis and ulcerative colitis are
associated with hypomotility (8).
Agents that possess the ability to ameliorate inflammation and
inflammation-related hypomotility and hypermotility disorders are
sorted after.
Irritable bowel syndrome (IBS) can be divided into
diarrhea-predominant irritable bowel syndrome (IBS-D),
constipation-predominant irritable bowel syndrome (IBS-C) and mixed
irritable bowel syndrome (IBS-M) according to symptoms (9). Previous studies suggested that
curcumin was beneficial for treating IBS-D, IBS-C and IBS-M
(10,11). Hyper- or hypomotility disorders have
been observed in different types of IBS (12). However, the potential effects of
curcumin on intestinal inflammation and inflammation-related
motility disorders remain to be elucidated.
In the present study, it was hypothesized that
curcumin ameliorates both intestinal inflammation and
inflammation-related motility disorders by returning the levels of
myosin light chain (MLC) phosphorylation in intestinal smooth
muscles to normal levels. Both diarrhea-prominent (DP)
hypermotility and constipation-prominent (CP) rat models were used
to investigate this hypothesis. The successful establishment of DP
and CP rat models was assessed not only by measuring the parameters
of the incidence of diarrhea or constipation, but also by measuring
the phosphorylation levels of MLC in the MLC kinase (MLCK) and Rho
kinase signaling pathways in the rat jejunal smooth muscle. In
addition, inflammatory markers, including serum TNF-α and IL-1β
levels and NF-κB translocation to the nucleus, were measured to
verify the extent of inflammation. The aforementioned parameters
were also used to determine whether curcumin could ameliorate both
intestinal inflammation and inflammation-related hypermotility in
DP rats and hypomotility in CP rats, with aims of unraveling the
mechanisms underlying the dual effects of curcumin.
Materials and methods
Animals
A total of 60 male Sprague-Dawley rats (age, 8–10
weeks; weight, 180–220 g) were obtained from the Experimental
Animal Center, Dalian Medical University [Certificate of
Conformity: no. SCXK (Liao) 2008–0002; Dalian, China]. The
experimental protocol was approved by the Dalian Medical University
Animal Care and Ethics Committee (Dalian, China) and the animal
care and treatment were conducted in accordance with the National
Institutes of Health Guide for Care and Use of Laboratory Animals
(Publication no. 85-23, revised 1985) (13). The rats were housed one per cage,
with all the animals housed in a room at 25°C with 60% humidity and
12-h light/dark cycles. The rats were fed a pelleted diet ad
libitum with free access to water and were fasted overnight
before the experiment.
Drugs
Curcumin (Purify speciation, ≥99%; cat. no.
458-37-7) was purchased from Shanghai Aladdin Biochemical
Technology Co., Ltd. Unless otherwise indicated, all other
chemicals were obtained from Sigma Aldrich, Merck KGaA.
Experimental models of diarrhea and
constipation
Sprague-Dawley rats were randomly divided into two
groups (n=10 per group): The DP and CP groups. The CP group
included sham rats, CP rats and curcumin-treated CP rats.
Similarly, the DP group included sham rats, DP rats and
curcumin-treated DP rats. CP rats were established by daily gavage
with cold water (0–4°C) for 2 weeks, whilst the CP sham rats were
treated by daily gavage with room-temperature water instead of cool
water (13). DP rats were
established by intracolonic acetic acid (4%) instillation using an
injector with hose for 2 weeks, whereas the DP sham rats received
intracolonic saline instillation (14,15).
The body weights of rats in each group were recorded every 2 days.
Fecal moisture content and granule number of each group were
measured at day 2 and day 7 post-CP and -DP induction to determine
whether the rat models were successfully established. After the
successful establishment of the CP and DP rat models, the rats were
treated with 200 mg/kg curcumin by gavage once daily. Following 7
days of consecutive administration, the rats were sacrificed.
Abdominal withdrawal reflex (AWR)
The rats were first anesthetized with ether before a
distension balloon catheter (6-Fr, 2 mm external diameter) was
placed in the descending colon of mildly sedated rats. After waking
up and adapting for 20 min, a round of distention was applied for
10 sec, followed by a 20 sec resting period. Distention at the
various quantities (0, 0.25 and 0.5 ml) were repeated twice,
following which the balloon was deflated and withdrawn after
measuring the AWR. Visceral sensory responses to rectal distension
were quantified by scoring the AWR, as described previously
(16). Briefly, 0 indicated no
behavioral response during colorectal distention (CRD); 1 indicated
an immobility response during CRD at the onset of the stimulus; 2
indicated mild contraction of the abdominal muscles without lifting
the abdomen off the platform; 3 indicated strong contraction of the
abdominal muscles without lifting the abdomen off the platform; and
4 indicated body arching, and lifting the pelvic structure and
scrotum off the platform.
Hematoxylin and eosin (H&E)
staining
The colon was fixed with 10% buffered formalin
solution for 30 min at room temperature and then dehydrated in
xylene followed by 75% ethanol overnight at room temperature,
followed by paraffin embedding. Serial 4-µm sections were cut. The
sections were stained with hematoxylin and eosin for 1 min at room
temperature for histologic analysis using a light microscope
(magnification, ×200).
Measurement of the serum TNF-α and
IL-1β levels
Blood samples (5 ml) were collected to measure the
serum levels of the cytokines TNF-α and IL-1β using rat TNF-α
Quantikine (cat. no. RTA00) and rat IL-1β Quantikine (cat. no.
RLB00) ELISA kits (both R&D Systems, Inc.).
Western blotting
The translocation and activity of NF-κB, the content
of Rho A, Rho-associated kinase 2 (ROCKII), phosphorylated
(p)-MLC20, p-myosin phosphates target subunit 1 (MYPT1),
130 kDa-MLCK and the expression of c-kit tyrosine kinase in rat
jejunal segments were determined using western blotting as
previously described (16). Jejunal
strips were teased along the natural line of cleavage from the
longitudinal smooth muscle and the mucosal layer was removed. The
samples were immediately weighed and minced on an ice-cold plate,
following which the protein was extracted using a total protein
extraction kit or a nuclear protein extraction kit (Nanjing KeyGen
Biotech Co., Ltd.) and quantified by a bicinchoninic acid protein
assay kit (Beyotime Institute of Biotechnology). Protein (50 µg)
from the tissue samples were separated by 7.5–20% SDS-PAGE and then
transferred onto PVDF membranes. The PVDF membrane was probed with
antibodies (all 1:1,000): IκB (cat. no. 40903; ProteinTech Group,
Inc.), p65 (cat. no. 66535-1-Ig; ProteinTech Group, Inc.), p-p65
(cat. no. 3039s; Cell Signaling Technology, Inc.), Rho A (cat. no.
27213-1-AP; ProteinTech Group, Inc.), ROCKII (cat. no. 8236s; Cell
Signaling Technology, Inc.), MLC20 (cat. no. 3672s; Cell Signaling
Technology, Inc.), p-MLC20 (cat. no. 3675s; Cell Signaling
Technology, Inc.), MYPT1 (cat. no. 2634; Cell Signaling Technology,
Inc.), p-MYPT1 (cat. no. 3040T; Cell Signaling Technology, Inc.),
MLCK (cat. no. 21642-1-AP; ProteinTech Group, Inc.) at 4°C
overnight. A primary antibody against β-actin (cat. no. bs-0061R;
BIOSS) or GAPDH (cat. no. 60004-1-Ig; ProteinTech Group, Inc.) for
total protein and a primary antibody against histone-3 (cat. no.
ab94817; Abcam) for nuclear protein was used as a loading control.
Horseradish peroxidase-conjugated anti-rabbit/mouse IgG (cat. no.
33101ES60; Shanghai Yeasen Biotechnology Co., Ltd.) was used as
secondary antibody at 1:1,000 for 60 min at room temperature. ECL
(Beyotime Institute of Biotechnology) detection was performed for
visualizing the protein bands, which were quantified by using the
MultiSpectral imaging system (Analytik Jena AG).
Analysis of MLCK and gene expression
by reverse transcription-quantitative (RT-q) PCR
Total RNA was extracted from the jejunal smooth
muscle layer using TRIzol® reagent (cat. no. 15596026;
Thermo Fisher Scientific, Inc.). cDNA was synthesized using the
PrimeScript One Step RT-PCR kit (cat. no. RR055B; Takara
Biotechnology Co., Ltd.) at 45°C for 60 min and 72°C for 5 min.
Oligo (dT) primers (Promega Corporation) were used for RT-q PCR.
The primers (Takara Biotechnology Co., Ltd.) were designed to
detect the transcripts of MLCK according to the published sequences
(2). The housekeeping gene β-actin
served as a comparative reference. The primer sequences were as
follows: MLCK forward, 5′-AATGGTGTTGCTGGAGATCGAGGT-3′ and reverse,
5′-GCTGGATCAAATTGCGGTGGTTCA-3′ and β-actin forward,
5′-GCAGGAGTACGATGAGTCCG-3′ and reverse, 5′-ACGCAGCTCAGTAACAGTCC-3′.
RT-qPCR was performed using SYBR Green-based fluorescence detection
(cat. no. AQ142-21; TransStart Tip Green qPCR SuperMIX; TransGen
Biotech Co., Ltd.). The PCR mixtures were denatured at 95°C for 30
sec, followed by 40 thermal cycles of 95°C for 5 sec, 60°C for 35
sec and 72°C for 30 sec. Relative gene expression was calculated
using to the 2−∆∆Ct method (17).
Statistical analysis
Data are expressed as the mean ± SD of ≥six
independent repeats. Comparisons between two groups were performed
using unpaired Student's t-test. To compare≥three groups,
one-way ANOVA followed by Tukey's post hoc test was performed. The
data were analyzed using SPSS (version 19.0; IBM Corp.). P<0.05
was considered to indicate a statistically significant
difference.
Results
Amelioration of diarrhea in DP rats
and constipation in CP rats
The DP rat model induced by acetic acid (4% v/v)
instillation and the CP rat model induced by cool water (4°C)
gavage were used to model hyper- and hypomotility (18,19).
The successful establishment of inflammation-related diarrhea in
the DP rats and inflammation-related constipation in the CP rats
was evaluated by determining the granules and moisture content of
feces. On days 2 and 7, the granules and moisture contents in the
feces from the CP and DP rats were significantly decreased and
increased, respectively, compared with those in sham rats (Table I). Curcumin significantly reversed
the reduction in granule and fecal moisture contents of the CP rats
whilst also significantly reversing the increased granule and fecal
moisture contents of the DP rats (Table
I). This suggest that curcumin can ameliorate the symptoms of
constipation and diarrhea in the CP and DP rats. Changes in AWR
scores were next measured. The AWR score for all experimental
groups during the recording period showed an increase in volume
dependence by colorectal distention (colorectal distention,
0.25–0.5 ml water; Table II).
However, no significant differences were observed among the groups.
Additionally, no significant changes of colonic morphology were
observed by H&E staining (Fig.
S1).
 | Table I.Granules and moisture content of
feces in rats. |
Table I.
Granules and moisture content of
feces in rats.
|
| Day 2 | Day 7 |
|---|
|
|
|
|
|---|
| Groups | Granules, % | Moisture content,
% | Granules, % | Moisture content,
% |
|---|
| DP sham | 100.0 | 100.0 | 100.0 | 100.0 |
| DP |
221.0±64.0a |
192.0±54.3a |
342.0±89.2a |
255.0±78.3a |
| DP + Cur |
120.0±32.2b |
175.0±45.5b |
102.8±61.2b |
154.0±74.9b |
| CP sham | 100.0 | 100.0 | 100.0 | 100.0 |
| CP |
31.0±20.2a |
45.0±14.4a |
25.0±12.3a |
33.0±21.7a |
| CP + Cur |
89.0±34.4b |
78.0±26.2b |
91.0±25.8b |
85.0±22.1b |
 | Table II.Score of abdominal withdrawal reflex
to colorectal distention. |
Table II.
Score of abdominal withdrawal reflex
to colorectal distention.
|
|
| Volume of colon
distention |
|---|
|
|
|
|
|---|
| Groups | N | 0.25 ml | 0.5 ml |
|---|
| CP related
group |
|
|
|
|
Sham | 10 | 0.22±0.34 | 2.70±0.33 |
|
Curcumin | 10 | 0.21±0.33 | 2.40±0.34 |
| CP | 10 | 0.30±0.42 | 2.60±0.39 |
| CP +
Curcumin | 10 | 0.24±0.25 | 2.50±0.28 |
| DP related
group |
|
|
|
|
Sham | 10 | 0.22±0.33 | 2.50±0.29 |
|
Curcumin | 10 | 0.21±0.29 | 2.60±0.26 |
| DP | 10 | 0.23±0.25 | 2.80±0.40 |
| DP +
Curcumin | 10 | 0.25±0.42 | 2.48±0.59 |
Amelioration of intestinal
inflammation in both CP and DP rats
The inflammatory cytokines TNF-α and IL-1β, which
serve important roles in the progression of both diarrhea and
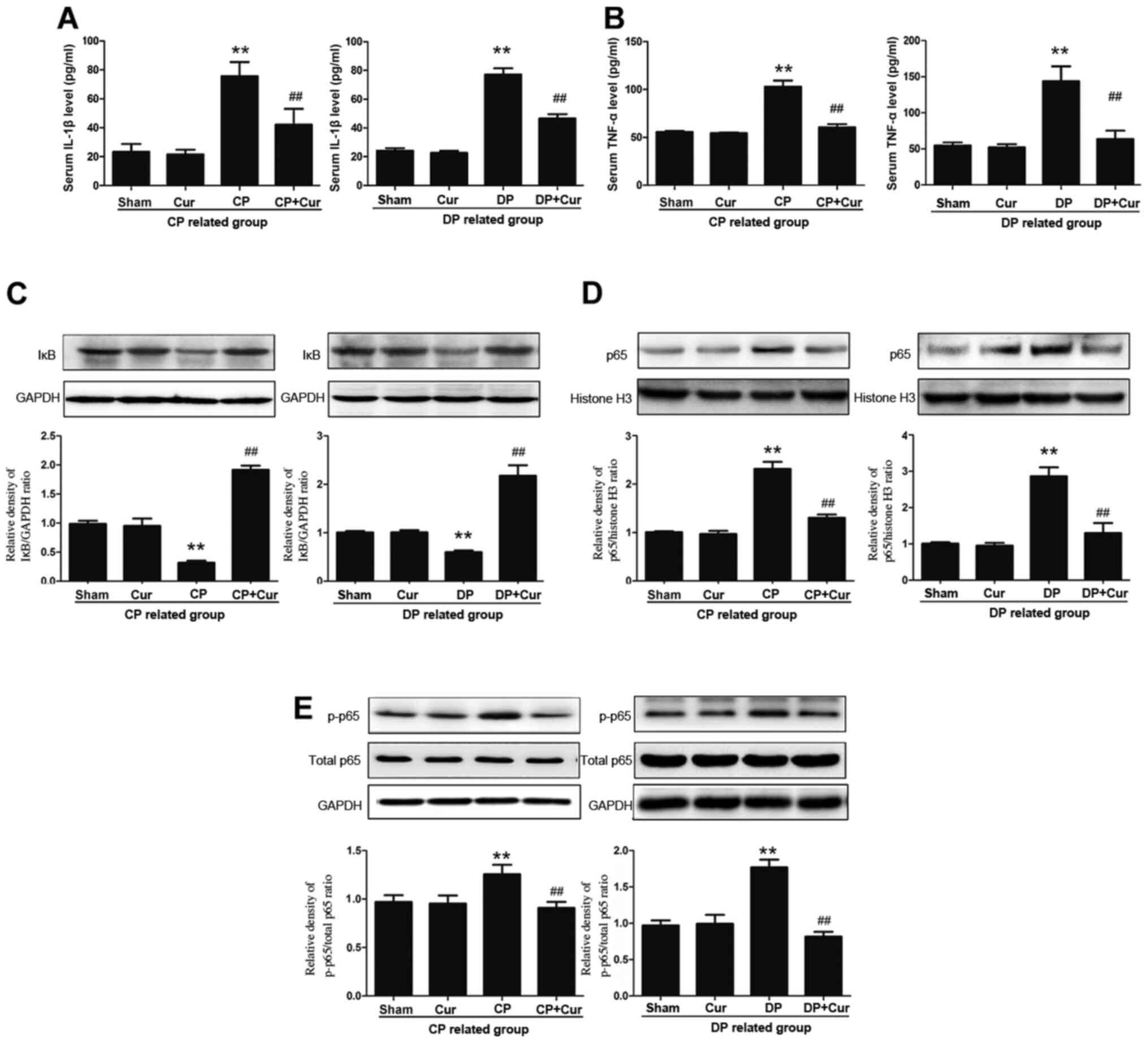
constipation, are associated with gut inflammation (20). In the present study, increased TNF-α
and IL-1β levels were observed in the serum of the DP and CP rats
compared with those in the Sham group (Fig. 2A and B), suggesting the presence of
the inflammatory processes in both the DP and CP rats. Curcumin
treatment significantly downregulated TNF-α and IL-1β release in
both the DP and CP rats (Fig. 2A and
B). The pro-inflammatory mediator NF-κB is a nuclear
transcription factor that serves a central role in inflammatory
responses, which increases in TNF-α and IL-1β serum levels
(1). NF-κB activation is
characterized by IκB degradation, p65 nuclear translocation and
increased p65 phosphorylation (20,21).
Compared with the sham group, the NF-κB signaling pathway was
activated in the CP and DP groups. The results of the present study
indicated that curcumin inhibited the NF-κB pathway by
significantly blocking IκB degradation (Fig. 2C), significantly inhibiting p65
nuclear translocation (Fig. 2D) and
significantly reducing p65 phosphorylation (Fig. 2E) in the intestinal smooth muscle
layer of the DP and CP rats. The aforementioned results indicated
that curcumin reduced intestinal inflammation in DP and CP
rats.
Curcumin induces MLC phosphorylation
to normal levels through the Rho A/ROCK2/MYPT1 pathway
Intestinal motility is positively associated with
MLC phosphorylation in the smooth muscle (22). Smooth muscle MLC phosphorylation is
regulated by the relative activities of MLCK and myosin light chain
phosphatase (MLCP) (23,24). The results of the present study
indicated significant reductions and increased MLC20
phosphorylation in the jejunal smooth muscle of the CP and DP rats,
respectively (Fig. 3A and B).
However, these were significantly reversed after 7 days of curcumin
administration compared with those of the CP and DP rats (Fig. 3A and B).
To determine the associated mechanisms, the MLCK and
MLCP activities were assessed. In the CP rats, reductions in 130
kDa MLCK mRNA and protein expression levels were not significantly
affected by the administration of curcumin (Fig. 4A and B). By contrast, in the DP
rats, the increased 130 kDa MLCK mRNA and expression levels were
significantly reversed (Fig. 4A and
B). Inhibition of MLCP activates the Ca2+
sensitivity pathway, leading to an increase in smooth muscle
contraction (25). MYPT1 is the
central subunit that regulates MLCP activity that can be activated
by the Rho kinase and the NF-κB pathways upstream (26). Significantly increased levels of Rho
A, ROCK II and phosphorylated MYPT1 expression were observed in the
DP and CP rats compared with those in Sham rats (Fig. 4C and D). Curcumin treatment for 7
consecutive days significantly reversed the increased expression of
Rho A, ROCK II and MYPT1 phosphorylation in both DP and CP rats
(Fig. 4C and D).
Curcumin restores the normal function
of interstitial cells of Cajal (ICCs)
Damage to the ICCs may induce changes in the
amplitude and duration of smooth muscle physical contractions
(27). The activity of c-kit
tyrosine kinase is associated with the function of ICCs in the gut
(28). Significantly increased
expression levels of c-kit tyrosine kinase in rat intestinal smooth
muscle tissue in the DP rats and significantly decreased expression
levels of c-kit tyrosine kinase in the CP rats were observed,
compared with those in Sham rats (Fig.
5). Curcumin significantly reversed the increased expression
levels of c-kit tyrosine kinase in the DP rats, but did not
significantly upregulate the decreased expression levels of c-kit
tyrosine kinase in the CP rats (Fig.
5), suggesting a potential role of curcumin in restoring the
normal function of ICCs.
Discussion
Gut inflammatory disorders are associated with both
diarrhea and constipation, with characteristics of intestinal
dysmotility (29). For instance,
diarrhea and constipation are associated with a phenotype or
potentially inflammatory conditions such as IBS and IBD (30). Endogenous substances, including
leptin (31) and oxytocin (32), have been reported to simultaneously
participate in the regulation of gastrointestinal inflammation and
motility. This suggests that ideal anti-inflammatory, naturally
occurring and health-promoting compounds with low toxicity may
exist that can simultaneously ameliorate both intestinal
inflammation and inflammation-related diarrhea and constipation.
Curcumin, which has been reported to be an anti-inflammatory and
anticarcinogenic agent, is a potent inhibitor of NF-κB (33,34)
that has been used to treat IBD (35). Curcumin has also been reported to
interfere with colonic inflammation partly through the inhibition
of chemokine expression and through the direct inhibition of
neutrophil chemotaxis and chemokines (36). Based on the aforementioned
information, it was hypothesized that curcumin could simultaneously
modulate gut inflammation and motility disorders.
The major characteristics of the curcumin-induced
bidirectional amelioration of diarrhea and constipation in DP and
CP rats were as follows. Firstly, curcumin ameliorated the symptoms
of diarrhea and constipation in the DP and CP rats. Secondly,
curcumin significantly downregulated the expression of the
inflammatory mediators TNF-α, IL-1β and NF-κB in the DP and CP
rats. Thirdly, curcumin reversed the decreased MLC20
phosphorylation in the jejunal smooth muscle of the CP rats and
reversed the increased MLC20 phosphorylation in the DP
rats possibly through Ca2+-sensitive pathways.
The results of the present study demonstrated that
curcumin ameliorated the symptoms and inflammation in the CP and DP
rats, laying the foundation for further investigation on the
effects of curcumin on smooth muscle motility-related
phosphorylation. Smooth muscle contractility is regulated by the
phosphorylation and dephosphorylation of MLC20, which is
mediated by both the MLCK signaling and Rho kinase pathways. These
pathways are known as Ca2+-dependent and
Ca2+-sensitive pathways (37). Motility disorders, which are related
to anomalous regulation of the Ca2+ and Rho A/ROCK
II/MYPT1 pathways, are observed in gastrointestinal inflammatory
disorders (38). The results of the
present study indicated that increased IL-1β, TNF-α and NF-κB are
found during intestinal inflammation, which were reversed after
curcumin administration in both CP and DP rats.
The decreased MLC20 phosphorylation in
the CP rats and increased MLC20 phosphorylation in the
DP rats were returned to normal levels by curcumin, indicating that
curcumin benefits both inflammation-related hyper- and
hypointestinal motility. The downregulation of the 130 kDa MLCK
mRNA and protein expression levels were observed only in the DP
rats, unlike the upregulation of the 130 kDa MLCK mRNA and protein
expression levels in the CP rats. Instead, the increased expression
levels of phosphorylated MYPT1 in the DP and CP rats were returned
to normal levels after the administration of curcumin. In both CP
and DP rats, curcumin inhibited the increased nuclear translocation
of p65 and the Ca2+-sensitive pathway was activated as
Rho A increased, followed by the upregulation of ROCK II and
phosphorylated MYPT1. Phosphorylation of MYPT1 inhibits MLCP
activity by directly interacting with protein phosphatase 1
catalytic subunit or by acting through a combination of molecular
and biochemical mechanisms (39,40).
Changes in intracellular Ca2+ result in dysfunction of
Ca2+-related proteins, such as c-kit tyrosine kinase,
which is necessary for the function of Cajal cells; and MLCK, which
promotes myosin phosphorylation (41). Results from the present study
indicated that curcumin reversed the increased c-kit tyrosine
kinase and MLCK expression levels, possibly through the inhibition
of NF-κB activity, in the DP rats. However, upregulation of MLCK in
CP rats was not observed, suggesting that the curcumin-induced
return of MLC20 phosphorylation to normal levels in the
CP rats is not mediated by the MLCK signaling pathway, but more
dependent on the Ca2+-sensitive pathway.
ICCs are pacemaker cells that generate electrical
slow waves in the gastrointestinal tract and express c-kit tyrosine
kinase, which is essential for ICC phenotype development and is
required for spontaneous electrical and mechanical activities
(42). Changes in the amplitude and
duration of smooth muscle physical contractions are likely due to
functional damage to ICCs (26).
Phenotypic changes in ICCs were previously observed in a
lipopolysaccharide-treated mouse model characterized by suppressed
c-kit protein and mRNA expression (43), indicating that ICCs serve a vital
role in motility changes during inflammation. The results of the
present study indicated that the increased and decreased expression
levels of c-kit tyrosine kinase in the CP and DP rats,
respectively, were returned to normal by the administration of
curcumin. This suggested that c-kit tyrosine kinase is involved in
the curcumin-induced return of inflammation-related hyper- and
hypojejunal contractility to normal.
Taken together, the present study demonstrated that
curcumin returned the inflammation-related hyper-contractility in
the DP rats and the hypo-contractility in the CP rats to normal
through the NF-κB/Rho kinase pathway, in addition to c-kit and MLCK
regulation. These results suggest that curcumin has potential value
in relieving the inflammation-related alternating symptoms of gut
dysmotility, where motility modulation during inflammatory
conditions is based more on the Ca2+-sensitive pathway
than the Ca2+-dependent pathway. However, although
curcumin can inhibit gut inflammation, the role of curcumin on gut
motility, the relationship between gut inflammation and motility
disorders, as well as the detailed regulatory mechanism require
further investigation. Since a number of studies have reported the
anti-inflammatory effects of curcumin (44,45),
the present study investigated the role of curcumin in regulating
gut motility based on the body status. Isolated smooth muscle
segments and smooth muscle cells were used to investigate the
mechanism of curcumin for intestinal inflammation and motility. The
results demonstrated that curcumin inhibited smooth muscle
contraction by blocking Ca2+ influx (46). Therefore, the effects of curcumin on
intestinal motility is a highly complex process, including the
regulation of the enteric nervous system and smooth muscle, but
also the central nervous system.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was supported by the Science and
Technology Research Program of Chongqing Municipal Education
Commission (grant nos. KJQN201902703 and KJZD- K202002701), the
Nursery Project of Chongqing Three Gorges Medical College (grant
no. 2016mpxz21) and the Natural Science Foundation of Chongqing
(grant no. cstc2019jcyj-msxmX0299).
Availability of data and materials
The datasets used and/ or analyzed during the
current study are available from the corresponding author on
reasonable requests.
Authors' contributions
YZ conceptualized the study; YY and RL performed the
experiments, analyzed the data and prepared the manuscript. SX and
CZ conceived the study, provided the reagents and materials, and
analyzed the data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All animals received humane care in compliance with
the Guide for the Care and Use of Laboratory Animals in China. The
present study was approved by the Dalian Medical University Animal
Care and Ethics Committee (approval no. 2019-4015).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
DP
|
diarrhea-prominent
|
|
CP
|
constipation- prominent
|
|
MLCK
|
myosin light chain kinase
|
|
MLC
|
myosin light chain
|
|
ROCK-II
|
Rho-associated kinase 2
|
|
MYPT1
|
myosin phosphates target subunit 1
|
|
IBD
|
inflammatory bowel disease
|
|
IBS
|
irritable bowel syndrome
|
|
MLCP
|
myosin light chain phosphatase
|
|
ICCs
|
interstitial cells of Cajal
|
References
|
1
|
Lestari ML and Indrayanto G: Curcumin.
Profiles Drug Subst Excip Relat Methodol. 39:113–204. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Prasad S, Gupta SC, Tyagi AK and Aggarwal
BB: Curcumin, a component of golden spice: From bedside to bench
and back. Biotechnol Adv. 32:1053–1064. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Aggarwal BB, Yuan W, Li S and Gupta SC:
Curcumin-free turmeric exhibits anti-inflammatory and anticancer
activities: Identification of novel components of turmeric. Mol
Nutr Food Res. 57:1529–1542. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Homayouni A, Amini M, Sohrabi M, Varshosaz
J and Nokhodchi A: Curcumin nanoparticles containing poloxamer or
soluplus tailored by high pressure homogenization using antisolvent
crystallization. Int J Pharm. 562:124–134. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ridzuan NRA, Rashid NA, Othman F, Budin
SB, Hussan F and Teoh SL: Protective role of natural products in
cisplatin-induced nephrotoxicity. Mini Rev Med Chem. 19:1134–1143.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wong KE, Ngai SC, Chan KG, Lee LH, Goh BH
and Chuah LH: Curcumin Nanoformulations for Colorectal Cancer: A
Review. Front Pharmacol. 10:1522019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ozaki H, Hori M, Kinoshita K and Ohama T:
Intestinal dysmotility in inflammatory bowel disease: Mechanisms of
the reduced activity of smooth muscle contraction.
Inflammopharmacology. 13:103–111. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Du X, Allwood G, Webberley KM, Inderjeeth
AJ, Osseiran A and Marshall BJ: Noninvasive diagnosis of irritable
bowel syndrome via bowel sound features: Proof of concept. Clin
Transl Gastroenterol. 10:e000172019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ng QX, Soh AYS, Loke W, Venkatanarayanan
N, Lim DY and Yeo WS: A Meta-analysis of the clinical use of
curcumin for irritable bowel syndrome (IBS). J Clin Med. 7:2982018.
View Article : Google Scholar
|
|
10
|
Goulart RA, Barbalho SM, Lima VM, Souza
GA, Matias JN, Araújo AC, Rubira CJ, Buchaim RL, Buchaim DV,
Carvalho ACA and Guiguer ÉL: Effects of the use of curcumin on
ulcerative colitis and Crohn's disease: A systematic review. J Med
Food. Nov 5–2020.(Epub of print). View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Horii K, Ehara Y, Shiina T, Naitou K,
Nakamori H, Horii Y, Shimaoka H, Saito S and Shimizu Y: Sexually
dimorphic response of colorectal motility to noxious stimuli in the
colorectum in rats. J Physiol. 2020.(Ahead of print). PubMed/NCBI
|
|
12
|
Chen D, Xiong Y, Wang L, Lv B and Lin Y:
Characteristics of emodin on modulating the contractility of
jejunal smooth muscle. Can J Physiol Pharmacol. 90:455–462. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zou BC, Dong L, Wang Y, Wang SH and Cao
MB: Expression and role of 5-HT7 receptor in brain and intestine in
rats with irritable bowel syndrome. Chin Med J (Engl).
120:2069–2074. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
La JH, Kim TW, Sung TS, Kang JW, Kim HJ
and Yang IS: Visceral hypersensitivity and altered colonic motility
after subsidence of inflammation in a rat model of colitis. World J
Gastroenterol. 9:2791–2795. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Peng HY, Yeh CM, Cheng JK, Chau YP, Ruan
T, Chen GD, Hsieh MC, Lai CY and Lin TB: Acute uterine irritation
provokes colonic motility via transient receptor potential
A(1)-dependent spinal NR2B phosphorylation in rats. Anesthesiology.
120:436–446. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Qi DB and Li WM: Effects of
electroacupuncture on expression of c-fos protein in the spinal
dorsal horn of rats with chronic visceral hyperalgesia. Zhong Xi Yi
Jie He Xue Bao. 10:1490–1496. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ohama T, Hori M, Momotani E, Iwakura Y,
Guo F, Kishi H, Kobayashi S and Ozaki H: Intestinal inflammation
downregulates smooth muscle CPI-17 through induction of TNF-alpha
and causes motility disorders. Am J Physiol Gastrointest Liver
Physiol. 292:G1429–G1438. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen DP, Xiong YJ, Tang ZY, Yao QY, Ye DM,
Liu SS and Lin Y: Characteristics of deslanoside-induced modulation
on jejunal contractility. World J Gastroenterol. 18:5889–5896.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim HH, Kim DS, Kim SW, Lim SH, Kim DK,
Shin TY and Kim SH: Inhibitory effects of Diospyros kaki in a model
of allergic inflammation: Role of cAMP, calcium and nuclear
factor-κB. Int J Mol Med. 32:945–951. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang W, Sun J, Shen X, Xue Y, Yuan S and
Wang X: Effect of PA-MSAH preprocessing on the expression of
TLR-4-NF-κB pathway and inflammatory factors in the intestinal
tract of rats with septic shock. Exp Ther Med. 17:2567–2574.
2019.PubMed/NCBI
|
|
22
|
Sun G, Xing C, Zeng L, Huang Y, Sun X and
Liu Y: Flemingia philippinensis Flavonoids Relieve Bone Erosion and
Inflammatory Mediators in CIA Mice by Downregulating NF-κB and MAPK
Pathways. Mediators Inflamm. 2019:57902912019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu Z, Liang H, Zhang M, Tao X, Dou D, Hu L
and Kang T: Ardipusilloside-I stimulates gastrointestinal motility
and phosphorylation of smooth muscle myosin by myosin light chain
kinase. Korean J Physiol Pharmacol. 21:609–616. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chang AN, Mahajan P, Knapp S, Barton H,
Sweeney HL, Kamm KE and Stull JT: Cardiac myosin light chain is
phosphorylated by Ca2+/calmodulin-dependent and -independent kinase
activities. Proc Natl Acad Sci USA. 113:E3824–E3833. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Somlyo AP and Somlyo AV: Ca2+ sensitivity
of smooth muscle and nonmuscle myosin II: Modulated by G proteins,
kinases, and myosin phosphatase. Physiol Rev. 83:1325–1358. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Martelli L, Ragazzi E, di Mario F,
Martelli M, Castagliuolo I, Dal Maschio M, Palu G, Maschietto M,
Scorzeto M, Vassanelli S and Brun P: A potential role for the
vanilloid receptor TRPV1 in the therapeutic effect of curcumin in
dinitrobenzene sulphonic acid-induced colitis in mice.
Neurogastroenterol Motil. 19:668–674. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Der T, Bercik P, Donnelly G, Jackson T,
Berezin I, Collins SM and Huizinga JD: Interstitial cells of cajal
and inflammation-induced motor dysfunction in the mouse small
intestine. Gastroenterology. 119:1590–1599. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jo HJ, Kim N, Nam RH, Kang JM, Kim JH,
Choe G, Lee HS, Park JH, Chang H, Kim H, et al: Fat deposition in
the tunica muscularis and decrease of interstitial cells of Cajal
and nNOS-positive neuronal cells in the aged rat colon. Am J
Physiol Gastrointest Liver Physiol. 306:G659–G669. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Szałwińska P, Włodarczyk J, Spinelli A,
Fichna J and Włodarczyk M: IBS-Symptoms in IBD
patients-manifestation of concomitant or different entities. J Clin
Med. 10:312020. View Article : Google Scholar
|
|
30
|
Kamiya T, Shikano M, Tanaka M, Ozeki K,
Ebi M, Katano T, Hamano S, Nishiwaki H, Tsukamoto H, Mizoshita T,
et al: Therapeutic effects of biobran, modified arabinoxylan rice
bran, in improving symptoms of diarrhea predominant or mixed type
irritable bowel syndrome: A pilot, randomized controlled study.
Evid Based Complement Alternat Med. 2014:8281372014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yarandi SS, Hebbar G, Sauer CG, Cole CR
and Ziegler TR: Diverse roles of leptin in the gastrointestinal
tract: Modulation of motility, absorption, growth, and
inflammation. Nutrition. 27:269–275. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Welch MG, Margolis KG, Li Z and Gershon
MD: Oxytocin regulates gastrointestinal motility, inflammation,
macromolecular permeability, and mucosal maintenance in mice. Am J
Physiol Gastrointest Liver Physiol. 307:G848–G862. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jobin C, Bradham CA, Russo MP, Juma B,
Narula AS, Brenner DA and Sartor RB: Curcumin blocks
cytokine-mediated NF-kappa B activation and proinflammatory gene
expression by inhibiting inhibitory factor I-kappa B kinase
activity. J Immunol. 163:3474–3483. 1999.PubMed/NCBI
|
|
34
|
Zhu T, Chen Z, Chen G, Wang D, Tang S,
Deng H, Wang J, Li S, Lan J, Tong J, et al: Curcumin attenuates
asthmatic airway inflammation and mucus hypersecretion involving a
PPARγ-Dependent NF-κB signaling pathway in vivo and in vitro.
Mediators Inflamm. 2019:49274302019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Torres J, Ellul P, Langhorst J,
Mikocka-Walus A, Barreiro-de Acosta M, Basnayake C, Ding NJS,
Gilardi D, Katsanos K, Moser G, et al: European crohn's and colitis
organisation topical review on complementary medicine and
psychotherapy in inflammatory bowel disease. J Crohns Colitis.
13:673–685e. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Larmonier CB, Midura-Kiela MT, Ramalingam
R, Laubitz D, Janikashvili N, Larmonier N, Ghishan FK and Kiela PR:
Modulation of neutrophil motility by curcumin: Implications for
inflammatory bowel disease. Inflamm Bowel Dis. 17:503–515. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Álvarez-Santos MD, Álvarez-González M,
Estrada-Soto S and Bazán-Perkins B: Regulation of myosin
light-chain phosphatase activity to generate airway smooth muscle
hypercontractility. Front Physiol. 11:7012020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rajagopal S, Kumar DP, Mahavadi S,
Bhattacharya S, Zhou R, Corvera CU, Bunnett NW, Grider JR and
Murthy KS: Activation of G protein-coupled bile acid receptor,
TGR5, induces smooth muscle relaxation via both Epac- and
PKA-mediated inhibition of RhoA/Rho kinase pathway. Am J Physiol
Gastrointest Liver Physiol. 304:G527–G535. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Moreno-Dominguez A, Colinas O, El-Yazbi A,
Walsh EJ, Hill MA, Walsh MP and Cole WC: Ca2+
sensitization due to myosin light chain phosphatase inhibition and
cytoskeletal reorganization in the myogenic response of skeletal
muscle resistance arteries. J Physiol. 591:1235–1250. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Grassie ME, Moffat LD, Walsh MP and
MacDonald JA: The myosin phosphatase targeting protein (MYPT)
family: A regulated mechanism for achieving substrate specificity
of the catalytic subunit of protein phosphatase type 1δ. Arch
Biochem Biophys. 510:147–159. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Drumm BT, Rembetski BE, Messersmith K,
Manierka MS, Baker SA and Sanders KM: Pacemaker function and neural
responsiveness of subserosal interstitial cells of Cajal in the
mouse colon. J Physiol. 598:651–681. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Somlyo AP and Somlyo AV: Smooth muscle:
Excitation- contraction coupling, contractile regulation, and the
cross-bridge cycle. Alcohol Clin Exp Res. 18:138–143. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wei J, Li N, Xia X, Chen X, Peng F, Besner
GE and Feng J: Effects of lipopolysaccharide-induced inflammation
on the interstitial cells of Cajal. Cell Tissue Res. 356:29–37.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Fallahi F, Borran S, Ashrafizadeh M,
Zarrabi A, Pourhanifeh MH, Khaksary Mahabady M, Sahebkar A and
Mirzaei H: Curcumin and inflammatory bowel diseases: From in vitro
studies to clinical trials. Mol Immunol. 130:20–30. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Qiao P, Ma J, Wang Y, Huang Z, Zou Q, Cai
Z and Tang Y: Curcumin prevents neuroinflammation by inducing
microglia to transform into the M2-phenotype via CaMKKβ-dependent
activation of the AMP-activated protein kinase signal pathway. Curr
Alzheimer Res. 17:735–752. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bardak H, Uğuz AC and Bardak Y: Curcumin
regulates intracellular calcium release and inhibits oxidative
stress parameters, VEGF, and caspase-3/-9 levels in human retinal
pigment epithelium cells. Physiol Int. 104:301–315. 2017.
View Article : Google Scholar : PubMed/NCBI
|