Introduction
Osteoporosis is a systemic osteopathy defined by
insufficiency in bone repair due to the impairment of bone
microstructure, the decrease of bone quality and density and the
increase of bone fragility (1,2).
Moreover, the osteoporotic microenvironment is not conducive to the
proliferation and osteogenic differentiation of bone marrow
mesenchymal stem cells (BMSCs), causing excessive bone loss and
decreased osteogenic capacity (3,4). These
factors lead to inadequate osseointegration within the bone and
implant surfaces, thus causing an increased risk of complications
in patients after joint replacement and dental implantation, due to
implant loosening and displacement (5–7).
Therefore, improving the osseointegration efficiency of implants
would be of high clinical benefit to solve these problems.
Titanium (Ti) alloy is a widely applied material for
orthopedic or dental implants with promising application prospects
due to its predominant mechanical strength and corrosion
resistance; however, it is restricted by its high stiffness which
consequently results in stress-shielding-induced osteolysis
(8,9). Three-dimensional (3D) printing
technology is a promising method for the generation of
individualized implants with complex components and porous sections
in a single process (10,11). Interconnected porous implants with
controlled pore size and porosity can significantly decrease the
stiffness, imitate the structure of natural bone tissue superiorly
and promote bone regeneration. Moreover, bone ingrowth into the
micropores of 3D-printed porous Ti (pTi) alloy could enhance the
osseointegration and improve the stability of the implants
(12–14). However, considering the biological
inertia, smooth surface and poor cellular adhesion of Ti alloys,
pTi scaffolds may fail due to insufficient osteointegration with
the surrounding bone, especially under pathological states, such as
osteoporosis (15,16). Therefore, improving the bioactivity
of osteoblast-related cells on the surface of titanium alloy is
anticipated to treat the postoperative complications of
osteoporosis patients. Bai et al (17) constructed a pTi/poloxamer 407
hydrogel system loaded with zoledronate, a bisphosphonate, thus
obtaining an optimized osseointegration effect in osteoporotic
defect models. In addition, Vladescu et al (10) coated 3D-printed Ti6Al4V scaffolds
with HA, and calcium phosphate nanoparticles to improve the
bioactivity and biocompatibility of the scaffolds. However, as it
is difficult to release the loaded drugs continuously and maintain
the stability of bio-coating, implanting pTi scaffolds into bone
defects followed by extracorporeal non-invasive and repetitive
therapy may be a potential strategy to promote bone integration
(18,19).
Pulse electromagnetic fields (PEMF), which are
transient electromagnetic fields produced in a coil when a pulse
current generated by a pulse generator passes through the coil, are
considered as a non-invasive treatment with the effect of promoting
bone regeneration in clinical applications (20). In terms of cytology, external PEMF
have could promote the early and late osteogenesis, enhance
mineralization of BMSCs (21),
improve cell viability and enhance alkaline phosphatase (ALP)
activity of osteoblasts (22), as
well as inhibit bone resorption by inhibiting the formation and
maturation of osteoclasts (23).
Although previous studies have indicated that PEMF therapy has
beneficial effects on bone regeneration and significantly enhances
the healing of fracture, non-union and other orthopedic diseases,
it has not been widely used to enhance osteointegration for
osteoporotic bone defects (24,25).
In the present study, it was hypothesized that the
combination therapy of 3D-printed pTi and PEMF may have a positive
effect on bone regeneration in vitro and in vivo in
osteoporotic bone defects. The pTi implant supports optimized pore
size and porosity, which match the mechanical properties of the
bone tissue to decrease stress shielding. Additionally, a previous
study has demonstrated that PEMF therapy (50 Hz; 1 mT) could induce
osteogenic differentiation and proliferation of MSCs (26). In the present study, PEMF were used
at the same frequency and intensity to detect whether PEMF therapy
affect the fate of osteoporosis-derived BMSCs (OP-BMSCs) and
enhance osseointegration in osteoporotic rabbits (Fig. 1). To the best of the authors'
knowledge, this is the first study to investigate the combination
of 3D-printed pTi and PEMF to promote osseointegration in
osteoporosis to reduce complications following implantation.
Materials and methods
Materials
Ti6Al4V powder was purchased from AK Medical Co.,
Ltd. Low glucose Dulbecco's Modified Eagle's Medium (LG-DMEM),
streptomycin-penicillin and fetal bovine serum (FBS) were obtained
from Gibco, Thermo Fisher Scientific, Inc. Cell Counting Kit-8
(CCK-8; cat. no. C0039) and Calcein acetoxymethyl ester
(Calcein-AM)/Propidium Iodide (PI; cat. no. C2015M) were purchased
from Beyotime Institute of Biotechnology. Rhodamine-phalloidin and
DAPI were supplied by Invitrogen (Thermo Fisher Scientific, Inc.).
The media for alizarin red staining (ARS) and osteogenic
differentiation of rabbit BMSCs (cat. no. RBXMX-90021) were
purchased from Cyagen Biosciences. The 4% paraformaldehyde solution
and PBS were obtained from Beijing Solarbio Science &
Technology. Eastep Super Total RNA Extraction Kit was supplied by
Promega Corporation. Perfect Real Time RT reagent kit, Prime Script
RT reagent kit, and SYBR Premix Ex Taq II kit were provided by
Takara Biotechnology. TRIzol® reagent was supplied by
Invitrogen (Thermo Fisher Scientific, Inc.). Masson stains were
obtained from Thermo Fisher Scientific, Inc. The estrogen ELISA kit
(cat. no. ML-Elisa-1434) was obtained from R&D Systems.
Preparation of pTi implants
Ti6Al4V porous scaffolds conducted by 3D printing
were prepared by using additive manufacturing with an electron beam
melting system (Q10, Arcam AB), as previously reported (27,28).
Briefly, pTi alloy implants (porosity, 70%; pore size, 600 µm) with
a pre-designed 3D template were translated to a standard
triangulation language (STL) file, which was loaded into the EBM
system. Spherical Ti alloy powder was melted layer by layer
according to the preset parameters (porosity, 70%; pore size, 600
µm), then solidified by cooling. Disk-shaped (φ10 mm × L3 mm) pTi
implants for microstructure identification in vitro
experiments, and columnar-shaped (φ6 mm × L10 mm) pTi implants for
osseointegration study in vivo. All prepared pTi implants
were ultrasonically cleaned and sequentially cleaned in acetone,
ethyl alcohol and deionized water for ~15 min for each
treatment.
Characterization of pTi implants
To verify whether the prepared implants match with
the pre-designed parameters, the porosity of samples was detected
by micro-computed tomography (CT) (SkyScan 1076 scanner, Bruker
micro-CT NV) and analyzed using NRecon software (version 1.6.6;
Bruker micro-CT). To calculate the average pore size and
distribution, the microstructure of pTi scaffolds was obtained
using a JSM-6700F scanning electron microscope (SEM; JEOL, Ltd.),
and images were quantitatively analyzed using ImageJ 1.50i
(National Institutes of Health).
Establishment of osteoporosis
models
The osteoporotic rabbit model was generated by
bilateral OVX. Five-month-old female New Zealand White rabbits
(n=30; 2.5 kg) were randomly separated into two groups, the OVX
group (n=27) and the sham group (n=3). Surgical operations were
conducted under general anesthesia using 3% (w/v) pentobarbital (50
mg/kg) intravenously. The median incision of the lower abdomen was
chosen for surgery after skin shaving and sterilizing. The rabbits
in the OVX group underwent bilateral OVX surgery, whereas those in
the sham group were pseudo-operated, closing to the abdominal
cavity after leaving the ovaries in situ. All rabbits were
maintained in a cage individually and fed with standard chow
(15–25°C, humidity 60–70%, 12-h light/dark cycle, fed twice a day,
and drank ad libitum). Penicillin (1.5 mg/kg) was
administered to post-operative rabbits by intramuscular injection
for three consecutive days to prevent infection.
Ten months after surgery, the concentration of serum
estrogen was measured using an ELISA kit. Furthermore, three
animals from each group were sacrificed by intravenous injection of
air under anesthesia by 3% (w/v) pentobarbital (150 mg/kg)
intravenously, and distal femurs were harvested for micro-CT
assessment to confirm the establishment of osteoporosis.
Scaffold implantation and PEMF
treatment
At 10 months post-OVX, osteoporotic rabbits were
used in vivo osseointegration experiments. After general
anesthesia using 3% (w/v) pentobarbital (50 mg/kg) and preparation
for operation, a longitudinal incision was performed in the distal
femur of the left hind limb. After exposing the bony surface of the
lateral condyle, cylindrical bone defects with a diameter of 6 mm
and a depth of 10 mm were prepared with a bone drill. The defects
were transplanted with pTi implants, then the incisions were closed
by absorbable sutures. Postoperative management was carried out as
aforementioned.
On the fourth day after the implantation, the 24
osteoporotic rabbits were randomly divided into two groups: i) pT1
group, which received pTi implants without PEMF; and ii) pTi +
PMEFs group, which received pTi implants with PEMF therapy. The
rabbits of the pTi + PEMF group were kept in a plastic fixer, with
their left legs placed within the scope of the coils of the PEMF
machine in order to expose them to the pulse electromagnetic waves.
The treatment parameters of PEMF were 50 Hz, 1 mT, 2 h per day.
After 6 and 12 weeks of treatment, rabbits were sacrificed by
intravenous injection of pentobarbital sodium 100 mg/kg, and femur
samples were collected and fixed in 4% polyformaldehyde solution
for further Micro-CT analysis and histological evaluation.
Isolation and culture of OP-BMSCs
OP-BMSCs were extracted from female rabbits (n=3) 10
months after bilateral ovariotomy (OVX) and cultured as previously
reported (27). Briefly, BMSCs
derived from the OVX rabbits were harvested from the marrow cavity
of long bone of extremities after centrifugation (996 × g, 20 min,
20°C) and cultured in LG-DMEM medium containing 10% FBS (v/v) and
1% penicillin and streptomycin in a humidified environment with
37°C and 5% CO2. Once OP-BMSCs grew to ~80% confluence,
the adherent cells were treated with 0.25% (w/v) trypsin/EDTA at
37°C for 3 min. The cell suspension was collected and centrifuged
(377 × g, 5 min, 20°C), then the obtained cell precipitates were
resuspended and passaged. The third generation of OP-BMSCs was used
in subsequent in vitro experiments.
In vitro cell experiments. To evaluate on
cell attachment, proliferation, survival rate, morphology, and
osteogenic differentiation of OP-BMSCs, CCK-8 assays, Calcein-AM/PI
and rhodamine phalloidin staining, and reverse transcription
quantitative-PCR (RT-qPCR) were carried out. Briefly, the pTi
implants were immersed into a 24-well plate with DMEM, and OP-BMSCs
(2×105 cells/well) were added into each sample. The
experiments consisted of two groups: i) pTi group, OP-BMSCs seeded
on pTi implants under the basal culture medium without PEMF; and
ii) pTi + PEMF group, OP-BMSCs seeded on the pTi implants with PEMF
therapy. The PEMF device (Prima PFM61009; Shanghai Prima
Electronics Co., Ltd.) consisted of a generator and its connected
coils. The OP-BMSCs in the pTi + PEMF group were placed on the
center of the coils in an incubator and received PEMF stimulation
during culture (50 Hz; 1 mT; 2 h per day). The medium was changed
every 3 days.
Evaluation of cell viability
After incubating for 24 h, the DMEM medium was
discarded, and the CCK-8 solution was then added into each well and
incubated for 2 h at 37°C and 5% CO2. The number of
OP-BMSCs adhering to the surface of implants was quantitatively
detected by measuring the absorbance at 450 nm using a microplate
reader (Multiskan EX; Thermo Fisher Scientific, Inc.). To evaluate
the cell proliferation, CCK-8 assays were conducted after 1, 4 and
7 days of OP-BMSC culture on implants with or without PEMF.
Live/Dead staining of the OP-BMSCs was detected at day 3 using a
Calcein-AM/PI Double Stain kit according to the manufacturer's
protocol, and observed under a FV1000 confocal laser scanning
microscope (CLSM) (Olympus Corporation). For morphological
evaluation at day 3, the samples were fixed with 4%
paraformaldehyde for 10 min at room temperature, then permeabilized
by 0.1% Triton X-100 for 5 min at room temperature, and washed with
PBS repeatedly for 3 times. Finally, the samples were stained with
rhodamine-phalloidin for 30 min and DAPI for 5 min at room
temperature in dark according to the manufacturer's instructions.
The photomicrographs of stained cells were collected under a
CLSM.
Evaluation osteogenic differentiation
in vitro
To detect the osteogenic ability of OP-BMSCs in the
presence of PEMF, basal culture medium was replaced by osteogenic
induction medium including LG-DMEM along with β-glycerol-phosphate
(10 mM), ascorbate-2-phosphate (50 µM), and dexamethasone (0.1 µM).
The protocols of cell culture and PEMF treatment were the same as
the cell viability investigation in the previous section. Following
osteogenic culture for 14 or 21 days, ARS (15 min, room
temperature) was conducted to assess calcium deposition according
to the manufacturer's protocols and observed by a stereoscopic
microscope (ZOOM-750; Tuming Optical Instrument Co., Ltd.).
Subsequently, 10% cetylpyridinium chloride was added into the dyed
mineralized nodules to dissolve the stained calcium nodules for
further semi-quantitative analysis. The obtained solution was
detected at 450 nm through a microplate reader to assess
unobservable calcium nodules inside the micropores.
Moreover, after treating with osteogenic induction
medium for 14 and 21 days, the expression levels of alkaline
phosphatase (ALP), runt-related transcription factor-2 (Runx-2),
osteocalcin (OCN), and bone morphogenetic protein-2 (BMP-2) in
OP-BMSCs in the different groups were measured using RT-qPCR. The
sequences of primers were listed in Table I. Briefly, total RNA was extracted
with TRIzol® (Thermo Fisher Scientific, Inc.) and 1 µg
total RNA per sample was reversely transcribed to cDNA using a
Prime Script RT reagent kit according to the manufacturer's
instructions. The expressions of target genes were quantified by
RT-qPCR using the SYBR Premix Ex Taq II kit according to the
manufacturer's protocol. The qPCR was conducted using 2X Fast
SYBR-Green Master Mix. Amplification was performed in 96-well
optical reaction plates (Roche Diagnostics) on LightCycler 480
(Roche Diagnostics) using the following program: 94°C for 1 min to
activate polymerase, 40 cycles at 94°C for 30 sec, 57°C for 20 sec
and 72°C for 20 sec; melting curve analysis was performed after
every run by heating up to 95°C to monitor presence of unspecific
products. The relative mRNA expression levels were normalized to
that of GAPDH and calculated using the 2−ΔΔCq method
(29).
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Primer name | Oligonucleotide
sequence (5′-3′) |
|---|
| ALP-F | F:
ATCGGACCCTGCCTTACC |
| ALP-R | R:
CTCTTGGGCTTGCTGTCG |
| Runx-2-F | F:
ACTACCAGCCACCGAGACCA |
| Runx-2-R | R:
ACTGCTTGCAGCCTTAAATGACTCT |
| OCN-F | F:
AGCCACCGAGACACCATGAGA |
| OCN-R | R:
AGCCACCGAGACACCATGAGA |
| BMP-2-F | F:
CAACACCGTGCTCAGCTTCC |
| BMP-2-R | R:
TTCCCACTCATTTCTGAAAGTTCC |
| GAPDH-F | F:
CAATGACCCCTTCATTGACC |
| GAPDH-R | R:
TGGACTCCACGACGTACTCA |
Micro-CT analysis
To explore the efficiency of bone formation, the
samples were screened by micro-CT (voltage, 90 kV voltage; current
intensity, 114 mA; pixel size, 18 µm). The cylindrical region of
the pTi was targeted as the region of interest (ROI; a cylinder
with diameter of 6 mm and height of 10 mm) for 3D reconstruction
and further parameter analysis. Quantitative morphometric analysis,
including bone volume/tissue volume ratio (BV/TV, %), trabecular
number (Tb.N, mm), trabecular thickness (Tb.Th, mm) and trabecular
separation (Tb.Sp, mm), was conducted using micro-CT auxiliary
software (NRecon version 1.6.6).
Histological evaluation
The femur specimens containing pTi implants were
embedded in methyl methacrylate, without undergoing decalcification
and sectioned into thin sections (150–300 µm thickness). The
sections were ground down and polished to 40–50 µm through the
transverse saw cuts and polishing machine (EXAKT Apparatebau GmbH
& Co. KG). After polishing, these hard tissue sections were
stained with Masson stain for 150 min at room temperature to
evaluate the bone regeneration and osseointegration with the porous
implants.
Statistical analysis
All data are presented as the mean ± standard
deviation (SD). Two independent groups were compared using unpaired
Student's t-test, while >2 groups were analyzed using one-way
ANOVA followed by Tukey's post hoc test using SPSS 19.0 (SPSS
Inc.). P<0.05 was considered to indicate a statistically
significant difference. All experiments were performed
independently at least three times and the detailed times are shown
in the figure legends.
Results and Discussion
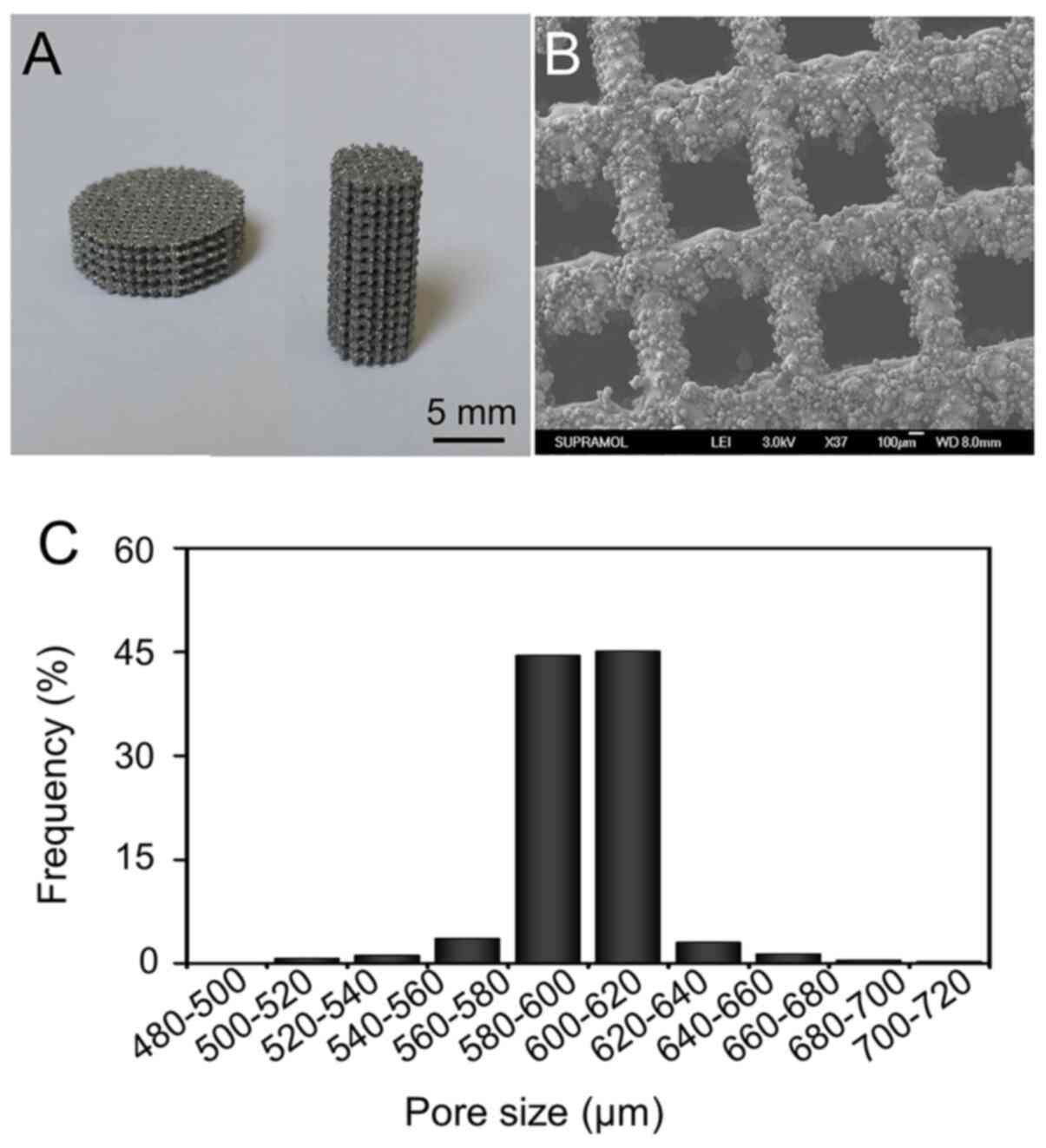
Characterization of pTi implants
pTi implants were successfully manufactured by EBM
technology. Representative optical images of the pTi implants are
presented in Fig. 2A. The micro-CT
quantitative analysis revealed the porosity of the pTi was
70.08±0.55%. The pore size detected based on the SEM pictures was
mainly distributed at 580–620 µm (Fig.
2B). The average pore size was 600.11±7.21 µm (Fig. 2C). Therefore, the actual porosity
and pore size were in accordance with the pre-designed parameters
(600 µm and 70%, respectively).
The osseointegrative capacity of implants is usually
determined by the osteoconductivity of the prosthesis, which is
further bound up with the porosity, pore size and distribution of
the scaffolds. The porosity of porous implants for bone tissue
engineering, is expected to be >50%, especially within the scope
of 65–75%, which is mechanically and structurally similar to human
trabecular bone. Additionally, a 300–700 µm pore size is beneficial
for osteoblast adhesion, differentiation and proliferation
(30). Thus, the porosity and pore
size of pTi implants generated in this study were optimal for bone
tissue engineering. It has been reported that the increased surface
area and porosity of the implants can enhance the initial
stability, bone ingrowth ability and the friction coefficient
between the bone and scaffolds, thus decreasing micromotion and
accelerating osseointegration after implanting in vivo
(31). Furthermore, interconnected
internal structure of the pTi scaffolds were favorable to oxygen
and nutrient exchange, therefore improving cell growth and
communication and further enhancing bone regeneration (32).
Cell attachment, proliferation,
survival and morphology
Under normal conditions, the bone is continuously
renewed through a coordinated progress involving complex stem cell
behaviors, including adhesion, proliferation, differentiation,
maturation and mineralization (33). By contrast, under the condition of
osteoporosis, BMSCs present decreased ability of proliferation and
osteoblastic differentiation leading to limited capacity of bone
regeneration (34). External PEMF
have been shown to promote cell proliferation, osteogenesis and
mineralization (21,22). Therefore, it was hypothesized that
PEMF could be applied as a potential target to treat osteoporosis
by modulating BMSC behavior. To test this hypothesis, the OP-BMSCs
harvested from the OVX rabbits were added onto the pTi and received
PEMF therapy.
The osseointegration between the implants and bone
tissue starts from osteogenesis-related cells adhering to the
surface of the prosthesis (28). As
shown in Fig. 3A, the absorbance,
which stood for the number of OP-BMSCs, was significantly increased
in the pTi + PEMF group compared with that in the pTi group.
Moreover, cell proliferation in the different groups was evaluated
using a CCK-8 assay. In the OP-BMSCs from the pTi + PEMF group,
cell proliferation was significantly increased compared with that
in the pTi group on day 4, and this difference was more distinct on
day 7 (Fig. 3B). OP-BMSCs seeded on
the implants treated with or without PEMF were stained with Calcein
AM/PI to measure cell viability. The fluorescent images
demonstrated that the OP-BMSCs possessed good cell viability in the
pTi and pTi + PEMF groups on day 3 (Fig. 3C). Furthermore, the viability rate
of OP-BMSCs was also quantified. The results showed that the
viability rates in the pTi and pTi + PEMF groups were 89.52±1.64,
92.34±2.00%, respectively, and there was no significant difference
between the two groups (Fig. 3D).
These results suggested that the pTi have good biocompatibility,
and that external PEMF could promote cell attachment and
proliferation, as well as and maintain viability of OP-BMSCs.
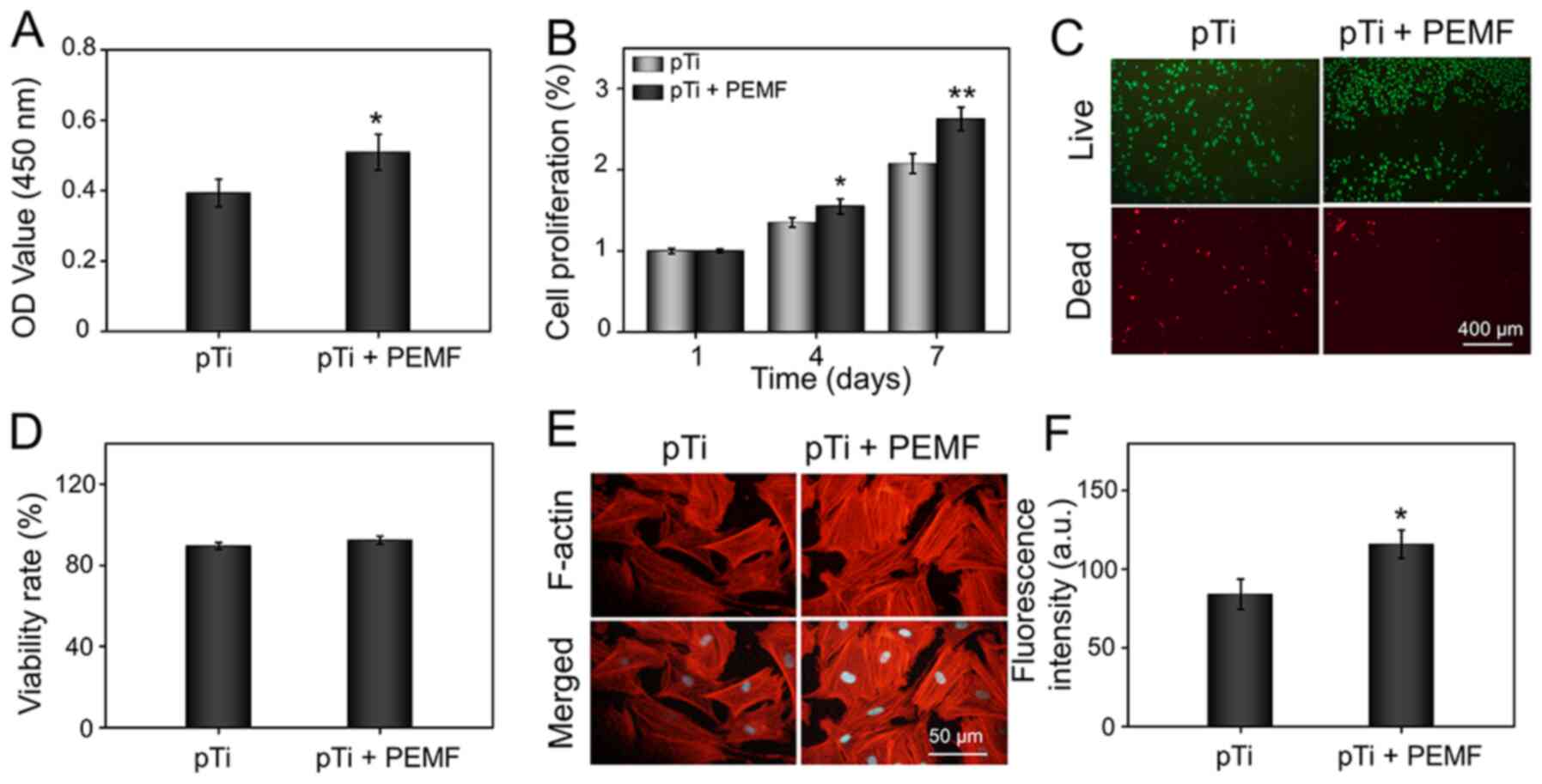 | Figure 3.Cell viability of OP-BMSCs in the pTi
and pTi + PEMF groups. (A) Cell attachment on the pTi scaffold
following 24-h incubation. (B) Cell proliferation at day 1, 4 and
7. (C) Calcein AM/PI staining of OP-BMSCs at day 3. The green
signal represents live cells, whereas the red signal represents
dead cells. (D) Quantitative analysis of Calcein AM/PI staining.
(E) Fluorescence images of cellular morphology in the pTi group and
pTi + PEMF group following 3-day culture. F-actin filaments of
BMSCs were stained with rhodamine-phalloidin, whereas nuclei were
stained with DAPI. (F) Quantitative analysis of F-actin.
*P<0.05, **P<0.01 vs. pTi. pTi, porous titanium; PEMF, pulse
electromagnetic field; OD, optical density; a.u., arbitrary units;
OP-BMSCs, osteoporosis-derived bone marrow mesenchymal stem cells;
AM/PI, calcein acetoxymethyl ester/propidium Iodide. |
Because the morphology of the cells on the implants
could dramatically influence cellular behavior, the cytoskeletal
distribution of cells attached on the hydrogel was then analyzed
(35). OP-BMSCs were double-stained
with rhodamine-phalloidin (indicating the F-actin filament) and
DAPI (indicating the nucleus) for observation. As demonstrated in
Fig. 3E, the F-actin filament
morphology of OP-BMSCs on the pTi implants with PEMF therapy showed
better spreading and displayed more lamellipodia extensions than
those on pTi scaffolds without PEMF therapy, indicating that the
external PEMF could induce cell maturation (36). The intensity of F-actin on the
OP-BMSCs was further quantitatively analyzed in the
immunofluorescent images. As shown in Fig. 3F, OP-BMSCs in pTi + PEMF group
showed increased F-actin fluorescence (1.38-fold) compared to that
in the pTi group. It is well-recognized that the F-actin filament
plays a fundamental role in the early maturation of BMSCs, and
helps improve bone cell function by modulating cell proliferation
and differentiation (36,37). Therefore, PEMF therapy can
significantly enhance the expression of F-actin filaments and
improve cytoskeletal organization, which is beneficial for the
early osteogenic differentiation of BMSCs.
Osteogenic differentiation of
OP-BMSCs
In addition to cell attachment, proliferation,
survival and morphology of OP-BMSCs and osteogenic differentiation
are critical factors for the initiation of bone regeneration
(28). In order to explore the
effect of external PEMF on cell osteogenic differentiation on the
pTi implants, ARS was used to assess mineralized matrix synthesis.
ARS is a histological staining method for mineralization, which
indicates calcium deposition. As demonstrated in Fig. 4A, calcium deposits in the pTi group
were limited at day 14. However, in the pTi + PEMF group, visible
calcified nodules were detected, which were more prominent on day
21. Furthermore, the semi-quantitative analysis confirmed that the
cells in pTi + PEMF group exhibited increased calcium deposition
compared with the pTi group at 14 and 21 days (Fig. 4B). Therefore, these results
indicated that PEMF could promote the formation of mineralized
matrix and further enhance the efficiency of mineralization.
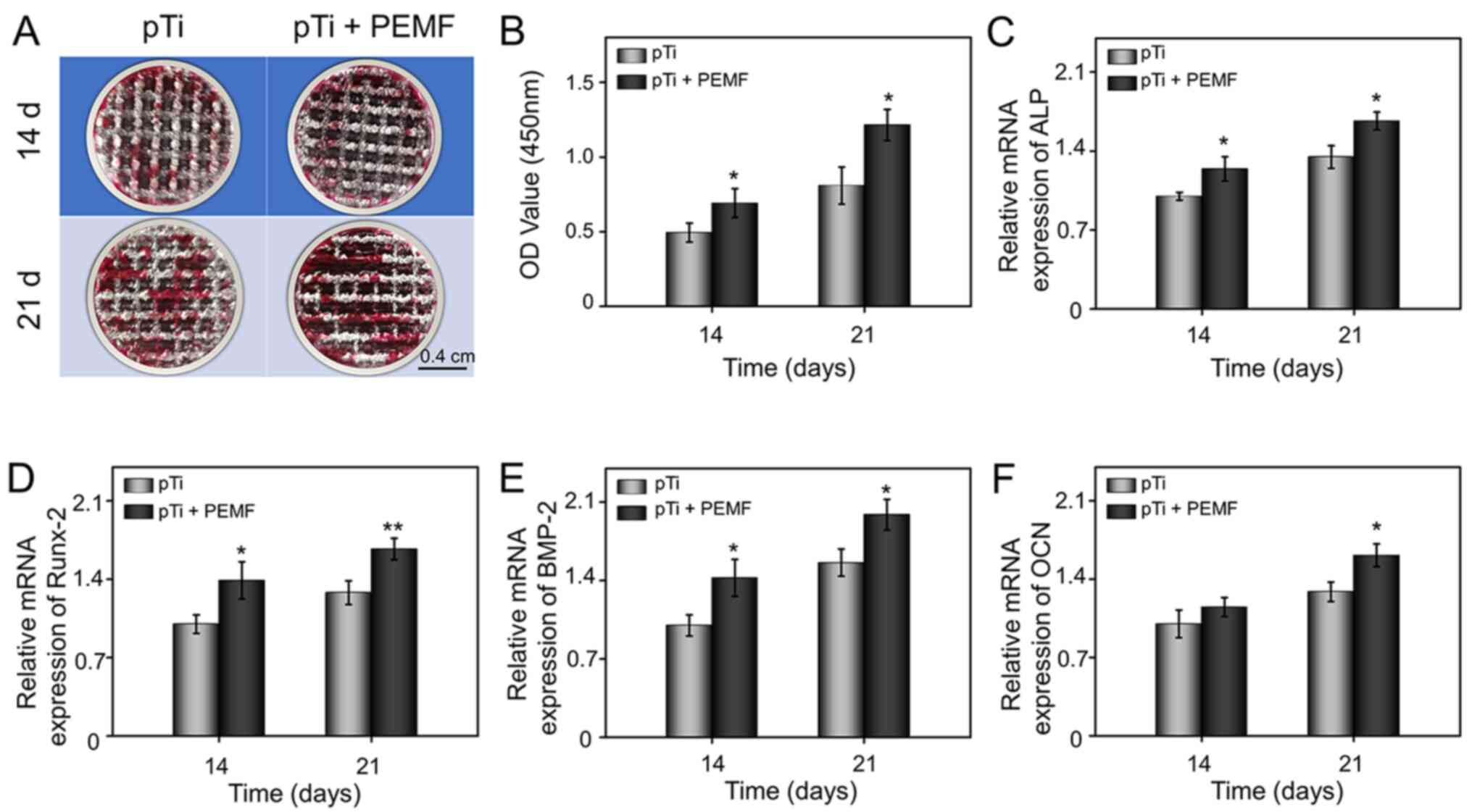 | Figure 4.Osteogenic differentiation of
OP-BMSCs in the pTi group and pTi + PEMF group. (A) Gross images
and (B) semi-quantitative analysis of alizarin red staining after
14 and 21 days of osteogenesis induction. (C-F) mRNA expression
levels of osteogenesis-related genes, ALP, RUNX-2, BMP-2, and OCN
in OP-BMSCs grown on pTi or pTi + PEMF after osteogenic induction
culture for 14 and 21 days. *P<0.05, **P<0.01 vs. pTi. pTi,
porous titanium; PEMF, pulse electromagnetic field; OD, optical
density; OP-BMSCs, osteoporosis-derived bone marrow mesenchymal
stem cells; ALP, alkaline phosphatase; Runx-2; runt-related
transcription factor-2; OCN, osteocalcin, BMP-2, bone morphogenetic
protein-2. |
In order to verify the effect of external PEMF on
osteogenic differentiation of OP-BMSCs on the pTi implants at the
mRNA level, multiple osteogenic differentiation markers, such as
ALP, Runx-2, OCN and BMP-2, were detected using RT-qPCR. In
general, ALP is marker for osteogenic differentiation, and the
stimulation of ALP activity is a critical event in early
osteogenesis (38). The results
indicated the mRNA level of ALP was significantly increased in the
pTi + PEMF group after treatment for either 14 days or 21 days,
compared with that of the pTi group (Fig. 4C). Runx-2 is a indicative of early
osteoblastic differentiation and can induce the expression of key
osteogenic genes, such as OCN and osteopontin (39). Compared with the pTi group, the
expression of Runx-2 in the pTi + PEMF group was upregulated
1.39-fold and 1.31-fold on day 14 and 21, respectively (Fig. 4D). BMP-2 is an essential
osteoinductive factors and recognized to induce the differentiation
of BMSCs into osteoblasts (40).
Compared with pTi group, the transcriptional level of BMP-2 was
upregulated significantly at day 14 and 21 in the presence of
external PEMF (Fig. 4E). OCN is a
bone-specific protein synthesized by osteoblasts that can enhance
osteogenic maturation and bone formation; it is expressed most
abundantly at the late stage of osteogenesis (41). The expression level of OCN was
measured in order to evaluate the osteogenic maturation of
OP-BMSCs. The expression of OCN in pTi + PEMF group did not differ
significantly at day 14, but was significantly upregulated at day
21, compared with the pTi group (Fig.
4F).
Based on the detection of ARS and quantification of
osteogenic-related gene expression, it may be hypothesized that
external PEMF could enhance the osteogenic differentiation of
OP-BMSCs on the pTi implants. However, the mechanism through which
PEMF can promote osteogenic differentiation remains unclear. For
the osteogenic induction of BMSCs by PEMF, previous studies have
obtained partial mechanism level explanation. Bagheri et al
(42) confirmed that PEMF activated
the osteogenic differentiation of BMSCs through the Notch pathway.
Zhou et al (43) suggested
that PEMF induced the osteogenic differentiation of BMSCs by
activating Wnt/β-catenin signaling. In addition, Selvamurugan et
al (44) showed that PEMF
induced the TGF-β signaling pathway and increased the expression of
microRNA-21-5p in the bone metabolism of BMSCs. Similarly,
beneficial osteogenic induction effects were also observed in the
present study in OP-BMSCs treated with external PEMF.
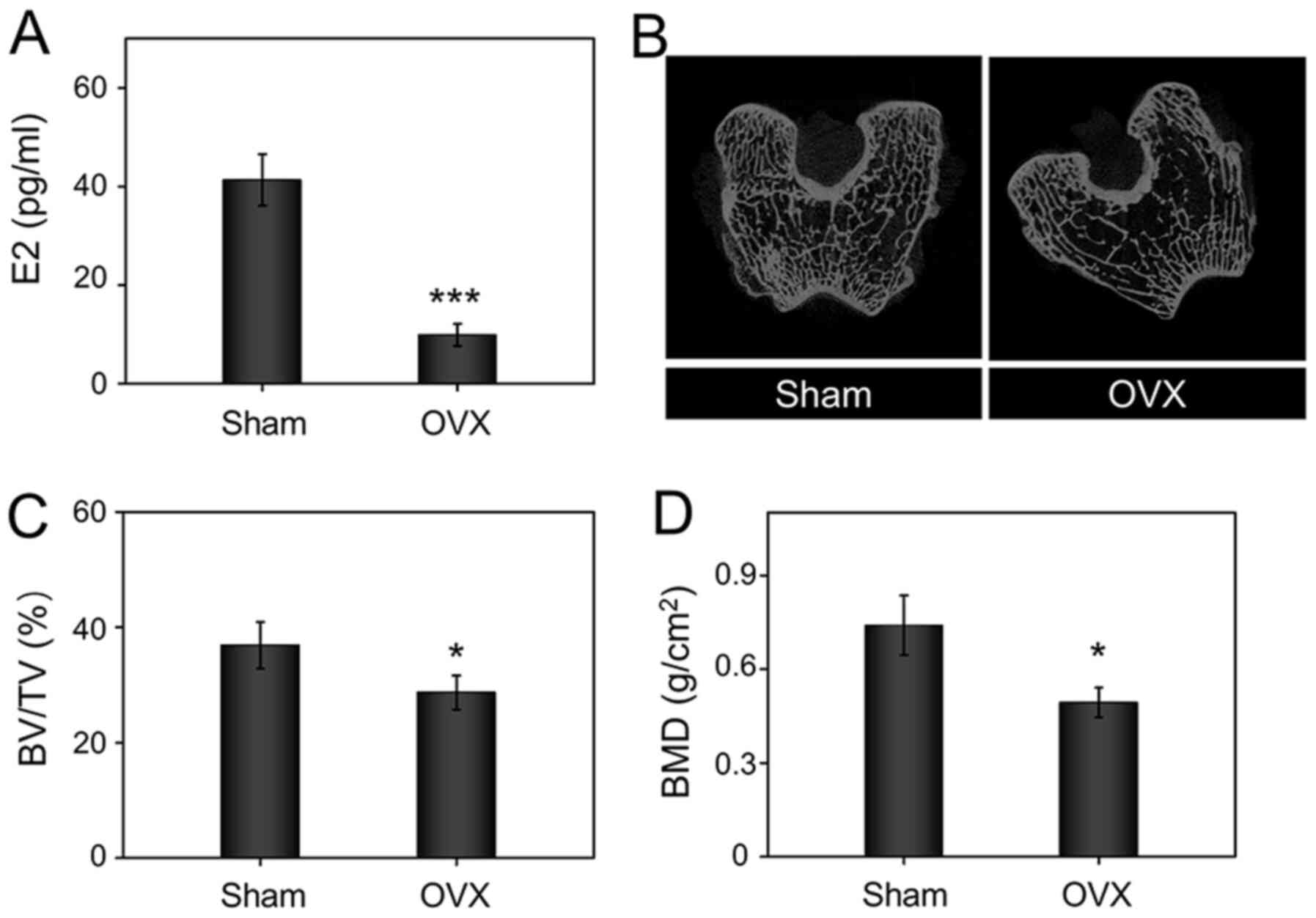
Establishment of osteoporosis
model
In osteoporosis, the imbalance between bone
formation and bone resorption leads to reduced bone mineral density
(BMD), which improves risk of fracture and obstructed the ability
of bone regeneration (1,2). It has previously been reported that
estrogen deficiency can result in increased bone resorption, thus
impairing osteoblast function (45). The serum levels of estrogen was
significantly inhibited in the OVX group compared with those of the
sham group (Fig. 5A). Moreover,
Micro-CT 2D slides showed the trabecular bone structure became
looser and thinner in the OVX group (Fig. 5B). The statistical analysis
suggested that the BMD (Fig. 5C)
and BV/TV (Fig. 5D) in the OVX
rabbits were significantly decreased compared with those in the
sham group. Thus, these results indicated that the osteoporosis
model was successfully established 10 months after bilateral
OVX.
Evaluation of bone regeneration and
osseointegration in vivo
Osteogenic-associated growth factors, such as BMP-2,
are downregulated in the osteoporotic microenvironment (3). Furthermore, BMSCs derived from
osteoporosis are few in number and deficient in osteogenic activity
(4). The microenvironment with low
osteogenic activity, and the imbalance between bone formation and
resorption in osteoporosis state increase the risk of implants
loosening and displacement after implantation. Developing novel
combination therapy that promotes the osteogenic differentiation of
OP-BMSCs and enhances osseointegration to decrease implant
loosening and displacement remains a challenge. In order to
evaluate bone regeneration and osseointegration induced by PEMF, an
in vivo study was performed in OVX rabbits.
New bone regeneration in the osteoporotic bone
defects was scanned using micro-CT. Reconstruction of the porous
implants and surrounding bone tissues are shown in Fig. 6A. The reconstructed spatial
distribution of the new bone formation revealed that with an
implantation time of 6–12 weeks, bone formation on the surface of
the pTi in the pTi + PEMF group were increased significantly
compared with the pTi group. To quantify the bone ingrowth, the ROI
scanning of the implanted region and empty defect area was carried
out. The BV/TV values of pTi and pTi + PEMF groups were 10.35±1.88
and 14.36±1.18% at 6 weeks, and 15.02±1.20 and 20.37±1.39% at 12
weeks (Fig. 6B), respectively,
consistent with the 3D reconstruction results. In parallel, pTi +
PEMF group was indicated with a higher Tb.Th and Tb.N value
(Fig. 6C and D) and lower Tb.Sp
value (Fig. 6E) compared to that in
the pTi group at 6 and 12 weeks. In general, the results of
micro-CT scanning indicated that PEMF could significantly enhance
bone ingrowth into the pTi implants in osteoporotic bone
defects.
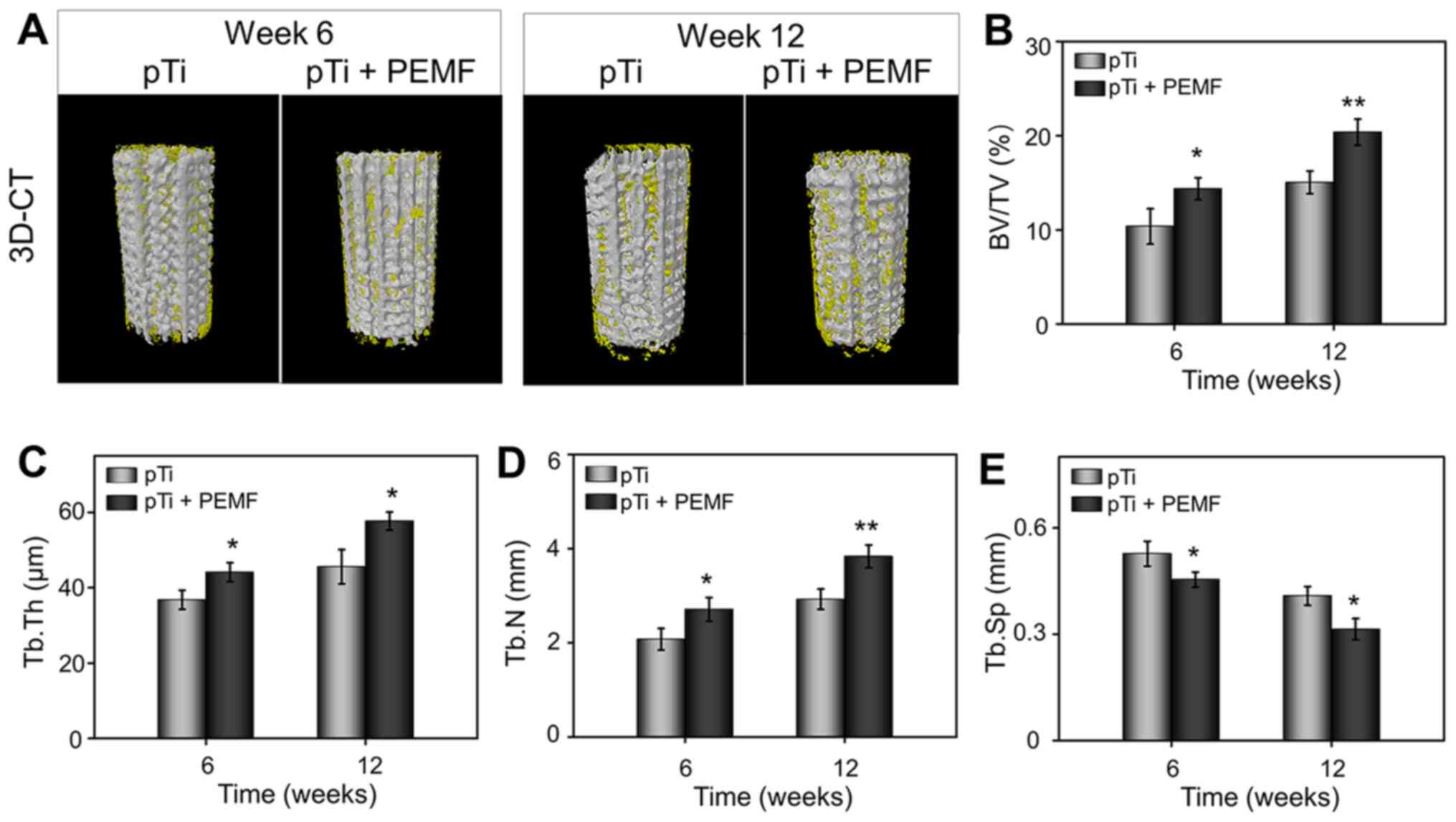 | Figure 6.Micro-CT evaluation of bone
regeneration in osteoporotic bone defects at 6 and 12 weeks after
pTi implantation. (A) 3D reconstruction images of porous implants
(white) and regenerated bone (yellow). Quantitative analysis of (B)
BV/TV, (C) Tb.Th, (D) Tb.N, and (E) Tb.Sp in the pTi and pTi + PEMF
groups using micro-CT. P<0.05, **P<0.01 vs. pTi. pTi, porous
titanium; PEMF, pulse electromagnetic field; BV, bone volume; TV,
tissue volume ratio; CT, computed tomography; Tb.N, trabecular
number; Tb.Th, trabecular thickness; Tb.Sp, trabecular
separation. |
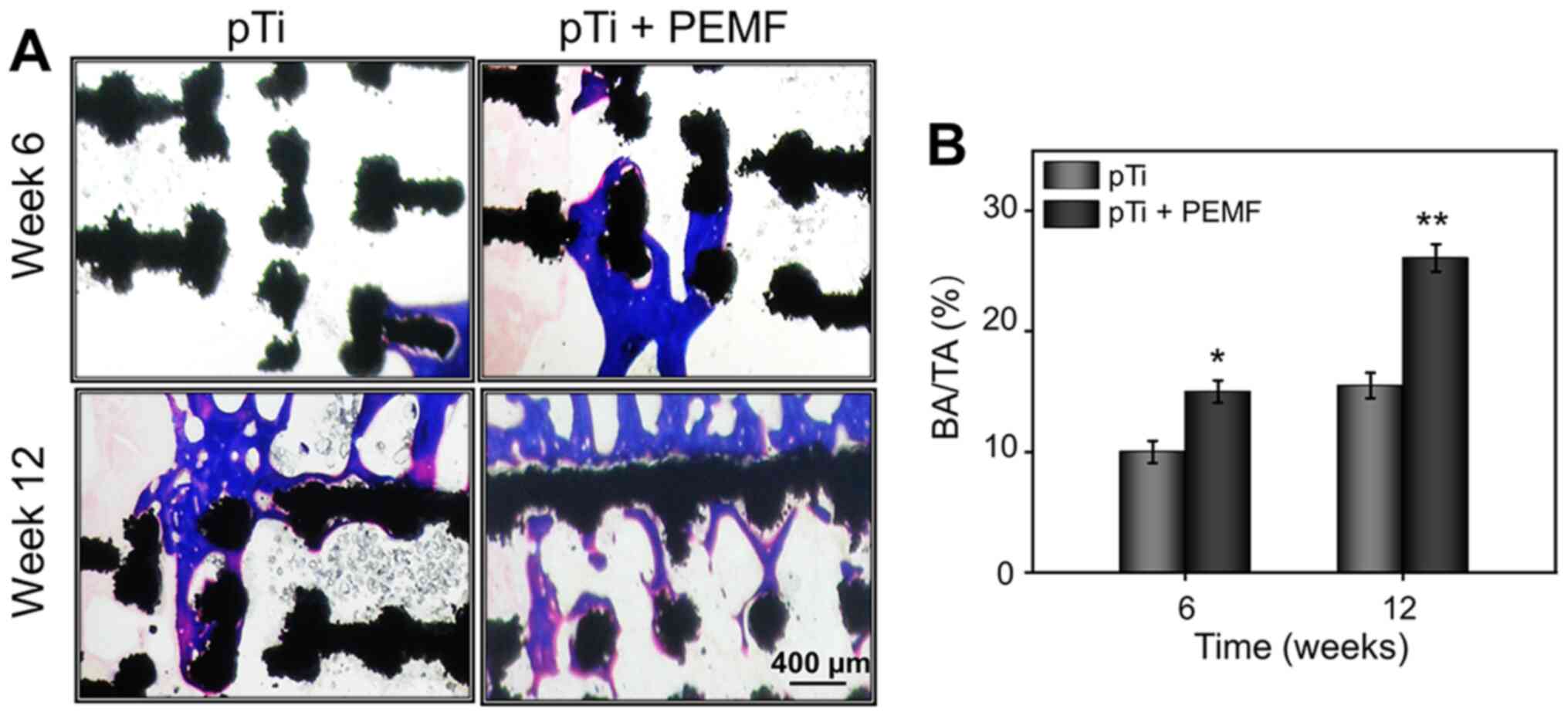
Good osseointegration between host bone tissues and
implants involved a series of complex biological processes,
including the migration and differentiation of BMSCs. The optimal
prosthesis is expected to conform to the surrounding bone tissue to
avoid complications including loosening and displacement after
implantation, as well as recover the function of bone. In the
present study, hard tissue sections were stained with Masson's
stain to evaluate bone osseointegration at the bone-implant
interface. As shown in Fig. 7A,
improved bone regeneration was observed between the newly formed
bone and pTi implants without gaps in the pTi + PEMF group, while
regenerated bone almost completely surrounded the surface of the
implants along with a part of bone tissue filling the inner pores,
especially after 12 weeks of PEMF treatment. Furthermore, there was
a certain amount of new bone tissue around implants of the pTi
group, but not as evident as that seen in the pTi + PEMF group. To
calculate the ratio of bone area/total area (BA/TA), the
histological pictures were analyzed using ImageJ. As shown in
Fig. 7B, the BA/TA of pTi + PEMF
group was significantly higher than pTi group at 6 weeks and 12
weeks, respectively.
PEMF are considered as a clinical physiotherapy that
can enhancing bone regeneration (46). In the present study, pTi scaffolds
were implanted into bone defects, and the addition of external PEMF
led to a positive result on bone regeneration in osteoporosis
models. This novel combination therapy of 3D-printed pTi and PEMF
exhibited potential to promote bone regeneration and
osseointegration of dental implants or artificial prostheses for
patients with osteoporosis. To summarize, 3D-printed pTi implants
could be customized according to desired parameters. External PEMF
can effectively promote the viability and osteogenic
differentiation of OP-BMSCs on the pTi surface and have great
prospects for applications in bone tissue engineering. The
combination of pTi and PEMF therapy resulted in improved repair
effects on osteoporotic bone defects. These findings provided
insight into the treatment of osseointegration under osteoporosis
state.
Acknowledgements
Not applicable.
Funding
This work was supported by the Cultivation Program
from the Renji Hospital of Shanghai Jiaotong University for
National Natural Science Foundation (grant no. 2018GZRPYQN06).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MY and WL designed the study, wrote the protocol,
collected the data, performed statistical analyses and contributed
to writing the manuscript. LY performed the technical work. SC
helped with data collection, study design and coordinated the
study. XL participated in the study design and helped to critically
revise the manuscript. SQ performed study design, analysis and
interpretation of data, critical revision of the manuscript and
supervised the study. MY and SQ confirm the authenticity of all the
raw data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All animal procedures were examined and approved by
The Animal Ethics Committee of Renji Hospital of Shanghai Jiaotong
University (approval no. SHJT-MRJ-2020-076).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lau RY and Guo X: A review on current
osteoporosis research: With special focus on disuse bone loss. J
Osteoporos. 2011:2938082011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Aghebati-Maleki L, Dolati S, Zandi R,
Fotouhi A, Ahmadi M, Aghebati A, Nouri M, Kazem Shakouri S and
Yousefi M: Prospect of mesenchymal stem cells in therapy of
osteoporosis: A review. J Cell Physiol. 234:8570–8578. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Boyce BF, Rosenberg E, de Papp AE and
Duong LT: The osteoclast, bone remodelling and treatment of
metabolic bone disease. Eur J Clin Invest. 42:1332–1341. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu W, Wang T, Yang C, Darvell BW, Wu J,
Lin K, Chang J, Pan H and Lu WW: Alkaline biodegradable implants
for osteoporotic bone defects-importance of microenvironment pH.
Osteoporos Int. 27:93–104. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brandi ML: Healing of the bone with
anti-fracture drugs. Expert Opin Pharmacother. 14:1441–1447. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Russell LA: Osteoporosis and orthopedic
surgery: Effect of bone health on total joint arthroplasty outcome.
Curr Rheumatol Rep. 15:3712013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bottai V, Dell'Osso G, Celli F, Bugelli G,
Cazzella N, Cei E, Guido G and Giannotti S: Total hip replacement
in osteoarthritis: The role of bone metabolism and its
complications. Clin Cases Miner Bone Metab. 12:247–250.
2015.PubMed/NCBI
|
|
8
|
Mei S, Wang H, Wang W, Tong L, Pan H, Ruan
C, Ma Q, Liu M, Yang H, Zhang L, et al: Antibacterial effects and
biocompatibility of titanium surfaces with graded silver
incorporation in titania nanotubes. Biomaterials. 35:4255–4265.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang L, Le Coz-Botrel R, Beddoes C,
Sjöström T and Su B: Gelatin freeze casting of biomimetic titanium
alloy with anisotropic and gradient pore structure. Biomed Mater.
12:0150142017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vladescu A, Vranceanu DM, Kulesza S,
Ivanov AN, Bramowicz M, Fedonnikov AS, Braic M, Norkin IA, Koptyug
A, Kurtukova MO, et al: Influence of the electrolyte's pH on the
properties of electrochemically deposited hydroxyapatite coating on
additively manufactured Ti64 alloy. Sci Rep. 7:168192017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chudinova EA, Surmeneva MA, Timin AS,
Karpov TE, Wittmar A, Ulbricht M, Ivanova A, Loza K, Prymak O,
Koptyug A, et al: Adhesion, proliferation, and osteogenic
differentiation of human mesenchymal stem cells on additively
manufactured Ti6Al4V alloy scaffolds modified with calcium
phosphate nanoparticles. Colloids Surf B Biointerfaces.
176:130–139. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Amin Yavari S, van der Stok J, Chai YC,
Wauthle R, Tahmasebi Birgani Z, Habibovic P, Mulier M, Schrooten J,
Weinans H and Zadpoor AA: Bone regeneration performance of
surface-treated porous titanium. Biomaterials. 35:6172–6181. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nune KC, Kumar A, Murr LE and Misra RD:
Interplay between self-assembled structure of bone morphogenetic
protein-2 (BMP-2) and osteoblast functions in three-dimensional
titanium alloy scaffolds: Stimulation of osteogenic activity. J
Biomed Mater Res A. 104:517–532. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pan S, Yin J, Yu L, Zhang C, Zhu Y, Gao Y
and Chen Y: 2D MXene-Integrated 3D-Printing scaffolds for augmented
osteosarcoma phototherapy and accelerated tissue reconstruction.
Adv Sci (Weinh). 7:19015112019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dimitriou R, Jones E, McGonagle D and
Giannoudis PV: Bone regeneration: Current concepts and future
directions. BMC Med. 9:662011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Z, Wang C, Li C, Qin Y, Zhong L, Chen
B, Li Z, Liu H, Chang F and Wang J: Analysis of factors influencing
bone ingrowth into three-dimensional printed porous metal
scaffolds: A review. J Alloy Compound. 717:271–285. 2017.
View Article : Google Scholar
|
|
17
|
Bai H, Cui Y, Wang C, Wang Z, Luo W, Liu
Y, Leng Y, Wang J, Li Z and Liu H: 3D printed porous biomimetic
composition sustained release zoledronate to promote
osteointegration of osteoporotic defects. Mater Des.
189:1085132020. View Article : Google Scholar
|
|
18
|
Ye D, Xu Y, Wang G, Feng X, Fu T, Zhang H,
Jiang L and Bai Y: Thermal effects of 2450 MHz microwave exposure
near a titanium alloy plate implanted in rabbit limbs.
Bioelectromagnetics. 36:309–318. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vayron R, Nguyen VH, Lecuelle B, Albini
Lomami H, Meningaud JP, Bosc R and Haiat G: Comparison of resonance
frequency analysis and of quantitative ultrasound to assess dental
implant osseointegration. Sensors (Basel). 18:13972018. View Article : Google Scholar
|
|
20
|
Liang H, Liu X, Pi Y, Yu Q, Yin Y, Li X,
Yang Y and Tian J: 3D-Printed β-tricalcium phosphate scaffold
combined with a pulse electromagnetic field promotes the repair of
skull defects in rats. ACS Biomater Sci Eng. 5:5359–5367. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu C, Yu J, Yang Y, Tang X, Zhao D, Zhao
W and Wu H: Effect of 1 mT sinusoidal electromagnetic fields on
proliferation and osteogenic differentiation of rat bone marrow
mesenchymal stromal cells. Bioelectromagnetics. 34:453–464. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tsai MT, Chang WH, Chang K, Hou RJ and Wu
TW: Pulsed electromagnetic fields affect osteoblast proliferation
and differentiation in bone tissue engineering.
Bioelectromagnetics. 28:519–528. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang P, Liu J, Yang Y, Zhai M, Shao X, Yan
Z, Zhang X, Wu Y, Cao L, Sui B, et al: Differential
intensity-dependent effects of pulsed electromagnetic fields on
RANKL-induced osteoclast formation, apoptosis, and bone resorbing
ability in RAW264.7 cells. Bioelectromagnetics. 38:602–612. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Adie S, Harris IA, Naylor JM, Rae H, Dao
A, Yong S and Ying V: Pulsed electromagnetic field stimulation for
acute tibial shaft fractures: A multicenter, double-blind,
randomized trial. J Bone Joint Surg Am. 93:1569–1576. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bagnato GL, Miceli G, Marino N, Sciortino
D and Bagnato GF: Pulsed electromagnetic fields in knee
osteoarthritis: A double blind, placebo-controlled, randomized
clinical trial. Rheumatology (Oxford). 55:755–762. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yin Y, Chen P, Yu Q, Peng Y, Zhu Z and
Tian J: The effects of a pulsed electromagnetic field on the
proliferation and osteogenic differentiation of human
adipose-derived stem cells. Med Sci Monit. 24:3274–3282. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Qiao S, Sheng Q, Li Z, Wu D, Zhu Y, Lai HC
and Gu Y: 3D-printed Ti6Al4V scaffolds coated with freeze-dried
platelet-rich plasma as bioactive interface for enhancing
osseointegration in osteoporosis. Mater Des. 194:1088252020.
View Article : Google Scholar
|
|
28
|
Bai H, Zhao Y, Wang C, Wang Z, Wang J and
Liu H, Feng Y, Lin Q, Li Z and Liu H: Enhanced osseointegration of
three-dimensional supramolecular bioactive interface through
osteoporotic microenvironment regulation. Theranostics.
10:4779–4794. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li Z, Wang C, Li C, Wang Z, Yang F, Liu H,
Qin Y and Wang J: What we have achieved in the design of 3D printed
metal implants for application in orthopedics? Personal experience
and review. Rapid Prototyp J. 24:1365–1379. 2018. View Article : Google Scholar
|
|
31
|
Raphel J, Holodniy M, Goodman SB and
Heilshorn SC: Multifunctional coatings to simultaneously promote
osseointegration and prevent infection of orthopaedic implants.
Biomaterials. 84:301–314. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kumar A, Nune KC and Misra RDK: Design and
biological functionality of a novel hybrid Ti-6Al-4V/hydrogel
system for reconstruction of bone defects. J Tissue Eng Regen Med.
12:1133–1144. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liang C, Peng S, Li J, Lu J, Guan D, Jiang
F, Lu C, Li F, He X, Zhu H, et al: Inhibition of osteoblastic
Smurf1 promotes bone formation in mouse models of distinctive
age-related osteoporosis. Nat Commun. 9:34282018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang X, Zhu Y, Cao L, Wang X, Zheng A,
Chang J, Wu J, Wen J, Jiang X, Li H and Zhang Z: Alginate-aker
injectable composite hydrogels promoted irregular bone regeneration
through stem cell recruitment and osteogenic differentiation. J
Mater Chem B. 6:1951–1964. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Feng YF, Wang L, Zhang Y, Li X, Ma ZS, Zou
JW, Lei W and Zhang ZY: Effect of reactive oxygen species
overproduction on osteogenesis of porous titanium implant in the
present of diabetes mellitus. Biomaterials. 34:2234–2243. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhao Y, Wang Z, Jiang Y, Liu H, Song S,
Wang C, Li Z, Yang Z, Liu H, Wang J, Yang B and Lin Q: Biomimetic
composite scaffolds to manipulate stem cells for aiding rheumatoid
arthritis management. Adv Funct Mater. 29:18078602019. View Article : Google Scholar
|
|
37
|
Yi H, Ur Rehman F, Zhao C, Liu B and He N:
Recent advances in nano scaffolds for bone repair. Bone Res.
4:160502016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee BN, Hong JU, Kim SM, Jang JH, Chang
HS, Hwang YC, Hwang IN and Oh WM: Anti-inflammatory and osteogenic
effects of calcium silicate-based root canal sealers. J Endod.
45:73–78. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sivashanmugam A, Charoenlarp P, Deepthi S,
Rajendran A, Nair SV, Iseki S and Jayakumar R: Injectable
shear-thinning CaSO(4)/FGF-18-Incorporated Chitin-PLGA hydrogel
enhances bone regeneration in mice cranial bone defect model. ACS
Appl Mater Interfaces. 9:42639–42652. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rashdan NA, Sim AM, Cui L, Phadwal K,
Roberts FL, Carter R, Ozdemir DD, Hohenstein P, Hung J, Kaczynski
J, et al: Osteocalcin regulates arterial calcification via altered
wnt signaling and glucose metabolism. J Bone Miner Res. 35:357–367.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ho SS, Vollmer NL, Refaat MI, Jeon O,
Alsberg E, Lee MA and Leach JK: Bone morphogenetic protein-2
promotes human mesenchymal stem cell survival and resultant bone
formation when entrapped in photocrosslinked alginate hydrogels.
Adv Healthc Mater. 5:2501–2509. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bagheri L, Pellati A, Rizzo P, Aquila G,
Massari L, De Mattei M and Ongaro A: Notch pathway is active during
osteogenic differentiation of human bone marrow mesenchymal stem
cells induced by pulsed electromagnetic fields. J Tissue Eng Regen
Med. 12:304–315. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhou YJ, Wang P, Chen HY, Liu C, Ji QD,
Yang XT, Gao Q and He CQ: Effect of pulsed electromagnetic fields
on osteogenic differentiation and Wnt/β-catenin signaling pathway
in rat bone marrow mesenchymal stem cells. Sichuan Da Xue Xue Bao
Yi Xue Ban. 46:347–353. 2015.(In Chinese). PubMed/NCBI
|
|
44
|
Selvamurugan N, He Z, Rifkin D, Dabovic B
and Partridge NC: Pulsed electromagnetic field regulates MicroRNA
21 expression to activate TGF-β signaling in human bone marrow
stromal cells to enhance osteoblast differentiation. Stem Cells
Int. 2017:24503272017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Rachner TD, Khosla S and Hofbauer LC:
Osteoporosis: Now and the future. Lancet. 377:1276–1287. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ehnert S, Schröter S, Aspera-Werz RH,
Eisler W, Falldorf K, Ronniger M and Nussler AK: Translational
insights into extremely low frequency pulsed electromagnetic fields
(ELF-PEMF) for bone regeneration after trauma and orthopedic
surgery. J Clin Med. 8:20282019. View Article : Google Scholar
|