Introduction
Oral squamous cell carcinoma (OSCC) is a common oral
disease. According to the most recent GLOBOCAN estimate, in Europe
between 2012 and 2015, there was an overall increasing incidence of
oral cancer and of oral cancer-associated mortality (1,2). At
present, OSCC treatment regimens include a combination of surgery,
radiotherapy and chemotherapy (3,4).
Despite the rapid development of modern medicine, the cure rate for
OSCC is still poor, displaying an overall 5-year survival rate of
60% (5). Therefore, identifying
additional safe and effective targeted drugs with low cytotoxicity
is important.
It has been reported that natural plant products
display tumor-inhibitory activities when used as chemopreventive or
therapeutic agents against human cancer cells (6,7).
Dioscin, which is extracted from the traditional Chinese medicinal
herb Dioscorea nipponica, has been reported to display
various biological effects, including renal ischemia/reperfusion
injury-alleviating, anti-inflammatory and antiallergic effects
(8,9). Additionally, in a nephrotoxicity and
cardiotoxicity model, dioscin displayed protective effects via
regulating oxidative stress (10–12).
Meanwhile, dioscin has been reported to display potent effects
against different types of cancer. For example, dioscin markedly
inhibited hepatocellular carcinoma proliferation and migration, but
induced apoptosis, autophagy and DNA damage (13). Si et al (14) reported that dioscin suppressed
laryngeal cancer cell proliferation via inducing cell cycle arrest
and inhibiting tumor invasion. Furthermore, dioscin inhibited human
lung cancer cell proliferation (15). However, to the best of our
knowledge, the effect of dioscin on OSCC has not been previously
reported.
The Hippo signaling pathway is highly conserved
across species. In mammals, the macrophage stimulating (MST)1/MST2
kinases can form a complex with salvador family WW domain
containing protein 1 and activate the downstream kinases large
tumor suppressor kinase (LATS)1 and LATS2 (16). One protein substrate of LATS is
yes-associated protein (YAP), which is an important effector
protein in the Hippo signaling pathway (17). Phosphorylated (p)-YAP is sequestered
in the cytoplasm, preventing its transcriptional activation
activity (18). Ras association
domain-containing protein 1 (RASSF1), a tumor suppressor gene
located on chromosome 3p21, is inactivated in numerous types of
cancer (19). Several protein
subtypes can be expressed from the RASSF1 gene via alternative
splicing and promoter usage, the predominant forms are RASSF1A and
RASSF1C, which have different N-termini (20). In a previous study, dioscin induced
RASSF1 demethylation in bladder cancer (21).
The aforementioned studies indicated that dioscin
may regulate the bioactivity of OSCC cells, which may involve the
Hippo/YAP signaling pathway. Therefore, the present study aimed to
evaluate the biological effects of dioscin on OSCC cells, as well
as the possible underlying effects. The results of the present
study suggested that dioscin may serve as a therapeutic agent for
OSCC.
Materials and methods
Cell line and culture
The tongue squamous cell line SCC15 was provided by
Professor Shaohua Liu (Qilu Hospital of Shandong University, Jinan,
China). SCC15 cells were cultured in DMEM (HyClone; Cytiva)
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.)
at 37°C with 5% CO2.
Reagents and antibodies
Dioscin (purity ≥98%) was purchased from Beijing
Solarbio Science & Technology Co., Ltd. Cells were treated with
different concentrations of dioscin (0, 0.5, 1 or 2 µM) for 24 or
48 h, or with 1 µM dioscin for different durations (0, 2, 4 or 8
h). Nuclear Protein Extraction kit (cat. no. R0050) was purchased
from Beijing Solarbio Science & Technology Co., Ltd and was
used to extract nuclear proteins prior to western blotting. Primary
antibodies targeted against GAPDH (cat. no. 60004-1-Ig) and histone
H3 (cat. no. 17168-1-AP) were obtained from ProteinTech Group, Inc.
Primary antibodies targeted against LATS1 (cat. no. 3477), p-LATS1
(Ser909; cat. no. 9157), p-MST (cat. no. 49332), MST2 (cat. no.
3952), YAP (cat. no. 4912), p-YAP (Ser397; cat. no. 13619), cyclin
D1 (cat. no. 92G2) and BAX (cat. no. 5023) were purchased from Cell
Signaling Technologies, Inc. The primary antibody targeted against
BCL-2 (cat. no. WL01556) was purchased from Wanleibio Co., Ltd. The
primary antibody targeted against RASSF1A (cat. no. ab91212) was
purchased from Abcam. The goat anti-mouse or anti-rabbit
HRP-conjugated secondary antibody (cat. nos. S0002 or S0001) was
obtained from Affinity Biosciences.
Western blotting
After washing twice with ice-cold PBS, the total
protein in cells were lysed using RIPA (cat. no. R0020; Beijing
Solarbio Science & Technology Co., Ltd.) lysis buffer for 30
min on ice. Cells were harvested and centrifuged at 20,664 × g at
4°C for 15 min. In order to extract nuclear protein from the cells,
cells were lysed using cytoplasmic protein extraction reagent lysis
buffer for 30 min on ice. The proteins were collected from the
supernatant after centrifugation at 20,664 × g at 4°C for 15 min,
and the nuclear protein extraction reagent was added to the cell
precipitation for centrifugation at 20,664 × g at 4°C for 15 min
and collection of nuclear protein. Protein concentrations were
determined using a BCA protein assay. Subsequently, proteins (20
µg) were separated via SDS-PAGE on 10% gels and transferred to PVDF
membranes (EMD Millipore). After blocking with non-fat milk for 1 h
at room temperature, the membranes were incubated overnight at 4°C
with the appropriate primary antibodies (all 1:1,000). After
washing three times with TBS-0.1% Tween-20 (TBST) for 15 min per
wash, the membranes were incubated with a goat anti-mouse or
anti-rabbit HRP-conjugated secondary antibody (1:20,000) for 1 h at
room temperature. The membranes were washed three times with TBST
and then protein bands were visualized using an enhanced
chemiluminescence kit (EMD Millipore) under using an Imager 600
(Amersham; Cytiva). GAPDH and histone H3 were used as the loading
controls. Protein expression levels were analyzed by ImageJ (1.8.0)
software (National Institutes of Health).
Coimmunoprecipitation
Following treatment with dioscin for 24 h, lysates
were prepared according to the manufacturer's instructions (Active
Motif, Inc.). Following centrifugation at 4°C for 5 min at 137 × g,
cells were suspended in 100 µl freshly prepared complete RIPA
buffer and incubated for 30 min on a rotating platform at 4°C.
Whole cell extract was combined with 5 µg IgG (cat. no. 3900) or
anti-MST2 (cat. no. 3952) antibodies (both from Cell Signaling
Technology, Inc.) in a final volume of 500 µl and incubated
overnight at 4°C on a rotating platform. Subsequently, 25 µl
protein G magnetic beads were added to the tube and incubated
overnight at 4°C on a rotating platform. The protein G magnetic
beads were washed carefully and the protein samples were obtained.
The samples were resuspended in loading buffer (cat. no. P1040;
Beijing Solarbio Science & Technology Co., Ltd.) and boiled for
subsequent analysis via western blotting, according to the
aforementioned protocol.
Cell proliferation
Cell proliferation was assessed by performing Cell
Counting Kit-8 (CCK-8) assays (Dojindo Molecular Technologies,
Inc.) according to the manufacturer's protocol. SCC15 cells
(3×103 cells/well) were seeded into a 96-well plate.
After treatment, cells were cultured for 1–4 days. Subsequently, 10
µl CCK-8 solution was added to each well with 100 µl DMEM and
incubated for 2 h at 37°C. Optical density values were measured at
a wavelength of 450 nm.
5-Ethynyl-2′-deoxyuridine (EdU)
proliferation assay
Cells (1×104/well) were seeded into a
48-well plate and cultured for 48 h. The EdU Apollo DNA in
vitro Kit (Guangzhou RiboBio Co., Ltd.) was used to assess cell
proliferation. Cells were cultured with 200 µl EdU for 2 h and were
then fixed with 4% paraformaldehyde for 30 min at room temperature.
Subsequently, cells were treated for 10 min with 2 mg/ml glycine
and 0.5% Triton X-100 at room temperature. Then, DAPI working
solution was added to each well and incubated for 30 min in the
dark at room temperature. Quantification of EdU+ cells
was expressed as a percentage of DAPI+ cells. The
percentage of EdU+ cells was determined by fluorescence
microscopy (Olympus Corporation).
Lentiviral (LV) transduction
cells (1×105/well) were seeded into a
6-well plate and cultured for 24 h. At ~70% confluence, SCC15 cells
were transduced with LV-homo-YAP particles [overexpression (OE) YAP
group] or LV-5-negative control (NC; empty vector; OE NC group) for
12 h. Similarly, SCC15 cells were transduced with LV-homo-YAP-short
hairpin (sh)RNA LV particles (sh YAP group) or a corresponding
empty LV vector (sh NC group) for 12 h. The MOI for transduction
was 20. All LV particles were purchased from Shanghai Genepharma
Co., Ltd. After transduction for 48 h, cells were treated with 1
µg/ml puromycin for 2 weeks. Transduction efficiency was assessed
via western blotting.
Flow cytometric analysis of the cell
cycle
Cells (1×106) were harvested, fixed in
75% ethanol overnight at 4°C, washed with cold PBS and incubated
with 0.5 ml propidium iodide/RNase (Tianjin Sungene Biotech Co.,
Ltd.) working solution for 30 min at room temperature.
Subsequently, the cell cycle distribution was determined using an
Accuri C6 flow cytometer (Becton, Dickinson and Company) and
analyzed using FlowJo software (version 10; FlowJo LLC).
Flow cytometric analysis of cell
apoptosis
The Annexin V-PI staining kit was used to assess
apoptosis [Multi Sciences (Lianke) Biotech Co., Ltd.]. Cells were
harvested, washed with cold PBS and suspended in 500 µl binding
buffer. Subsequently, cells were incubated with 5 µl Annexin-V
allophycocyanin working solution for 10 min in the dark at room
temperature. Then, cells were incubated with 5 µl
7-aminoactinomycin D working solution for 5 min in the dark at room
temperature. Within 1 h, early and late cell apoptosis was
determined using an Accuri C6 flow cytometer (Becton, Dickinson and
Company) and analyzed using FlowJo software (version 10; FlowJo,
LLC).
Statistical analysis
Data are presented as the mean ± SD of three
independent experiments. Statistical analyses were performed using
GraphPad Prism software (version 6; GraphPad Software, Inc.).
Comparisons among multiple groups were analyzed using one-way ANOVA
followed by Tukey's post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
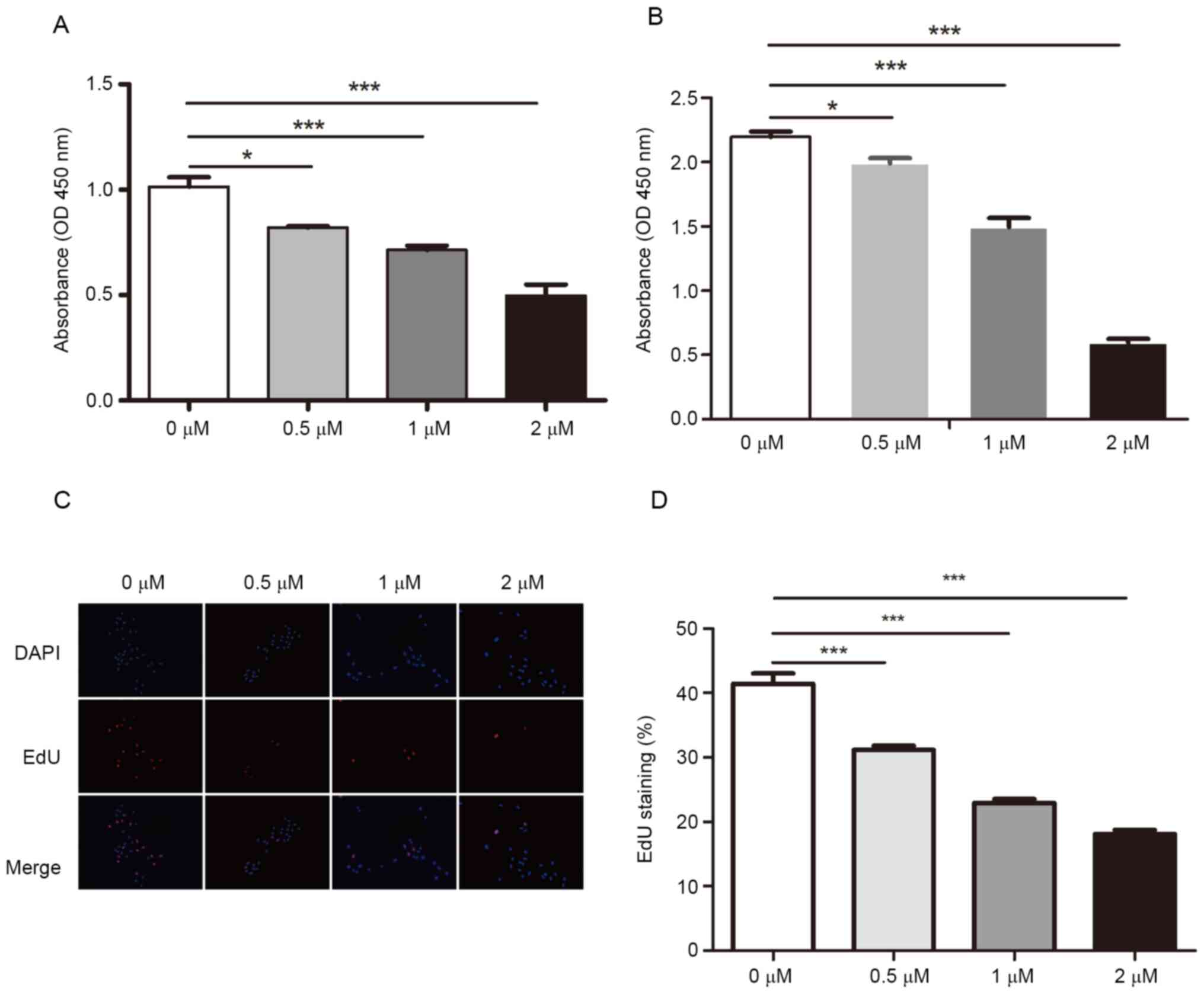
Dioscin reduces SCC15 cell
proliferation
To investigate the effects of dioscin on
proliferation, SCC15 cells were treated with different
concentrations of dioscin for 24 or 48 h, and cell proliferation
was determined by performing CCK-8 assays. Compared with the
control group (1.014±0.026), dioscin treatment significantly
inhibited cell proliferation, even at 0.5 µM (0.820±0.004)
(Fig. 1A). Similar results were
obtained following treatment for 48 h (Fig. 1B). Moreover, the EdU incorporation
assay results indicated that dioscin significantly decreased the
ratio of EdU+ cells in a concentration-dependent manner
(31.150±0.382, 22.92±0.352 and 18.100±0.311% at 0.5, 1 and 2 µM,
respectively) compared with the control group (41.410±0.813%)
(Fig. 1C and D). The results
indicated that dioscin inhibited SCC15 cell proliferation in a
dose-dependent manner.
Dioscin induces SCC15 cell cycle
arrest and apoptosis
To explore the cellular mechanism underlying the
antiproliferative effect of dioscin on SCC15 cells, flow cytometry
was performed to detect alterations in the cell cycle and
apoptosis. Following treatment with 0.5, 1 or 2 µM dioscin, the
number of cells in the G2/M phase was significantly
decreased (5.663±0.350, 1.816±0.304 and 0.5667±0.173%,
respectively) compared with the control group (7.283±0.238%)
(Fig. 2A and B). Moreover,
following treatment with 1 or 2 µM dioscin, the number of cells in
the G0/G1 phase was significantly increased
(69.740±1.3960 and 74.200±0.305%, respectively) compared with the
control group (65.830±0.644%). Subsequently, the effect of dioscin
on cell apoptosis was evaluated. Compared with the control group
(early apoptosis, 2.464±0.110%; late apoptosis, 6.254±0.078%),
dioscin (0.5, 1 or 2 µM) significantly increased the population of
early (3.100±0.112, 5.624±0.248 and 7.790±0.330%, respectively) and
late (8.142±0.072, 15.100±0.353 and 21.020±0.213%, respectively)
apoptotic cells (Fig. 2C and D).
The apoptosis-inducing effect of dioscin was enhanced with
increasing concentrations.
Dioscin-induced inhibition of cell
proliferation is mediated by the Hippo signaling pathway
Previous research has demonstrated that RASSF1
supports the phosphorylation of MST2 and SAV, both of which are
upstream molecules of YAP (22). A
previous study reported that dioscin induced RASSF1 demethylation
and inhibited the proliferative activity of bladder cancer cell
lines (21). Therefore, the present
study aimed to investigate the role of the Hippo signaling pathway
in dioscin-treated cells. SCC15 cells were treated with different
concentrations of dioscin (0, 0.5, 1 or 2 µM) for 24 h or with 1 µM
dioscin for different durations (0, 2, 4 or 8 h). The ratios of
p-LATS (Ser909)/LATS, p-MST/MST2 and p-YAP (Ser397)/YAP were
significantly increased by dioscin treatment in a concentration-
and time-dependent manner compared with the control group (Fig. 3A-D). Compared with the control
group, dioscin did not notably alter the expression levels of total
LATS1 and MST2, but markedly decreased the expression levels of
total YAP (Fig. 3A-D). In addition,
the expression levels of cell cycle- and apoptosis-related proteins
were assessed. Cyclin D1 and BCL-2 expression levels were markedly
decreased by dioscin in a concentration- and time-dependent manner
compared with the control group (Fig.
3E and F). However, BAX expression levels displayed the
opposite pattern. To investigate whether dioscin treatment altered
the localization of YAP, nuclear and cytoplasmic proteins were
extracted using the Nuclear Protein Extraction kit. As dioscin
concentrations increased, nuclear YAP expression levels were
markedly decreased, whereas YAP cytoplasmic accumulation was
notably increased compared with the control group (Fig. 3G).
 | Figure 3.Dioscin inhibits SCC15 cell
proliferation via inactivating the Hippo signaling pathway.
Following treatment with (A) 0, 0.5, 1 or 2 µM dioscin for 24 h or
(B) 1 µM dioscin for 0, 2, 4 or 8 h, Hippo signaling
pathway-related protein expression levels were measured via western
blotting. Hippo signaling pathway-related protein expression levels
were semi-quantified in SCC15 cells treated with (C) 0, 0.5, 1 or 2
µM dioscin for 24 h or (D) 1 µM dioscin for 0, 2, 4 or 8 h.
Following treatment with (E) 0, 0.5, 1 or 2 µM dioscin for 24 h or
(F) 1 µM dioscin for 0, 2, 4 or 8 h, cell cycle and
apoptosis-related protein expression levels were determined via
western blotting. (G) Following treatment with 0, 0.5, 1 or 2 µM
dioscin for 24 h, nuclear and cytoplasmic proteins were extracted
and detected via western blotting. (H) Following treatment with 1
µM dioscin for 24 h, MST2 immunoprecipitation was detected via
coprecipitation with RASSF1A and LATS1 followed by western blotting
analysis. Data are presented as the mean ± SD of three independent
experiments. *P<0.05 and ***P<0.001. RASSF1A, Ras association
domain-containing protein 1A; p, phosphorylated; LATS, large tumor
suppressor kinase; MST, macrophage stimulating; YAP, yes-associated
protein; Cyto, cytoplasmic; ip, immunoprecipitation. |
To detect Hippo signaling pathway activation
following dioscin treatment, the expression levels of RASSF1A, a
protein consistently linked to tumor onset and poor cancer
prognosis (23), were detected. The
western blotting results indicated that RASSF1A expression levels
were markedly increased by dioscin treatment in a concentration-
and time-dependent manner compared with the control group (Fig. 3A and B). However, the mechanism
underlying dioscin-induced inactivation of the Hippo signaling
pathway is not completely understood. RASSF1A has been reported to
form a complex with MST2 and SAV, which phosphorylates LATS1,
leading to YAP phosphorylation (24). The aforementioned results indicated
that dioscin treatment resulted in RASSF1A accumulation (Fig. 3A and B), and endogenous RASSF1Aor
LATS1 were coimmunoprecipitated with the MST2protein (Fig. 3H). As shown in Fig. 3H, RASSF1A and LATS1 were detected in
the anti-MST2 precipitate, but not in the IgG precipitate.
YAP overexpression abrogates
dioscin-induced inhibition of cell proliferation
The aforementioned results indicated that the
RASSF1A/MST2/YAP axis was involved in dioscin-induced inhibition of
cell proliferation. To evaluate the hypothesis, SCC15 cells were
transduced with OE YAP or OE NC and then treated with 1 µM dioscin.
The flow cytometry results demonstrated that, compared with the OE
NC group (3.450±0.886%), dioscin treatment significantly decreased
the number of cells in the G2/M phase (1.097±0.112%),
whereas the number of cells in the proliferative phases was
significantly increased in the OE YAP group (7.887±0.206%) compared
with the OE NC group. (Fig. 4A and
B). Meanwhile, compared with the OE NC + dioscin group
(1.097±0.112%), the increase of proliferation activity of OE YAP +
dioscin group (6.890±0.237%), indicated that overexpression of YAP
gene abrogated dioscin-induced inhibition of cell proliferation.
(Fig. 4B). The apoptosis assay
results demonstrated that dioscin treatment significantly increased
the number of apoptotic cells (39.010±0.464%), whereas in the OE
YAP group this increase was attenuated (19.150±0.831%) compared
with the OE NC group (24.770±0.382%) (Fig. 4C and D). The EdU incorporation
(Fig. 4E and F) and CCK-8 (Fig. 4G) assay results demonstrated that,
compared with the OE NC group or OE YAP group, dioscin treatment
significantly suppressed SCC15 cell proliferation in the OE NC +
dioscin and OE YAP + dioscin groups, whereas YAP overexpression in
the OE YAP or OE YAP + dioscin groups enhanced cell proliferation
compared with the OE NC or OE NC + dioscin groups, respectively.
The dioscin-induced suppression of proliferation was inhibited in
the OE YAP group. The protein expression levels of cell cycle- and
apoptosis-related proteins were measured via western blotting. The
western blotting results further verified the alterations in the
cell cycle and apoptosis (Fig.
4H).
YAP knockdown enhances the antitumor
activity of dioscin
To further understand the role of YAP in mediating
the suppressive effects of dioscin on SCC15 cells, SCC15 cells were
transduced with sh YAP or sh NC and then treated with 1 µM dioscin.
Flow cytometry was performed to assess alterations in cell cycle
distribution and apoptosis. Both YAP knockdown and dioscin
treatment led to a decrease of cells in G2/M phase, and
the combination of these interventions augmented this effect
(G2/M phase cells: sh NC, 11.770±0.0880%; sh NC +
dioscin, 11.000±0.152%; sh YAP, 10.300±0.152%; and sh YAP +
dioscin, 6.263±0.489%) (Fig. 5A and
B). Moreover, compared with the sh NC group (19.860±0.474%),
the proportion of apoptotic cells was increased following dioscin
treatment (21.600±0.462%) (not statistically significant) or YAP
knockdown (22.010±0.353%), and the combination of these two
interventions was synergistic (27.020±0.277%) (Fig. 5C and D). Subsequently, SCC15 cell
proliferation was evaluated by performing EdU incorporation
(Fig. 5E and F) and CCK-8 (Fig. 5G) assays. Compared with the sh NC
group, both dioscin and YAP knockdown significantly decreased cell
proliferation. In addition, the combination of dioscin treatment
and YAP knockdown resulted in further inhibition of cell
proliferation compared with either intervention alone, as
determined by flow cytometry, EdU staining, western blotting and
CCK-8 assay at 24 h.
Subsequently, the expression levels of YAP, cyclin
D1, BCL-2 and BAX were measured. Similarly, the combination of
dioscin treatment and YAP knockdown led to more notable suppressive
effects on protein expression compared with either intervention
alone. However, the expression levels of the proapoptotic protein
BAX displayed the opposite trends (Fig.
5H).
Discussion
OSCC is one of the most common and malignant tumors
in the oral and maxillofacial regions; however, following systemic
treatment, the prognosis of patients with OSCC is still poor
(25). In addition to the health
and functional abnormalities that typically result from tumors,
OSCC often affects the appearance of patients, leading to a heavy
psychological burden (26).
Dioscin is a natural steroidal saponin that can be
extracted from various traditional Chinese medicines, including
Dioscorea. Dioscin has been reported to exert a variety of
biological activities, including protection against liver damage
(27) and anti-inflammatory
activity (28). Moreover, dioscin
may produce anticancer effects on various types of cancer cells
(29–32). Previous studies have indicated that
dioscin induced cervical carcinoma cell apoptosis (33) and suppressed ovarian cancer cell
viability (34). The present study
demonstrated that, compared with the control group, dioscin
resulted in cell cycle arrest and increased apoptosis, which was
consistent with a previous study that indicated that dioscin
protected against cancer progression (35). The present study also assessed cell
cycle- and apoptosis-related protein expression levels, including
cyclin D1, BCL-2 and BAX. The alterations in protein expression
levels were consistent with the alterations in biological
activities.
YAP is the hub protein in the Hippo signaling
pathway (36). Previous studies
have demonstrated that the Hippo signaling pathway is inactivated
and YAP protein expression is increased in numerous types of
cancer, including cervical cancer, urothelial cell carcinoma and
oral squamous cell carcinoma (37–39).
Direct inhibition of the YAP oncoprotein is an anticancer
therapeutic strategy (40). A
previous study reported that dioscin downregulated the expression
of tafazzin, a homolog protein of YAP, in hepatocellular carcinoma;
however, alterations in YAP expression were not investigated
(41). The present study
demonstrated that, compared with the control group, dioscin notably
deactivated YAP protein expression by increasing its
phosphorylation, and nuclear YAP accumulation was markedly
decreased following dioscin treatment. Therefore, it was
hypothesized that dioscin inhibited OSCC cell viability by
downregulating YAP expression. To further investigate whether the
effects of dioscin on the biological characteristics of OSCC cells
were mediated via YAP, YAP expression was altered in OSCC cells via
LV transduction. YAP overexpression and knockdown reduced and
enhanced dioscin-induced antitumor effects, respectively.
RASSF1A is one of the most commonly inactivated
tumor suppressor genes in sporadic human malignant tumors (42). RASSF1A activation is an important
factor in the pathogenesis and progression of solid tumors
(43,44). A previous study reported that
dioscin demethylated the RASSF1A gene in bladder cancer cells, thus
inhibiting cancer cell proliferation (21). The present study demonstrated that
RASSF1A protein expression levels were increased by dioscin
treatment in a concentration- and time-dependent manner compared
with the control group. Increases in RASSF1A expression provided a
potential explanation for the inhibitory effects of dioscin on OSCC
cell proliferation. A previous study demonstrated that ATM
serine/threonine kinase directly phosphorylated the Ser131 site in
RASSF1A, activating MST2 and LATS1 (22), which then phosphorylated YAP and
induced cell apoptosis. The results of the present study
demonstrated that dioscin treatment enhanced the binding of MST2
with RASSF1A and LATS1, which could be explained by the
augmentation of YAP phosphorylation.
To conclude, the present study demonstrated that
dioscin resulted in OSCC cell cycle arrest and apoptosis, which
involved the RASSF1A/MST2/YAP signaling axis. The present study
indicated that dioscin may serve as a useful therapeutic agent for
OSCC or other types of tumors.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Shandong
Provincial Natural Science Foundation (grant no. ZR2018MH018),
China Postdoctoral Science Foundation (grant no. 2017M610432) and
Young Scholars Program of Shandong University (grant no.
2015WLJH53).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HT and XC performed the experiments, analyzed the
data, prepared the figures and tables, and wrote the manuscript. YZ
and YWe assisted in the data collection and analyses. YWa and XF
assisted in data analyses. HT and XC confirm the authenticity of
all the raw data. WG was involved in designing the experiments and
drafting the manuscript. YWe agreed to be accountable for all
aspects of the work, supervised the work, edited the manuscript and
gave final approval of the version to be published. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Omar E: Current concepts and future of
noninvasive procedures for diagnosing oral squamous cell carcinoma
- a systematic review. Head Face Med. 11:62015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Haddad RI and Shin DM: Recent advances in
head and neck cancer. N Engl J Med. 359:1143–1154. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ferris RL, Blumenschein G Jr, Fayette J,
Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE,
Even C, et al: Nivolumab for recurrent squamous-cell carcinoma of
the head and neck. N Engl J Med. 375:1856–1867. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chestnut C, Subramaniam D, Dandawate P,
Padhye S, Taylor J III, Weir S and Anant S: Targeting major
signaling pathways of bladder cancer with phytochemicals: A review.
Nutr Cancer. 11:1–23. 2020. View Article : Google Scholar
|
|
7
|
Khan T, Ali M, Khan A, Nisar P, Jan SA,
Afridi S and Shinwari ZK: Anticancer plants: A review of the active
phytochemicals, applications in animal models, and regulatory
aspects. Biomolecules. 10:472019. View Article : Google Scholar
|
|
8
|
Kalailingam P, Kannaian B, Tamilmani E and
Kaliaperumal R: Efficacy of natural diosgenin on cardiovascular
risk, insulin secretion, and beta cells in streptozotocin
(STZ)-induced diabetic rats. Phytomedicine. 21:1154–1161. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zheng L, Yin L, Xu L, Qi Y, Li H, Xu Y,
Han X, Liu K and Peng J: Protective effect of dioscin against
thioacetamide-induced acute liver injury via FXR/AMPK signaling
pathway in vivo. Biomed Pharmacother. 97:481–488. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Y, Xu Y, Qi Y, Xu L, Song S, Yin L,
Tao X, Zhen Y, Han X, Ma X, et al: Protective effects of dioscin
against doxorubicin-induced nephrotoxicity via adjusting
FXR-mediated oxidative stress and inflammation. Toxicology.
378:53–64. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao L, Tao X, Qi Y, Xu L, Yin L and Peng
J: Protective effect of dioscin against doxorubicin-induced
cardiotoxicity via adjusting microRNA-140-5p-mediated myocardial
oxidative stress. Redox Biology. 16:189–198. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang Y, Tao X, Yin L, Xu L, Xu Y, Qi Y,
Han X, Song S, Zhao Y, Lin Y, et al: Protective effects of dioscin
against cisplatin-induced nephrotoxicity via the
microRNA-34a/sirtuin 1 signalling pathway. Br J Pharmacol.
174:2512–2527. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mao Z, Han X, Chen D, Xu Y, Xu L, Yin L,
Sun H, Qi Y, Fang L, Liu K and Peng J: Potent effects of dioscin
against hepatocellular carcinoma through regulating TP53-induced
glycolysis and apoptosis regulator (TIGAR)-mediated apoptosis,
autophagy, and DNA damage. Br J Pharmacol. 176:919–937. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Si L, Zheng L, Xu L, Yin L, Han X, Qi Y,
Xu Y, Wang C and Peng J: Dioscin suppresses human laryngeal cancer
cells growth via induction of cell-cycle arrest and MAPK-mediated
mitochondrial-derived apoptosis and inhibition of tumor invasion.
Eur J Pharmacol. 774:105–117. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wei Y, Xu Y, Han X, Qi Y, Xu L, Xu Y, Yin
L, Sun H, Liu K and Peng J: Anti-cancer effects of dioscin on three
kinds of human lung cancer cell lines through inducing DNA damage
and activating mitochondrial signal pathway. Food Chem Toxicol.
59:118–128. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guo C, Zhang X and Pfeifer GP: The tumor
suppressor RASSF1A prevents dephosphorylation of the mammalian
STE20-like kinases MST1 and MST2. J Biol Chem. 286:6253–6261. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang J, Wu S, Barrera J, Matthews K and
Pan D: The Hippo signaling pathway coordinately regulates cell
proliferation and apoptosis by inactivating Yorkie, the
Drosophila Homolog of YAP. Cell. 122:421–434. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Harvey KF, Zhang X and Thomas DM: The
Hippo pathway and human cancer. Nat Rev Cancer. 13:246–257. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dammann R, Li C, Yoon JH, Chin PL, Bates S
and Pfeifer GP: Epigenetic inactivation of a RAS association domain
family protein from the lung tumour suppressor locus 3p21.3. Nat
Genet. 25:315–319. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Agathanggelou A, Cooper WN and Latif F:
Role of the Ras-association domain family 1 tumor suppressor gene
in human cancers. Cancer Res. 65:3497–508. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou Q, Song W and Xiao W: Dioscin induces
demethylation of DAPK-1 and RASSF-1alpha genes via the antioxidant
capacity, resulting in apoptosis of bladder cancer T24 cells. EXCLI
J. 16:101–112. 2017.PubMed/NCBI
|
|
22
|
Hamilton G, Yee KS, Scrace S and O'Neill
E: ATM regulates a RASSF1A-dependent DNA damage response. Curr
Biol. 19:2020–2025. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu G, Zhou X, Xing J, Xiao Y, Jin B, Sun
L, Yang H, Du S, Xu H and Mao Y: Identification of RASSF1A promoter
hypermethylation as a biomarker for hepatocellular carcinoma.
Cancer Cell Int. 20:5472020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guo C, Tommasi S, Liu L, Yee JK, Dammann R
and Pfeifer GP: RASSF1A is part of a complex similar to the
Drosophila Hippo/Salvador/Lats tumor-suppressor network.
Curr Biol. 17:700–705. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schwam ZG and Judson BL: Improved
prognosis for patients with oral cavity squamous cell carcinoma:
Analysis of the National Cancer Database 1998–2006. Oral Oncol.
52:45–51. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Metzger K, Moratin J, Horn D, Pilz M,
Ristow O, Hoffmann J, Freier K, Engel M and Freudlsperger C:
Treatment delay in early-stage oral squamous cell carcinoma and its
relation to survival. J Craniomaxillofac Surg. Feb 12–2021.(Epub
ahead of print). doi: 10.1016/j.jcms.2021.02.007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lu B, Xu Y, Xu L, Cong X, Yin L, Li H and
Peng J: Mechanism investigation of dioscin against CCl4-induced
acute liver damage in mice. Environ Toxicol Pharmacol. 34:127–135.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu S, Xu H, Peng J, Wang C, Jin Y, Liu K,
Sun H and Qin J: Potent anti-inflammatory effect of dioscin
mediated by suppression of TNF-α-induced VCAM-1, ICAM-1and EL
expression via the NF-κB pathway. Biochimie. 110:62–72. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hsieh MJ, Tsai TL, Hsieh YS, Wang CJ and
Chiou HL: Erratum to: Dioscin-induced autophagy mitigates cell
apoptosis through modulation of PI3K/Akt and ERK and JNK signaling
pathways in human lung cancer cell lines. Arch Toxicol.
91:2495–2496. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gao LL, Li FR, Jiao P, Yang MF, Zhou XJ,
Si YH, Jiang WJ and Zheng TT: Paris chinensis dioscin induces G2/M
cell cycle arrest and apoptosis in human gastric cancer SGC-7901
cells. World J Gastroenterol. 17:4389–4395. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Song X, Wang Z, Liang H, Zhang W, Ye Y, Li
H, Hu Y, Zhang Y, Weng H, Lu J, et al: Dioscin induces gallbladder
cancer apoptosis by inhibiting ROS-Mediated PI3K/AKT signalling.
Int J Biol Sci. 13:782–793. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hsu WH, Lee BH and Pan TM: Effects of red
mold dioscorea on oral carcinogenesis in DMBA-induced hamster
animal model. Food Chem Toxicol. 49:1292–1297. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhao X, Tao X, Xu L, Yin L, Qi Y, Xu Y,
Han X and Peng J: Dioscin induces apoptosis in human cervical
carcinoma HeLa and SiHa cells through ROS-Mediated DNA damage and
the mitochondrial signaling pathway. Molecules. 21:7302016.
View Article : Google Scholar
|
|
34
|
Guo X and Ding X: Dioscin suppresses the
viability of ovarian cancer cells by regulating the VEGFR2 and
PI3K/AKT/MAPK signaling pathways. Oncol Lett. 15:9537–9542.
2018.PubMed/NCBI
|
|
35
|
Si L, Xu L, Yin L, Qi Y, Han X, Xu Y, Zhao
Y, Liu K and Peng J: Potent effects of dioscin against pancreatic
cancer via miR-149-3P-mediated inhibition of the Akt1 signalling
pathway. Br J Pharmacol. 174:553–568. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hansen CG, Moroishi T and Guan KL: YAP and
TAZ: A nexus for Hippo signaling and beyond. Trends Cell Biol.
25:499–513. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
He C, Mao D, Hua G, Lv X, Chen X,
Angeletti PC, Dong J, Remmenga SW, Rodabaugh KJ, Zhou J, et al: The
Hippo/YAP pathway interacts with EGFR signaling and HPV
oncoproteins to regulate cervical cancer progression. EMBO Mol Med.
7:1426–1449. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ciamporcero E, Shen H, Ramakrishnan S, Yu
Ku S, Chintala S, Shen L, Adelaiye R, Miles KM, Ullio C, Pizzimenti
S, et al: YAP activation protects urothelial cell carcinoma from
treatment-induced DNA damage. Oncogene. 35:1541–1553. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen X, Gu W, Wang Q, Fu X, Wang Y, Xu X
and Wen Y: C-MYC and BCL-2 mediate YAP-regulated tumorigenesis in
OSCC. Oncotarget. 9:668–679. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bae JS, Kim SM and Lee H: The Hippo
signaling pathway provides novel anti-cancer drug targets.
Oncotarget. 8:16084–16098. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen Z, Xu J, Wu Y, Lei S, Liu H, Meng Q
and Xia Z: Diosgenin inhibited the expression of TAZ in
hepatocellular carcinoma. Biochem Biophys Res Commun.
503:1181–1185. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Grawenda AM and O'Neill E: Clinical
utility of RASSF1A methylation in human malignancies. Br J Cancer.
113:372–381. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhou SL, Cui J, Fan ZM, Li XM, Li JL, Liu
BC, Zhang DY, Liu HY, Zhao XK, Song X, et al: Polymorphism of A133S
and promoter hypermethylation in Ras association domain family 1A
gene (RASSF1A) is associated with risk of esophageal and gastric
cardia cancers in Chinese population from high incidence area in
northern China. BMC Cancer. 13:2592013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Klacz J, Wierzbicki PM, Wronska A,
Rybarczyk A, Stanislawowski M, Slebioda T, Olejniczak A,
Matuszewski M and Kmiec Z: Decreased expression of RASSF1A tumor
suppressor gene is associated with worse prognosis in clear cell
renal cell carcinoma. Int. J Oncol. 48:55–66. 2016.
|