Introduction
Endometriosis (EM) is a common gynecological
disease, induced by the presence of an active endometrium on the
lateral side of the uterine cavity (1). The pathological processes of EM
include periodic bleeding, the migration of uterine cells and their
attachment to other organs (2).
Although EM is a benign lesion, it exhibits biological behavior
similar to that of a malignant tumor, including invasion, distant
metastasis and spread (3–5). Therefore, the early treatment and
prevention of EM are crucial.
Berberine (BBR) is widely available in Rhizoma
coptidis, Phellodendron amurense and other plants, and is a
member of a class of isoquinoline alkaloids widely used in clinical
practice against pathogenic microorganisms, to reduce blood sugar
levels, to regulate blood lipid levels, and to protect the heart
and fight inflammation (6). BBR has
been reported to exert inhibitory effects on various types of
tumors (7–9). BBR has been demonstrated to inhibit
the growth and metastasis of endometrial cancer via the microRNA
(miRNA/miR)-101/cyclooxygenase-2 (COX-2)/prostaglandin E2 signaling
pathway, indicating that BBR is a potential anticancer drug for the
treatment of endometrial cancer (10). In addition, BBR has been reported to
exert an antitumor effect by inhibiting the proliferation and
inducing the apoptosis of ovarian cancer cells (11). Therefore, it was hypothesized that
BBR may exert a regulatory effect on the proliferation, invasion
and migration of endometrial stromal cells in EM.
Studies have revealed that the expression of miR-429
is abnormal in a variety of cancer types. It has been reported that
regulating the expression of miR-429 can regulate the
proliferation, invasion and apoptosis of tumor cells (12–14).
Therefore indicating that miR-429 can be used as a potential target
for the regulation of cancer cells in drug therapy. The expression
of miR-429 in colon cancer tissues was previously revealed to be
significantly upregulated. However, following the administration of
BBR, the expression of miR-429 in colon cancer tissues was shown to
be markedly decreased, indicating that BBR may be a potential
therapeutic drug for colon cancer and that miR-429 may be a target
of BBR (15). In addition, it has
been reported that the expression of miR-429 in the tissues of
patients with EM is upregulated (16). Therefore, the present study examined
the effects of BBR on the proliferation, invasion and migration of
endometrial stromal cells in EM, and explored the potential
underlying mechanisms involved, so as to provide a theoretical
basis for BBR treatment of EM.
Materials and methods
Cells and cell culture
Immortalized human endometrial stromal cells (HESCs;
CRL-2615) were purchased from The Cell Bank of Type Culture
Collection of the Chinese Academy of Science, and incubated in DMEM
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS
(Gibco; Thermo Fisher Scientific, Inc.) at 37°C with 5%
CO2.
Reagent
BBR (cat. no. B3251-10G; Sigma-Aldrich; Merck KGaA)
was dissolved in pure methanol (100%) and then diluted in PBS to
obtain the desired concentration (20, 40, 60, 80 µM) before being
added to the cells. The final methanol concentration was <0.1%.
BBR at different concentrations (20, 40, 60 and 80 µM) was added to
the cells, and they were incubated for 24 h. Cells in the control
group were treated with PBS at the same dose. Moreover, 80 µM BBR
was added to the cells at 24, 48 and 72 h to select the most ideal
conditions for subsequent experiments (10).
Cell transfection
One day prior to transfection, cells
(5×104 cells/well) were seeded into six-well plates and
cultured at 37°C in an atmosphere containing 5% CO2.
Cells were pretreated with BBR for 2 h and then transfected with
miR-429 mimic or negative control (mimic NC), which were purchased
from Guangzhou RiboBio Co., Ltd. All transfections were performed
at a concentration of 20 nM miR-429 mimics and mimic NC using
Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.),
according to the manufacturer's instructions. The sequences of
miRNAs used in the present study were as follows: miR-429 mimic,
sense 5′-UAAUACUGUCUGGUAAUGCCGU-3′ and antisense
5′-GGCAUUACCAGACAGUAUUAUU-3′; NC, sense 5′-UUCUCCGAACGUGUCACGUTT-3′
and antisense 5′-ACGUGACACGUUCGGAGAATT-3′. Follow-up experiments
were performed 48 h after cell transfection. miR-429 was
overexpressed using transfection of miR-429 mimics, and cells were
then divided into the following groups: i) Control; ii) BBR; iii)
BBR + mimic-NC (BBR was administered after transfection with empty
vector); and iv) BBR + miR-429 mimic groups (BBR was administered
after miR-429 overexpression).
Cell Counting Kit-8 (CCK-8) assay
Cells were seeded into 96-well plates at a density
of 1×104 cells/well. Following treatment with BBR, 10 µl
CCK-8 solution (Dojindo Molecular Technologies, Inc.) was added to
each well and the cells were incubated at 37°C for 4 h, according
to the manufacturer's instructions. The absorbance was measured at
450 nm using a VersaMax™ microplate reader.
Edu staining
Cells were transferred into 96-well plates at a
density of 1×104 cells/well and cultured at 37°C
overnight. After the corresponding treatment, cells were incubated
with 5′-Ethynl-2′deoxyuridine (Edu; 10 µM) for 4 h, and 50 µl cell
fixation solution (PBS containing 4% paraformaldehyde) was added to
the cells at room temperature for 30 min. Then, 100 µl penetrant
(PBS containing 0.5% Triton X-100) was added to each well for cell
permeabilization for 10 min. The Click-iT™ Edu Alexa Fluor™ 488
imaging kit (Thermo Fisher Scientific, Inc.) was used according to
the manufacturer's instructions. Images were captured using a light
microscope (magnification, ×200; Nikon Eclipse Ti-S; Nikon
Corporation).
Colony formation assay
Cells were seeded in 6-well plates at a density of
1×106 cells/well and cultured at 37°C for ~21 days until
visible colonies appeared. The cells were fixed with pure methanol
for 15 min at room temperature and stained with 0.1% (w/v) crystal
violet for 20 min at room temperature. The images of colonies
growing in the plates were taken at day 21. The cells were counted
under a light contrast microscope (magnification, ×100; BX51;
Olympus Corporation) using Image-Pro Plus 5.0 software (Media
Cybernetics, Inc.). The experiments were repeated at least 3
times.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from the cells using
RNAzol® RT (Sigma-Aldrich; Merck KGaA), following the
manufacturer's protocols. The concentration and purity of the RNA
were measured using a Nanodrop™ 2000 spectrophotometer (Thermo
Fisher Scientific, Inc.). cDNA was synthesized using a RevertAid
First Strand cDNA Synthesis kit (cat. no. K1622; Thermo Fisher
Scientific, Inc.) at ~65°C for 10 min. FastStart™ Universal
SYBR-Green Master (Rox) (Roche Diagnostics) was used for
quantitative PCR on a StepOnePlus™ Real-Time PCR System (Thermo
Fisher Scientific, Inc.), according to the manufacturer's
protocols. The amplification conditions were as follows: 95°C for
10 min, followed by 40 cycles of 95°C for 10 sec and 60°C for 60
sec. Primer sequences used in PCR were obtained from GenScript. RNA
expression was normalized to the expression of U6. Primer sequences
used for qPCR were as follows: miR-429 forward,
5′-ACCTCGCCACCGCCTCCCATTGTCCCGTCG-3′ and reverse,
5′-TGCCAGGCCCGGGTGGGTGTGAACCGGCTTC-3′; U6 forward,
5′-CTCGCTTCGGCAGCACA-3′ and reverse, 5′-ACGCTTCACGAATTTGC-3′;
proliferation marker protein Ki-67 (Ki-67) forward,
5′-AATTCAGACTCCATGTGCCTGAG-3′ and reverse,
5′-CTTGACACACACATTGTCCTCAGC-3′; proliferating cell nuclear antigen
(PCNA) forward, 5′-CACCTTAGCACTAGTATTCGAAGCAC-3′ and reverse,
5′-CACCCGACGGCATCTTTATTAC-3′; and GAPDH forward,
5′-GGAGCGAGATCCCTCCAAAAT-3′ and reverse,
5′-GGCTGTTGTCATACTTCTCATGG-3′. The experiments were repeated at
least 3 times. The relative expression of target genes was
calculated using the 2−ΔΔCq method (17).
Western blotting
Protein was extracted from the cells with RIPA lysis
buffer (Beyotime Institute of Biotechnology). Total protein was
quantified using a BCA protein assay kit. Following extraction of
the total protein from the cells, 10% SDS-PAGE was used to separate
the proteins (30 µg), and then separated proteins were transferred
to PVDF membranes. Membranes were blocked with 5% skimmed milk for
1 h at room temperature, and incubated with the following primary
antibodies overnight at 4°C: Anti-Ki-67 (1:1,000; cat. no.
ab245113; Abcam), anti-PCNA (1:1,000; cat. no. ab92552; Abcam),
anti-matrix metalloproteinase (MMP)2 (1:1,000; cat. no. ab92536;
Abcam), anti-MMP4 (1:1,000; cat. no. ab232865; Abcam),anti-MMP9
(1:1,000; cat. no. ab76003; Abcam) and anti-GAPDH (1:1,000; cat.
no. ab8245; Abcam). Following which, membranes were incubated with
a horseradish peroxidase-conjugated secondary antibody (1:5,000;
cat. no. ab6721; Abcam) for 2 h at room temperature. Proteins were
detected by the ECL™ Western Blotting Analysis System (GE
Healthcare). The experiments were repeated at least 3 times.
Protein expression levels were semi-quantified using ImageJ
software (version 146; National Institutes of Health).
Wound healing assay
Cells at a density of 1×106 cells/well
were allowed to grow until 90% confluency, and a gap was then
created using a 200-µl pipette tip. The cells were then cultured
with fresh DMEM medium. The distance of wound closure was measured
at 0 and 24 h with a light contrast microscope (magnification,
×100; BX51; Olympus Corporation) and cells at the edges of the
scratch were monitored. The percentage wound closure was determined
using the following equation:
(A0-At)/A0 ×100, where
A0 is the wound area at the h of the wound generation,
and At is the wound area at the h of observation. The
experiments were repeated at least 3 times.
Transwell assay
For the cell invasion assay, 24-well Transwell
plates (Corning, Inc.) with 8-µm pore inserts coated with Matrigel
(BD Biosciences) were used. Plates had been precoated with Matrigel
at 37°C for 30 min. Cells (~2×104) were seeded into the
Matrigel matrix-coated (Corning, Inc.) upper chambers with cell
culture fluid, and DMEM supplemented with 10% FBS was added to the
lower chambers. After 24 h, the Matrigel and cells were removed
using a cotton-tipped swab. The filters were fixed in 4%
formaldehyde for 15 min at 25°C and stained with 0.1% crystal
violet solution for 30 min at room temperature. Cells in five
random fields were observed under a light contrast microscope
(magnification, ×100; BX51; Olympus Corporation). The experiments
were repeated at least 3 times.
Statistical analysis
Data are presented as the mean ± standard deviation.
SPSS 17.0 statistical software (SPSS, Inc.) was used for all
statistical analyses. Comparisons between groups were performed
using one-way analysis of variance followed by Tukey's post hoc
test. P<0.05 was considered to indicate a statistically
significant difference. All experiments were repeated at least 3
times.
Results
BBR inhibits the viability of
HESCs
A CCK-8 assay was performed to determine the effects
of various concentrations of BBR (20, 40, 60 and 80 µM) on the
survival rate of HESCs. It was found that treatment with 80 µM BBR
significantly inhibited the viability of the cells; thus, the
concentration of 80 µM BBR was selected for use in the following
experiments (Fig. 1A).
Subsequently, the HESCs were treated with 80 µM BBR for 24, 48 and
72 h. Compared with the control group, the cell survival rate
decreased following treatment with BBR (Fig. 1B). The colony formation assay then
showed that there was a notable decrease in cell proliferation
following BBR treatment compared with the control group (Fig. 1C). Edu staining was performed to
detect cell proliferation, and the results demonstrated that cell
proliferation in the BBR group notably decreased compared with the
control (Fig. 1D). In addition, the
expression levels of the proliferation-related proteins, Ki-67 and
PCNA, were also detected, and it was found that the expression
levels of Ki-67 and PCNA proteins significantly decreased following
BBR treatment compared with the control group (Fig. 1E and F).
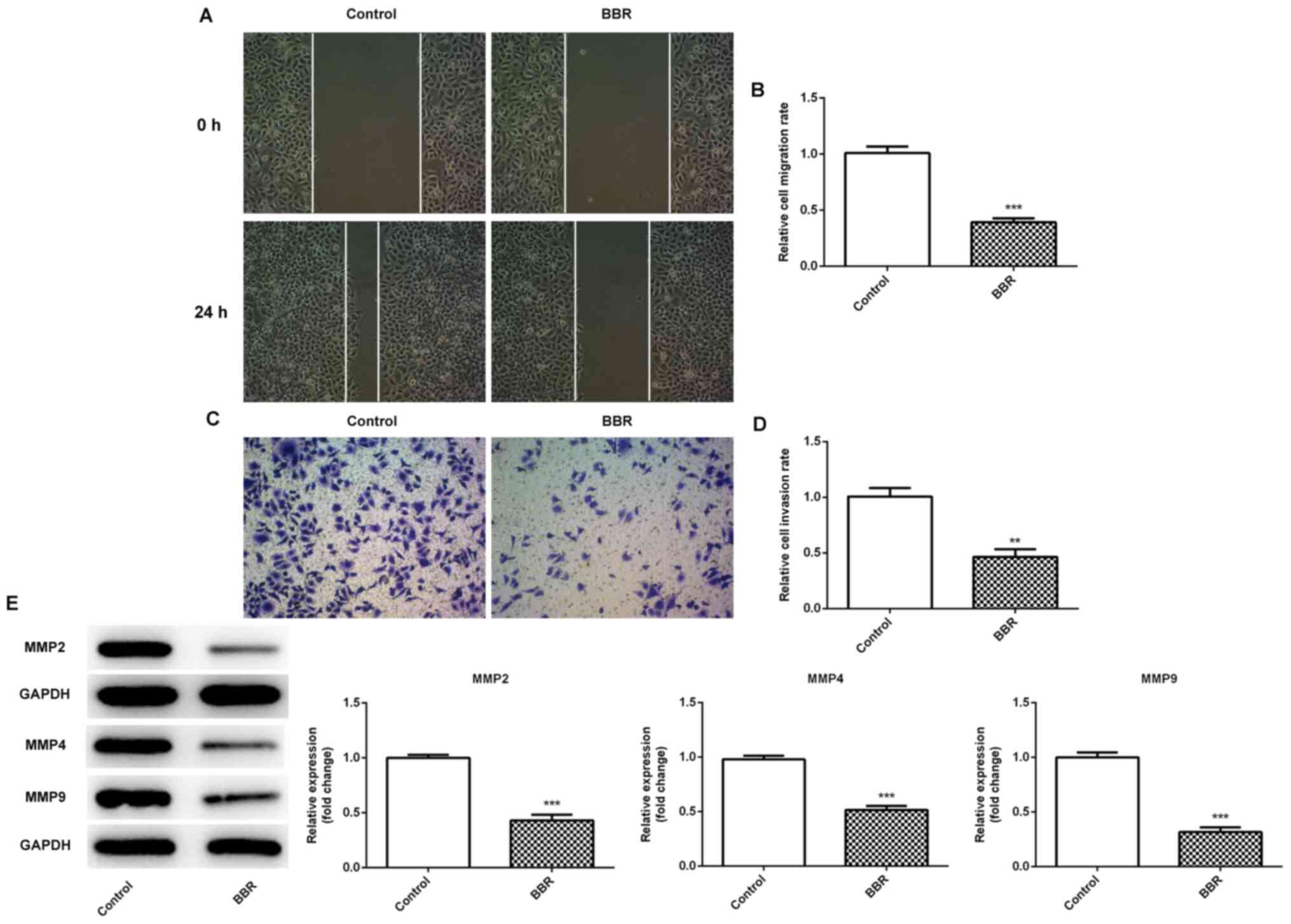
BBR inhibits the migration and
invasion of HESCs
Wound healing and Transwell assays revealed that the
cells treated with BBR exhibited a significant decrease in cell
migratory (Fig. 2A and B) and
invasive (Fig. 2C and D) activity
compared with the control group, which was also accompanied by a
decrease in the expression levels of the invasion- and
migration-related proteins, MMP2 and MMP9 (Fig. 2E). The experimental results revealed
that BBR significantly inhibited the migration and invasion of
endometrial stromal cells.
BBR inhibits the proliferation,
migration and invasion of HESCs by downregulating miR-429
During the experiment, it was found that the
expression of miR-429 in the BBR-treated cells significantly
decreased compared with the control group (Fig. 3A). Therefore, it was hypothesized
that miR-429 may be a target of BBR in the treatment of EM. To
provide evidence for this hypothesis, miR-429 was overexpressed
using transfection of miR-429 mimics, and the transfection
efficiency was detected by RT-qPCR (Fig. 3B). The results of the CCK-8 and
colony formation assay revealed that compared with the BBR +
mimic-NC group, the overexpression of miR-429 in the BBR + miR-429
mimic group reversed the inhibitory effects of BBR on the survival
rate and proliferation of HESCs (Fig.
4A and B). The results of the EdU staining showed that the
overexpression of miR-429 reversed the inhibitory effect of BBR on
the proliferation of HESCs compared with BBR + mimic-NC (Fig. 4C). The protein expression levels of
Ki-67 and PCNA in the BBR + miR-429 mimic group were also
significantly increased compared with the BBR + mimic-NC group
(Fig. 4D). The results of RT-qPCR
(Fig. 4E) were consistent with
those obtained for western blotting in Fig. 4D.
Wound healing and Transwell assays revealed that the
overexpression of miR-429 in the BBR + miR-429 mimic group reversed
the inhibitory effects of BBR on the migration (Fig. 5A and B) and invasion (Fig. 5C and D) of HESCs, which was
accompanied by an increase in Ki-67 and PCNA protein expression
(Fig. 5E). These findings indicated
that BBR inhibited the proliferation, invasion and migration of
HESCs by downregulating miR-429.
Discussion
Although EM is not a malignant type of tumor, its
pathological process, which is characterized by the infiltration,
migration and invasion of uterine cells, is similar to that of
cancer (18,19). Therefore, regulating the
proliferation, migration and invasion of uterine cells may be an
important breakthrough in the treatment of EM.
BBR has a wide range of pharmacological activities,
among which BBR exerts antitumor effects. Wang and Zhang (10) found that BBR suppressed the growth
and metastasis of endometrial cancer cells via miR-101/COX-2. BBR
and metformin have been revealed to prevent endometrial cancer cell
migration and invasion by inhibiting the expression of
lipolysis-stimulated lipoprotein receptor (20). Moreover, there are numerous links
between endometrial cancer and EM (21–23).
However, whether BBR exerts a therapeutic effect in EM was not
reported in these previous studies. In the present study, the
concentration of BBR used was 80 µM and the incubation time was 24
h. At present, the effect of this concentration has not been
studied clinically, so the dose response experiment and further
in vivo experiments need to be explored by our laboratory in
future studies. It was found that BBR inhibited the proliferation,
invasion and migration of HESCs.
It would be of interest to determine the underlying
mechanisms of BBR in the regulation of proliferation, invasion and
migration of HESCs. The multiple pharmacological actions of BBR are
the result of different molecular targets of this phytochemical.
Certain studies have reported that BBR plays a therapeutic role in
cancer by regulating the expression of miRNAs (24–26).
In the present study, it was found that the expression of miR-429
decreased significantly following treatment of the cells with BBR.
miR-429 has been reported to promote the progression of a variety
of cancers. miR-429 has been demonstrated to competitively combine
with long non-coding RNA SNHG22 to increase zinc finger
E-box-binding homeobox 1 expression and promote the malignant
development of thyroid papillary carcinoma (27). Increased proliferation and migration
following the overexpression of miR-429 was previously observed in
breast cancer cells, whereas the silencing of miR-429 attenuated
tumor growth (28). Therefore, in
the present study it was hypothesized that miR-429 plays a role in
promoting cell proliferation, migration and invasion in EM.
Following the overexpression of miR-429, it was found that the
proliferative, invasive and migratory ability of HESCs were
promoted, thus indicating that miR-429 successfully reversed the
inhibitory effects of BBR on cell proliferation, invasion and
migration. This indicated that BBR inhibited cell proliferation,
migration and invasion by downregulating miR-429.
However, the present study only verified that BBR
could inhibit the proliferation, invasion and migration of HESCs by
regulating miR-429 at the cellular level. These findings were not
validated in vivo or clinically, so it cannot be determined
whether 80 µM BBR is clinically applicable. Therefore, this study
can only provide a theoretical basis for BBR in the clinical
treatment of EM.
In conclusion, the present study demonstrated that
BBR inhibited the proliferation, invasion and migration of HESCs by
downregulating miR-429. The findings of the present study may
provide the basis for the drug treatment of EM.
Acknowledgements
Not applicable.
Funding
Scientific and technological research project of
education department of Jiangxi province (grant no. GJJ190640).
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YG wrote the manuscript and analyzed the data. ZZ
carried out the experiments, supervised the present study, searched
the literature and revised the manuscript. YG and ZZ confirm the
authenticity of all the raw data. Both authors read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patients consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gordts S, Koninckx P and Brosens I:
Pathogenesis of deep endometriosis. Fertil Steril. 108:872–885.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Czyzyk A, Podfigurna A, Szeliga A and
Meczekalski B: Update on endometriosis pathogenesis. Minerva
Ginecol. 69:447–461. 2017.PubMed/NCBI
|
|
3
|
Li J, Ma J, Fei X, Zhang T, Zhou J and Lin
J: Roles of cell migration and invasion mediated by twist in
endometriosis. J Obstet Gynaecol Res. 45:1488–1496. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kodaman PH: Current strategies for
endometriosis management. Obstet Gynecol Clin North Am. 42:87–101.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Grimstad FW and Carey E: Periclitoral
endometriosis: The dilemma of a chronic disease invading a rare
location. J Minim Invasive Gynecol. 22:684–686. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jin Y, Khadka DB and Cho WJ:
Pharmacological effects of berberine and its derivatives: A patent
update. Expert Opin Ther Pat. 26:229–243. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu L, Fan J, Ai G, Liu J, Luo N, Li C and
Cheng Z: Berberine in combination with cisplatin induces
necroptosis and apoptosis in ovarian cancer cells. Biol Res.
52:372019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Du J, Sun Y, Lu YY, Lau E, Zhao M, Zhou QM
and Su SB: Berberine and evodiamine act synergistically against
human breast cancer MCF-7 cells by inducing cell cycle arrest and
apoptosis. Anticancer Res. 37:6141–6151. 2017.PubMed/NCBI
|
|
9
|
Abrams SL, Follo MY, Steelman LS,
Lertpiriyapong K, Cocco L, Ratti S, Martelli AM, Candido S, Libra
M, Murata RM, et al: Abilities of berberine and chemically modified
berberines to inhibit proliferation of pancreatic cancer cells. Adv
Biol Regul. 71:172–182. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang Y and Zhang S: Berberine suppresses
growth and metastasis of endometrial cancer cells via
miR-101/COX-2. Biomed Pharmacother. 103:1287–1293. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jin P, Zhang C and Li N: Berberine
exhibits antitumor effects in human ovarian cancer cells.
Anticancer Agents Med Chem. 15:511–516. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu G, Zheng H, Xu J, Guo Y, Zheng G, Ma C,
Hao S, Liu X, Chen H, Wei S, et al: miR-429 suppresses cell growth
and induces apoptosis of human thyroid cancer cell by targeting
ZEB1. Artif Cells Nanomed Biotechnol. 47:548–554. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dai W, He J, Zheng L, Bi M, Hu F, Chen M,
Niu H, Yang J, Luo Y, Tang W and Sheng M: miR-148b-3p, miR-190b,
and miR-429 regulate cell progression and act as potential
biomarkers for breast cancer. J Breast Cancer. 22:219–236. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Han Y, Zhao Q, Zhou J and Shi R: miR-429
mediates tumor growth and metastasis in colorectal cancer. Am J
Cancer Res. 7:218–233. 2017.PubMed/NCBI
|
|
15
|
Liu H, Huang C, Wu L and Wen B: Effect of
evodiamine and berberine on miR-429 as an oncogene in human
colorectal cancer. Onco Targets Ther. 9:4121–4127. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Braicu OL, Budisan L, Buiga R, Jurj A,
Achimas-Cadariu P, Pop LA, Braicu C, Irimie A and Berindan-Neagoe
I: miRNA expression profiling in formalin-fixed paraffin-embedded
endometriosis and ovarian cancer samples. Onco Targets Ther.
10:4225–4238. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Meng X, Liu J, Wang H, Chen P and Wang D:
MicroRNA-126-5p downregulates BCAR3 expression to promote cell
migration and invasion in endometriosis. Mol Cell Endocrinol.
494:1104862019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Garcia-Solares J, Dolmans MM, Squifflet
JL, Donnez J and Donnez O: Invasion of human deep nodular
endometriotic lesions is associated with collective cell migration
and nerve development. Fertil Steril. 110:1318–1327. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shimada H, Satohisa S, Kohno T, Takahashi
S, Hatakeyama T, Konno T, Tsujiwaki M, Saito T and Kojima T: The
roles of tricellular tight junction protein lipolysis-stimulated
lipoprotein receptor in malignancy of human endometrial cancer
cells. Oncotarget. 7:27735–27752. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim JJ, Kurita T and Bulun SE:
Progesterone action in endometrial cancer, endometriosis, uterine
fibroids, and breast cancer. Endocr Rev. 34:130–162. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen JJ, Xiao ZJ, Meng X, Wang Y, Yu MK,
Huang WQ, Sun X, Chen H, Duan YG, Jiang X, et al: MRP4 sustains
Wnt/β-catenin signaling for pregnancy, endometriosis and
endometrial cancer. Theranostics. 9:5049–5064. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kajiyama H, Suzuki S, Yoshihara M,
Tamauchi S, Yoshikawa N, Niimi K, Shibata K and Kikkawa F:
Endometriosis and cancer. Free Radic Biol Med. 133:186–192. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
McCubrey JA, Lertpiriyapong K, Steelman
LS, Abrams SL, Yang LV, Murata RM, Rosalen PL, Scalisi A, Neri LM,
Cocco L, et al: Effects of resveratrol, curcumin, berberine and
other nutraceuticals on aging, cancer development, cancer stem
cells and microRNAs. Aging (Albany NY). 9:1477–1536. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dai W, Mu L, Cui Y, Li Y, Chen P, Xie H
and Wang X: Berberine promotes apoptosis of colorectal cancer via
regulation of the long non-coding RNA (lncRNA) cancer
susceptibility candidate 2 (CASC2)/AU-binding factor 1
(AUF1)/B-cell CLL/lymphoma 2 (Bcl-2) axis. Med Sci Monit.
25:730–738. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lo SN, Wang CW, Chen YS, Huang CC, Wu TS,
Li LA, Lee IJ and Ueng YF: Berberine activates aryl hydrocarbon
receptor but suppresses CYP1A1 induction through miR-21-3p
stimulation in MCF-7 breast cancer cells. Molecules. 22:18472017.
View Article : Google Scholar
|
|
27
|
Gao H, Sun X, Wang H and Zheng Y: Long
noncoding RNA SNHG22 increases ZEB1 expression via competitive
binding with microRNA-429 to promote the malignant development of
papillary thyroid cancer. Cell Cycle. 19:1186–1199. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cava C, Novello C, Martelli C, Lodico A,
Ottobrini L, Piccotti F, Truffi M, Corsi F, Bertoli G and
Castiglioni I: Theranostic application of miR-429 in HER2+ breast
cancer. Theranostics. 10:50–61. 2020. View Article : Google Scholar : PubMed/NCBI
|