Introduction
The annual incidence of liver cancer increased by
75% between 1990 and 2015 worldwide (1). Hepatocellular carcinoma (HCC) comprise
~75–85% of liver cancers (2), which
constitute a huge health burden, especially in Asia where 72% of
cases occur (3). Surgical resection
is one of the main treatment methods for HCC; however, it cannot
prevent postoperative recurrence (4). The HCC recurrence rate at 2 years
post-surgery is 61.6% according to the statistics provided by the
World Health Organization (2018) (2). At present, the metastasis of liver
cancer cells cannot be prevented by surgical resection, and there
are a lack of effective drugs for the prevention and treatment of
metastasis (5).
The epithelial-mesenchymal transition (EMT) induces
cancer metastasis by promoting the separation and invasion of
cancer cells from homologous cells (6). E-cadherin has been reported to
maintain the tight connection between cells and prevent cell
invasion and metastasis, and the decrease in the levels of
E-cadherin is a common EMT biomarker (7). The upregulation of Vimentin and the
transcription factor Snail are also commonly used EMT biomarkers
(8,9). Recent studies have emphasized the
vital role of EMT in the aggressive progression of HCC (10–13),
in this regard further efforts for identification of EMT regulatory
mechanisms will facilitate the development of novel strategies to
combat metastasis. Chemokines and chemokine receptors serve key
roles in the invasion and metastasis of malignant tumours (14–16).
CC-chemokine receptor 10 (CCR10) has been demonstrated to be
significantly upregulated in HCC specimens compared with the normal
liver specimens (10). Furthermore,
PI3K/Akt/GSK-3β has been demonstrated to be a classical signalling
pathway via which CCR10 activates phosphorylation of Akt (16).
Plasmodium is a genus of single-cell protozoa
that causes malaria, which may enter hepatocytes for dormancy or
reproduction and lead to cell rupture, release merozoites and
invade red blood cells (17).
Although Plasmodium stays in the liver and causes black
colouring of the tissue due to the deposition of the
Plasmodium pigment, it does not damage liver function
(18). Our previous studies have
demonstrated that Plasmodium infection inhibits tumour
development and metastasis in a murine Lewis lung cancer model
(19–24). However, although Plasmodium
infection has been demonstrated to suppress HCC angiogenesis by
decreasing the infiltration of tumour-associated macrophages and
reducing their matrix metalloproteinase 9 levels (25), the effects of Plasmodium
infection on HCC metastasis and recurrence remain unclear.
To gain further insight into the effects of
Plasmodium infection on HCC metastasis and recurrence, the
antitumor effects of Plasmodium infection using two murine
orthotopic models were studied, with an emphasis on the regulation
of Plasmodium infection on EMT of HCC. Here, it was
demonstrated that Plasmodium infection prevented recurrence
and metastasis of HCC potentially via CCR10-mediated
PI3K/Akt/GSK-3β/Snail signalling.
Materials and methods
Ethics statement
Ethical approval was provided by Guangzhou Institute
of Biomedicine and Health, Chinese Academy of Sciences (approval
no. N2019014; Guangzhou, China). All animal experiments in this
study were performed following the standard guidelines for the care
of animals, which were approved by the Welfare Committee of the
Center of Experimental Animals. According to the international
regulations, the mice were euthanised when the tumour diameter
reached 2 cm. All efforts were made to minimise animal
suffering.
Antibodies
Western blotting was performed using the following
primary antibodies: GAPDH (cat. no. ab9385; Abcam), E-cadherin
(cat. no. BS1098; Bioworld Technology, Inc.), Snail (cat. no.
3879S; Cell Signaling Technology, Inc.), PI3K (cat. no. ab151549;
Abcam), phosphorylated (p)-PI3K p85 (Tyr458)/p55 (Tyr199) (cat. no.
4228S), Akt (cat. no. 9272S), p-Akt (Ser473) (cat. no. 4060S),
GSK-3β (cat. no. 12456S), p-GSK-3β (Ser9(cat. no. 5558S) (all Cell
Signaling Technology, Inc.) and CCR10 (cat. no. ab3904; Abcam).
Animals, cells and parasites
Female C57BL/6 mice (age, 6–8 weeks) purchased from
Beijing Vital River Laboratory Animal Technology Co., Ltd. were
bred in the Animal Center of Guangzhou Institute of Biomedicine and
Health (GIBH; Guangzhou, China) according to the Guide to the Care
and Use of Laboratory Animal Committee of the Institute. Mice were
housed under specific pathogen-free conditions with a 12-h
light/dark cycle and were provided food and water ad
libitum. The Hepa1-6 luciferase liver cancer cell line derived
from C57BL/6 mice was obtained from The First Affiliated Hospital
of Sun Yat-Sen University and cultured in Dulbecco's modified
Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% foetal bovine serum (FBS; Gibco; Thermo
Fisher Scientific, Inc.), and was incubated in a humidified
atmosphere with 5% CO2 at 37°C. The Plasmodium
yoelii nonlethal strain (Py17XNL) was obtained from the Malaria
Research and Reference Reagent Resource Center (MR4).
Animal models
Mice undergoing surgery were anaesthetised by
intraperitoneal injection of 240 mg/kg 1.25% Avertin. Two animal
models were established. The tumour volumes were calculated as
follows: Volume=(length × width2)/2. Mice were
euthanized by exsanguination under anaesthesia with 240 mg/kg
Avertin.
Non-resection model
The non-resection mouse model was established by
intrahepatic injection of 5×105 Hepa1-6 luciferase cells
into the left liver lobe. Female C57BL/6 mice (n=56) were randomly
divided into two groups (n=28 mice/group). The control group (Hep)
received 5×105 Hepa1-6 luciferase cells only. The mice
in the Plasmodium-infected group (Hep + Py) were
orthotopically implanted with 5×105 Hepa1-6 luciferase
cells and intraperitoneally injected with 5×105
Plasmodium yoelii 17XNL-parasitised erythrocytes.
Subsequently, 26 mice (n=13 mice/group) were randomly selected for
observation of survival, dynamics of parasitaemia and weight
changes. The tumours in the remaining 30 mice (n=15 mice/group)
were harvested on day 15 post-inoculation for further
experiments.
Resection model
To investigate the effects of Plasmodium
infection on postoperative recurrence, the resection mouse model
was established by an intrahepatic injection of tumour pieces and
tumour resection on day 21 post-implantation. Tumours were derived
from subcutaneous tumour-bearing mice, which had been
subcutaneously injected with 5×105 Hepa1-6 luciferase
cells. The tumours were harvested and cut into 1-mm3
pieces on day 14 post-inoculation. The tumour pieces were implanted
into the livers (left liver lobe) of 20 mice, and in vivo
luciferase imaging was used to monitor the survival of the
transplanted tumours once a week in the following days. The mice
underwent resection on day 21 post-inoculation to completely remove
the transplanted tumour mass and were randomly divided into two
groups according to the inoculation with or without
Plasmodium on day 3 post-surgery. The primary outcomes were
death and intraperitoneal metastasis caused by HCC in both
groups.
In vivo luciferase imaging
To evaluate tumour survival, the luminescence
properties of luciferase were used to track HCC in vivo.
Each mouse was intraperitoneally injected with 10 µl/g potassium
fluorescein as a substrate. The IVIS Spectrum (PerkinElmer, Inc.)
in vivo imaging system was used to monitor the fluorescence
emitted by Hepa1-6 luciferase at 10–20 min post-injection.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from tumour tissues using
TRIzol® reagent (cat. No. 15596018; Invitrogen; Thermo
Fisher Scientific, Inc.) and reverse-transcribed (37°C for 15 min,
85°C for 5 sec and 4°C hold) using a cDNA Synthesis kit (cat. No.
RR047A; Takara Bio, Inc.). qPCR was performed using TB Green Premix
Ex Taq (cat. no. RR820A; Takara Bio, Inc.) with CFX96 Touch PCR
machine (Bio-Rad Laboratories, Inc.). The qPCR procedures were as
follows: Predenaturation at 95°C for 30 sec; denaturation at 95°C
for 5 sec, annealing and extension at 60°C for 30 sec (39 cycles);
denaturation at 95°C for 10 sec; and finally melt curve at 65–95°C
for 5 sec. The mRNA levels of the genes of interest were normalised
to those of β-actin. The primer sequences used were as follows:
β-Actin forward, 5′-TCTGGCACCACACCTTCTAC-3′ and reverse,
5′-TCATCTTTTCACGGTTGGCCT-3′; Vimentin forward,
5′-CTGCGAGAGAAATTGCAGGAG-3′ and reverse,
5′-CTTTCATACTGCTGGCGCAC-3′; CCR10 forward,
5′-CAAGCCCACAGAGCAGGTCTC-3′ and reverse,
5′-GATCGGGTAGTTCGTCTGGC-3′. The 2−ΔΔCq method was used
to quantify the relative changes in gene expression (26). Each sample was tested in at least
three independent replicates.
Protein extraction and western
blotting
Tissues were lysed in RIPA lysis buffer (cat. no.
KGP702-100; Nanjing KeyGen Biotech Co., Ltd.) containing 1%
phenylmethanesulphonyl fluoride (Beyotime Institute of
Biotechnology) and phosphatase inhibitor cocktail (cat. no.
HY-K0021; MedChem Express) on ice for 30 min and subsequently
centrifuged at 16,000 × g for 15 min at 4°C to collect the
supernatants. After protein determination using a BCA assay,
protein samples containing 15–20 µg protein were separated using
10% polyacrylamide gel electrophoresis (SurePAGE; cat. no. M00665;
GenScript) and transferred to polyvinylidene difluoride membranes
(cat. no. ISEQ00010; EMD Millipore). The membrane was probed with
anti-E-cadherin (1:500), anti-Snail1 (1:1,000), anti-p-PI3K
(1:1,000), anti-PI3K (1:1,000), anti-p-AktSer473
(1:500), anti-Akt (1:300), anti-p-GSK3βSer9 (1:500),
anti-GSK3β (1:500) and anti-β-actin (1:2,000) antibodies at room
temperature for 2 h. Then, membranes were washed three times, 5 min
each, with Tris-buffered saline with 0.1% Tween-20 (TBST). After
incubation with HRP-conjugated Goat anti-Mouse IgG (cat. no.
ab205719; Abcam) or HRP-conjugated Goat anti-Rabbit IgG (cat. no.
ab205718; Abcam) at room temperature for 1 h (1:5,000), membranes
were washed four times, 5 min each, with TBST. Then, the bands were
detected using ECL reagents (cat. no. WBULS0500; EMD Millipore).
Densitometry of band signals was quantified using Quantity One
software (version 4.6.6; Bio-Rad Laboratories, Inc.). Data are
presented as the ratio of the target protein to GAPDH densitometry
values.
Giemsa stain
Giemsa stain was performed for determination of the
ratio of Plasmodium-infected RBC in the blood. A small drop
of mouse tail blood was collected on a glass slide to make a thin
layer of blood smear. Then, using a Pasteur pipette, the thin film
was fixed by carefully dropping methanol onto the thin film. The
slides were placed on a drying rack on a flat surface for ~2 min to
let the blood film dry completely. Then, Giemsa working solution
(cat. no. 48900; Sigma-Aldrich; Merck KGaA) was poured slowly on
the slide until the blood films were covered. After 40 min of
staining, the stain was washed from the slides and then the slides
were allowed to air-dry. Finally, the ratio of parasitaemia was
calculated using the following formula: Parasitaemia (%)=(the
number of Plasmodium-infected RBC per 1,000 RBC/1,000)
×100%.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 8.0 (GraphPad Software, Inc.) and the R software (version
3.6.3; http://www.R-project.org) (27). Data of normally distributed
variables are presented as the mean ± standard deviation.
Categorical data are presented as frequency and percentages. An
independent sample Student's t-test was used to compare the means
of two groups. The Fisher's exact test was used to compare the rate
of metastasis between two groups. Overall survival (OS) in the two
groups was estimated using the Kaplan-Meier method, and the OS
differences between the two groups were compared using the log-rank
test. P<0.05 was considered to indicate a statistically
significant difference. All of above experiments were repeated at
least twice and the results were similar.
Results
Plasmodium infection significantly
inhibits HCC progression and metastasis in a non-resection mouse
model
In this experiment, luciferase imaging results
revealed that Hepa1-6 cells were successfully implanted into all
mice, and the fluorescence signal was significantly lower in Hep +
Py group compared with that in the Hep group on day 15-post
inoculation (Fig. 1A). The peak of
infection appeared on ~day 18 post-intraperitoneal injection of the
parasite in both models; the highest Plasmodium density
reached on average 55–60% and decreased to ~5% on day 24 (Figs. S1A and B and S2). There was no significant difference
in body weight between the two groups during the course of
Plasmodium infection (in the treated group), whereas the
weight was not comparable between the two groups thereafter since
the majority of the mice in the control group died (Fig. 1B). Notably, the tumour-bearing mice
treated with Plasmodium exhibited a higher survival rate
(53.8%, 7/13) and a longer OS time (until to the end of the study)
compared with that observed in the control group (median interval,
21 days) (Fig. 1C). The tumour
volume on day 15 post-inoculation in the Hep + Py group (0.23±0.05
cm3) was significantly lower compared with that in the
Hep group (1.85±0.7 cm3) (Fig. 1E and F). The tumour weight on day 15
post-inoculation in the Hep + Py group was also significantly lower
compared with that in the Hep group (Fig. 1G and H). In addition, only 2/15 of
mice were observed to have tumour metastasis in the intraperitoneal
organs of the Hep + Py group, whereas severe intestinal and
abdominal metastasis were present in 14/15 of mice in the Hep group
(Fig. 1D).
Plasmodium infection suppresses HCC
recurrence in a resection mouse model
The resection mouse model was established by
intrahepatic implantation of tumour pieces, followed by complete
tumour resection on day 21 post-implantation (Fig. 2A). In the early stage, no
significant differences were observed in the body weight between
the mice in the two groups. Following tumour resection, weight loss
and short-term weight recovery occurred in the Hep + Py group.
During the period of infection, the food intake of mice in the
Plasmodium infection group was poor; thus, the weight gain
in the Plasmodium infection group was slower compared with
that in the control group. Following self-healing from infection,
the weight in the Plasmodium infection group continued to
increase, whereas that of the control group decreased due to tumour
development (Fig. 2B). The effect
of surgical resection followed by Plasmodium infection on
weight loss was notable when comparing the infected group in the
resection model with the infected group in the non-resection model
(Figs. 1B and 2B). A phenomenon of hemozoin accumulation
(dark colour) (28) was observed in
the liver in the Plasmodium-infected group in the resection
(Fig. 2D) and the non-resection
(Fig. 1E) models. Notably, the
results demonstrated that the Plasmodium-treated
tumour-bearing mice survived longer compared with the control mice
(Fig. 2C). The cumulative
recurrence rate in the Plasmodium infection group (20%,
2/10) was significantly lower compared with that in the control
group (80%, 8/10) on day 75 post-inoculation (Fig. 2D).
Plasmodium infection inhibits HCC
metastasis possibly through EMT suppression
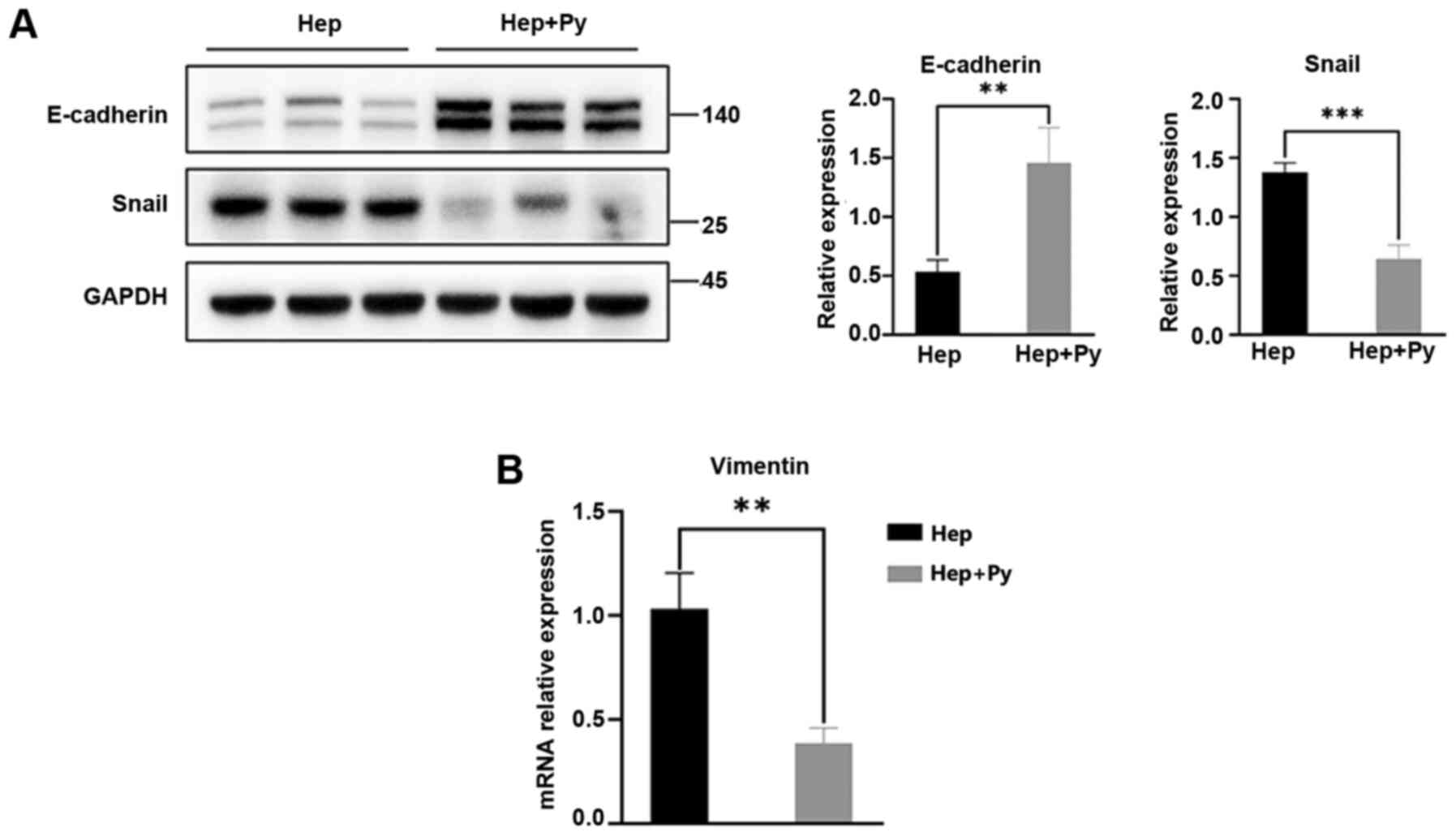
E-cadherin is the key cadherin serves an
antimetastatic role and is the most important EMT biomarker
(6). In the present study, the
expression levels of E-cadherin in the Hep + Py group were
significantly higher compared with those in the Hep group (Fig. 3A). As one of the biomolecules that
binds to the E-boxes in the E-cadherin promoter, Snail directly
downregulates the expression of E-cadherin to promote the
occurrence of EMT (29). The
results of the present study demonstrated that the expression
levels of Snail were significantly lower in the Hep + Py group
compared with those in the Hep group (Fig. 3A). The RT-qPCR results showed that
Vimentin expression, one of the molecular markers of EMT, was
significantly decreased in the Hep + Py group compared with in the
Hep group (Fig. 3B).
Plasmodium infection suppresses the
activation of the PI3K/Akt/GSK-3β signalling pathway
Previous studies have reported that the
PI3K/Akt/GSK-3β signalling pathway and the transcriptional
repressor Snail are required for EMT in HCC (14,16).
Therefore, the effects of Plasmodium infection on the
PI3K/Akt/GSK-3β signalling pathway was tested in HCC cells. Western
blot analysis demonstrated that the phosphorylation of Akt (Ser473)
(Fig. 4A and C) and GSK-3β (Ser9)
(Fig. 4A and D) was downregulated
in the Plasmodium-treated group compared with that in the
control group, although the difference in PI3K phosphorylation
levels between the two groups was not statistically significant
(Fig. 4B).
Plasmodium infection downregulates the
expression of CCR10
The chemokine/chemokine receptor axis has been
demonstrated to activate the PI3K/Akt/GSK-3β/Snail signalling
pathway in HCC cells (14,16). As expected, the mRNA expression
levels of CCR10 were significantly lower in the
Plasmodium-treated group compared with those in the control
group (Fig. 5B). Western blotting
results revealed that the protein expression levels of CCR10 were
also significantly lower in the infected group compared with those
in the control group (Fig. 5A).
Discussion
Primary HCC accounts for 75–85% of liver cancer
(8.2% of mortality rates in 2018), which is the fourth most common
cause of cancer-related death worldwide (2,30).
Recurrence is the leading cause of postoperative death in patients
with liver cancer (31). The
results of the present study demonstrated that Plasmodium
infection significantly inhibited tumour growth and abdominal
metastasis in two mouse models, including a murine HCC resection
model. The underlying mechanisms of action may involve the
inhibition of the PI3K/Akt/GSK-3β/Snail signalling pathway by
downregulating the expression levels of CCR10, reducing the
accumulation of Snail and upregulating the levels of
E-cadherin.
The EMT has been implicated in carcinoma invasion
and metastasis (32). Previous
studies have suggested that the downregulation of E-cadherin is a
prominent feature of the EMT. For example, the transcriptional
activity and protein expression of E-cadherin has been reported to
be enhanced by preventing the binding of the E-boxes of the
E-cadherin promoter to transcriptional barriers, including
zinc-finger transcriptional repressors snail and slug, the
repressor zinc-finger E-box-binding homeobox (ZEB)-2, ZEB-1, as
well as the basic helix-loop-helix, transcription factors twist and
E12/E47, which prevent the EMT (32,33).
The results of the current study demonstrated that
Plasmodium infection increased the expression levels of
E-cadherin and downregulated the levels of Snail and Vimentin
compared with those in the control group, which suggested that
Plasmodium infection may prevent the EMT in HCC.
PI3K activates the secondary messenger PIP3, which
activates Akt via phosphorylation at Ser308 (34). GSK-3β is a downstream target of
p-Akt (35), which promotes the
degradation of Snail (36), whereas
the phosphorylation of GSK-3β at Ser9 by PI3K/Akt leads to its
inactivation (37) and upregulation
of Snail. Snail, which downregulates E-cadherin, is downstream to
GSK-3β; GSK-3β inhibits the expression of Snail and increases the
expression levels of E-cadherin (38). The results of the current study
demonstrated that Plasmodium infection inhibited the
phosphorylation of Akt, which subsequently inhibited the conversion
of GSK-3β to p-GSK-3β and reduced the expression levels of Snail.
Therefore, the inhibitory effects of Snail on E-cadherin expression
were eliminated, and the EMT was prevented.
Chemokines exert their action through seven
trans-membrane spanning G-protein-linked chemokine receptors
(39,40). Previous studies have suggested that
chemokines and chemokine receptors are involved in inflammatory
reactions and wound healing (41–43). A
recent study has demonstrated that chemokines and their receptors
serve a crucial role in tumour cell growth and metastasis in
melanoma, lung cancer, gastric carcinoma, pancreatic cancer,
colorectal carcinoma and HCC (44).
CCR10 activation has been reported to stimulate the invasion and
migration of HCC, breast cancer and melanoma cells (16,45,46).
CCR10 and the downstream PI3K/Akt signalling pathway serve a role
in HCC progression (16). The
results of the present study demonstrated that Plasmodium
infection downregulated the expression levels of CCR10 compared
with those in the control group. The inhibition of CCR10-mediated
PI3K/Akt/GSK-3β/Snail signalling may result in the suppression of
the EMT programming in HCC. However, one limitation of the present
study was that experiments were only repeated twice.
In conclusion, the results of the current study
identified a potential novel mechanism of Plasmodium
infection against HCC in mice (Fig.
6), providing evidence for the prevention of recurrence and
distant metastasis of HCC by regulating the EMT through infection
with Plasmodium parasites. In addition, our previous studies
have demonstrated that Plasmodium infection displays
antitumor effects by activating the innate and acquired antitumor
immunity, remodelling the tumour immunosuppressive microenvironment
and inhibiting angiogenesis (20–25).
Based on these studies, three clinical trials are ongoing in China
(trial nos. NCT02786589, NCT03474822 and NCT04165590). These
findings may be important for developing novel strategies for the
treatment and prevention of metastasis and recurrence of HCC,
especially for patients in the perioperative period.
 | Figure 6.Potential mechanism of
Plasmodium infection against recurrence and metastasis of
HCC in mice. Plasmodium infection induces: i) The
downregulation of CCR10 and p-Akt, but not PI3K; ii) the
downregulation of p-GSK-3β, but not GSK-3β; iii) the downregulation
of Snail, but not p-Snail; and iv) the upregulation of E-cadherin.
Notably, the downregulation of Snail and the upregulation of
E-cadherin may contribute to the suppression of the EMT in murine
HCC resection and non-resection models. HCC, hepatocellular
carcinoma; CCR10, CC-chemokine receptor 10; Py, Plasmodium
yoelii 17XNL; p-, phosphorylated; EMT, epithelial-mesenchymal
transition; CCL, C-C motif chemokine ligand. |
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Dr Zhenhui Li (The
Third Affiliated Hospital of Kunming Medical University, Yunnan
Cancer Hospital, Yunnan Cancer Center, Kunming, China) and Dr Wei
Guo (Guangzhou University of Chinese Medicine, Guangzhou, China)
for their assistance in the animal experiments.
Funding
The present study was supported by the Key Regional
Project of Science and Technology Service Network Program of The
Chinese Academy of Sciences (grant no. KFJ-STS-QYZX-042), the
Science and Technology Program of Guangzhou, China (grant no.
201707010447), The National Natural Science Foundation of China
(grant no. 81673003), the Clinical Research Program of High Level
University of Guangzhou Medical University 2017, the State Key
Laboratory of Respiratory Diseases (grant no. SKLRD-OP-201802) and
the Applied Basic Research Projects of Yunnan Province, China
(grant no. 2019FE001-054).
Availability of data and materials
All data generated or analysed during this study are
included in this published article. The manuscript has been
published as a preprint.
Authors' contributions
XPC, XWZ, YL, XC and LQ conceived and designed the
study. YL, XC, MM and LQ completed in vitro culture
experiments and performed the in vivo operations. ZT, WTD
and LLD assisted in the in vivo operations and conducted the
staining experiments. YL, MM, DA and SF carried out the western
blotting and PCR assays. YL, MM, XC, STZ and XFL performed the data
analysis and interpretation. XFL, XWZ, XPC and LQ made substantial
contributions to the writing of the manuscript. All authors agreed
to be accountable for all aspects of the work. YL, XC, XPC and XWZ
confirmed the authenticity of all the raw data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The specific procedures used during the animal study
were approved by the Ethics Committee of The Animal Laboratory
Center of Guangzhou Institutes of Biomedicine and Health, Chinese
Academy of Sciences, and obtained the corresponding IACUC (approval
no. N2019014). All efforts were made to minimise animal
suffering.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
HCC
|
hepatocellular carcinoma
|
|
EMT
|
epithelial-mesenchymal transition
|
|
OS
|
overall survival
|
|
CCR10
|
CC-chemokine receptor 10
|
|
Py
|
Plasmodium yoelii 17XNL
|
References
|
1
|
Global Burden of Disease Liver Cancer
Collaboration, ; Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu
MA, Allen C, Al-Raddadi R, Alvis-Guzman N, Amoako Y, et al: The
burden of primary liver cancer and underlying etiologies from 1990
to 2015 at the global, regional, and national level: Results from
the global burden of disease study 2015. JAMA Oncol. 3:1683–1691.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sharma SA, Kowgier M, Hansen BE, Brouwer
WP, Maan R, Wong D, Shah H, Khalili K, Yim C, Heathcote EJ, et al:
Toronto HCC risk index: A validated scoring system to predict
10-year risk of HCC in patients with cirrhosis. J Hepatol. Aug
24–2017.(Online ahead of print).
|
|
5
|
Zhou L, Rui JA, Wang SB, Chen SG and Qu Q:
Risk factors of microvascular invasion, portal vein tumor
thrombosis and poor post-resectional survival in HBV-related
hepatocellular carcinoma. Hepatogastroenterology. 61:1696–1703.
2014.PubMed/NCBI
|
|
6
|
Giannelli G, Koudelkova P, Dituri F and
Mikulits W: Role of epithelial to mesenchymal transition in
hepatocellular carcinoma. J Hepatol. 65:798–808. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Y, Shi J, Chai K, Ying X and Zhou BP:
The role of snail in EMT and tumorigenesis. Curr Cancer Drug
Targets. 13:963–972. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kaufhold S and Bonavida B: Central role of
Snail1 in the regulation of EMT and resistance in cancer: A target
for therapeutic intervention. J Exp Clin Cancer Res. 33:622014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yuan K, Xie K, Lan T, Xu L, Chen X, Li X,
Liao M, Li J, Huang J, Zeng Y and Wu H: TXNDC12 promotes EMT and
metastasis of hepatocellular carcinoma cells via activation of
β-catenin. Cell Death Differ. 27:1355–1368. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ma M, Xu H, Liu G, Wu J, Li C, Wang X,
Zhang S, Xu H, Ju S, Cheng W, et al: Metabolism-induced tumor
activator 1 (MITA1), an energy stress-inducible long noncoding RNA,
promotes hepatocellular carcinoma metastasis. Hepatology.
70:215–230. 2019.PubMed/NCBI
|
|
12
|
Peng JM, Bera R, Chiou CY, Yu MC, Chen TC,
Chen CW, Wang TR, Chiang WL, Chai SP, Wei Y, et al: Actin
cytoskeleton remodeling drives epithelial-mesenchymal transition
for hepatoma invasion and metastasis in mice. Hepatology.
67:2226–2243. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wan S, Meyer AS, Weiler SME, Rupp C, Tóth
M, Sticht C, Singer S, Thomann S, Roessler S, Schorpp-Kistner M, et
al: Cytoplasmic localization of the cell polarity factor scribble
supports liver tumor formation and tumor cell invasiveness.
Hepatology. 67:1842–1856. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou SL, Zhou ZJ, Hu ZQ, Li X, Huang XW,
Wang Z, Fan J, Dai Z and Zhou J: CXCR2/CXCL5 axis contributes to
epithelial-mesenchymal transition of HCC cells through activating
PI3K/Akt/GSK-3β/Snail signaling. Cancer Lett. 358:124–135. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cui D, Zhao Y and Xu J: Activated
CXCL5-CXCR2 axis promotes the migration, invasion and EMT of
papillary thyroid carcinoma cells via modulation of β-catenin
pathway. Biochimie. 148:1–11. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu Q, Chen JX, Chen Y, Cai LL, Wang XZ,
Guo WH and Zheng JF: The chemokine receptor CCR10 promotes
inflammation-driven hepatocarcinogenesis via PI3K/Akt pathway
activation. Cell Death Dis. 9:2322018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tavares J, Formaglio P, Thiberge S,
Mordelet E, Van Rooijen N, Medvinsky A, Ménard R and Amino R: Role
of host cell traversal by the malaria sporozoite during liver
infection. J Exp Med. 210:905–915. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Odedra A, Webb L, Marquart L, Britton LJ,
Chalon S, Moehrle JJ, Anstey NM, William T, Grigg MJ, Lalloo DG, et
al: Liver function test abnormalities in experimental and clinical
Plasmodium vivax infection. Am J Trop Med Hyg.
103:1910–1917. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qin L, Chen C, Chen L, Xue R, Ou-Yang M,
Zhou C, Zhao S, He Z, Xia Y, He J, et al: Worldwide malaria
incidence and cancer mortality are inversely associated. Infect
Agent Cancer. 12:142017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Adah D, Yang Y, Liu Q, Gadidasu K, Tao Z,
Yu S, Dai L, Li X, Zhao S, Qin L, et al: Plasmodium
infection inhibits the expansion and activation of MDSCs and Tregs
in the tumor microenvironment in a murine Lewis lung cancer model.
Cell Commun Signal. 17:322019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shi X, Qin L, Liu G, Zhao S, Peng N and
Chen X: Dynamic balance of pSTAT1 and pSTAT3 in C57BL/6 mice
infected with lethal or nonlethal Plasmodium yoelii. Cell
Mol Immunol. 5:341–348. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen L, He Z, Qin L, Li Q, Shi X, Zhao S,
Chen L, Zhong N and Chen X: Antitumor effect of malaria parasite
infection in a murine Lewis lung cancer model through induction of
innate and adaptive immunity. PLoS One. 6:e244072011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu Q, Yang Y, Tan X, Tao Z, Adah D, Yu S,
Lu J, Zhao S, Qin L, Qin L and Chen X: Plasmodium parasite
as an effective hepatocellular carcinoma antigen glypican-3
delivery vector. Oncotarget. 8:24785–24796. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang Y, Liu Q, Lu J, Adah D, Yu S, Zhao S,
Yao Y, Qin L, Qin L and Chen X: Exosomes from
Plasmodium-infected hosts inhibit tumor angiogenesis in a
murine Lewis lung cancer model. Oncogenesis. 6:e3512017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang B, Li Q, Wang J, Zhao S, Nashun B,
Qin L and Chen X: Plasmodium infection inhibits tumor
angiogenesis through effects on tumor-associated macrophages in a
murine implanted hepatoma model. Cell Commun Signal. 18:1572020.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
R Core Team: R: A language and environment
for statistical computing. R Foundation for Statistical Computing;
Vienna: 2014, PubMed/NCBI
|
|
28
|
Levesque MA, Sullivan AD and Meshnick SR:
Splenic and hepatic hemozoin in mice after malaria parasite
clearance. J Parasitol. 85:570–573. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Barrallo-Gimeno A and Nieto MA: The Snail
genes as inducers of cell movement and survival: Implications in
development and cancer. Development. 132:3151–3161. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Villanueva A: Hepatocellular carcinoma. N
Engl J Med. 380:1450–1462. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Buonaguro L, Mauriello A, Cavalluzzo B,
Petrizzo A and Tagliamonte M: Immunotherapy in hepatocellular
carcinoma. Ann Hepatol. 18:291–297. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang J and Weinberg RA:
Epithelial-mesenchymal transition: At the crossroads of development
and tumor metastasis. Dev Cell. 14:818–829. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Eger A, Aigner K, Sonderegger S, Dampier
B, Oehler S, Schreiber M, Berx G, Cano A, Beug H and Foisner R:
DeltaEF1 is a transcriptional repressor of E-cadherin and regulates
epithelial plasticity in breast cancer cells. Oncogene.
24:2375–2385. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Elich M and Sauer K: Regulation of
hematopoietic cell development and function through
phosphoinositides. Front Immunol. 9:9312018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang C, Wei S, Sun WP, Teng K, Dai MM,
Wang FW, Chen JW, Ling H, Ma XD, Feng ZH, et al:
Super-enhancer-driven AJUBA is activated by TCF4 and involved in
epithelial-mesenchymal transition in the progression of
hepatocellular carcinoma. Theranostics. 10:9066–9082. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lee YJ and Han HJ: Troglitazone
ameliorates high glucose-induced EMT and dysfunction of SGLTs
through PI3K/Akt, GSK-3β, Snail1, and β-catenin in renal proximal
tubule cells. Am J Physiol Renal Physiol. 298:F1263–F1275. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liang Y, Jing Z, Deng H, Li Z, Zhuang Z,
Wang S and Wang Y: Soluble epoxide hydrolase inhibition ameliorates
proteinuria-induced epithelial-mesenchymal transition by regulating
the PI3K-Akt-GSK-3β signaling pathway. Biochem Biophys Res Commun.
463:70–75. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhou BP, Deng J, Xia W, Xu J, Li YM,
Gunduz M and Hung MC: Dual regulation of Snail by
GSK-3beta-mediated phosphorylation in control of
epithelial-mesenchymal transition. Nat Cell Biol. 6:931–940. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Amarandi RM, Hjortø GM, Rosenkilde MM and
Karlshøj S: Probing biased signaling in chemokine receptors.
Methods Enzymol. 570:155–186. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zweemer AJM, Toraskar J, Heitman LH and
Ijzerman AP: Bias in chemokine receptor signalling. Trends Immunol.
35:243–252. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Roh YS and Seki E: Chemokines and
chemokine receptors in the development of NAFLD. Adv Exp Med Biol.
1061:45–53. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ridiandries A, Tan JTM and Bursill CA: The
role of chemokines in wound healing. Int J Mol Sci. 19:32172018.
View Article : Google Scholar
|
|
43
|
Kieseier BC, Tani M, Mahad D, Oka N, Ho T,
Woodroofe N, Griffin JW, Toyka KV, Ransohoff RM and Hartung HP:
Chemokines and chemokine receptors in inflammatory demyelinating
neuropathies: A central role for IP-10. Brain. 125:823–834. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Marcuzzi E, Angioni R, Molon B and Calì B:
Chemokines and chemokine receptors: Orchestrating tumor
metastasization. Int J Mol Sci. 20:962018. View Article : Google Scholar
|
|
45
|
Lin HY, Sun SM, Lu XF, Chen PY, Chen CF,
Liang WQ and Peng CY: CCR10 activation stimulates the invasion and
migration of breast cancer cells through the ERK1/2/MMP-7 signaling
pathway. Int Immunopharmacol. 51:124–130. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Monteagudo C, Ramos D, Pellín-Carcelén A,
Gil R, Callaghan RC, Martín JM, Alonso V, Murgui A, Navarro L,
Calabuig S, et al: CCL27-CCR10 and CXCL12-CXCR4 chemokine
ligand-receptor mRNA expression ratio: New predictive factors of
tumor progression in cutaneous malignant melanoma. Clin Exp
Metastasis. 29:625–637. 2012. View Article : Google Scholar : PubMed/NCBI
|