Introduction
Hepatocellular carcinoma (HCC) is one of the
severest types of human cancer and is the third-leading cause of
cancer-related mortality worldwide (1). Although the treatment of HCC has
improved, the long-term prognosis of patients with HCC is still
poor, mainly due to the advanced stages of the disease at the time
of diagnosis and its high metastasis and recurrence rates (2). Therefore, potential drugs with fewer
side effects that could significantly inhibit the growth of
existing tumors and prevent cancer cell metastasis, invasion and
angiogenesis are required for treating HCC (3).
The epithelial-mesenchymal transition (EMT) is an
important mechanism for epithelial-derived tumor cells to become
malignant and acquire an invasive phenotype (4,5). It is
accompanied by the downregulation of the epithelial marker
E-cadherin and the upregulation of the mesenchymal markers vimentin
and N-cadherin (6,7). Previous studies have identified that
metastatic cancer cells induce EMT by modulating their cell shape
or adhesive properties, thereby affecting cell adhesion and
migration (8,9). Therefore, EMT serves an important role
in tumorigenesis, invasion and metastasis.
VEGF is a signal protein released by epithelial
cells and serves an important role in cell proliferation and
neovascularization in several cancers, including HCC (10). In addition to the well-known effects
of VEGF on angiogenesis, VEGF signaling serves an important role in
promoting the proliferation and inhibiting the apoptosis in tumor
cells (11). In HCC, multivariate
analyses suggests that only a strong VEGF expression in tissue is
significantly associated with metastatic recurrence (12). VEGF can stimulate the activation of
the VEGFR2-dependent mTOR pathway to promote angiogenesis in lung
cancer cells (13). Furthermore,
there is evidence that the proliferation, migration, invasion and
adhesion of non-small cell lung cancer (NSCLC) cells are
significantly inhibited by blocking the VEGF/VEGFR2 pathway
(11).
Apatinib is a tyrosine kinase inhibitor that
selectively inhibits VEGFR2, resulting in the blocking of the
intracellular VEGF signaling pathway (14). As a new oral antiangiogenic agent,
apatinib has shown encouraging clinical results in treating various
solid tumors (15–17). Apatinib has been identified as the
only effective drug for patients with terminal gastric cancer
without chemotherapy indications in a phase III clinical trial
(18). In a phase II clinical
trial, apatinib monotherapy is effective and safe in advanced HCC
(19). However, the underlying
mechanism of apatinib against HCC remains to be elucidated.
The present study explored the effect and potential
mechanism of apatinib in HCC cell migration, invasion and
angiogenesis using the Hep3b cell line. It was found that apatinib
reduced the proliferation, migration and invasion of Hep3b cells by
regulating VEGF and PI3K-AKT signaling pathways.
Materials and methods
Cell culture and reagents
The human HCC cell line Hep3b was purchased from the
Cell Resource Center of Shanghai Institutes for Biological
Sciences, Chinese Academy of Sciences and cultured in RPMI-1640
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml
penicillin and 100 µg/ml streptomycin. Human umbilical vein
endothelial cells (HUVECs) were purchased from the American Type
Culture Collection and maintained in Dulbecco's modified Eagle's
medium (DMEM)/F12 (Gibco; Thermo Fisher Scientific, Inc.)
containing 10% FBS supplemented with 100 U/ml penicillin and 100
µg/ml streptomycin. All cells were cultured in a humidified chamber
at 37°C with 5% CO2. Apatinib was purchased from Jiangsu
Hengrui Medicine Co., Ltd. and was dissolved in dimethyl sulfoxide
(DMSO).
Cell transfection
VEGFR2 overexpression vector and empty vector (EV)
were purchased from VectorBuilder. VEGFR2 were amplified by using
the sense primer 5′-TGTCGTTGTAGGGTATAGGATTTATGAT-3′ and the
anti-sense primer 5′-ATACTTGTCGTCTGATTCTCCAGGTTTC-3′. Following the
manufacturer's instructions for Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.), Hep3b cells at 80%
confluence were transfected with 1 µg/ml VEGFR2 expression vector
or EV. After incubated at 37°C with 5% CO2 for 24 h for
24 h, cells successfully transfected with the vectors were used for
subsequent experiments; the transfection efficiency was verified by
fluorescence microscopy (Fig.
S1).
MTT assay
After Hep3b cells were cultured overnight in 96-well
plates (1×105 cells/well), the following three treatment
conditions were set: i) Cells treated with 0, 20, 40 and 60 µM
apatinib for 24 h; ii) cells treated with 40 µM apatinib for 12,
24, 48 and 72 h; and iii) cells transfected with VEGF
overexpression vector or EV for 2 h following treatment with 40 µM
apatinib for 24 h. Following culture for another 24 h, 10 mg/ml MTT
was added into each well and incubated for another 4 h. Cells were
centrifuged at 1,000 × g for 5 min at room temperature. DMSO (100
µl) was added into each well and incubated for 30 min to dissolve
the formazan product. The absorbance value of each well was
measured at a 490 nm wavelength.
Transwell assay
Hep3b cells were resuspended in RPMI-1640
supplemented with 1% FBS and 1×104 Hep3b cells were
seeded into the top chamber and treated under two conditions: i)
Cells treated with 40 µM apatinib for 24 h; and ii) cells
transfected with VEGF overexpression vector or EV for 2 h following
treatment with 40 µM apatinib for 24 h. Cells in the upper chamber
were then gently removed and the invaded cells were collected and
fixed for 30 min using 4% paraformaldehyde, stained with 0.1%
crystal violet for 30 min at room temperature and washed three
times with PBS. The number of cells was then counted under an
optical microscope (×100 magnification; Olympus Corporation).
Wound healing assay
Hep3b cells were seeded into 6-well plates at
2×105 cells/well and two treatment conditions were set:
i) Cells treated with 40 µM apatinib for 24 h; and ii) cells
transfected with VEGF overexpression vector or EV for 2 h following
treatment with 40 µM apatinib for 24 h. The wound gap on the cell
monolayer was created using a 200 µl pipette tip and cultured in
serum-free RPMI-1640. An optical microscope at ×100 magnification
was used for imaging and the migration of cells was observed at 24
h after wound scratching.
Matrigel in vitro HUVEC tube formation
assay
The conditioned media (CM) of Hep3b cells were
collected and stored at −80°C. HUVECs were trypsinised and seeded
(5.0×104 cells per well) into Matrigel-coated wells
(coated with Matrigel at 4°C, then incubated for 30 min at 37°C)
with 250 µl of CM from Hep3b cells. Following incubation at 37°C
for 24 h, six different fields were randomly chosen in each well
and images were captured.
Western blotting analysis
Hep3b cells were washed in PBS and lysed using the
protein extraction reagent RIPA (Invitrogen; Thermo Fisher
Scientific, Inc.). The concentration of proteins was measured by
BCA kit (cat. no. ab102536; Abcam). Equivalent amounts of proteins
(30 µg) from each sample were electrophoresed on SDS-polyacrylamide
gel (SDS-PAGE) and transferred onto a polyvinylidene fluoride
membrane, blocked in 4% skim milk for 2 h at room temperature and
incubated with the following specific primary antibodies:
E-cadherin antibody (ab219332 1:1,000; Abcam), α-catenin antibody
(ab51032 1:2,000; Abcam), N-cadherin (ab76011, 1:5000 dilution,
Abcam), vimentin (ab92547 1:1,000; Abcam), p-PI3K (ab182651
1:1,000; Abcam), PI3K (ab227204 1:1,000; Abcam), p-AKT (ab38449,
1:500 dilution, Abcam), AKT (ab18785 1:1,000; Abcam), VEGF
(ab214424 1:1,000; Abcam), VEGFR2 (ab221679 1:1,000; Abcam), Snail
(ab53519 1:1,000; Abcam), Slug (ab27568 1:1,000; Abcam) and MPP9
(ab38898 1:1,000; Abcam) overnight at 4°C. β-actin (ab8277 1:1,000;
Abcam) was used as internal reference. Then, the membranes were
incubated in HRP-linked goat anti-rabbit IgG secondary antibody
(ab97051; 1:10,000; Abcam) for 2 h at room temperature.
Immunoreactivity was visualized by a colorimetric reaction using an
ECL substrate buffer (EMD Millipore) and membranes were scanned
with Gel Doz EZ imager (Bio-Rad Laboratories, Inc.).
Statistical analysis
Data are shown as the mean ± standard deviation.
Statistical analysis was performed using Tukey's post hoc test for
one-way analysis of variance using the SPSS 16.0 software package
(SPSS, Inc.). P<0.05 was considered to indicate a statistically
significant difference.
Results
Apatinib inhibits the proliferation of
Hep3b cells
Compared with the control group, 10, 20, 40 and 60
µM apatinib treatment significantly reduced the proliferation of
Hep3b cells (Fig. 1A). Treatment
with 40 µM apatinib for 24 h inhibited the proliferation of Hep3b
cells and a greater decrease in cell proliferation was observed
upon treatment with apatinib for 48 and 72 h (Fig. 1B). These results indicated that
apatinib decreases the proliferation of Hep3b cells in a dose- and
time-dependent manner.
Apatinib inhibits the migration and
invasion of Hep3b cells
Previous studies have revealed that apatinib reduces
tumor cell metastasis by inhibiting EMT (20,21).
The present study investigated the metastatic ability of Hep3b
cells following treatment with 40 µM apatinib using the Transwell
and wound healing assays. It was found that apatinib treatment was
sufficient to reduce invasion of Hep3b cells (Fig. 2A) and inhibit migration (Fig. 2B). EMT-related protein levels in
Hep3b cells treated with or without 40 µM apatinib were detected by
western blotting. The results showed that apatinib significantly
induced protein expression of E-cadherin and α-catenin and reduced
protein expression of N-cadherin, vimentin, Snail, Slug and MMP9 in
Hep3b cells (Fig. 2C). The PI3K/Akt
pathway activation is known to be involved in tumor cell invasion
and metastasis in response to various growth factors, including EMT
(22,23). In the present study, the treatment
of 40 µM apatinib significantly inhibited the activation of the
PI3K/AKT pathway (Fig. 2D). These
data demonstrated that apatinib inhibited migration and invasion by
suppressing the PI3K/AKT pathway-dependent EMT in Hep3b cells.
 | Figure 2.Apatinib inhibited the migration and
invasion of Hep3b cells. (A) Invasion of Hep3b cells incubation
with apatinib for 24 was determined by Transwell assay
(magnification, ×100). (B) Migration of Hep3b cells in response to
apatinib was determined by wound scratch assay at 0 and 24 h under
microscope (magnification, ×100). (C) Western blotting was used to
measure the expression level of EMT-related proteins E-cadherin,
α-catenin, N-cadherin, Vimentin, Snail, Slug and MMP9 (D) Western
blotting was used to measure the expression level of p-PI3K, PI3K,
p-AKT and AKT. Values are shown as mean ± standard deviation (n=3);
**P<0.01 vs. Con group. EMT, epithelial-mesenchymal transition;
p-, phosphorylated; Con, control; Ap, apatinib. |
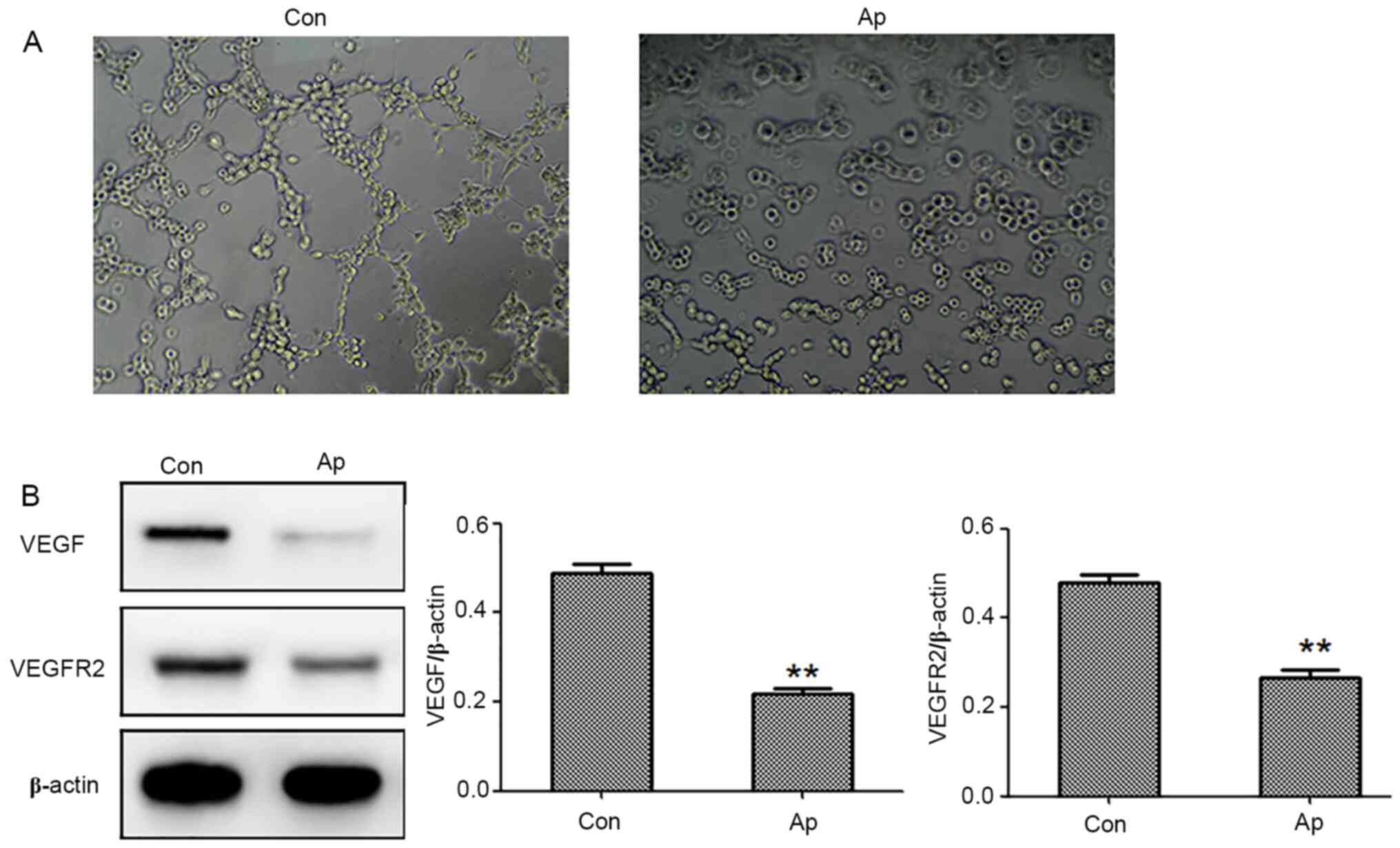
Apatinib inhibits the angiogenesis of
HUVEC cells
Next, it was determined whether the conditioned
medium from Hep3b cells could regulate tube formation in HUVECs.
After 24 h of incubation, CM from Hep3b cells treated with apatinib
has decreased the extent of tube formation by HUVECs compared with
the control group (Fig. 3A). To
further elucidate the underlying molecular mechanism of apatinib on
angiogenesis, protein expression levels of VEGF and VEGFR2 were
determined upon cell treatment. It was found that treatment of
Hep3b cells with 40 µM apatinib led to decreases in VEGF and VEGFR2
protein levels (Fig. 3B).
VEGFR2 overexpression abolishes the
inhibitory effect of apatinib on Hep3b cell migration and
invasion
Knowing that apatinib inhibited expression of VEGF
and VEGFR2 and signaling activity in Hep3b cells, the role of VEGF
signaling in apatinib-treated Hep3b cells was next determined.
VEGFR2 overexpression significantly counteracted the inhibitory
effects of apatinib on the proliferation, migration and invasion of
Hep3b cells (Fig. 4A-C). VEGFR2
overexpression significantly induced EMT in apatinib-treated Hep3b
cells via the downregulation of E-cadherin and α-catenin and
upregulation of N-cadherin, vimentin, Snail, Slug and MMP9
(Fig. 4D). In addition, VEGFR2
overexpression significantly induced the activation of the PI3K/AKT
pathway by increasing the levels of phosphorylated PI3K and AKT in
Hep3b cells (Fig. 4E).
 | Figure 4.VEGFR2 overexpression suppresses the
inhibitory effect of apatinib on Hep3b cell proliferation,
migration and invasion. (A) The proliferation of Hep3b cells were
detected by MTT assay. (B) Invasion of Hep3b cells was determined
by Transwell assay (magnification, ×100). (C) Migration of Hep3b
cells was determined by wound scratch assay at 0 and 24 h under
microscope (magnification, ×100). (D) Western blotting was used to
measure the expression level of EMT-related proteins E-cadherin,
α-catenin, N-cadherin, Vimentin, Snail, Slug and MMP9. (E) Western
blotting was used to measure the expression level of p-PI3K, PI3K,
p-AKT and AKT. Values are shown as mean ± standard deviation (n=3);
*P<0.05 or **P<0.01 vs. EV group; #P<0.05 or
##P<0.01 vs. Ap group. EMT, epithelial-mesenchymal
transition; p-, phosphorylated; EV, empty vector; Ap, apatinib. |
VEGFR2 overexpression abolishes the
inhibitory effect of apatinib on the angiogenesis of HUVEC
cells
After 24 h of incubation, CM from Hep3b cells
transfected with VEGFR2 overexpression vector combined with
apatinib treatment increased the extent of tube formation by HUVECs
compared the Ap group (Fig. 5A).
The protein expression levels of VEGF and VEGFR2 in Hep3b cells
transfected with the VEGF overexpression vector combined with
apatinib treatment were higher than the Ap group (Fig. 5B).
Discussion
Liver cancer is one of the three most lethal
cancers. Although patients with HCC exhibit increased survival
rates following curative resection, the prognosis of patients with
HCC remains poor due to tumor metastasis and invasiveness (24). Apatinib is a highly selective
inhibitor of multiple tyrosine kinases and one of the latest agents
with encouraging preclinical and clinical data in treating solid
tumors (25). Previous studies have
shown that apatinib treatment leads to apoptosis and autophagy and
inhibits EMT and metastasis in osteosarcoma cells (26,27).
The present study found that apatinib significantly inhibited the
proliferation, invasion and metastasis of Hep3b cells. This was
consistent with a previous study on cholangiocarcinoma, which found
that apatinib inhibits cellular migration and invasion via the
PI3K/AKT pathway (28). The
PI3K/AKT signaling pathway serves an important role in regulating
tumor growth, angiogenesis, apoptosis, invasion and metastasis
(29). Abnormal activation of the
PI3K/Akt/mTOR signaling pathway occurs in ~45% of HCC cases and is
associated with the poor prognosis in patients with HCC through
related independent prognostic factors, such as vascular invasion,
metastasis stage and tumor differentiation (30,31).
The activation of the PI3K/AKT pathway is reported to enhance the
invasion and metastasis of HCC cells (32). The present study found that apatinib
treatment reduced the phosphorylation of PI3K and AKT in Hep3b
cells, indicating that a strong inhibitory effect of apatinib on
Hep3b cell migration and invasion is associated with the inhibition
of the PI3K/AKT signaling pathway. Similar results have found in
NSCLC cells, in which the apatinib treatment synergistically
reduced proliferation and inhibited the migration and invasion of
NSCLC cells (33).
A previous study reported that apatinib treatment
significantly attenuated macrophage infiltration and EMT of lung
tissue (20). EMT is a fundamental
process during which tumor cells acquire the capacity of migration
and invasion (34). It is mainly
characterized by the downregulation of cell adhesion molecules
[including E-cadherin, α-catenin and zonula occludens-1 (ZO-1)],
transformation of the cytokeratin cytoskeleton into vimentin and
morphological characteristics of mesenchymal cells (35). E-cadherin, ZO-1 and α-catenin are
necessary to form stable adherens junctions (36). During the EMT process, epithelial
cells lose E-cadherin and transform into spindle shaped mesenchymal
cells by acquiring N-cadherin. Vimentin and fibronectin are
mesenchymal markers that are overexpressed in cancer cells,
demonstrating that these factors promote tumor growth, metastasis
and recurrence (37). Zheng et
al (27) suggested that
apatinib inhibits the migration and invasion of osteosarcoma by
targeting STAT3 pathway to inhibit EMT. The present study found
that the apatinib treatment increased the expressions of the
epithelial hallmarks E-cadherin and α-catenin and decreased the
expressions of the mesenchymal hallmarks N-cadherin, Vimentinin,
Snail, Slug and MMP9 in Hep3b cells. These results indicated that
apatinib attenuates the migration and invasion of Hep3b cells by
regulating EMT.
HCC is a typical hypervascular tumor with a high
VEGFR expression (38). VEGF
promotes angiogenesis by inducing proliferation and migration of
endothelial cells (39). Tumor
angiogenesis provides essential growth that requires nutrients and
oxygen for tumor occurrence, development and metastasis and which
are closely associated with tumor stage and prognosis (40–42).
Therefore, studies have been conducted to explore agents that
target the VEGF axis in advanced HCC (43–45).
It has been reported that apatinib effectively inhibits the
proliferation, migration and tube formation of HUVEC by blocking
the VEGF axis (14). Chen et
al (21) reported that the
inhibitory effects of apatinib on EMT and tumorigenesis may be
associated with the downregulation of the expression levels of
MMP2/9, VEGF and VEGFR2 in HCC. Similarly, apatinib had an
inhibitory effect on the VEGF/VEGFR pathway, Hep3b cell migration,
invasion and tube formation in the present study (46). A previous study reported that the
PI3K/Akt/NF-κB pathway can regulate the invasion of carcinoma cells
via upregulation of VEGF, indicating that VEGF is the downstream
target of the PI3K/AKT pathway in regulating cancer invasion and
metastasis (47). In addition,
apatinib treatment inhibits tumor growth and angiogenesis in
anaplastic thyroid carcinoma via suppression of the AKT pathway way
(48). The present study found that
apatinib inhibited the expressions of VEGF and VEGFR2 and reduced
the activation of PI3K/AKT. Conversely, the VEGFR2 overexpression
markedly increased the activation of the PI3K/AKT pathway. These
results indicated that the anti-angiogenic effect of apatinib in
Hep3b cells may be mediated, at least in part, by preventing the
VEGF expression and subsequent decrease in PI3K/AKT pathway
activation.
In conclusion, the data presented in the current
study revealed that apatinib suppressed the proliferation,
migration and invasion of Hep3b cells by inhibiting the PI3K-AKT
pathway-mediated EMT. In addition, it suppressed the expression and
activity of pro-angiogenic factors VEGF and VEGFR-2, which may also
involve the activation of the downstream PI3K-AKT signaling
pathway, resulting in the inhibition of angiogenesis. These
findings indicated that apatinib has a great potential for use as
an antitumor agent in patients with HCC by inhibiting cell
migration, invasion and angiogenesis by blocking the VEGF and
PI3K/AKT pathways. A further study on the effect apatinib effect on
other liver cancer cell lines and in vivo animal experiment
will be of great interest to fully elucidate the underlying
signaling pathways involved in its anti-cancer effect.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was supported by the Qingdao Health
Science and Technology Project (grant no. 2016-WJZD076).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JS, ZG and YZ designed the experiments. JS, CS and
ML performed most of the experiments. JS drafted the manuscript. ZG
contributed to the analysis and interpretation of the data. YZ
modified the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Forner A, Reig M and Bruix J:
Hepatocellular carcinoma. Lancet. 391:1301–1314. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang N, Ekanem NR, Sakyi CA and Ray SD:
Hepatocellular carcinoma and microRNA: New perspectives on
therapeutics and diagnostics. Adv Drug Deliv Rev. 81:62–74. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bruix J, Gores GJ and Mazzaferro V:
Hepatocellular carcinoma: Clinical frontiers and perspectives. Gut.
63:844–855. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Radisky DC: Epithelial-mesenchymal
transition. J Cell Sci. 118:4325–4326. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang SH, Wu XC, Zhang MD, Weng MZ, Zhou D
and Quan ZW: Upregulation of H19 indicates a poor prognosis in
gallbladder carcinoma and promotes epithelial-mesenchymal
transition. Am J Cancer Res. 6:15–26. 2015.PubMed/NCBI
|
|
7
|
Huang Q, Han J, Fan J, Duan L, Guo M, Lv
Z, Hu G, Chen L, Wu F, Tao X, et al: IL-17 induces EMT via Stat3 in
lung adenocarcinoma. Am J Cancer Res. 6:440–451. 2016.PubMed/NCBI
|
|
8
|
Li M, Zhang B, Sun B, Wang X, Ban X, Sun
T, Liu Z and Zhao X: A novel function for vimentin: The potential
biomarker for predicting melanoma hematogenous metastasis. J Exp
Clin Cancer Res. 29:1092010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Han J, Wang F, Lan Y, Wang J, Nie C, Liang
Y, Song R, Zheng T, Pan S, Pei T, et al: KIFC1 regulated by
miR-532-3p promotes epithelial-to-mesenchymal transition and
metastasis of hepatocellular carcinoma via gankyrin/AKT signaling.
Oncogene. 38:406–420. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang X, Zhang XF, Lu X, Jia HL, Liang L,
Dong QZ, Ye QH and Qin LX: MicroRNA-26a suppresses angiogenesis in
human hepatocellular carcinoma by targeting hepatocyte growth
factor-cMet pathway. Hepatology. 59:1874–1885. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu Y, Qiao Y, Hu C, Liu L, Zhou L, Liu B,
Chen H and Jiang X: VEGFR2 inhibition by RNA interference affects
cell proliferation, migration, invasion, and response to radiation
in Calu-1 cells. Clin Transl Oncol. 18:212–219. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Minata M, Harada KH, Kudo M, Ikai I and
Nishida N: The prognostic value of vascular endothelial growth
factor in hepatocellular carcinoma for predicting metastasis after
curative resection. Oncology. 84 (Suppl 1):75–81. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chatterjee S, Heukamp LC, Siobal M,
Schöttle J, Wieczorek C, Peifer M, Frasca D, Koker M, König K,
Meder L, et al: Tumor VEGF:VEGFR2 autocrine feed-forward loop
triggers angiogenesis in lung cancer. J Clin Invest. 123:1732–1740.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tian S, Quan H, Xie C, Guo H, Lü F, Xu Y,
Li J and Lou L: YN968D1 is a novel and selective inhibitor of
vascular endothelial growth factor receptor-2 tyrosine kinase with
potent activity in vitro and in vivo. Cancer Sci. 102:1374–1380.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li F, Liao Z, Zhao J, Zhao G, Li X, Du X,
Yang Y and Yang J: Efficacy and safety of Apatinib in stage IV
sarcomas: Experience of a major sarcoma center in China.
Oncotarget. 8:64471–64480. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li J, Qin S, Xu J, Xiong J, Wu C, Bai Y,
Liu W, Tong J, Liu Y, Xu R, et al: Randomized, double-blind,
placebo-controlled phase III trial of apatinib in patients with
chemotherapy-refractory advanced or metastatic adenocarcinoma of
the stomach or gastroesophageal junction. J Clin Oncol.
34:1448–1454. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hu X, Zhang J, Xu B, Jiang Z, Ragaz J,
Tong Z, Zhang Q, Wang X, Feng J, Pang D, et al: Multicenter phase
II study of apatinib, a novel VEGFR inhibitor in heavily pretreated
patients with metastatic triple-negative breast cancer. Int J
Cancer. 135:1961–1969. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li J, Qin S, Xu J, Guo W, Xiong J, Bai Y,
Sun G, Yang Y, Wang L, Xu N, et al: Apatinib for
chemotherapy-refractory advanced metastatic gastric cancer: Results
from a randomized, placebo-controlled, parallel-arm, phase II
trial. J Clin Oncol. 31:3219–3225. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qin S, Bai Y, Ouyang X, Cheng Y, Li J, Xu
J, Liang J, Li Q, Wu W and Liu W: Apatinib for patients with
advanced hepatocellular carcinoma: A randomised, open-label,
multicentre, phase II clinical trial. Lin Chuang Zhong Liu Xue Za
Zhi. 22:1057–1065. 2017.(In Chinese).
|
|
20
|
Liu S, Su L, Mu X, Shi Y, Zhang A and Ge
X: Apatinib inhibits macrophage-mediated epithelial - mesenchymal
transition in lung cancer. RSC Advances. 8:21451–21459. 2018.
View Article : Google Scholar
|
|
21
|
Chen Y, Chen X, Ding X and Wang Y:
Afatinib, an EGFR inhibitor, decreases EMT and tumorigenesis of Huh
7 cells by regulating the ERK VEGF/MMP9 signaling pathway. Mol Med
Rep. 20:3317–3325. 2019.PubMed/NCBI
|
|
22
|
Zhang Y, Wang SJ, Han ZH, Li YQ, Xue JH,
Gao DF, Wu XS and Wang CX: PI3K/AKT signaling pathway plays a role
in enhancement of eNOS activity by recombinant human angiotensin
converting enzyme 2 in human umbilical vein endothelial cells. Int
J Clin Exp Pathol. 7:8112–8117. 2014.PubMed/NCBI
|
|
23
|
Xu W, Yang Z and Lu N: A new role for the
PI3K/Akt signaling pathway in the epithelial-mesenchymal
transition. Cell Adhes Migr. 9:317–324. 2015. View Article : Google Scholar
|
|
24
|
Yu X, Zheng Y, Zhu X, Gao X, Wang C, Sheng
Y, Cheng W, Qin L, Ren N, Jia H, et al: Osteopontin promotes
hepatocellular carcinoma progression via the PI3K/AKT/Twist
signaling pathway. Oncol Lett. 16:5299–5308. 2018.PubMed/NCBI
|
|
25
|
Scott AJ, Messersmith WA and Jimeno A:
Apatinib: A promising oral antiangiogenic agent in the treatment of
multiple solid tumors. Drugs Today (Barc). 51:223–229. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu K, Ren T, Huang Y, Sun K, Bao X, Wang
S, Zheng B and Guo W: Apatinib promotes autophagy and apoptosis
through VEGFR2/STAT3/BCL-2 signaling in osteosarcoma. Cell Death
Dis. 8:e30152017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zheng B, Ren T, Huang Y and Guo W:
Apatinib inhibits migration and invasion as well as PD-L1
expression in osteosarcoma by targeting STAT3. Biochem Biophys Res
Commun. 495:1695–1701. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang M, Huang B, Li G and Zeng S:
Apatinib affect VEGF-mediated cell proliferation, migration,
invasion via blocking VEGFR2/RAF/MEK/ERK and PI3K/AKT pathways in
cholangiocarcinoma cell. BMC Gastroenterol. 18:1692018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Saxena NK, Sharma D, Ding X, Lin S, Marra
F, Merlin D and Anania FA: Concomitant activation of the JAK/STAT,
PI3K/AKT, and ERK signaling is involved in leptin-mediated
promotion of invasion and migration of hepatocellular carcinoma
cells. Cancer Res. 67:2497–2507. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhou Q, Lui VW and Yeo W: Targeting the
PI3K/Akt/mTOR pathway in hepatocellular carcinoma. Future Oncol.
7:1149–1167. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fathi Maroufi N, Rashidi MR, Vahedian V,
Akbarzadeh M, Fattahi A and Nouri M: Therapeutic potentials of
Apatinib in cancer treatment: Possible mechanisms and clinical
relevance. Life Sci. 241:1171062020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lou L, Ye W, Chen Y, Wu S, Jin L, He J,
Tao X, Zhu J, Chen X, Deng A, et al: Ardipusilloside inhibits
survival, invasion and metastasis of human hepatocellular carcinoma
cells. Phytomedicine. 19:603–608. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liao X, Tao L, Guo W, Wu ZX, Du H, Wang J,
Zhang J, Chen H, Chen ZS, Lin L, et al: Combination of Cordycepin
and Apatinib Synergistically Inhibits NSCLC Cells by
Down-Regulating VEGF/PI3K/Akt Signaling Pathway. Front Oncol.
10:17322020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen T, You Y, Jiang H and Wang ZZ:
Epithelial-mesenchymal transition (EMT): A biological process in
the development, stem cell differentiation, and tumorigenesis. J
Cell Physiol. 232:3261–3272. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Song L, Li XX, Liu XY, Wang Z, Yu Y, Shi
M, Jiang B and He XP: EFEMP2 suppresses the invasion of lung cancer
cells by inhibiting epithelial-mesenchymal transition (EMT) and
down-regulating MMPs. OncoTargets Ther. 13:1375–1396. 2020.
View Article : Google Scholar
|
|
36
|
Piao HL, Yuan Y, Wang M, Sun Y, Liang H
and Ma L: α-catenin acts as a tumour suppressor in
E-cadherin-negative basal-like breast cancer by inhibiting NF-κB
signalling. Nat Cell Biol. 16:245–254. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Niknami Z, Eslamifar A, Emamirazavi A,
Ebrahimi A and Shirkoohi R: The association of vimentin and
fibronectin gene expression with epithelial-mesenchymal transition
and tumor malignancy in colorectal carcinoma. EXCLI J.
16:1009–1017. 2017.PubMed/NCBI
|
|
38
|
Yamaguchi R, Yano H, Nakashima Y,
Ogasawara S, Higaki K, Akiba J, Hicklin DJ and Kojiro M: Expression
and localization of vascular endothelial growth factor receptors in
human hepatocellular carcinoma and non-HCC tissues. Oncol Rep.
7:725–729. 2000.PubMed/NCBI
|
|
39
|
Chen H, Shen YF, Gong F, Yang GH, Jiang YQ
and Zhang R: Expression of VEGF and its effect on cell
proliferation in patients with chronic myeloid leukemia. Eur Rev
Med Pharmacol Sci. 19:3569–3573. 2015.PubMed/NCBI
|
|
40
|
Podar K and Anderson KC: Inhibition of
VEGF signaling pathways in multiple myeloma and other malignancies.
Cell Cycle. 6:538–542. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lin D and Wu J: Hypoxia inducible factor
in hepatocellular carcinoma: A therapeutic target. World J
Gastroenterol. 21:12171–12178. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Song P, Gao J, Inagaki Y, Kokudo N,
Hasegawa K, Sugawara Y and Tang W: Biomarkers: Evaluation of
screening for and early diagnosis of hepatocellular carcinoma in
Japan and China. Liver Cancer. 2:31–39. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Buijs N, Oosterink JE, Jessup M,
Schierbeek H, Stolz DB, Houdijk AP, Geller DA and van Leeuwen PA: A
new key player in VEGF-dependent angiogenesis in human
hepatocellular carcinoma: Dimethylarginine dimethylaminohydrolase
1. Angiogenesis. 20:557–565. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bhoori S and Mazzaferro V: Combined
immunotherapy and VEGF-antagonist in hepatocellular carcinoma: A
step forward. Lancet Oncol. 21:740–741. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yao Y, Wang T, Liu Y and Zhang N:
Co-delivery of sorafenib and VEGF-siRNA via pH-sensitive liposomes
for the synergistic treatment of hepatocellular carcinoma. Artif
Cells Nanomed Biotechnol. 47:1374–1383. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yang C and Qin S: Apatinib targets both
tumor and endothelial cells in hepatocellular carcinoma. Cancer
Med. 7:4570–4583. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Shen K, Ji L, Gong C, Ma Y, Yang L, Fan Y,
Hou M and Wang Z: Notoginsenoside Ft1 promotes angiogenesis via
HIF-1α mediated VEGF secretion and the regulation of PI3K/AKT and
Raf/MEK/ERK signaling pathways. Biochem Pharmacol. 84:784–792.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jin Z, Cheng X, Feng H, Kuang J, Yang W,
Peng C, Shen B and Qiu W: Apatinib inhibits angiogenesis via
suppressing Akt/GSK3β/ANG signaling pathway in anaplastic thyroid
cancer. Cell Physiol Biochem. 44:1471–1484. 2017. View Article : Google Scholar : PubMed/NCBI
|