Introduction
The incidence of pituitary prolactinoma is
reportedly 27 cases per million individuals per year (1,2).
Abnormal levels of prolactin (PRL) and growth hormone (GH) in
patients with prolactinoma can lead to dysregulation of the central
gonadal axis (3,4) and central precocious puberty in
children (5). Current treatments of
pituitary tumors are mainly based on hormone therapy and surgery,
with auxiliary chemotherapy and radiotherapy (6). However, the risks of surgical
complications and side effects of drugs can limit the benefits of
such treatment regimens (7,8).
Insulin-like growth factor (IGF)-1 is a polypeptide
that regulates growth and metabolism (9,10), and
was previously found to promote tumor development (11). IGF-1 can increase the resistance of
gliomas to temozolomide via the Wnt/β-catenin pathway (12), promote the proliferation of tumor
cells, stimulate the endocrine effect of prolactinoma (13), and accelerate mitosis of
prolactinoma cells (14). However,
the cancer-promoting mechanism of IGF-1 remains unclear.
MicroRNA (miRNA/miR) is involved in the
post-transcriptional regulation of gene expression and can induce
the degradation of mRNA by targeting the 3′-untranslated region
(UTR) (15). In prolactinoma,
miRNAs, such as miR-93-5p (16),
miR-145-5p (17) and miR-1299
(18), have regulatory functions.
miR-29-3p has tumor suppressor effects in stomach (19), bone (20) and breast (21) cancer. Moreover, miR-29 can affect
the growth of rats by inhibiting the expression of IGF-1 (22). However, the role of miR-29-3p in
prolactinoma is still unclear.
Therefore, the aim of the present study was to
determine the effects of miR-29a-3p on the proliferation and
apoptosis of prolactinoma cells, as well as the secretion of PRL
and GH via the IGF-1 and β-catenin pathways.
Materials and methods
Bioinformatics analysis
The relationship between miR-29a-3p and the survival
of patients with prolactinoma (i.e., pheochromocytoma and
paraganglioma) was analyzed using the log-rank test via
Kaplan-Meier (http://kmplot.com/analysis/index.php?p=background). In
The Cancer Genome Atlas (TCGA) database, a total of 179 patients
with pheochromocytoma and paraganglioma were included. However, the
database does not separate the two tumors. Of the 179 patients, 69
had low expression of miR-29a-3p and 110 had high expression.
Cell culture and transfection
Rat pituitary adenoma RC-4B/C cells [American Type
Culture Collection (ATCC); ATCC® CRL-1903] and
prolactinoma MMQ (ATCC® CRL-10609) and GH3
(ATCC® CCL-82.1) cells were cultured in F-12K medium
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with 2.5%
fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) and 15%
horse serum (HyClone; Cytiva) at 37°C under a humidified atmosphere
of 5% CO2/95% air.
MMQ and GH3 cells were divided into 4 groups: NC,
mimic, mimic + IGF-1 and IGF-1. According to the group, miR-29a-3p
and/or IGF-1 were overexpressed by transfection. Plasmids encoding
the full-length human IGF-1 (pcDNA3.1), a miR-29a-3p mimic and
corresponding negative control (NC, 3′-GGACACUAUCUGACAUCGACUA-5′)
were purchased from Shanghai GenePharma Co., Ltd. Cells
(1×105) were transfected with 100 pmol pcDNA3.1 or 50 nM
mimic (7°C, 5% CO2, 48 h) using
Lipofectamine® 2000 transfection reagent (Invitrogen;
Thermo Fisher Scientific, Inc.). All subsequent experiments were
performed within 24 h after the completion of transfection.
Dual-luciferase reporter assay
Mutated (Mut) IGF-1 was designed based on binding
sites that were predicted with the TargetScan v7.2 (http://www.targetscan.org/vert_72/). Cells
(1×104) were seeded in 96-well plates. After 24 h of
culture, the cells were transfected with wild-type (Wt)/Mut IGF-1
and miR-29a-3p mimic/NC cloned into pMIR-REPORT™ luciferase vectors
(Ambion; Thermo Fisher Scientific, Inc.) using Lipofectamine 2000
transfection reagent. Mut IGF-1 was induced by rapid site-directed
mutagenesis kit (Beyotime Institute of Biotechnology). The
concentration of Wt/Mut IGF-1 and miR-29a-3p mimic/NC were 1 µg and
50 nM, respectively. After 48 h, the Dual-Luciferase Reporter 1000
Assay System (Promega Corporation) was used to evaluate
Luciferase/Renilla values.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was isolated from cells using
TRIzol® reagent (Sigma-Aldrich; Merck KGaA). For
miR-29a-3p, complementary DNA was synthesized using the miScript
kit (60 min at 42°C, 5 min at 70°C, and then stored at 4°C) and
amplified with the miScript SYBR Green PCR kit (40 cycles at 95°C
for 10 min, 94°C for 15 sec, 60°C for 1 min, 60°C for 1 min and
then stored at 4°C) (both from Qiagen GmbH), RT kit was used
according to the manufacturer's protocol. The quantity of the
RT-qPCR products was compared to that of U6 as a standardized
reference. IGF-1 mRNA was reverse transcribed (60 min at 42°C, 5
min at 70°C, and then stored at 4°C) and amplified by RT-qPCR (40
cycles at 95°C for 10 min, 94°C for 15 sec, 60°C for 1 min, 60°C
for 1 min, and then stored at 4°C) with the PrimeScript RT kit and
SYBR Premix Ex Taq™ kit (both purchased from Takara Bio, Inc.),
respectively. The primers used for RT-qPCR analysis were
manufactured by Wuhan GeneCreate Biological Engineering Co., Ltd.
Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an
internal reference for mRNA. IGF-1 mRNA levels were quantified
using the 2−ΔΔCq method (23). The primer sequences were as follows:
IGF-1 forward, 5′-TGGTGGACGCTCTTCAGTTC-3′ and reverse,
5′-TCCGGAAGCAACACTCATCC-3′; GAPDH forward, 5′-GATGCTGGTGCTGAG-3′
and reverse, 5′-GTGGTGCAGGATGCATTGCTCTGA-3′; miR-29a-3p forward,
5′-ACCCCTTAGAGGATGACTGAT-3′ and reverse,
5′-AACCGATTTCAGATGGTGCT-3′; and U6 forward, 5′-GCTTCGGCAGCACATA-3′
and reverse, 5′-ATGGAACGCTTCACGA-3′.
Western blotting
Total protein was extracted from cell lysates. In
order to detect the expression levels of β-catenin in the nuclear
and cytoplasmic fractions, the proteins were extracted separately
with Nuclear-Cytosol Extraction kits (Applygen Technologies, Inc.).
Protein concentration was measured with a bicinchoninic acid kit
and 40 µg total protein was separated via sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (10%, 120 V, 90 min),
and then separated proteins were transferred to polyvinylidene
fluoride membranes (90 V, 90 min). Membranes were then blocked with
5% non-fat milk for 1 h at room temperature, and then incubated
overnight at 4°C with primary antibodies (all purchased from Abcam)
against IGF-1 (1:800; cat. no. ab182408), β-catenin (1:500; cat.
no. ab16051), caspase-3 (1:5,000; cat. no. ab32351), Bax (1:500;
cat. no. ab243140), GAPDH (1:5,000; cat. no. ab8245) and
TATA-box-binding protein (TBP; 1:2,000; cat. no. ab171969). The
next day, the membranes were incubated with horseradish
peroxidase-labeled goat anti-rabbit secondary antibody (1:5,000;
ab7090; Abcam) for 2 h at room temperature. The blots were detected
with Pierce™ ECL Western Blotting Substrate (Pierce; Thermo Fisher
Scientific, Inc.) and imaged with a ChemiDoc MP Imaging System
(Bio-Rad Laboratories, Inc.). GAPDH was used as an internal
reference for the total protein contents of the nuclear and
cytoplasmic fractions, while TBP was used as an internal reference
for nuclear proteins.
Cell counting kit-8 (CCK-8) assay
Briefly, 5×104 cells/well (100 µl) were
cultured in 96-well plates. After 48 h, the medium was replaced
with medium supplemented with 10 µl CCK-8 reagent (Beyotime
Institute of Biotechnology) and the cells were cultured for an
additional 2 h at 37°C. The optical density at 450 nm was measured
using a microplate reader (model 680; Bio-Rad Laboratories,
Inc.).
Colony formation assay
The cells in the logarithmic growth phase were
digested with 0.25% trypsin and pipetted into single wells, and the
cells were suspended in DMEM medium with 10% fetal bovine serum for
later use. Then, 200 cells were seeded in a 10 ml dish with
pre-warmed culture medium at 37°C, and gently rotated to evenly
disperse the cells. The medium was changed twice a week. After 2
weeks, the medium was poured and the cells carefully washed twice
with PBS. Paraformaldehyde at 4% was added to fix the cells for 15
min at room temperature. GIMSA staining solution was added and for
30 min at 2°C. After washing, the sample was observed under a light
microscope (magnification, ×40) and the number of colonies with
more than 50 cells was counted.
5-Ethynyl-2′-deoxyuridine (EdU)
assay
The proliferative ability of cells was detected via
an EdU assay. Briefly, 1×104 cells were seeded into the
wells of a 96-well plate and cultured under an atmosphere of 5%
CO2/95% air at 37°C. EdU (Guangzhou RiboBio Co., Ltd.)
was diluted to 10 µM with culture medium and added to the wells.
After incubation for 2 h, the medium was discarded and the cells
were washed, and then fixed with 4% paraformaldehyde in
phosphate-buffered saline and incubated for 30 min at room
temperature. After washing, 100 µl penetrant (0.5% Triton-X-100 in
phosphate-buffered saline) was added to each well and the plate was
incubated in a decolorizing shaker for 10 min. Afterward, the cells
were washed, then 100 µl Apollo567 staining reaction solution
(Guangzhou RiboBio Co., Ltd.) was added to the wells and the plate
was incubated for 30 min at room temperature in the dark. After
washing, the cells were fixed (4% paraformaldehyde, room
temperature, 1 h) and observed under a fluorescence microscope
(Olympus Corporation) at an excitation wavelength of 550 nm and
emission wavelength of 565 nm (magnification, ×200).
Flow cytometry assay
Briefly, the cells were treated with 5 µl Annexin
V-fluorescein isothiocyanate and propidium iodide (Tianjin Sungene
Biotech Co., Ltd.) for 15 min in the dark, and then subjected to
flow cytometry with a BD FACSCalibur flow cytometer (BD
Biosciences) to measure apoptosis rates. FlowJo software version
7.6.2 (FlowJo LLC) was used for analysis.
Enzyme-linked immunosorbent assay
(ELISA)
After the cells were cultured for 48 h, the culture
medium was collected by centrifugation (3,000 × g; 10 min; room
temperature) and the levels of PRL (ab272780; Abcam) and GH
(F15621; Shanghai Xitang Biotechnology Co., Ltd.) were detected
with ELISA kits. In accordance with the manufacturer's
instructions, antibodies and chromogenic reagents were added
sequentially to the wells of the ELISA plates and incubated for the
recommended periods of time. Finally, the optical density at 450 nm
was measured and standard curves were generated to measure the
concentrations of PRL and GH.
Statistical analysis
Experiments were repeated three times for each
sample. All statistical analyses were conducted using GraphPad
Prism 7.0 software (GraphPad Software, Inc.). The data are
expressed as the mean ± standard error of the mean. Variance among
multiple groups was identified by one-way analysis of variance
(ANOVA). Pairwise comparisons between groups were conducted using
the Tukey's test following ANOVA. P<0.05 was considered to
indicate a statistically significant difference.
Results
miR-29a-3p expression is low in
prolactinoma cells and targets IGF-1
In order to preliminarily analyze the significance
of miR-29a-3p in prolactinoma, analysis of TCGA database showed
that low expression of miR-29a-3p was associated with poor
prognosis of patients with pheochromocytomas and paragangliomas
(P=0.02, Fig. 1A). The prediction
results showed that there were binding sites between miR-29a-3p and
the 3′-UTR of IGF-1 mRNA, and both are conserved (Fig. 1B). The target binding relationship
was verified with the dual-luciferase reporter assay. After
transfection with the miR-29a-3p mimic, miR-29a-3p expression
significantly increased (P<0.05, Fig. 1C and D), transfection with
miR-29a-3p mimic and Wt-IGF-1 decreased the relative luciferase
activity in MMQ and GH3 cells, thereby confirming targeted binding
(Fig. 1E and F). To initially
explore the expression characteristics of miR-29a-3p and IGF-1 in
prolactinoma, benign pituitary adenoma RC-4B/C cells were used as a
control. The results showed that miR-29a-3p expression was
significantly downregulated and IGF-1 mRNA and protein levels were
significantly upregulated in MMQ and GH3 prolactinoma cells
(P<0.05, Fig. 1G-J). The
aforementioned data suggested that miR-29a-3p targeted IGF-1 and
may play an important regulatory role in prolactinoma.
miR-29a-3p targets the inhibition of
IGF-1 expression in prolactinoma cells
To further analyze the effects of miR-29a-3p/IGF-1
on prolactinoma, MMQ and GH3 cells were divided into four treatment
groups: NC, mimic, mimic + IGF-1, and IGF-1. The results showed
that overexpression of miR-29a-3p significantly inhibited the mRNA
and protein levels of IGF-1 (P<0.05), while transfection of
IGF-1 pcDNA3.1 blocked the inhibitory effects of miR-29a-3p on
IGF-1 (Fig. 2A-F). These findings
suggested that miR-29a-3p strongly inhibited IGF-1 expression at
the mRNA and protein levels in MMQ and GH3 cells.
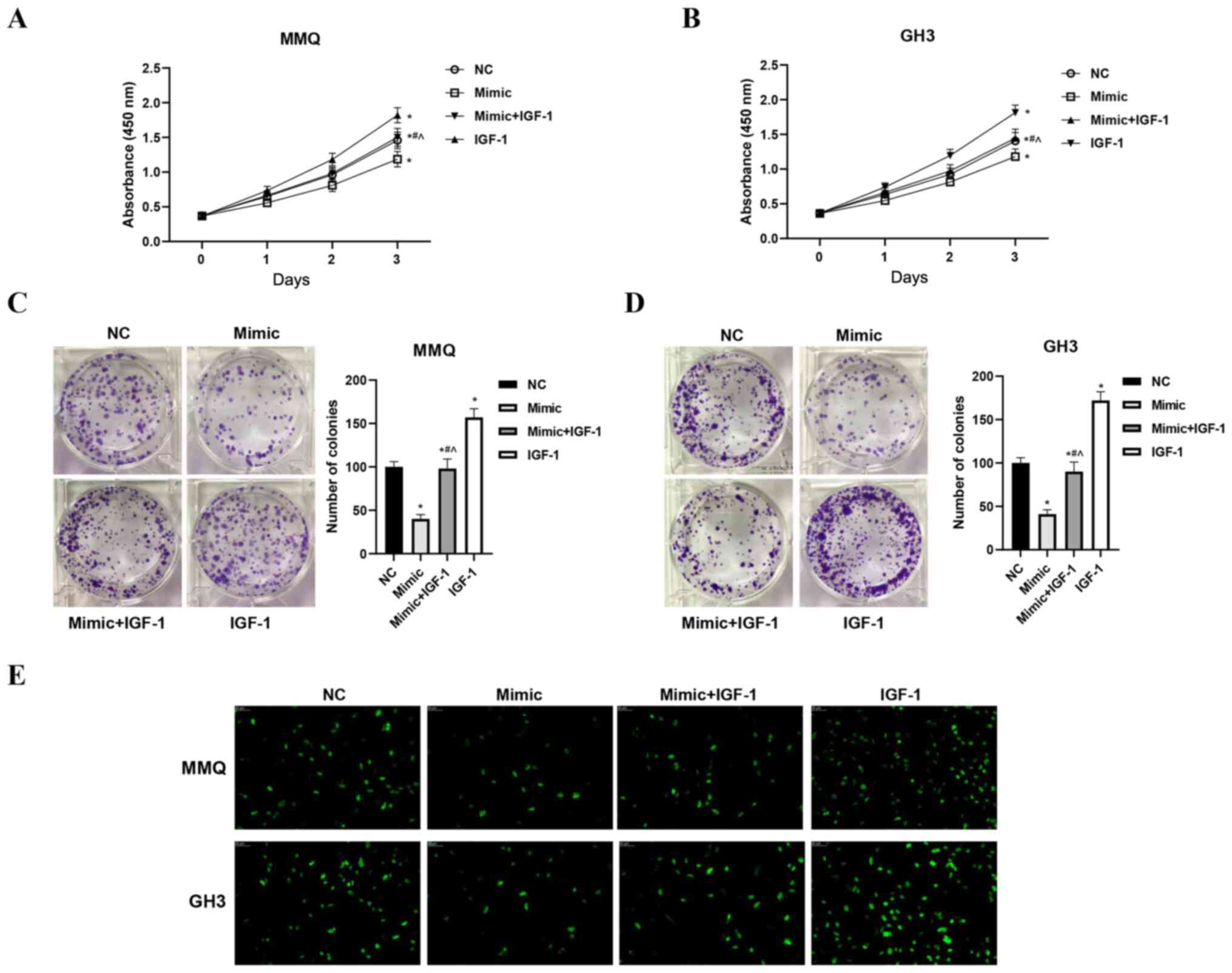
miR-29a-3p inhibits the proliferation
and promotes apoptosis of prolactinoma cells by inhibiting
IGF-1
The viability, proliferation and apoptosis of cells
in the four treatment groups were assessed with CCK-8, EdU and flow
cytometry assays, respectively. The results showed that
overexpression of miR-29a-3p inhibited the viability and
proliferation of MMQ and GH3 cells. Overexpression of IGF-1 not
only increased cell viability and promoted proliferation, but also
significantly alleviated the inhibitory effects of miR-29a-3p
(P<0.05, Fig. 3A-E). Moreover,
increased expression of miR-29a-3p significantly increased the
apoptosis rates of MMQ and GH3 cells, while overexpression of IGF-1
had an opposite effect on apoptosis by neutralizing the effect of
miR-29a-3p (P<0.05, Fig. 4A-C).
Correspondingly, the levels of apoptosis marker proteins caspase-3
and Bax increased correspondingly after miR-29a-3p was
overexpressed. The increased expression of IGF-1 significantly
decreased the caspase-3 and Bax protein levels of MMQ and GH3
cells, thus indicating that it reduced the apoptosis-promoting
effect of miR-29a-3p (P<0.05, Fig.
4D-F). These results suggested that miR-29a-3p inhibited
proliferation and induced apoptosis of prolactinoma cells, which
was closely related to the inhibition of IGF-1.
miR-29a-3p inhibits PRL and GH
secretion of prolactinoma cells by targeting IGF-1
In order to further analyze the effect of miR-29a-3p
on prolactinoma cells, PRL mRNA levels in the cells and the PRL
concentration in the culture medium were detected by RT-qPCR and
ELISA, respectively. The results showed that the PRL mRNA
expression and culture medium levels were significantly lower in
the mimic group, and higher in the IGF-1 group than in the NC
group, and significantly higher in the mimic + IGF-1 group compared
with in the mimic group (P<0.05, Fig. 5A-D). GH is mainly secreted by GH3
cells, with lower levels produced by MMQ cells (24). Overexpression of miR-29a-3p
significantly lowered GH mRNA levels in GH3 cells and GH
concentration in the medium as compared with the NC group.
Overexpression of IGF-1 had the opposite effects and blocked the
inhibitory effects of miR-29a-3p on the secretory function of GH3
cells (P<0.05, Fig. 5E and F).
These results suggested that miR-29a-3p inhibited the expression
and secretion of PRL and/or GH in MMQ and GH3 cells by inhibiting
IGF-1.
Regulatory effects of miR-29a-3p/IGF-1
on prolactinoma is related to β-catenin
To further analyze the mechanism used by
miR-29a-3p/IGF-1 to regulate the progression and secretion of
prolactinoma, β-catenin levels were measured. The results showed
that increasing miR-29a-3p expression significantly increased
β-catenin levels in the cytoplasmic fractions and significantly
decreased levels in the nuclear fractions of MMQ and GH3 cells. On
the contrary, IGF-1 promoted β-catenin activation and translocation
into the nucleus. In addition, overexpression of IGF-1 reversed the
inhibitory effects of miR-29a-3p on β-catenin (P<0.05, Fig. 6A-D). Collectively, these results
suggested that the effects of miR-29a-3p/IGF-1 on the
proliferation, metastasis and secretion of MMQ and GH3 cells were
related to β-catenin.
Discussion
Current treatment regimens for pituitary tumors
involve the use hormones, which limits applications for the
treatment of prolactinoma (25).
Pituitary tumors secrete PRL and GH, which can cause additional
physiological effects (26,27). Therefore, it is of great
significance to identify novel targets for the diagnosis and
treatment of prolactinoma.
IGF-1, also known as somatomedin C, is produced by
autocrine and paracrine cells, and is necessary for the
physiological effects of GH. In a rat model of prolactinoma induced
by 17-β estradiol, inhibition of IGF-1 alleviated serum levels of
PRL and reduced the blood vessel density of prolactinoma (28). Imatinib is an IGF-1 inhibitor that
also can inhibit the secretion of GH by GH3 cells in vitro
(29). Clinical studies have
reported that increased IGF-1 expression is associated with the
proliferation of pituitary tumor cells and a poorer prognosis
(30). The results of the present
study suggested that inhibition of IGF-1 by exogenous methods could
inhibit the growth of prolactinoma and reduce the secretion of PRL
and GH. In vivo, IGF-1 is also targeted by miRNAs (31,32).
In polycystic ovary syndrome, IGF-1 is targeted by miR-323-3p and
miR-323-3p/IGF-1 is involved in the regulation of steroid secretion
and human cumulus cell function (33). miR-135a inhibits the proliferation
and metastasis of lung cancer cells by targeting IGF-1 to inhibit
the AKT pathway (34). In the
present study, miR-29a-3p was downregulated and IGF-1 was
upregulated in prolactinoma cells. Moreover, it was predicted and
verified that miR-29a-3p was an upstream regulator of IGF-1 and,
thus, presents a possible target to reduce IGF-1 mRNA and protein
levels. These results also suggested that miR-29a-3p might play a
role in the development and progression of prolactinoma by
regulating IGF-1.
In order to further analyze the effects of
miR-29a-3p/IGF-1 on prolactinoma, miR-29a-3p and/or IGF-1 was
overexpressed in MMQ and GH3 cells. The results showed that
miR-29a-3p inhibited the proliferation and induced apoptosis of
prolactinoma cells, while IGF-1 blocked the inhibitory effects of
miR-29a-3p. miR-29a participates in inflammatory-induced damage and
apoptosis of cardiomyocytes caused by obesity or oxidative stress
via targeting the expression of IGF-1 (35,36).
Regarding the biological behavior of tumor cells, members of the
miR-29 family inhibit the proliferation and induce apoptosis of
breast cancer cells by inhibiting IGF-1 (37), as well as inhibiting the migration
of vascular endothelial cells (38). Taken together, the results of the
present and previous studies indicate that miR-29a-3p can inhibit
the proliferation and induce apoptosis of prolactinoma cells, and
these functions are related to the targeted inhibition of IGF-1
expression.
β-catenin, which is a key protein in the Wnt
pathway, can enter the nucleus to regulate gene transcription.
Inhibition of Wnt/β-catenin can reduce PRL secretion by MMQ cells
(39,40). Inhibiting the nuclear translocation
and inactivating the function of β-catenin can restore the
proliferative ability of prolactinoma cells (41). The results of the present study
showed that increasing the expression of miR-29a-3p inhibited the
nuclear translocation of β-catenin in MMQ and GH3 cells, while
inhibiting the secretion of PRL and/or GH. Overexpression of IGF-1
restored the retention of β-catenin in the cytoplasm caused by
miR-29a-3p. A study by Lei et al (42) showed that miR-137 inhibited the
proliferation and tumorigenesis of GH3 cells by inhibiting the
expression of microphthalmia-associated transcription factor, and
inhibiting the nuclear translocation of β-catenin can further
strengthen the inhibitory effect of miR-137 on GH3 cells,
suggesting that the inhibitory effect of miR-29a-3p on the
biological behavior and secretory function of prolactinoma may be
achieved by inhibiting β-catenin via a molecular mechanism that is
inseparable from IGF-1. However, in addition to miR-29a-3p, there
are other miRNAs that can target IGF-1. This has not yet been
reported in prolactinoma, and thus we will focus on this area in
future research. Moreover, the expression level and clinical
significance of miR-29-3p in prolactinoma tissue samples still need
to be further explored.
In conclusion, the level of miR-29a-3p in
prolactinoma was suppressed, thus increasing miR-29a-3p expression
may inhibit the proliferation of prolactinoma cells and the
secretion of PRL and GH by targeting IGF-1 via mechanisms that may
be related to the β-catenin pathway. However, further studies are
needed to elucidate the relationship between miR-29a-3p and
prolactinoma, and the underlying mechanism regulating
IGF-1/β-catenin in prolactinoma cells must be verified in
vivo.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WY and JX contributed to the conception of the
study. WY and JX confirm the authenticity of all the raw data. WY,
SL, DM, WG, HL and JX performed the experiment. WY, SL, WG, DM, HL
and JX contributed significantly to data analysis and manuscript
preparation, including writing of the manuscript and constructive
discussions. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Glezer A and Bronstein MD: Prolactinomas.
Endocrinol Metab Clin North Am. 44:71–78. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vroonen L, Daly AF and Beckers A:
Epidemiology and management challenges in prolactinomas.
Neuroendocrinology. 109:20–27. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Blanco AM: Hypothalamic- and
pituitary-derived growth and reproductive hormones and the control
of energy balance in fish. Gen Comp Endocrinol. 287:1133222020.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Silveira MA, Zampieri TT, Furigo IC,
Abdulkader F, Donato J Jr and Frazão R: Acute effects of
somatomammotropin hormones on neuronal components of the
hypothalamic-pituitary-gonadal axis. Brain Res. 1714:210–217. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kubo T, Furujo M, Mori S, Imai K, Ueda Y,
Tsukahara K, Morita H, Ogura K, Fukuhara S, Shimizu J, et al: An
infant case of macroprolactinemia with transient idiopathic central
precocious puberty. Endocr J. 54:825–828. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tirosh A and Shimon I: Current approach to
treatments for prolactinomas. Minerva Endocrinol. 41:316–323.
2016.PubMed/NCBI
|
|
7
|
Faltermeier CM, Magill ST, Blevins LS Jr
and Aghi MK: Molecular biology of pituitary adenomas. Neurosurg
Clin N Am. 30:391–400. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Donoho DA and Laws ER Jr: The role of
surgery in the management of prolactinomas. Neurosurg Clin N Am.
30:509–514. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen T, Zheng F, Tao J, Tan S, Zeng L,
Peng X and Wu B: Insulin-like growth factor-1 contributes to
mucosal repair by β-arrestin2-mediated extracellular signal-related
kinase signaling in experimental colitis. Am J Pathol.
185:2441–2453. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yoneyama Y, Lanzerstorfer P, Niwa H,
Umehara T, Shibano T, Yokoyama S, Chida K, Weghuber J, Hakuno F and
Takahashi SI: IRS-1 acts as an endocytic regulator of IGF-I
receptor to facilitate sustained IGF signaling. Elife.
7:e328932018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lyons A, Coleman M, Riis S, Favre C,
O'Flanagan CH, Zhdanov AV, Papkovsky DB, Hursting SD and O'Connor
R: Insulin-like growth factor 1 signaling is essential for
mitochondrial biogenesis and mitophagy in cancer cells. J Biol
Chem. 292:16983–16998. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen KC, Chen PH, Ho KH, Shih CM, Chou CM,
Cheng CH and Lee CC: IGF-1-enhanced miR-513a-5p signaling
desensitizes glioma cells to temozolomide by targeting the
NEDD4L-inhibited Wnt/β-catenin pathway. PLoS One. 14:e02259132019.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cónsole GM, Hereñú CB, Camihort GA, Luna
GC, Ferese C and Goya RG: Effect of insulin-like growth factor-I
gene therapy on the somatotropic axis in experimental
prolactinomas. Cells Tissues Organs. 190:20–26. 2009. View Article : Google Scholar
|
|
14
|
Castillo AI and Aranda A: Differential
regulation of pituitary-specific gene expression by insulin-like
growth factor 1 in rat pituitary GH4C1 and GH3 cells.
Endocrinology. 138:5442–5451. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Simonson B and Das S: MicroRNA
therapeutics: The next magic bullet? Mini Rev Med Chem. 15:467–474.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu B, Mao Z, Du Q, Jiang X, Wang Z, Xiao
Z, Zhu D, Wang X, Zhu Y and Wang H: miR-93-5p targets Smad7 to
regulate the transforming growth factor-β1/Smad3 pathway and
mediate fibrosis in drug-resistant prolactinoma. Brain Res Bull.
149:21–31. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jian M, Du Q, Zhu D, Mao Z, Wang X, Feng
Y, Xiao Z, Wang H and Zhu Y: Tumor suppressor miR-145-5p sensitizes
prolactinoma to bromocriptine by downregulating TPT1. J Endocrinol
Invest. 42:639–652. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xiao Z, Wang Z, Hu B, Mao Z, Zhu D, Feng Y
and Zhu Y: MiR-1299 promotes the synthesis and secretion of
prolactin by inhibiting FOXO1 expression in drug-resistant
prolactinomas. Biochem Biophys Res Commun. 520:79–85. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao X, Hou Y, Tuo Z and Wei F:
Application values of miR-194 and miR-29 in the diagnosis and
prognosis of gastric cancer. Exp Ther Med. 15:4179–4184.
2018.PubMed/NCBI
|
|
20
|
Xu W, Li Z, Zhu X, Xu R and Xu Y: miR-29
family inhibits resistance to methotrexate and promotes cell
apoptosis by targeting COL3A1 and MCL1 in osteosarcoma. Med Sci
Monit. 24:8812–8821. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gao G, Liang X and Ma W: Sinomenine
restrains breast cancer cells proliferation, migration and invasion
via modulation of miR-29/PDCD-4 axis. Artif Cells Nanomed
Biotechnol. 47:3839–3846. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Habibi P, Alihemmatti A, Alipour M,
Nourazar A, Yousefi H, Andalib S and Ahmadiasl N: Effects of
exercise on miR-29 and IGF-1 expression and lipid profile in the
heart of ovariectomized rat. Acta Endocrinol (Buchar). 12:130–136.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tang H, Zhu D, Zhang G, Luo X and Xie W:
AFAP1-AS1 promotes proliferation of pituitary adenoma cells through
miR-103a-3p to activate PI3K/AKT signaling pathway. World
Neurosurg. 130:e888–e898. 2019. View Article : Google Scholar
|
|
25
|
Molitch ME: Diagnosis and treatment of
pituitary adenomas: A review. JAMA. 317:516–524. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lamberts SW, de Quijada M and Klijn JG:
The effect of tamoxifen on GH and PRL secretion by human pituitary
tumors. J Endocrinol Invest. 3:343–347. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Watanabe D, Yagasaki H, Kojika S, Ogiwara
M, Kinouchi H, Nakane T and Inukai T: GH/PRL-secreting pituitary
macroadenoma associated with GNAS p.Gln227Leu mutation: Pediatric
case report and review. Endocr J. 66:403–408. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Console GM, Herenu CB, Camihort GA, Luna
GC, Bracamonte MI, Morel GR and Goya RG: Insulin-like growth
factor-I gene therapy reverses morphologic changes and reduces
hyperprolactinemia in experimental rat prolactinomas. Mol Cancer.
7:132008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gupta P, Rai A, Mukherjee KK, Sachdeva N,
Radotra BD, Punia RPS, Vashista RK, Hota D, Srinivasan A,
Dhandapani S, et al: Imatinib inhibits GH secretion from
somatotropinomas. Front Endocrinol (Lausanne). 9:4532018.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kempf J, Schmitz A, Meier A, Delfs N,
Mueller B, Fandino J, Schuetz P and Berkmann S: Adenoma size and
postoperative IGF-1 levels predict surgical outcomes in acromegaly
patients: Results of the Swiss pituitary registry (SwissPit). Swiss
Med Wkly. 148:w146532018.PubMed/NCBI
|
|
31
|
O'Neill BT, Lee KY, Klaus K, Softic S,
Krumpoch MT, Fentz J, Stanford KI, Robinson MM, Cai W, Kleinridders
A, et al: Insulin and IGF-1 receptors regulate FoxO-mediated
signaling in muscle proteostasis. J Clin Invest. 126:3433–3446.
2016. View
Article : Google Scholar
|
|
32
|
Cui X, Li M, He Z, Hu L, Liu J, Yan J and
Hua L: MiR-302b-5p enhances the neuroprotective effect of IGF-1 in
methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson's
disease by regulating inducible nitric-oxide synthase. Cell Biochem
Funct. 38:1025–1035. 2020. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang T, Liu Y, Lv M, Xing Q, Zhang Z, He
X, Xu Y, Wei Z and Cao Y: miR-323-3p regulates the steroidogenesis
and cell apoptosis in polycystic ovary syndrome (PCOS) by targeting
IGF-1. Gene. 683:87–100. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhou Y, Li S, Li J, Wang D and Li Q:
Effect of microRNA-135a on cell proliferation, migration, invasion,
apoptosis and tumor angiogenesis through the IGF-1/PI3K/Akt
signaling pathway in non-small cell lung cancer. Cell Physiol
Biochem. 42:1431–1446. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu Y, Hu Q, Ao J, Li H and Li M: Role of
miR-92a-3p/PTEN axis in regulation of pancreatic cancer cell
proliferation and metastasis. Zhong Nan Da Xue Xue Bao Yi Xue Ban.
45:280–289. 2020.(In English, Chinese). PubMed/NCBI
|
|
36
|
Wang Y, Zhao R, Liu W, Wang Z, Rong J,
Long X, Liu Z, Ge J and Shi B: Exosomal circHIPK3 released from
hypoxia-pretreated cardiomyocytes regulates oxidative damage in
cardiac microvascular endothelial cells via the miR-29a/IGF-1
pathway. Oxid Med Cell Longev. 2019:79546572019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shastri AA, Saleh A, Savage JE, DeAngelis
T, Camphausen K and Simone NL: Dietary alterations modulate the
microRNA 29/30 and IGF-1/AKT signaling axis in breast cancer liver
metastasis. Nutr Metab (Lond). 17:232020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li Z, Jiang R, Yue Q and Peng H:
MicroRNA-29 regulates myocardial microvascular endothelial cells
proliferation and migration in association with IGF1 in type 2
diabetes. Biochem Biophys Res Commun. 487:15–21. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cao L, Gao H, Li P, Gui S and Zhang Y: The
Wnt/β-catenin signaling pathway is involved in the antitumor effect
of fulvestrant on rat prolactinoma MMQ cells. Tumour Biol.
35:5121–5127. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chauvet N, Romanò N, Meunier AC, Galibert
E, Fontanaud P, Mathieu MN, Osterstock G, Osterstock P, Baccino E,
Rigau V, et al: Combining cadherin expression with molecular
markers discriminates invasiveness in growth hormone and prolactin
pituitary adenomas. J Neuroendocrinol. 28:123522016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang C, Tan C, Wen Y, Zhang D, Li G, Chang
L, Su J and Wang X: FOXP1-induced lncRNA CLRN1-AS1 acts as a tumor
suppressor in pituitary prolactinoma by repressing the autophagy
via inactivating Wnt/β-catenin signaling pathway. Cell Death Dis.
10:4992019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lei C, Jing G, Jichao W, Xiaohui L, Fang
Q, Hua G, Yazhou M and Zhang Y: MiR-137′s tumor suppression on
prolactinomas by targeting MITF and modulating Wnt signaling
pathway. J Clin Endocrinol Metab. 104:6391–6402. 2019. View Article : Google Scholar : PubMed/NCBI
|