Introduction
Sepsis is a type of clinical disorder characterized
by dysregulated immune and systemic inflammatory response to
infections (1) and is induced by
pathogens such as lipopolysaccharide (LPS)-releasing gram-negative
bacteria. Sepsis can cause severe injuries in multiple organs,
leading to a mortality rate ranging 15–25% (2). Without timely diagnosis and treatment,
sepsis can cause irreversible septic shock, tissue damage and
failure of multiple organs. Therefore, early diagnosis remains the
key for the survival of patients with sepsis (3). Myocardial dysfunction (MD) is a common
complication of sepsis (4,5). MD is mainly caused by global
myocardial ischemia in sepsis (5).
The early onset of sepsis-induced MD lacks obvious and classic
clinical symptoms and the early detection is poor, leading to
inferior treatment outcomes (6).
Studies on the pathogenesis of sepsis have
identified many molecular factors involved in the occurrence and
progression of sepsis (7,8). The development of sepsis requires the
involvement of non-coding RNAs (ncRNAs), such as long (>200 nt)
ncRNAs (lncRNAs) (9). Some lncRNAs
have been demonstrated to be critical factors in sepsis and
regulation of their expression is considered as a potential
therapeutic target for sepsis (10,11).
However, the functions of most lncRNAs in sepsis remain to be
elucidated. LncRNA NR024118 has been characterized as an inhibitor
of LPS-induced inflammatory injury (12), a key player in sepsis (13). Preliminary RNA-seq data in the
present study revealed an inverse correlation between NR024118 and
IL-6, which promotes inflammatory responses in patients with sepsis
(14). Therefore, it was
hypothesized that NR024118 may also participate in sepsis. The
present study was therefore performed to investigate the
interaction between NR024118 and IL-6 in sepsis.
Materials and methods
Patients with sepsis and healthy
controls
The present study was approved by the Ethics
Committee of Guangxi Zhuang Autonomous National Hospital (China;
approval no. 35765323). Research subjects included 82 patients with
sepsis but without MD (Sepsis group; 50 males and 32 females, age
range 37–67 years old, mean age 52.1±6.0 years old), 35 patients
with sepsis and MD (MD group; 23 males and 12 females, age range
37–68 years old, mean age 51.8±6.4 years old) and 82 healthy
controls (Control group; 50 males and 32 females, age range 37–67
years old, mean age 52.3±6.3 years old). In all patients with
sepsis included in the present study sepsis was caused by bacterial
infection. Patients with sepsis were diagnosed by blood test. MD
was diagnosed by echocardiogram (ejection fraction below 45%),
blood tests and symptoms, such as shoulder or arm pain, shortness
of breath and fatigue. The present study excluded patients with MD
caused by factors other than sepsis. All participants were enrolled
at aforementioned hospital between March 2017 and March 2019.
Patients were all newly diagnosed cases and recurrent cases were
excluded. Exclusion criteria included: i) Patients afflicted with
other diseases, such as cancer and metabolic diseases; and ii)
patients who were treated by any therapies prior to admission. All
patients signed informed consent before this study. Baseline
clinical data of three groups of participants are listed in
Table I.
 | Table I.Baseline clinical data of three groups
of participants. |
Table I.
Baseline clinical data of three groups
of participants.
|
| Controls (82) | Sepsis patients
(82) | MD patients (35) |
|---|
| Age (year) | 52.3±6.3 years
old | 52.1±6.0 years
old | 51.8±6.4 years
old |
| Sex
(male/female) | 50/32 | 50/32 | 23/12 |
| Body mass index
(kg/m2) | 21.04±1.34 | 20.99±1.48 | 20.87±1.43 |
| White blood cells
(×109/l) | 6.99±1.83 | 19.34±6.77 | 19.98±6.98 |
| Serum creatinine
(mg/dl) | 1.09±0.31 | 1.72±0.43 | 1.75±0.46 |
| C-reactive protein
(mg/l) | 6.00±3.11 | 89.23±23.77 | 93.29±20.03 |
| Procalcitonin
(ng/ml) | 0.03±0.01 | 9.99±3.78 | 7.98±3.12 |
| Albumin (g/l) | 41.56±4.22 | 24.74±4.49 | 25.03±4.23 |
| APACHE II score | – | 12.77±3.45 | 12.57±3.67 |
| SOFA score | – | 5.5±1.49 | 5.7±1.72 |
Plasma preparation
On the day of admission all participants were
subjected to blood (5 ml) extraction under fasting conditions. All
blood samples were transferred to BD Vacutainer® PPT™
plasma preparation tubes (BD Diagnostics; Becton, Dickinson and
Company) to be centrifuged at 1,200 × g at room temperature for 12
h to prepare plasma samples. Plasma samples were stored at −80°C
before use.
Cardiomyocytes and cell culture
The human cardiomyocyte cell line AC16 was purchased
from Sigma-Aldrich (Merck KGaA). Cell culture medium was composed
of 10% FBS and 90% cardiomyocyte growth medium (ScienCell Research
Laboratories, Inc.). Cells were cultivated in a 5% CO2
incubator at 37°C to reach the confluence of ~80%. AC16 cells were
treated with LPS at different concentrations (0, 150, 300 and 500
ng/ml) for 24 h to induce sepsis models.
Vector construction and transient
transfections
PcDNA3.1 vector was used as the backbone to
establish the expression vector of NR024118 (NCBI ID: NR_024118.1).
The vector construction service was provided by Invitrogen (Thermo
Fisher Scientific, Inc.). The short interfering RNAs (siRNAs)
targeting NR024118, si-NR024118 (CCACCACCATCTTCCTCAATGGCAA), were
designed and synthesized by Guangzhou RiboBio Co., Ltd., as were
the negative control siRNAs for knockdown experiments. Transient
transfections were used to transfect 10 nM vector (10 nM empty
vector transfection as negative control group, NC; 10 nM small
interfering empty vector transfection as negative control group,
si-NC) into 106 AC16 cells. Untransfected cells were
used as the control (C) cells. Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) was used to perform
transient transfections with plasmids or siRNAs (40 nM). The vector
was first mixed with Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) to prepare transfection mixture.
Cells were then incubated with the transfection mixture for 6 h,
followed by washing with fresh medium. Cells were cultivated in
fresh medium for another 48 h prior to LPS treatment. The cell
transfection rates were detected by reverse
transcription-quantitative (RT-q) PCR.
RNA extraction
TRIzol® (Thermo Fisher Scientific, Inc.)
was used to extract total RNAs from plasma and AC16 cells
(106 cell/well) according to manufacturer's protocol. In
the cases of LPS treatment, AC16 cells were cultivated in medium
containing 0, 150, 300 or 500 ng/ml LPS for 24 h before use. All
RNA samples were incubated with gDNA eraser (Takara Biotechnology
Co., Ltd.) at 37°C for 1 h to remove genomic DNA.
RT-qPCR
Precision nanoScript2 Reverse Transcription kit
(Primerdesign Ltd.) was used to reverse transcribe RNA samples into
cDNA and SYBR® Green Quantitative RT-qPCR kit
(Sigma-Aldrich; Merck KGaA) was used to prepare all qPCR reactions
all according to the manufacturers' protocols. The expression
levels of NR024118 were determined with 18S rRNA as endogenous
control. The PCR conditions were; 95°C for 30 sec, 95°C for 10 sec
and 60°C for 35 sec for a total of 40 cycles. Overexpression of
NR024118 in AC16 cells was confirmed by RT-qPCR. In all, three
replicate qPCR reactions were included in each experiment and the
2−ΔΔCq method was used to calculate the fold changes of
gene expression levels (15).
Primer sequences were: 5′-AGGTTGGCTGGTGTTCCAGC-3′ (forward) and
5′-CACACGCATAGAGTAGTCTC-3′ (reverse) for NR024118;
5′-CTACCACATCCAAGGAAGCA-3′ (forward) and
5′-TTTTTCGTCACTACCTCCCCG-3′ (reverse) for human 18S rRNA.
Electrophysiological study
All whole-cell patch-clamp recordings were made from
single AC16 cells in the presence of LPS, using the Axopatch 200B
patch clamp amplifier (Axon Instruments; Molecular Devices, LLC) at
5 kHz in the fast-current clamp mode at 35±1°C.
ELISA
The levels of TNF-α, IL-1β and IL-6 (presented as
ng/ml) in plasma and the cell culture medium of AC16 cells
(centrifuged at 1,000 × g for 20 min at 4°C to remove cells,)
collected at 48 h post-transfection were detected using human TNF-α
(cat. no. MTA00B; Abcam), IL-1β (cat. no. MLB00C; Abcam) and IL-6
(cat. no. ab46042; Abcam) ELISA kits.
Cell apoptosis analysis
The effects of transfection on the apoptosis of AC16
cells were analyzed by cell apoptosis assay at 48 h
post-transfection. AC16 cells were transferred to fresh cell
culture medium supplemented with LPS at a dose of 500 ng/ml.
Subsequently, pre-cold phosphate-buffered saline (PBS) was used to
resuspend cells, followed by staining with Annexin V-FITC/PI
Apoptosis Detection kit (Beijing Solarbio Science & Technology
Co., Ltd.) for 20 min in dark at room temperature. Flow cytometry
(FACStar PLUS; Becton, Dickinson and Company) was then used to
detect the apoptotic cells. The apoptotic rate was calculated by
the percentage of early + late apoptotic cells. FlowJo software
(version 7.6.3; FlowJo, LLC) was used to analyze the results.
Luciferase report assays
Luciferase assay for the transcriptional activity of
NF-κB was implemented by co-transfection of a NF-κB TransLucent
reporter vector (NF-κB/Luc; Panomics, Inc.; Thermo Fisher
Scientific, Inc.) and a plasmid construct for β-galactosidase
(β-gal; Clontech Laboratories, Inc.) expression in AC16 cells, as a
transfection control, using Lipofectamine® 2000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). Cell lysates were
prepared 24 h after transfection, and luciferase activity was
measured with the Luciferase Assay System (Promega Corporation)
following the manufacturer's instructions. NF-κB activity were
expressed after normalized to β-gal activity. BAY treatment was
introduced into NR024118 overexpressing cells to inhibit the NF-κB
pathway, with DMSO as control.
Statistical analyses
Each experiment was performed in triplicate and mean
values were calculated. GraphPad Prism 6 (GraphPad Software, Inc.)
software was used to perform all statistical analyses. One-way
analysis of variance (ANOVA) combined with Tukey's post hoc test
was used to compare differences among multiple groups. Correlations
were analyzed by Pearson's correlation coefficient. P<0.05 was
considered to indicate a statistically significant difference.
Results
NR024118 is downregulated in sepsis
and further downregulated in sepsis combined with MD
The expression levels of NR024118 in plasma
collected from Sepsis group (n=82), MD group (n=35) and the Control
group (n=82) were measured by performing RT-qPCR. Compared with the
Control group, the expression levels of NR024118 were significantly
lower in Sepsis and MD groups (Fig.
1; P<0.05). In addition, the expression levels of NR024118
were also significantly lower in MD group than that in Sepsis group
(Fig. 1; P<0.05).
IL-6 is upregulated in sepsis and
further upregulated in sepsis combined with MD
The expression levels of IL-6 in plasma collected
from Sepsis group (n=82), MD group (n=35) and the Control group
(n=82) were measured by ELISA. Compared with the Control group, the
expression levels of IL-6 were significantly increased in Sepsis
and MD groups (Fig. 2; P<0.05).
In addition, the expression levels of IL-6 were also significantly
higher in MD group than that in Sepsis group (Fig. 2; P<0.05).
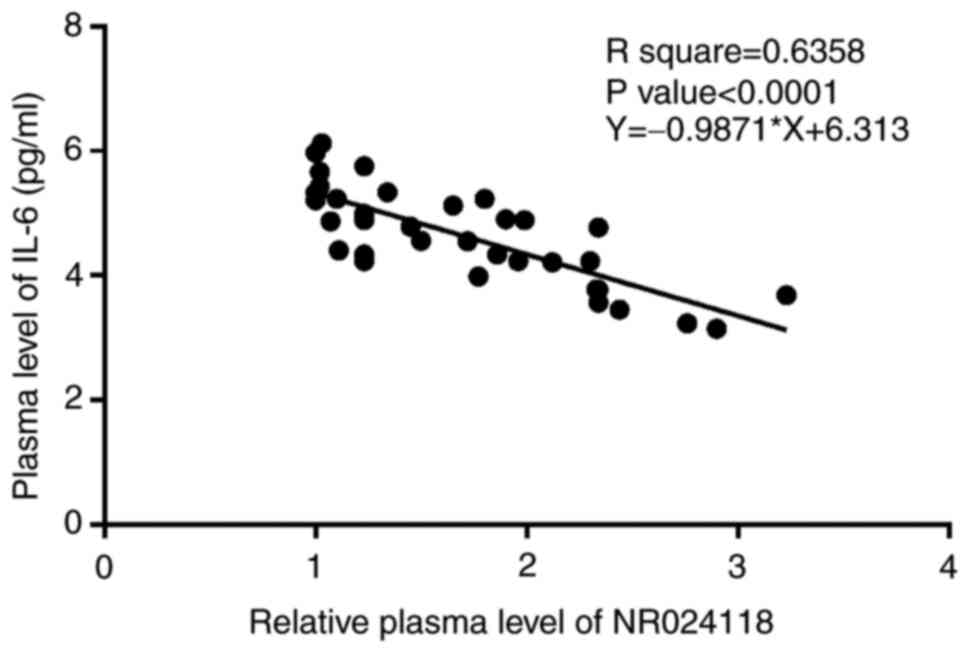
NR024118 and IL-6 are inversely
correlated in sepsis with MD
The correlation between the expression levels of
NR024118 and IL-6 across plasma samples from MD group (n=35) were
analyzed by Pearson's correlation coefficient. It was observed that
NR024118 and IL-6 were inversely and significantly correlated
(Fig. 3). The close correlation
indicated possible interaction.
LPS can induce MD
To determine the effect of LPS on myocardial cells,
AC-16 cells were treated with different concentrations (0, 150, 300
and 500 ng/ml) of LPS. Flow cytometry results demonstrated that the
apoptosis ability of AC-16 cells was enhanced with the increase of
LPS concentration (Fig. 4A).
Simultaneously, the APD in the LPS group were significantly shorter
compared with that in the control group (Fig. 4B). In addition, to determine whether
myocardial cells had an inflammatory response, the contents of
inflammatory cytokines were measured. ELISA demonstrated that the
amount of TNF-α, IL-1β and IL-6 were enhanced with the increase of
LPS concentration, indicating the increased inflammatory response
of AC-16 cells (Fig. 4B-E). These
data confirmed that LPS could induce the expression of inflammatory
factors and shorten APD to construct sepsis-induced MD.
Overexpression of NR024118 reduces the
secretion of IL-6 from cardiomyocytes and ameliorated LPS-induced
myocardial APD duration and cell injury
AC16 cells were cultivated in medium containing LPS
(0, 150, 300 or 500 ng/ml) for 24 h, followed by measurement of the
expression levels of NR024118 by RT-qPCR. It was observed that LPS
treatment decreased the expression levels of NR024118 in AC16 cells
in a dose-dependent manner (Fig.
5A; P<0.05). Subsequently, AC16 cells were transfected with
NR024118 expression vector. Overexpression of NR024118 in AC16
cells were confirmed by RT-qPCR. Compared with the C (untransfected
cells) and NC (empty vector transfection) groups, the expression
levels of NR024118 were significantly increased at 48 h
post-transfection (Fig. 5B;
P<0.05). In addition, Compared with the C and si-NC (small
interfering empty vector transfection) group, the expression levels
of NR024118 were significantly decreased by si-NR024118. In
addition, APD90 duration following the overexpression of
NR024118 in AC16 cells were longer (Fig. 5C; P<0.05). ELISA was performed to
analyze the secretion of IL-16 from AC16 cells in each transfection
group. Compared with C and NC groups, IL-6 secretion was
significantly reduced in AC16 cells with the overexpression of
NR024118, while silencing of NR024118 demonstrated the opposite
results (Fig. 5D; P<0.05). Cell
apoptosis assay was performed to assess the effects of NR024118 on
LPS-induced apoptosis of AC16 cells. Compared with C and NC groups,
overexpression of NR024118 significantly decreased the apoptosis of
AC16 cells, while silencing of NR024118 had the opposite effect
(Fig. 5E; P<0.05).
NR024118 regulates LPS-induced MD
through the NF-κB signaling pathway
The NF-κB signaling pathway is a classic signaling
pathway associated with inflammatory response. To determine whether
LPS-induced cellular inflammation response could be achieved by
regulating the NF-κB signaling pathway, the effect of NR02411 on
the NF-κB signaling pathway in LPS-treated AC16 cells was
evaluated. Western blot analysis demonstrated that p65
phosphorylation (Fig. 6A) and NF-κB
activation (Fig. 6B) were inhibited
by overexpression of NR02411 while promoted by inhibition of
NR02411. In addition, IL-6 production was also elevated in
cardiomyocytes with the silencing of NR02411, while this effect can
be counteracted by further treatment with BAY (Fig. 6C). These results demonstrated that
NR02411 could negatively regulate IL-6 production by inhibiting p65
phosphorylation and the NF-κB signaling pathway.
Discussion
The involvement of NR024118 in sepsis and
sepsis-induced MD was investigated in the present study. It was
found that NR024118 was downregulated in sepsis and further
downregulated in sepsis with MD. NR024118 may suppress the
secretion of IL-16 and cell apoptosis to improve sepsis.
The functions of NR024118 have been mainly
investigated in inflammatory responses (12,16).
It has been reported that NR024118 can interact with the NF-κB/Nrf2
signaling to suppress LPS-induced cell apoptosis and inflammatory
responses in chondrocytes (12). In
a rheumatoid arthritis rat model, the expression of NR024118 in
synovial fibroblasts can be induced by Shikonin to suppress
inflammatory responses (16). These
two studies have characterized NR024118 as an inhibitor of
inflammation. Sepsis is essentially a type of inflammatory disease
(1). Consistently, the present
study also observed the downregulation of NR024118 in sepsis,
indicating the involvement of NR024118 in sepsis. A previous study
reported that in cardiac fibrosis angiotensin II induces the
downregulation of NR024118 to promote disease progression (17), indicating the involvement of
NR024118 in heart diseases. In the present study further
downregulation of NR024118 was observed in patients with sepsis
combined with MD. In addition, overexpression of NR024118 decreased
heart cell apoptosis. Therefore, NR024118 may have protective
effects on sepsis induced MD.
IL-6 is a major contributor to inflammatory
responses in patients with sepsis (14). The present study also observed
further upregulation of IL-6 in patients with sepsis with MD
compared with patients with sepsis without MD. Therefore, the
further upregulation of IL-6 may promote the development of MD. The
present study observed an inverse correlation between NR024118 and
IL-6 across plasma samples from patients with sepsis complicated
with MD. LPS is often used to induce cellular sepsis models in
vitro due to its ability to bind to Toll-like receptor 4 and
activate inflammatory response (18). In the LPS-treated myocytes, APD was
significantly shortened under sepsis (19). The effect of LPS on myocardial cells
was evaluated and it was found that LPS could promote apoptosis as
well as the amounts of TNF-α, IL-1β and IL-6, indicating the
increased inflammatory response of AC16 cells. Simultaneously, the
APD in the LPS group was shorted significantly compared with that
in the control group.
In summary, the results of the present study
demonstrated that NR024118 was significantly inhibited in
LPS-induced AC16 cells and overexpression of NR024118 reduced IL-6
secretion and increased APD, while silencing of NR024118 had the
opposite effect. It has been well established that IL-6 can promote
inflammatory responses in patients with sepsis to aggregate disease
conditions (14). Therefore,
NR024118 suppresses the secretion of IL-6 to relieve sepsis and
prevent sepsis induced MD. In addition, p65 phosphorylation and
NF-κB activation were inhibited by overexpression of NR02411 and
promoted by inhibition of NR02411. This suggested that NR02411
alleviated the inflammatory response of LPS-induced myocardial
cells mainly by inhibiting the NF-κB signaling pathway. Therefore,
it was concluded that NR024118 was suppressed in sepsis and
inhibited LPS-induced apoptosis of cardiomyocytes. However, the
present study only included in vitro cell experiments, which
may not fully reflect the disease conditions in the heart of
patients with sepsis. Future studies may explore the expression of
NR02411 in sepsis-induced myocardial tissues and analyze the
function of NR02411 in vivo through animal models.
In conclusion, NR024118 was downregulated in sepsis
and sepsis-induced MD. NR024118 may interact with IL-6 and regulate
heart cell apoptosis to participate in sepsis induced MD.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
GQ and LW were responsible for study design, writing
the manuscript, literature searches, performing experiments and
data analysis. FJ and JL were responsible for literature searches,
performing experiments and data analysis. BZ, DP and XL were
responsible for data acquisition and statistical analysis. GQ and
LW confirmed the authenticity of all the raw data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee Guangxi Zhuang Autonomous National Hospital. The present
study was performed in accordance with the World Medical
Association Declaration of Helsinki. All patients provided written
informed consent prior to their inclusion within the present
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
MD
|
myocardial dysfunction
|
|
ncRNAs
|
non-coding RNAs
|
|
lncRNAs
|
long (>200 nt) ncRNAs
|
References
|
1
|
Hotchkiss RS, Moldawer LL, Opal SM,
Reinhart K, Turnbull IR and Vincent JL: Sepsis and septic shock.
Nat Rev Dis Primers. 2:160452016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Moore JX, Donnelly JP, Griffin R, Howard
G, Safford MM and Wang HE: Defining sepsis mortality clusters in
the United States. Crit Care Med. 44:1380–1387. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Resch B, Gusenleitner W and Müller WD:
Procalcitonin and interleukin-6 in the diagnosis of early-onset
sepsis of the neonate. Acta Paediatr. 92:243–245. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Krishnagopalan S and Kumar A, Parrillo JE
and Kumar A: Myocardial dysfunction in the patient with sepsis.
Curr Opin Crit Care. 8:376–388. 2020. View Article : Google Scholar
|
|
5
|
Kakihana Y, Ito T, Nakahara M, Yamaguchi K
and Yasuda T: Sepsis-induced myocardial dysfunction:
Pathophysiology and management. J Intensive Care. 4:222016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ibrahim MH, Azab AA, Kamal NM, Salama MA,
Ebrahim SA, Shahin AM, El-Sadek AE, Abdulghany WE, Sherief LM and
Abdallah EA: Early detection of myocardial dysfunction in poorly
treated pediatric thalassemia children and adolescents: Two Saudi
centers experience. Ann Med Surg (Lond). 9:6–11. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Englert JA, Bobba C and Baron RM:
Integrating molecular pathogenesis and clinical translation in
sepsis-induced acute respiratory distress syndrome. JCI Insight.
4:e1240612019.(Online ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rossaint J and Zarbock A: Pathogenesis of
multiple organ failure in sepsis. Crit Rev Immunol. 35:277–291.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang TN, Li D, Xia J, Wu QJ, Wen R, Yang
N and Liu CF: Non-coding RNA: A potential biomarker and therapeutic
target for sepsis. Oncotarget. 8:91765–91778. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fang Y, Hu J, Wang Z, Zong H, Zhang L,
Zhang R and Sun L: LncRNA H19 functions as an Aquaporin 1
competitive endogenous RNA to regulate microRNA-874 expression in
LPS sepsis. Biomed Pharmacother. 105:1183–1191. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shen J, Zhang J, Jiang X, Wang H and Pan
G: LncRNA HOX transcript antisense RNA accelerated kidney injury
induced by urine-derived sepsis through the miR-22/high mobility
group box 1 pathway. Life Sci. 210:185–191. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mei X, Tong J, Zhu W and Zhu Y:
lncRNA-NR024118 overexpression reverses LPS-induced inflammatory
injury and apoptosis via NF-κB/Nrf2 signaling in ATDC5
chondrocytes. Mol Med Rep. 20:3867–3873. 2019.PubMed/NCBI
|
|
13
|
He G, Zhang X, Chen Y, Chen J, Li L and
Xie Y: Isoalantolactone inhibits LPS-induced inflammation via NF-κB
inactivation in peritoneal macrophages and improves survival in
sepsis. Biomed Pharmacother. 90:598–607. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hou T, Huang D, Zeng R, Ye Z and Zhang Y:
Accuracy of serum interleukin (IL)-6 in sepsis diagnosis: A
systematic review and meta-analysis. Int J Clin Exp Med.
8:15238–15245. 2015.PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang KY and Chen DL: Shikonin inhibits
inflammatory response in rheumatoid arthritis synovial fibroblasts
via lncRNA-NR024118. Evid Based Complement Alternat Med.
2015:6317372015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jiang X, Zhang F and Ning Q: Losartan
reverses the down-expression of long noncoding RNA-NR024118 and
Cdkn1c induced by angiotensin II in adult rat cardiac fibroblasts.
Pathol Biol (Paris). 63:122–125. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wei JL, Wu CJ, Chen JJ, Shang FT, Guo SG,
Zhang XC and Liu H: LncRNA NEAT1 promotes the progression of
sepsis-induced myocardial cell injury by sponging miR-144-3p. Eur
Rev Med Pharmacol Sci. 24:851–861. 2020.PubMed/NCBI
|
|
19
|
Tai BY, Wen ZH, Cheng PY, Yang HY, Duh CY,
Chen PN and Hsu CH: Lemnalol modulates the electrophysiological
characteristics and calcium homeostasis of atrial myocytes. Mar
Drugs. 17:6192019. View Article : Google Scholar : PubMed/NCBI
|