Introduction
Preeclampsia (PE) is the presence of new-onset
hypertension and proteinuria or other end organ damage that occurs
after 20 weeks of pregnancy (1). In
the clinical setting, this disease is characterized by systolic
blood pressure (SBP) ≥140 mmHg or diastolic BP ≥90 mmHg, as well as
proteinuria (≥0.3 g/24 h) (2). In
humans, it is a hypertensive multisystem disorder, which can lead
to maternal and fetal mortality and morbidity (3), and substantially contributes to
prematurity of the fetus and long-term cardiovascular disease in
the mother (4).
It has been proposed that the development of PE is
closely associated with placenta formation, particularly in early
pregnancy stages (5). Previous
studies have reported that there are several alterations that
contribute to the development of PE, including vascular
dysfunction, oxidative stress and metabolic abnormalities (6,7). In
particular, it has been demonstrated that placental vascular
development is considerably altered (8–10), and
this vascular dysfunction in placenta tissues plays a critical role
in BP increase and PE (11,12). However, the molecular mechanism
underlying PE remains largely unknown.
Sphingosine-1-phosphate receptors (S1PR) are a class
of G-protein-coupled receptors, which participate in different
cellular responses, such as proliferation and apoptosis (13,14).
The S1PRs, S1PR1, S1PR2 and S1PR3 are widely expressed in various
tissues, while S1P4 is only found in lymphoid and hematopoietic
tissues, and S1P5 is mainly expressed in the central nervous system
(15). Increasing evidence suggests
that S1P is involved in BP, such as pulmonary arterial hypertension
(PAH) (16). For example, Chen
et al (16) reported that
S1P levels were upregulated in the lungs of patients with PAH, and
that the pharmacological inhibition of sphingosine kinase 1 and
S1PR2 prevents the development of hypoxia-mediated pulmonary
hypertension in rats. Thus, it was speculated that S1P may regulate
portal pressure. Ikeda et al (17) demonstrated that S1P increases portal
pressure in isolated rat perfused liver, and this effect is
mediated by an increase in the activity of Rho via a
S1PR2-dependent mechanism.
Based on the critical role of S1P in BP regulation,
it was hypothesized that S1PRs may play a role in the development
of PE. Thus, the present study aimed to assess changes in the
levels of S1P, S1PR1, S1PR2 and S1PR3 in placenta tissues, and
determine whether S1P plays a role in BP and angiogenesis imbalance
using a PE rat model.
Materials and methods
Ethics statement
The present study was approved by the Institutional
Animal Care and Use Committee (IACUC) of Shaoxing People's Hospital
(Jinhua, China; approval no. 20190713). The animal experiments were
conducted according to the IACUC Care and Use of Laboratory Animals
guidelines (18).
Animals
A total of 60 Sprague Dawley (SD) rats (30 males and
30 females; weight, 250–300 g; age, 7–9 weeks) were obtained from
Shanghai SLAC Laboratory Animal Co., Ltd. The rats were housed at
22±2°C with a 12-h light-dark cycle and free access to food and
water. The ratio of male to female was 1:1. Appearance of the sperm
plug or sperms detected in vaginal smear was regarded as
gestational day (GD) 0.
In the first set of experiments, 20 pregnant SD rats
were randomly divided into four groups (n=5), as follows: i)
Control group; ii) model group; iii) S1P group; and iv) model + S1P
group. In the model group, on GD 14, pregnant rats were
anesthetized (~3% isoflurane in 2 l/min O2), the uterus
was exteriorized and silver clips were used to clamp the abdominal
aorta (above the kidneys) and branches of the ovarian arteries to
construct the reduced uterine perfusion pressure (RUPP) rat model,
as previously described (19).
Whereas, pregnant rats that underwent a sham procedure were used as
the control on GD 14. A 1 mM stock of S1P (Sigma-Aldrich; Merck
KGaA) was prepared in 10 mM NaOH and diluted to desired S1P
concentration in saline with 0.1% BSA (pH 7.8-8.0; Beyotime
Institute of Biotechnology). In the S1P group, pregnant rats were
only administered with S1P (0.1 mg/kg body weight) by intravenous
injection in the tail vein for 3 consecutive days after GD 14
(20). In the model + S1P group,
RUPP rats were administered with S1P (0.1 mg/kg body weight) by
intravenous injection in the tail vein for 3 consecutive days after
GD 14.
After 7 days (GD 21), the rats were euthanized with
CO2 (CO2 displacement rate was 25% vol/min) 3
weeks after the last injection, followed by cervical dislocation.
Caesarean sections were performed on the rats and the placental
tissues were stored at −80°C for further assessment. The fetal
weights, placental weights, litter size and/or number of
resorptions/pregnancy losses were not obviously changed in this
RUPP model.
In the second set of experiments, twenty pregnant SD
rats were randomly divided into four groups (n=5) on GD 14, as
follows: i) Control group; ii) model group; iii) model + S1PR2
antagonist low (JTE-013, 2.5 mg/kg body weight) group; and iv)
model + S1PR2 antagonist high (JTE-013, 5 mg/kg body weight) group.
The control and model groups consisted of the same mice as those
used for these groups in the first set of experiments. JTE-013 was
intraperitoneally administrated at the indicated concentration once
every other day, until GD 21. When the aforementioned animal
experiments were finished, animals were euthanized by 25%
CO2.
BP measurement
SBP was measured using the BP-2000 Series II
non-invasive tail-artery pressure measuring instrument (Visitech
Systems) every day from days 15–21 of the pregnancy. Briefly, rats
were fixed on the pre-heated plate at 37°C, and the pressure-cuff
was attached to the rat tail. Rat tails were connected to the
pressure sensor of the BP-2000 Series II instrument. SBP of the
tail artery was measured in each pregnant rat at least three times,
and the average value of each measurement was calculated.
Hematoxylin and eosin (H&E)
staining
Harvested placental tissues were immersed in 10%
(v/v) buffered formalin overnight at room temperature and
subsequently embedded in paraffin. Paraffin-embedded tissue samples
were cut into 5-µm thick sections and stained with H&E for 15
min at room temperature, according to standard protocols. Stained
sections were observed using a light microscope. The number of
immune cells was quantified using ImageJ software (version 1.8.0;
National Institutes of Health).
Enzyme-linked immunosorbent assay
(ELISA)
ELISA was performed to detect serum S1P, inducible
nitric oxide synthase (iNOS), tumor necrosis factor-α (TNF-α),
interleukin (IL)-1β and IL-6 levels. The kits for S1P (cat. no.
ML-Elisa-0470) and iNOS (cat. no. CS-E01909) were respectively
purchased from Shanghai enzyme-linked Biotechnology Co., Ltd. and
Shanghai Ulva Biotechnology Co., Ltd. The kits for TNF-α (cat. no.
MTA00B), IL-1β (cat. no. MLB00C) and IL-6 (cat. no. D6050) were
purchased from R&D Systems, Inc.
Measurement of NO
Serum NO was measured using the NO assay kit (cat.
no. EMSNO; Invitrogen; Thermo Fisher Scientific, Inc.), according
to the manufacturer's instructions. Total NOx (nitrite + nitrate)
was used as an indicator of NO synthesis in serum (20).
Reverse transcription-quantitative
(RT-q)PCR
Following treatment, placental tissues were
collected from pregnant rats and total RNA was extracted using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). Total RNA was reverse transcribed into cDNA using the SYBR
PrimeScript™ RT-PCR kit (cat. no. RR066A; Takara Bio, Inc.). qPCR
was subsequently performed for the S1PR1, S1PR2, S1PR3, vascular
endothelial growth factor (VEGF) and fms-like tyrosine kinase 1
(Flt-1) genes using the Applied Biosystems 7500 Standard system
(Thermo Fisher Scientific, Inc.). cDNA equivalent to 100 ng total
RNA was used for qPCR, using the TaqMan Universal PCR Master Mix
(Applied Biosystems; Thermo Fisher Scientific, Inc.). PCR
thermocycling conditions were as follows: 4 min at 95°C, followed
by 30 sec at 95°C, 20 sec at 65°C and 30 sec at 72°C, for 35
cycles. The primer sequences were as follows: S1PR1 forward,
5′-CAGCAAATCGGACAATTCCT-3′ and reverse, 5′-GCCAGCGACCAAGTAAAGAG-3′;
S1PR2 forward, 5′-TGTATGGCAGCGACAAGAGC-3′ and reverse,
5′-ACCGAGGACCAGCGAGATG-3′; S1PR3 forward, 5′-GCCACCCGCCAGTCTTG-3′
and reverse, 5′-GCCAGCTTCCCCACGTAAT-3′; VEGF forward,
5′-ACCATGAACTTTCTGCTC-3′ and reverse, 5′-GGACGGCTTGAAGATATA-3′;
Flt-1 forward, 5′-TTTGCATAGCTTCCAATAAAGTTG-3′ and reverse,
5′-CATGACAGTCTAAAGTGGTGGAAC-3′; and GAPDH forward,
5′-CACCACCATGGAGAAGGC-3′ and reverse, 5′-CCATCCACAGTCTTCTGA-3′. The
relative expression levels of mRNAs were normalized to GAPDH, and
were calculated with 2−ΔΔCq method (21).
Western blotting
Total protein was extracted from placenta tissues
using RIPA lysis buffer (Beyotime Institute of Biotechnology),
according to the manufacturer's instructions. Total protein was
quantified using the BCA Protein Assay kit (cat. no. P0012S;
Beyotime Institute of Biotechnology) and 10 µg protein/lane was
separated via SDS-PAGE on a 8% gel. The separated proteins were
subsequently transferred onto nitrocellulose membranes (EMD
Millipore) and blocked with 0.5% non-fat milk for 1 h at room
temperature. The membranes were incubated with primary antibodies
against S1PR2 (cat. no. 21180-1-AP; 1:1,000; ProteinTech Group,
Inc.), VEGF (cat. no. 66828-1-AP; 1:1,000; ProteinTech Group,
Inc.), Flt-1 (cat. no. 13687-1-AP; 1:1,000; ProteinTech Group,
Inc.), endothelial (e)NOS (cat. no. ab76198; 1:1,000; Abcam) and
β-actin (cat. no. ab8226; 1:1,000; Abcam) overnight at 4°C.
Following the primary incubation, membranes were incubated with
anti-rabbit (cat. no. SA00001-2; 1:1,000; ProteinTech Group, Inc.)
or anti-mouse (cat. no. SA00001-1; 1:1,000; ProteinTech Group,
Inc.) HRP-conjugated secondary antibodies for 2 h at room
temperature. Protein bands were visualized using the enhanced
chemiluminescence kit (cat. no. GERPN2105; Millipore Sigma; Merck
KGaA) and semi-quantified using ImageJ 1.8.0 software (National
Institutes of Health).
Statistical analysis
Statistical analysis was performed using SPSS 21.0
software (IBM Corp.). Data are presented as the mean ± standard
deviation. The Kolmogorov-Smirnov test was used to detect the
normality of all data. Unpaired Student's t-test was used to
compare differences between two groups. One-way analysis of
variance and Tukey's post hoc test were used to compare differences
between multiple groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
S1PR2 is increased in the serum and
placental tissues of PE rats
The expression levels of S1P, S1PR1, S1PR2 and S1PR3
were detected in PE rats. As presented in Fig. 1A, S1P serum expression was
significantly upregulated in PE rats compared with the control
rats. As presented in Fig. 1B,
S1PR1 and S1PR3 mRNA expression levels were significantly
downregulated, whereas S1PR2 mRNA expression was significantly
upregulated in the serum of PE rats compared with the control rats.
Consistently, S1PR2 expression levels in the serum and placental
tissues were significantly upregulated in PE rats compared with the
control rats, and S1P significantly increased S1PR2 expression
levels in PE rats (Fig. 1C and
D).
Inhibition of S1PR2 with JTE-013
decreases BP in PE rats
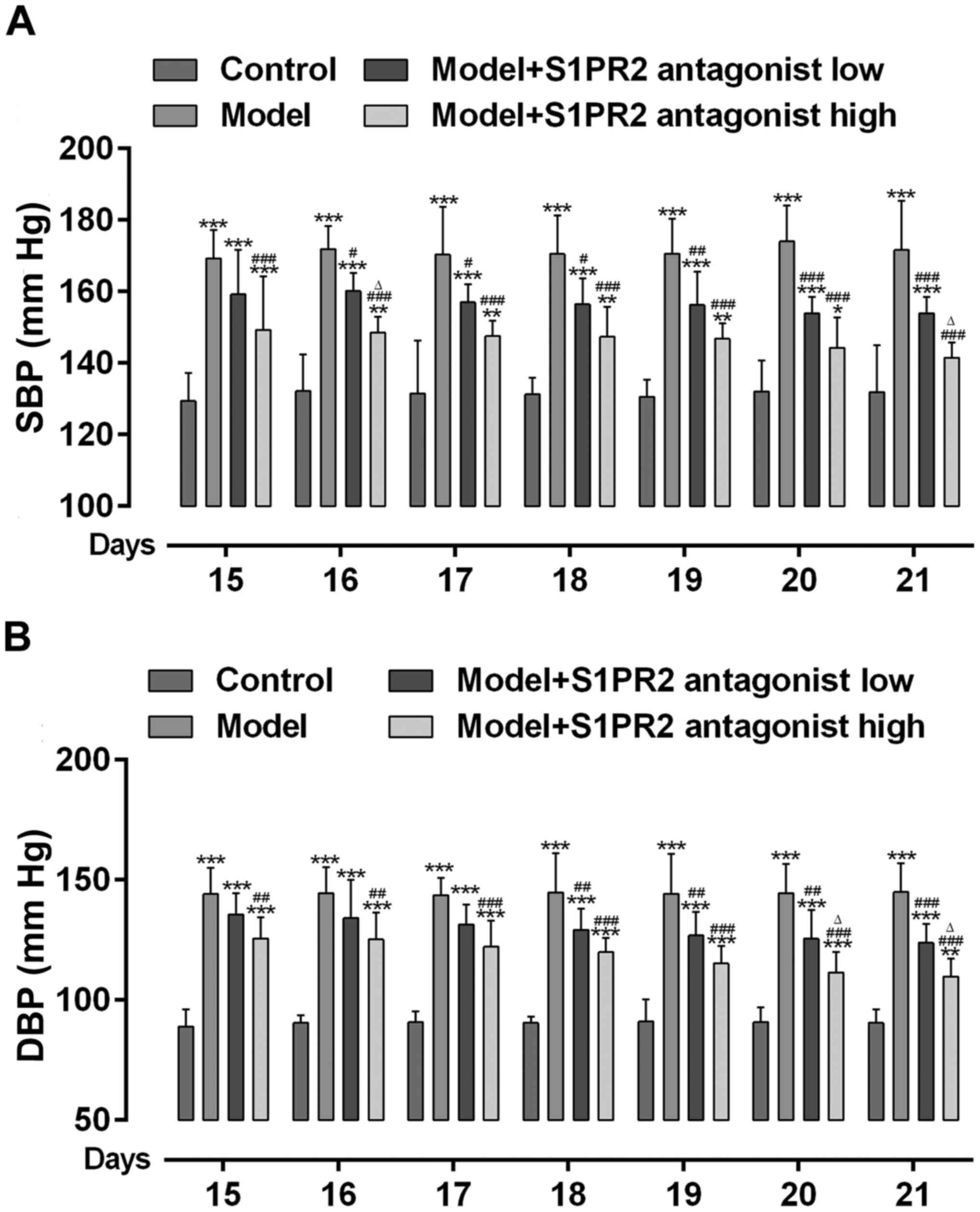
The present study assessed whether S1PR2 activation
was involved in increased BP in PE. As presented in Fig. 2A and B, BP significantly increased
in PE rats compared with the control rats. However, BP
significantly decreased in PE rats pretreated with JTE-013. High
dose JTE-013 displayed an enhanced effect on decreasing BP compared
with low dose.
Inhibition of S1PR2 with JTE-013
prevents iNOS activation and increases eNOS
The effect of S1PR2 inhibition on the NO (Fig. 3A), iNOS (Fig. 3B) and eNOS (Fig. 3C) signaling pathways was assessed.
The serum NO and iNOS levels were significantly upregulated in PE
rats. Conversely, eNOS expression in the placenta tissue was
significantly downregulated in PE rats. Notably, these effects were
reversed following treatment with JTE-013. High dose JTE-013
displayed an enhanced effect on decreasing serum NO and iNOS levels
compared with low dose.
JTE-013 regulates VEGF and Flt-1
expression in placental tissues
The effect of S1PR2 inhibition on the changes in
VEGF and Flt-1 expression levels in placental tissues was assessed.
As presented in Fig. 4A and B, VEGF
mRNA and protein expression levels were significantly downregulated
in the placental tissues of PE rats. Conversely, Flt-1 mRNA and
protein expression levels were significantly upregulated in the
placental tissues of PE rats. Notably, these effects were reversed
following treatment with JTE-013. High dose JTE-013 displayed an
enhanced effect on increasing VEGF expression and decreasing Flt-1
expression compared with low dose.
JTE-013 attenuates pathological
changes in placental tissues and increases the expression of
inflammatory cytokines
The effect of S1PR2 inhibition on inflammation and
pathological changes in placental tissues in PE was assessed. As
presented in Fig. 5A, there was
significant inflammation in the placental tissues of PE rats
compared with the control rats, as indicated by the increased
expression levels of inflammatory cytokines, including TNF-α, IL-1β
and IL-6. As presented in Fig. 5B,
there was significant infiltration of inflammatory cells in the
placental tissues of PE rats, as demonstrated by H&E staining.
Notably, these effects were reversed following treatment with
JTE-013. High dose JTE-013 displayed an enhanced effect on
suppressing inflammation compared with low dose.
 | Figure 5.JTE-013 attenuates pathological
changes in placental tissues and decreases inflammation in PE rats.
(A) Summarized data showing the inhibitory effect of JTE-013 on the
increased expression of serum TNF-α, IL-1β and IL-6, as determined
by an enzyme-linked immunosorbent assay. (B) Summarized data
showing JTE-013 attenuated the infiltration of inflammatory cells
in placental tissues, as detected by hematoxylin and eosin staining
(magnification, ×100 or 200). n=3. *P<0.05, **P<0.01 and
***P<0.001 vs. Control group; ##P<0.01 and
###P<0.001 vs. Model group; ∆P<0.05 and
∆∆P<0.01 vs. Model + S1PR2 antagonist low group. PE,
preeclampsia; TNF-α, tumor necrosis factor-α; IL-, interleukin;
S1PR2, sphingosine-1-phosphate receptor 2; lab, labyrinth; JZ,
junctional zone; de, decidua. |
Discussion
The results of the present study demonstrated that
S1PR2 expression was upregulated in placental tissues, and that the
inhibition of S1PR2 with JTE-013 decreased BP, inflammation and the
infiltration of inflammatory cells. These results are consistent
with previous findings, suggesting that S1P can induce its
receptor, S1PR2, to inhibit the migration of trophoblast cells
(22). In addition, another study
demonstrated that expression of the anti-angiogenic factor, S1PR2,
in villi tissue was upregulated in patients with PE (23).
Previous studies have reported that VEGF plays a key
role in vasculogenesis and angiogenesis, both of which are
important in the development of the placenta (24,25).
Increasing evidence suggests that VEGF expression is downregulated
(26,27), and the placenta produces elevated
levels of VEGF receptor (Flt-1), which captures free VEGF (28). It has also been reported that eNOS
expression is downregulated in PE (29). These changes result in insufficient
placental VEGF and eNOS expression, and endothelial dysfunction,
thereby resulting in the initiation and development of PE (26–28).
Amaral et al (30)
demonstrated that iNOS expression is upregulated in PE rats, and
that inhibition of iNOS significantly decreases RUPP-induced
increase of plasma 8-isoprostane. Consistent with these findings,
the results of the present study demonstrated that S1PR2 inhibition
significantly increased VEGF and eNOS expression levels, and
decreased the expression of iNOS and Flt-1 in placental
tissues.
It has been reported in previous literature that
oxidative stress is higher in women with PE, and at the same time,
the relationship between PE and the inflammatory response has also
attracted more attention (31).
Notably, it has been reported that oxidative stress and
inflammation increase in PE, and it may be a cause and consequence
of the cellular pathology (32,33).
PE is the excessive inflammatory response of women to pregnancy.
Generally, there are different degrees of inflammatory responses in
patients with PE and normal pregnancy, but the inflammatory
reaction in PE is overactivated, and the level of inflammatory
factors is significantly higher than that in women undergoing a
normal pregnancy (34). Serum
levels of inflammatory mediators, such as IL-6, INF-γ, CRP and
TNF-α, in patients with PE during early stages of pregnancy are
significantly higher than those in healthy pregnant women (35). In the present study, it was also
have found that the inflammatory cytokines (TNF-α, IL-1β and IL-6)
were increased in PE rats, which was reversed by S1PR2
inhibition.
In conclusion, the results of the present study
suggested that S1PR2 played a critical role in the initiation and
development of PE by modulation of VEGF, eNOS and iNOS expression
levels. Taken together, these results provide a novel
pharmacological target for the prevention and treatment of PE. A
key limitation of the present study is that Flt-1 expression was
only detected in placental tissues, not in plasma. The measured
plasma Flt-1 level may further strengthen the present
conclusion.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TZ, DG and WZ acquired the data, confirmed the
authenticity of all the raw data and contributed to the analysis
and interpretation of data. TZ and QD contributed to the design of
the study. TZ drafted the manuscript, which was revised by QD. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Animal Care and Use Committee of Shaoxing People's Hospital
(Jinhua, China; approval no. 20190713).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hypertension in pregnancy, . Report of the
American College of Obstetricians and Gynecologists' Task Force on
Hypertension in Pregnancy. Obstet Gynecol. 122:1122–1131.
2013.PubMed/NCBI
|
|
2
|
Shu W, Li H, Gong H, Zhang M, Niu X, Ma Y,
Zhang X, Cai W, Yang G, Wei M, et al: Evaluation of blood vessel
injury, oxidative stress and circulating inflammatory factors in an
L-NAME-induced preeclampsia-like rat model. Exp Ther Med.
16:585–594. 2018.PubMed/NCBI
|
|
3
|
Kemse NG, Kale AA and Joshi SR:
Supplementation of maternal omega-3 fatty acids to pregnancy
induced hypertension Wistar rats improves IL10 and VEGF levels.
Prostaglandins Leukot Essent Fatty Acids. 104:25–32. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kuklina EV, Ayala C and Callaghan WM:
Hypertensive disorders and severe obstetric morbidity in the United
States. Obstet Gynecol. 113:1299–1306. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lisonkova S and Joseph KS: Incidence of
preeclampsia: Risk factors and outcomes associated with
early-versus late-onset disease. Am J Obstet Gynecol.
209:544.e1–544 e12. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hladunewich M, Karumanchi SA and Lafayette
R: Pathophysiology of the clinical manifestations of preeclampsia.
Clin J Am Soc Nephrol. 2:543–549. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Roberts JM and Gammill HS: Preeclampsia:
Recent insights. Hypertension. 46:1243–1249. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Llurba E, Crispi F and Verlohren S: Update
on the pathophysiological implications and clinical role of
angiogenic factors in pregnancy. Fetal Diagn Ther. 37:81–92. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Verlohren S, Stepan H and Dechend R:
Angiogenic growth factors in the diagnosis and prediction of
pre-eclampsia. Clin Sci (Lond). 122:43–52. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Maynard SE and Karumanchi SA: Angiogenic
factors and preeclampsia. Semin Nephrol. 31:33–46. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Henao DE and Saleem MA: Proteinuria in
preeclampsia from a podocyte injury perspective. Curr Hypertens
Rep. 15:600–605. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Roberts JM and Escudero C: The placenta in
preeclampsia. Pregnancy Hypertens. 2:72–83. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen W, Xiang H, Chen R, Yang J, Yang X,
Zhou J, Liu H, Zhao S, Xiao J, Chen P, et al: S1PR2 antagonist
ameliorate high glucose-induced fission and dysfunction of
mitochondria in HRGECs via regulating ROCK1. BMC Nephrol.
20:1352019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cheng JC, Wang EY, Yi Y, Thakur A, Tsai SH
and Hoodless PA: S1P stimulates proliferation by upregulating CTGF
expression through S1PR2-Mediated YAP activation. Mol Cancer Res.
16:1543–1555. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sanchez T and Hla T: Structural and
functional characteristics of S1P receptors. J Cell Biochem.
92:913–922. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen J, Tang H, Sysol JR, Moreno-Vinasco
L, Shioura KM, Chen T, Gorshkova I, Wang L, Huang LS, Usatyuk PV,
et al: The sphingosine kinase 1/sphingosine-1-phosphate pathway in
pulmonary arterial hypertension. Am J Respir Crit Care Med.
190:1032–1043. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ikeda H, Nagashima K, Yanase M, Tomiya T,
Arai M, Inoue Y, Tejima K, Nishikawa T, Watanabe N, Omata M and
Fujiwara K: Sphingosine 1-phosphate enhances portal pressure in
isolated perfused liver via S1P2 with Rho activation. Biochem
Biophys Res Commun. 320:754–759. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schapiro SA and Everitt JI: Preparation of
animals for use in the laboratory: Issues and challenges for the
Institutional Animal Care and Use Committee (IACUC). ILAR J.
47:370–375. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
LaMarca BB, Bennett WA, Alexander BT,
Cockrell K and Granger JP: Hypertension produced by reductions in
uterine perfusion in the pregnant rat: Role of tumor necrosis
factor-alpha. Hypertension. 46:1022–1025. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chawla S, Rahar B and Saxena S: S1P
prophylaxis mitigates acute hypobaric hypoxia-induced molecular,
biochemical, and metabolic disturbances: A preclinical report.
IUBMB Life. 68:365–375. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Westwood M, Al-Saghir K, Finn-Sell S, Tan
C, Cowley E, Berneau S, Adlam D and Johnstone ED: Vitamin D
attenuates sphingosine-1-phosphate (S1P)-mediated inhibition of
extravillous trophoblast migration. Placenta. 60:1–8. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dobierzewska A, Palominos M, Sanchez M,
Dyhr M, Helgert K, Venegas-Araneda P, Tong S and Illanes SE:
Impairment of Angiogenic Sphingosine
Kinase-1/Sphingosine-1-Phosphate receptors pathway in preeclampsia.
PLoS One. 11:e01572212016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pavlov N, Frendo JL, Guibourdenche J,
Degrelle SA, Evain-Brion D and Badet J: Angiogenin expression
during early human placental development; association with blood
vessel formation. Biomed Res Int. 2014:7816322014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Santos TC, Oliveira MF, Papa PC, Dantzer V
and Miglino MA: VEGF system expression by immunohistochemistry and
real-time RT-PCR study on collared peccary placenta.
Theriogenology. 82:834–843. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu Y, Su Z, Li J, Wang Q, Meng G, Zhang Y,
Yang W, Zhang J and Gao P: Role of RNA-binding protein 5 in the
diagnosis and chemotherapeutic response of lung cancer. Oncol Lett.
17:2013–2019. 2019.PubMed/NCBI
|
|
27
|
Adu-Bonsaffoh K, Antwi DA, Gyan B and Obed
SA: Endothelial dysfunction in the pathogenesis of pre-eclampsia in
Ghanaian women. BMC Physiol. 17:52017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Luttun A and Carmeliet P: Soluble VEGF
receptor Flt1: The elusive preeclampsia factor discovered? J Clin
Invest. 111:600–602. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Motta-Mejia C, Kandzija N, Zhang W, Mhlomi
V, Cerdeira AS, Burdujan A, Tannetta D, Dragovic R, Sargent IL,
Redman CW, et al: Placental vesicles carry active endothelial
nitric oxide synthase and their activity is reduced in
preeclampsia. Hypertension. 70:372–381. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Amaral LM, Pinheiro LC, Guimaraes DA,
Palei AC, Sertório JT, Portella RL and Tanus-Santos JE:
Antihypertensive effects of inducible nitric oxide synthase
inhibition in experimental pre-eclampsia. J Cell Mol Med.
17:1300–1307. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ramos JGL, Sass N and Costa SHM:
Preeclampsia. Rev Bras Ginecol Obstet. 39:496–512. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Terlecky SR, Terlecky LJ and Giordano CR:
Peroxisomes, oxidative stress, and inflammation. World J Biol Chem.
3:93–97. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Roy S, Dhobale M, Dangat K, Mehendale S,
Lalwani S and Joshi S: Differential oxidative stress levels in
mothers with preeclampsia delivering male and female babies. J
Matern Fetal Neonatal Med. 28:1973–1980. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Raio L, Bersinger NA, Malek A, Schneider
H, Messerli FH, Hürter H, Rimoldi SF and Baumann MU: Ultra-high
sensitive C-reactive protein during normal pregnancy and in
preeclampsia: A pilot study. J Hypertens. 37:1012–1017. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Black KD and Horowitz JA: Inflammatory
markers and preeclampsia: A systematic review. Nurs Res.
67:242–251. 2018. View Article : Google Scholar : PubMed/NCBI
|