Introduction
At present, the prevalence of diabetes mellitus (DM)
is rapidly increasing worldwide, and the number of people living
with DM has quadrupled in the past three decades (1,2). DM is
a metabolic disorder that primarily manifests as abnormal glucose
metabolism with complications that seriously impair the quality of
life of patients, including retinopathy, nephropathy and neuropathy
(3). Previous studies have
demonstrated that diabetes can cause male reproductive dysfunction
(4–6). Hyperglycaemic male rats display
symptoms of impaired fertility and decreased sperm motility
(7,8). A high-glucose (HG) environment
associated with diabetes led to impaired testicular Sertoli cell
function, which subsequently affected spermatogenesis, resulting in
testicular spermatogenesis dysfunction (9). However, the specific mechanism
underlying HG-induced impairment of testicular Sertoli cell
function is not completely understood.
It has been widely reported that the reproductive
function of vertebrates is primarily regulated by the hypothalamus-
pituitary-gonadal axis (HPG) (10,11).
The hypothalamus regulates reproductive function by synthesizing
and secreting gonadotropin-releasing hormone (GnRH), which
stimulates the pituitary to secrete two gonadotropins, luteinizing
hormone and follicle stimulating hormone (12). Kisspeptins, which are encoded by the
KiSS-1 metastasis suppressor (KISS1) gene, serve a role upstream of
GnRH and are effective stimulators of the HPG axis in several
species (13,14). Increasing evidence indicates that
KISS1/KISS1 receptor (KISS1R) serves critical roles in the female
reproductive process (15,16), but few studies in the male
reproductive system have been conducted. A previous study reported
that the level of kisspeptin in serum from infertile men was
significantly lower compared with the serum from fertile control
individuals (17). Another study
implied that KISS1/KISS1R can affect sperm motility, whereas KISS1
receptor antagonists can reduce sperm motility (18). The aforementioned studies suggested
that KISS1/KISS1R was closely related to male reproductive
function. However, KISS1/KISS1R has not been reported to regulate
testicular Sertoli cell viability and apoptosis; therefore,
investigating the related mechanisms is of interest.
AKT is a serine/threonine kinase that is recognized
as the primary mediator of the downstream effects of PI3K (19). The PI3K/AKT signalling pathway
coordinates multiple signals, controlling how cells respond to
external stimuli to regulate cell proliferation and survival
(20). AKT is activated via
phosphorylation within the carboxy terminus at Ser473 (21). KISS1 has been reported to regulate
PI3K/AKT (22,23). In porcine ovarian granulosa cells,
KISS1 can regulate the cell cycle and inhibit apoptosis by
affecting the PI3K signalling pathway (24). The majority of the current research
on the PI3K signalling pathway has focused on tumour research as
the PI3K signalling pathway serves a critical role in the
development of tumours and has been identified as a novel
therapeutic target for tumours; however, the function of the PI3K
signalling pathway in reproduction is not completely
understood.
The aim of the present study was to examine the role
of KISS1/KISS1R in Sertoli cells. The mechanism underlying
KISS1/KISS1R-regulated Sertoli cell apoptosis under HG conditions
was also evaluated.
Materials and methods
Cell culture
The present study was approved by The Animal Ethics
Committee of The Second Affiliated Hospital of Nanchang University
[approval no. (2018) 031]. A total of five male adult C57BL/6 mice
(age, 8 weeks; weight, 25±3 g) were purchased from Hunan SJA
Laboratory Animal Co., Ltd. Mice were maintained with 12-h
light/dark cycles, housed at 60% humidity at 22°C and free water
and food. Mice were euthanized by inhalation of 5% isoflurane
(Merck Sharp & Dohme-Hoddesdon). Death was verified by
monitoring cardiac arrest, respiratory arrest and loss of reflexes.
Mouse Sertoli cells were obtained according to the following
protocol. Briefly, dealbuginized testes were digested sequentially
with trypsin and collagenase. Tissue explants were placed in tissue
culture dishes with serum-free minimum essential medium
(Sigma-Aldrich; Merck KGaA) supplemented with glutamine. Cells were
allowed to adhere and form confluent monolayers for 3 days at 37°C
with 5% CO2. Subsequently, the remaining germ cells were
removed with hypotonic solution and then 1 mM dibutyryl adenosine
3′:5′ cyclic monophosphate (Sigma-Aldrich; Merck KGaA) was added to
the medium. Cells were cultured with RPMI 1640 medium (Gibco;
Thermo Fisher Scientific, Inc.) supplemented with 10% FBS (Gibco;
Thermo Fisher Scientific, Inc.) at 37°C with 5% CO2 for
24 h, cells were treated with 5, 25 or 50 mM D-glucose
(Sigma-Aldrich; Merck KGaA) for 48 h at 37°C. A dose of 5 µM
PI3K/AKT pathway inhibitor (LY294002; cat. no. PZH1144; Invitrogen;
Thermo Fisher Scientific, Inc.) was used to treat cells at 37°C for
24 h.
Plasmid constructs and
transfection
Plasmids with DNA encoding KISS1 and KISS1R were
constructed by inserting the cDNA clone of KISS1 and KISS1R into
the pcDNA3.1 vector (Invitrogen; Thermo Fisher Scientific, Inc.).
The pcDNA3.1 empty vector was as a control. Small interfering
(si)RNA targeting KISS1 (si-KISS1, 5′-GCAGGAGAGUGAAGAUUAAAU-3′),
siRNA targeting KISS1R (si-KISS1R, 5′-CACUUGGUUGAUUAAUCAACU-3′) and
the negative control (NC) siRNA (si-NC; non-targeting control;
5′-GUCGAGCUGACCUAUCCGACG-3′) were synthesized by Sangon Biotech
Co., Ltd. Cells (1×105 cells/well) were transfected with
1 µg plasmid vector or with 50 nM siRNA using
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) at 37°C for 6 h. Cells were harvested for further
experiments after 24 h.
Reverse transcription-quantitative PCR
(qPCR)
Total RNA was extracted from cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). Total RNA was reverse transcribed into cDNA using the
ReverTra Ace™ qPCR RT Kit (Toyobo Life Science) according to the
manufacturer's protocol. Subsequently, qPCR was performed on a qPCR
instrument (Eppendorf) using SYBR® Green Real-Time PCR
Master Mix (Toyobo Life Science). The program was listed as
following: 95°C for 30 sec, followed by 40 cycles of 95°C for 5
sec, 60°C for 10 sec, and 72°C for 30 sec and a final extension at
72°C for 5 min. The following primers were used for qPCR: KISS1
forward, 5′-TTTCCTCTGTGCCACCCAC-3′ and reverse,
5′-AGGGATTCTAGCTGCTGGCC-3′; KISS1R forward,
5′-CCCACCCTCTGGACATTCAC-3′ and reverse,
5′-CCTAGAAGTGCCTTGAGGCTTG-3′; GAPDH forward,
5′-GAGTCAACGGATTTGGTCGTT-3′ and reverse,
5′-TTGATTTTGGAGGGATCTCG-3′. mRNA expression levels were quantified
using the 2−ΔΔCq method (25) and normalized to the internal
reference gene GAPDH.
Cell viability assay
Cells were seeded into 96-well plates at a density
of 1×104 cells/well. Cell viability was assessed by
adding 10 µl Cell Counting Kit-8 (CCK-8) solution (Beijing Solarbio
Science & Technology Co., Ltd.) to each well and incubating for
2 h. Absorbance was measured at a wavelength of 450 nm using a
microplate spectrophotometer.
Detection of apoptosis by flow
cytometry
Cells were seeded into 6-well plates at a density of
1×106 cells/well. Cell apoptosis was assessed using the
Annexin V-FITC/PI Apoptosis Detection Kit (Shanghai Yeasen
Biotechnology Co., Ltd.) according to the manufacturer's protocol.
Cells were digested and harvested. Cells were washed twice with
prechilled PBS and then resuspended in 100 µl 1X binding buffer.
Subsequently, cells were incubated with 5 µl Annexin V-FITC and 10
µl PI staining solution in the dark at room temperature for 10 min.
The data were acquired using a BD FACSCanto™ II (BD Biosciences)
flow cytometer. Apoptotic cells (early and late apoptosis) were
analysed using FlowJo software (version 7; FlowJo LLC).
TUNEL staining
Cells were fixed with DiffQuik Fixative (Baxter
International, Inc.) at 37°C for 30 sec. To conduct TUNEL staining,
cells were treated according to the manufacturer's protocol by
using DeadEnd™ Fluorometric TUNEL System (cat. no. G3250; Promega
Corporation). Cells were permeabilized using 0.1% Triton X-100
solution (Sigma-Aldrich; Merck KGaA) for 30 min at 37°C. A volume
of 50 µl TdT reaction mix was then added to the cells at 37°C for 1
h in the dark. Subsequently, 2X saline sodium citrate (300 mM NaCl;
30 mM sodium citrate; pH 7.0) for 15 min to stop reaction.
Following washing with PBS, cells were stained with 1 µg/ml DAPI
staining solution (cat. no. E607303; Sangon Biotech Co., Ltd.) for
10 min at 37°C. Cells were washed with PBS, then treated with
anti-Fade Mounting Medium (cat. no. E675011; Sangon Biotech Co.,
Ltd.) and observed in five randomly selected fields of view using a
fluorescence microscope (Olympus Corporation; magnification
×40).
Western blotting
Total protein was extracted from cells using RIPA
buffer (Sigma-Aldrich; Merck KGaA) supplemented with 1% protease
inhibitor and phosphorylase inhibitor. Protein concentrations were
determined using the BCA Kit (Beyotime Institute of Biotechnology).
Cell lysates were mixed with 5X SDS sample buffer and boiled for 10
min. The protein samples (50 µg/lane) were separated by SDS-PAGE on
12% gels and transferred to PVDF membranes. Following blocking with
BlockPro blocking solution (Energenesis Biomedical Co., Ltd.) for 1
h at 37°C, the membranes were incubated overnight at 4°C with
primary antibodies targeted against: Kisspeptin (cat. no. ab19028;
1:1,000; Abcam), KISS1R (cat. no. 13776; 1:1,000; Cell Signalling
Technology, Inc.), phosphorylated (p)-PI3K (cat. no. 17366;
1:1,000; Cell Signalling Technology, Inc.), PI3K (cat. no. 4249;
1:1,000; Cell Signalling Technology, Inc.), p-AKT (cat. no. 4060;
1:1,000; Cell Signalling Technology, Inc.), AKT (cat. no. ab8805;
1:1,000; Abcam), Bad (cat. no. ab32445; 1:1,000; Abcam), Bcl-2
(cat. no. ab182858; 1:1,000; Abcam), Bax (cat. no. ab32503;
1:1,000; Abcam), total caspase-3 (cat. no. ab13847; 1:1,000;
Abcam), cleaved caspase-3 (cat. no. ab2302; 1:1,000; Abcam) and
GAPDH (cat. no. ABS16; 1:2,000; Sigma-Aldrich; Merck KGaA). After
washing with 0.1% Tween-20 in PBS-T, the membranes were incubated
with the corresponding goat anti-Rabbit IgG H&L HRP antibody
(cat. no. ab205718; 1:2,000; Abcam) at room temperature for 1 h.
Protein bands were visualized using ECL Reagents (Beyotime
Institute of Biotechnology) and a GEL imaging system (Bio-Rad
Laboratories, Inc.). Protein expression was quantified using ImageJ
software (V1.8.0; National Institutes of Health) with GAPDH as the
loading control.
Statistical analysis
Each experiment was repeated three times. Data are
presented as the mean ± SD. Statistical analyses were performed
using GraphPad Prism 8.0 software (GraphPad Software, Inc.).
Comparisons among multiple groups were analysed using one-way ANOVA
followed by Tukey's post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
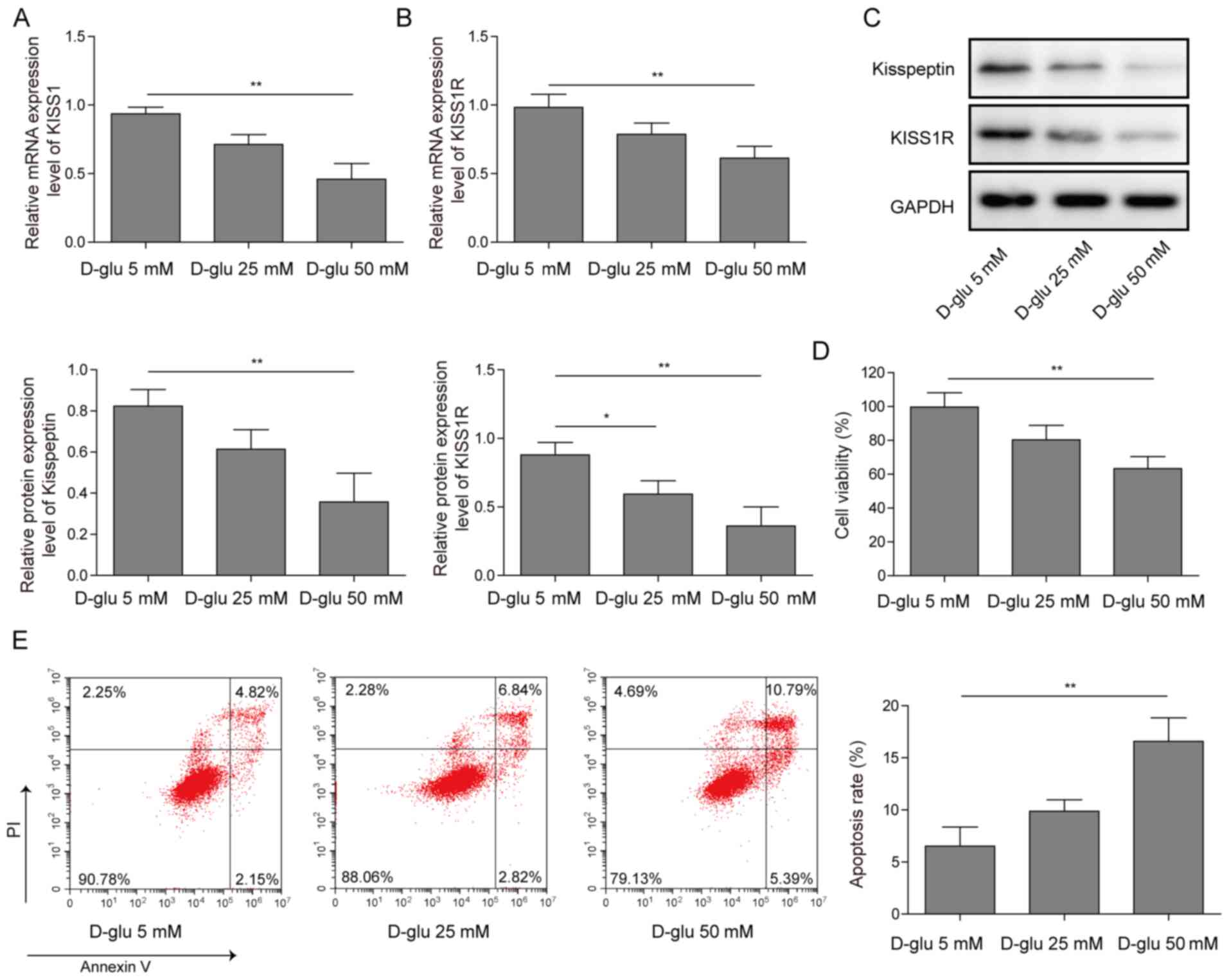
KISS1/KISS1R expression is reduced and
cell apoptosis is increased in HG-induced mouse Sertoli cells
Mouse Sertoli cells were treated with 5, 25 or 50 mM
D-glucose. KISS1 and KISS1R mRNA expression levels were decreased
by glucose treatment in a concentration-dependent manner (Fig. 1A and B). Similar trends were
observed for kisspeptin and KISS1R protein expression levels
(Fig. 1C). Cell viability was
decreased by glucose treatment in a concentration-dependent manner,
as determined by performing the CCK-8 assay (Fig. 1D). The flow cytometry results
demonstrated that the number of apoptotic cells was increased by
glucose treatment in a concentration-dependent manner (Fig. 1E). The results suggested that KISS1
and KISS1R expression levels were decreased and cell apoptosis was
increased by glucose treatment in a concentration-dependent manner
in mouse Sertoli cells.
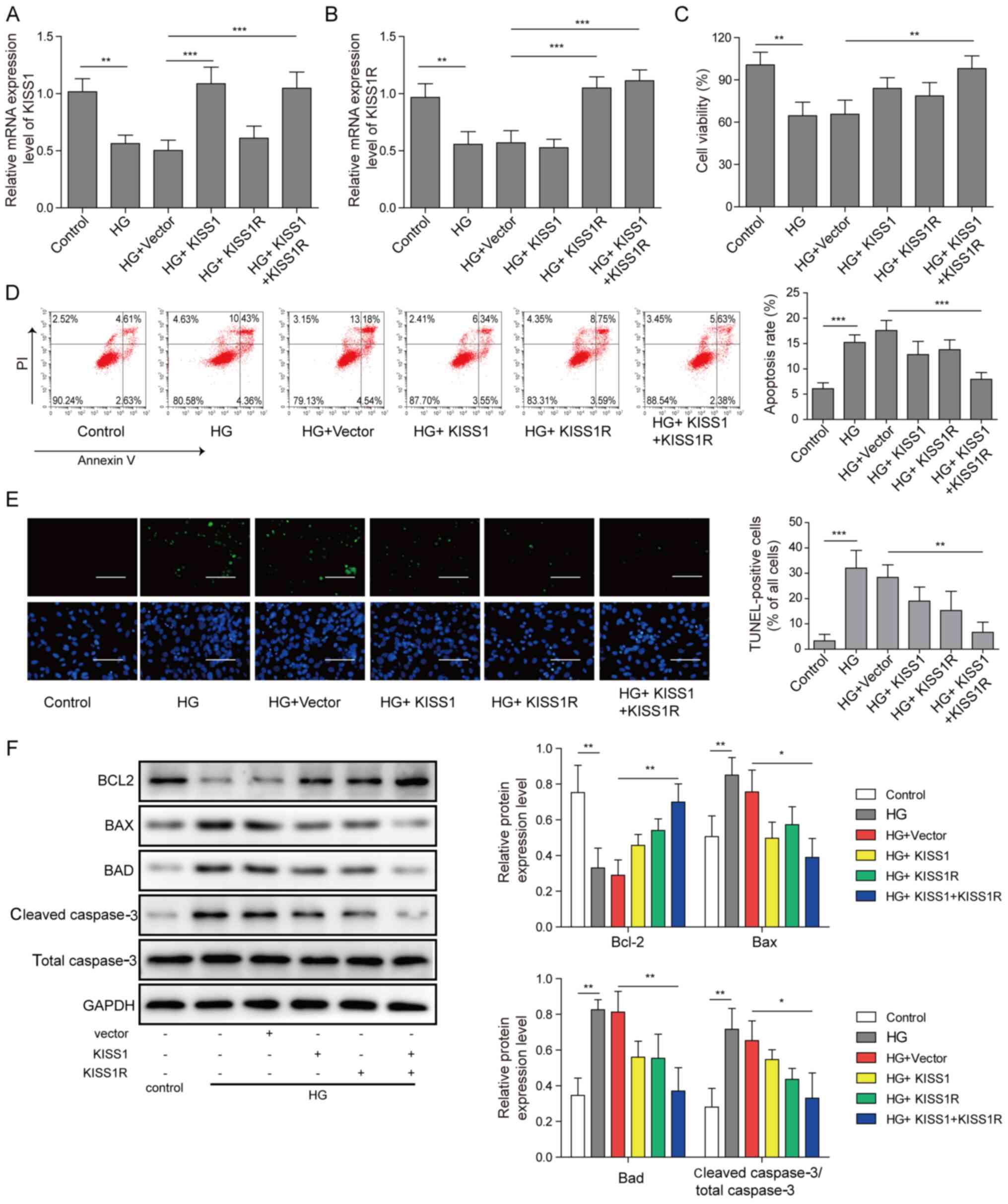
KISS1/KISS1R overexpression reduces
mouse Sertoli cell apoptosis under HG conditions
Mouse Sertoli cells were transfected with KISS1 and
KISS1R overexpression plasmids, and then treated with 50 mM
D-glucose. KISS1 and KISS1R mRNA expression levels were
significantly decreased in the HG group compared with the control
group. Moreover, KISS1 and KISS1R mRNA expression levels were
significantly increased by KISS1 or KISS1R overexpression,
respectively, compared with the HG + vector group. Compared with
the HG + vector group, KISS1 and KISS1R mRNA expression levels were
significantly upregulated by KISS1 and KISS1R overexpression
(Fig. 2A and B). According to the
CCK-8 assay results, compared with the HG + vector group, cell
viability was notably increased by KISS1 or KISS1R overexpression
alone, whereas cell viability was significantly increased by KISS1
and KISS1R overexpression (Fig.
2C). Cell apoptosis was increased in the HG group compared with
the control group. Following KISS1 and KISS1R overexpression,
apoptosis significantly decreased compared with the HG + vector
group. Consistent trends were observed for cell apoptosis via flow
cytometry (Fig. 2D) and TUNEL
staining (Fig. 2E). The protein
expression levels of Bad, Bcl-2, Bax and caspase-3 were assessed
via western blotting. Compared with the control group, Bcl-2
protein expression was significantly downregulated, and Bad, Bax
and caspase-3 protein expression levels were significantly
upregulated in the HG group. Compared with the HG group, KISS1 or
KISS1R overexpression alone slightly upregulated Bcl-2 expression,
and slightly downregulated Bad, Bax and caspase-3 protein
expression levels. Following KISS1 and KISS1R overexpression, Bcl-2
protein expression was significantly upregulated, and Bad, Bax and
caspase-3 protein expression levels were significantly decreased
compared with the HG + vector group (Fig. 2F). The results indicated that
KISS1/KISS1R overexpression inhibited HG-induced Sertoli cell
apoptosis.
KISS1/KISS1R regulates the expression
of PI3K/AKT signalling pathway-related proteins in mouse Sertoli
cells
Mouse Sertoli cells were transfected with KISS1 and
KISS1R overexpression plasmids or si-KISS1 and si-KISS1R. Compared
with the vector group, KISS1 and KISS1R mRNA expression levels were
significantly upregulated by KISS1 or KISS1R overexpression,
respectively. KISS1 and KISS1R simultaneous overexpression
significantly increased the KISS1 and KISS1R mRNA expression levels
compared with the vector group (Fig. 3A
and B). However, there were no significant differences observed
among the other groups. The protein expression levels of p-PI3K,
PI3K, p-AKT and AKT were assessed via western blotting. Compared
with the vector group, p-PI3K/PI3K and p-AKT/AKT protein expression
levels were slightly increased by KISS1 or KISS1R overexpression
alone (Fig. 3C). KISS1 and KISS1R
overexpression significantly increased p-PI3K/PI3K and p-AKT/AKT
protein expression levels compared with the vector group. Compared
with the si-NC group, KISS1 and KISS1R mRNA expression levels were
significantly downregulated by KISS1 or KISS1R knockdown,
respectively (Fig. 3D and E).
Compared with the si-NC group, the protein expression levels of
p-PI3K/PI3K and p-AKT/AKT were slightly downregulated by KISS1 or
KISS1R knockdown alone. KISS1 and KISS1R knockdown significantly
decreased p-PI3K/PI3K and p-AKT/AKT protein expression levels
compared with the si-NC group (Fig.
3F). The aforementioned results demonstrated that KISS1 and
KISS1R expression affected the expression levels of PI3K/AKT
pathway-related proteins.
 | Figure 3.KISS1/KISS1R regulates the expression
of PI3K/AKT signalling pathway-related proteins in mouse Sertoli
cells. Mouse Sertoli cells were transfected with KISS1 and KISS1R
overexpression plasmids or si-KISS1 and si-KISS1R. (A) KISS1 and
(B) KISS1R mRNA expression levels in mouse Sertoli cells following
transfection with KISS1 or KISS1R overexpression plasmids were
detected via RT-qPCR. (C) PI3K, AKT, p-PI3K and p-AKT protein
expression levels in mouse Sertoli cells were assessed via western
blotting. (D) KISS1 and (E) KISS1R mRNA expression levels in mouse
Sertoli cells following transfection with si-KISS1 or si-KISS1R
were detected via RT-qPCR. (F) PI3K, AKT, p-PI3K and p-AKT protein
expression levels in mouse Sertoli cells were assessed via western
blotting. *P<0.05, **P<0.01 and ***P<0.001. KISS1, KiSS-1
metastasis suppressor; KISS1R, KISS1 receptor; si, small
interfering RNA; p, phosphorylated; RT-qPCR, reverse
transcription-quantitative PCR; NC, negative control. |
PI3K/AKT inhibitor reverses
KISS1/KISS1R-mediated protective effects
Mouse Sertoli cells were transfected with KISS1 and
KISS1R overexpression plasmids, and then treated with PI3K/AKT
pathway inhibitor. Compared with the HG + vector group, KISS1 and
KISS1R mRNA expression levels were significantly increased by KISS1
or KISS1R overexpression, respectively (Fig. 4A and B). Compared with the HG +
vector group, the protein expression levels of p-PI3K/PI3K and
p-AKT/AKT were significantly upregulated by KISS1 and KISS1R
overexpression, whereas PI3K/AKT inhibitor LY294002 treatment
significantly reversed this effect (Fig. 4C). Compared with the HG + vector
group, Bcl-2 expression was significantly upregulated, and Bad, Bax
and caspase-3 expression levels were significantly decreased by
KISS1 and KISS1R overexpression, which indicated a decrease in
apoptosis (Fig. 4D). However, KISS1
and KISS1R overexpression-mediated effects on apoptosis-related
protein expression levels were significantly reversed by PI3K/AKT
inhibitor treatment. The flow cytometry results demonstrated that
compared with the HG + vector group, the number of apoptotic cells
was significantly downregulated by KISS1 and KISS1R overexpression,
whereas PI3K/AKT inhibitor treatment significantly reversed this
effect (Fig. 4E). Similar trends
were observed for the analysis of cell apoptosis via TUNEL staining
(Fig. 4F). Collectively, the
results demonstrated that KISS1/KISS1R regulated HG-induced cell
apoptosis by altering the PI3K/AKT signalling pathway.
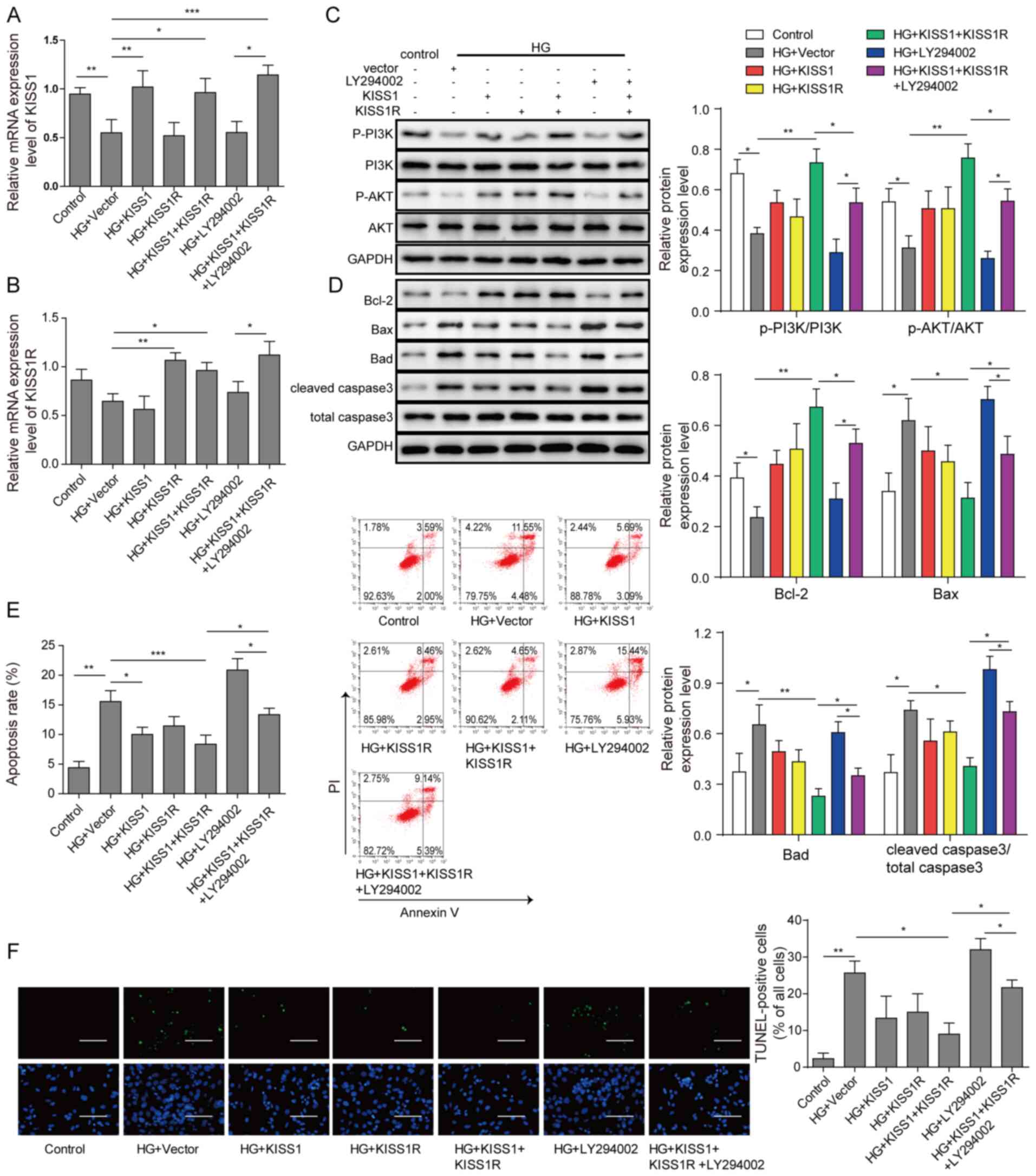 | Figure 4.PI3K/AKT inhibitor reverses
KISS1/KISS1R-mediated protective effects. Mouse Sertoli cells were
transfected with KISS1 and KISS1R overexpression plasmids, and then
treated with the PI3K/AKT signalling pathway inhibitor LY294002.
(A) KISS1 and (B) KISS1R mRNA expression levels in mouse Sertoli
cells were detected via reverse transcription-quantitative PCR. (C)
PI3K, AKT, p-PI3K, p-AKT (D) Bad, Bcl-2, Bax and caspase-3 protein
expression levels in mouse Sertoli cells were assessed via western
blotting. Mouse Sertoli cell apoptosis was evaluated via (E) flow
cytometry and (F) TUNEL staining. Scale bar, 100 µm. *P<0.05,
**P<0.01 and ***P<0.001. KISS1, KiSS-1 metastasis suppressor;
KISS1R, KISS1 receptor; p, phosphorylated; HG, high glucose. |
Discussion
It was estimated that 451 million people lived with
DM in 2017, with a prevalence rate of 8.8% (26). Diabetes is associated with diverse
clinical complications, including reproductive dysfunction
(27–29). Given the multifactorial nature of
DM, the mechanisms underlying DM-induced reproductive dysfunction
are not completely understood. As hyperglycaemia has a major effect
on disease pathophysiology, in vitro approaches have been
used to explore the effect of HG on human sperm function (30). The HG environment may cause impaired
testicular support cell function, affect spermatogenesis and cause
testicular spermatogenesis dysfunction (30). In addition, Liu et al
(31) reported that the motility
and viability of spermatozoa were markedly reduced after incubation
with glucose. In the present study, the results demonstrated that
compared with the control group, HG conditions significantly
decreased cell viability and significantly increased cell apoptosis
in mouse Sertoli cells, which was consistent with a previous study
(32). The present study lacked
verification of Sertoli cell purity, but this did not have an
impact on the conclusions of the present study.
KISS1 and KISS1R are involved in regulating
mammalian sexual maturity and development of the reproductive
system, and serve an important role in female reproduction
(33,34). However, few studies on the role of
KISS1/KISS1R in the male reproductive system have been conducted.
Previous studies have reported reduced sperm motility and
kisspeptin expression in male patients with diabetes (4,35). The
results of the present study demonstrated that KISS1/KISS1R
expression levels were significantly downregulated in HG-induced
mouse Sertoli cells compared with the control group, suggesting
that KISS1/KISS1R might serve a significant role in regulating
reproductive dysfunction in male patients with diabetes. Therefore,
the present study aimed to examine the specific mechanism
underlying KISS1/KISS1R-mediated regulation of the reproductive
function in male patients with diabetes.
Apoptosis serves a critical role in male
reproductive dysfunction (36).
Increased testicular cell apoptosis was observed in male mice with
reproductive disorders (37).
Moreover, increased endoplasmic reticulum stress and apoptosis were
observed in murine Leydig tumour cell line 1 cells treated with
palmitic acid (38). Yang et
al (39) reported that
decreased testosterone secretion accompanied by increased apoptosis
was observed in mouse Leydig cells after natriuretic peptide
receptor 2 inhibition. In the present study, compared with the
control group, cell apoptosis was significantly increased in mouse
Sertoli cells under HG conditions, which was consistent with a
previous study. KISS1 has been reported to regulate PI3K/AKT
(22), which was consistent with
the results of the present study that demonstrated that
KISS1/KISS1R overexpression activated the PI3K/AKT signalling
pathway, which regulates apoptosis (40). The typical AKT phosphorylation
targets include Bad and caspase-3, which are closely related to
apoptosis (41). The present study
demonstrated that KISS1/KISS1R mediated Sertoli cell apoptosis via
the PI3K/AKT signalling pathway under HG conditions. Although the
present study investigated the mechanism underlying KISS1/KISS1R
in vitro HG-induced cell models, future studies should
investigate the effects of KISS1/KISS1R on Sertoli cells using
in vivo diabetic mouse models.
The prevalence of diabetes is high in modern
society, and male patients with diabetes are usually affected by
reproductive dysfunction (42);
therefore, identifying the specific mechanism underlying the
regulation of reproductive dysfunction in male patients with
diabetes is important. The present study investigated the specific
mechanism underlying the regulation of reproductive dysfunction in
male patients with diabetes, providing potential targets for the
treatment of reproductive dysfunction in male patients with
diabetes.
Acknowledgements
Not applicable.
Funding
The present study was supported by The Medical and
Health Research Project of Zhejiang Province (grant no.
2019KY620).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL and DMG analysed the data and confirm their
authenticity. DMG conceived the study and assisted in drafting the
manuscript. JF designed the study. PPZ performed the literature
search. JPZ performed the experiments and acquired and analysed the
data. SXD assisted in the experimental plan formulation and data
analysis, and drafted, edited and reviewed the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by The Animal Ethics
Committee of The Second Affiliated Hospital of Nanchang University
[approval no. (2018) 031].
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
HG
|
high glucose
|
|
DM
|
diabetes mellitus
|
|
HPG
|
hypothalamus-pituitary-gonadal
axis
|
|
GnRH
|
gonadotropin- releasing hormone
|
References
|
1
|
Chen L, Magliano DJ and Zimmet PZ: The
worldwide epidemiology of type 2 diabetes mellitus-present and
future perspectives. Nat Rev Endocrinol. 8:228–236. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zheng Y, Ley SH and Hu FB: Global
aetiology and epidemiology of type 2 diabetes mellitus and its
complications. Nat Rev Endocrinol. 14:88–98. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nathan DM: Long-term complications of
diabetes mellitus. N Engl J Med. 328:1676–1685. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kyathanahalli C, Bangalore S,
Hanumanthappa K and Muralidhara: Experimental diabetes-induced
testicular damage in prepubertal rats. J Diabetes. 6:48–59. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Maresch CC, Stute DC, Alves MG, Oliveira
PF, de Kretser DM and Linn T: Diabetes-induced hyperglycemia
impairs male reproductive function: A systematic review. Hum Reprod
Update. 24:86–105. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jangir RN and Jain GC: Diabetes mellitus
induced impairment of male reproductive functions: A review. Curr
Diabetes Rev. 10:147–157. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Frenkel GP, Homonnai Z, Drasnin N, Sofer
A, Kaplan R and Kraicer PF: Fertility of the
streptozotocin-diabetic male rat. Andrologia. 10:127–136. 1978.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kühn-Velten N, Waldenburger D and Staib W:
Evaluation of steroid biosynthetic lesions in isolated leydig cells
from the testes of streptozotocin-diabetic rats. Diabetologia.
23:529–533. 1982. View Article : Google Scholar
|
|
9
|
Amaral S, Moreno AJ, Santos MS, Seiça R
and Ramalho-Santos J: Effects of hyperglycemia on sperm and
testicular cells of Goto-Kakizaki and streptozotocin-treated rat
models for diabetes. Theriogenology. 66:2056–2067. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shukla KK, Mahdi AA, Ahmad MK, Shankhwar
SN, Rajender S and Jaiswar SP: Mucuna pruriens improves male
fertility by its action on the hypothalamus-pituitary-gonadal axis.
Fertil Steril. 92:1934–1940. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li ZM, Liu N, Jiang YP, Yang JM, Zheng J,
Sun M, Li YX, Sun T, Wu J and Yu JQ: Vitexin alleviates
streptozotocin-induced sexual dysfunction and fertility impairments
in male mice via modulating the hypothalamus-pituitary-gonadal
axis. Chem Biological Interact. 297:119–129. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fink G: Neuroendocrine regulation of
pituitary function. Neuroendocrinology in Physiology and Medicine.
Conn PM and Freeman ME: Humana Press; Totowa, NJ: https://doi.org/10.1007/978-1-59259-707-9_7
|
|
13
|
Caraty A, Franceschini I and Hoffman GE:
Kisspeptin and the preovulatory gonadotrophin-releasing
hormone/luteinising hormone surge in the ewe: Basic aspects and
potential applications in the control of ovulation. J
Neuroendocrinol. 22:710–715. 2010.PubMed/NCBI
|
|
14
|
Dhillo WS, Chaudhri OB, Thompson EL,
Murphy KG, Patterson M, Ramachandran R, Nijher GK, Amber V,
Kokkinos A, Donaldson M, et al: Kisspeptin-54 stimulates
gonadotropin release most potently during the preovulatory phase of
the menstrual cycle in women. J Clin Endocrinol Metab.
92:3958–3966. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hu KL, Zhao H, Min Z, He Y, Li T, Zhen X,
Ren Y, Chang HM, Yu Y and Li R: Increased expression of KISS1 and
KISS1 receptor in human granulosa lutein cells-potential
pathogenesis of polycystic ovary syndrome. Reprod Sci.
26:1429–1438. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu KL, Zhao H, Chang HM, Yu Y and Qiao J:
Kisspeptin/kisspeptin receptor system in the ovary. Front
Endocrinol (Lausanne). 8:3652018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yu H, Liu J, Han Y, Chen C and Meng F:
Correlation between serum Kisspeptin and spermatogenic function in
men. bioRxiv. Oct 18–2019.doi: https://doi.org/10.1101/810572.
|
|
18
|
Funes S, Hedrick JA, Vassileva G,
Markowitz L, Abbondanzo S, Golovko A, Yang S, Monsma FJ and
Gustafson EL: The KiSS-1 receptor GPR54 is essential for the
development of the murine reproductive system. Biochem Biophys Res
Commun. 312:1357–1363. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Franke TF: PI3K/Akt: Getting it right
matters. Oncogene. 27:6473–6488. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Foster FM, Traer CJ, Abraham SM and Fry
MJ: The phosphoinositide (PI) 3-kinase family. J Cell Sci.
116:3037–3040. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin A, Piao H, Zhuang L, Sarbassov dos D,
Ma L and Gan B: FoxO transcription factors promote AKT Ser473
phosphorylation and renal tumor growth in response to pharmacologic
inhibition of the PI3K-AKT pathway. Cancer Res. 74:1682–1693. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Beymer M, Negrón AL, Yu G, Wu S, Mayer C,
Lin RZ, Boehm U and Acosta-Martínez M: Kisspeptin cell-specific
PI3K signaling regulates hypothalamic kisspeptin expression and
participates in the regulation of female fertility. Am J Physiol
Endocrinol Metab. 307:E969–E982. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen S, Chen W, Zhang X, Lin S and Chen Z:
Overexpression of KiSS-1 reduces colorectal cancer cell invasion by
downregulating MMP-9 via blocking PI3K/Akt/NF-κB signal pathway.
Int J Oncol. 48:1391–1398. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xin X, Li Z, Zhong Y, Li Q, Wang J, Zhang
H, Yuan X, Li J and Zhang Z: KISS1 suppresses apoptosis and
stimulates the synthesis of E2 in porcine ovarian Granulosa cells.
Animals (Basel). 9:542019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dowarah J and Singh VP: Anti-diabetic
drugs recent approaches and advancements. Bioorg Med Chem.
28:1152632020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Birben E, Sahiner UM, Sackesen C, Erzurum
S and Kalayci O: Oxidative stress and antioxidant defense. World
Allergy Organ J. 5:9–19. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Adedara IA, Okpara ES, Busari EO, Omole O,
Owumi SE and Farombi EO: Dietary protocatechuic acid abrogates male
reproductive dysfunction in streptozotocin-induced diabetic rats
via suppression of oxidative damage, inflammation and caspase-3
activity. Eur J Pharmacol. 849:30–42. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Johnson A, Cheng SC, Tsou D and Kong ZL:
Attenuation of reproductive dysfunction in diabetic male rats with
timber cultured Antrodia cinnamomea ethanol extract. Biomed
Pharmacother. 112:1086842019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jiang YP, Ye RJ, Yang JM, Liu N, Zhang WJ,
Ma L, Sun T, Niu JG, Zheng P and Yu JQ: Protective effects of
Salidroside on spermatogenesis in streptozotocin induced type-1
diabetic male mice by inhibiting oxidative stress mediated
blood-testis barrier damage. Chem Biol Interact. 315:1088692020.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu J, Wang Y, Gong L and Sun C: Oxidation
of glyceraldehyde- 3-phosphate dehydrogenase decreases sperm
motility in diabetes mellitus. Biochem Biophys Res Commun.
465:245–248. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Luo D, Zhang M, Su X, Liu L, Zhou X, Zhang
X, Zheng D, Yu C and Guan Q: High fat diet impairs spermatogenesis
by regulating glucose and lipid metabolism in Sertoli cells. Life
Sci. 257:1180282020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gahete MD, Vázquez-Borrego MC,
Martínez-Fuentes AJ, Tena-Sempere M, Castaño JP and Luque RM: Role
of the Kiss1/Kiss1r system in the regulation of pituitary cell
function. Mol Cell Endocrinol. 438:100–106. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ke R, Ma X and Lee LTO: Understanding the
functions of kisspeptin and kisspeptin receptor (Kiss1R) from
clinical case studies. Peptides. 120:1700192019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
George JT, Millar RP and Anderson RA:
Hypothesis: Kisspeptin mediates male hypogonadism in obesity and
type 2 diabetes. Neuroendocrinology. 91:302–307. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li X, Zhu Y, Zhang C, Liu J, Zhou G, Jing
L, Shi Z, Sun Z and Zhou X: BDE-209 induces male reproductive
toxicity via cell cycle arrest and apoptosis mediated by DNA damage
response signaling pathways. Environ Pollut. 255:1130972019.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Khodamoradi K, Amini-Khoei H,
Khosravizadeh Z, Hosseini SR, Dehpour AR and Hassanzadeh G:
Oxidative stress, inflammatory reactions and apoptosis mediated the
negative effect of chronic stress induced by maternal separation on
the reproductive system in male mice. Reprod Biol. 19:340–348.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen Z, Wen D, Wang F, Wang C and Yang L:
Curcumin protects against palmitic acid-induced apoptosis via the
inhibition of endoplasmic reticulum stress in testicular Leydig
cells. Reprod Biol Endocrinol. 17:712019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang L, Lei L, Zhao Q, Gong Y, Guan G and
Huang S: C-Type natriuretic peptide/natriuretic peptide receptor 2
is involved in cell proliferation and testosterone production in
mouse leydig cells. World J Mens Health. 37:186–198. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ma Y, Qin H and Cui Y: MiR-34a targets
GAS1 to promote cell proliferation and inhibit apoptosis in
papillary thyroid carcinoma via PI3K/Akt/Bad pathway. Biochem
Biophys Res Commun. 44:958–963. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang XH, Chen SY, Tang L, Shen YZ, Luo L,
Xu CW, Liu Q and Li D: Myricetin induces apoptosis in HepG2 cells
through Akt/p70S6K/bad signaling and mitochondrial apoptotic
pathway. Anticancer Agents Med Chem. 13:1575–1581. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Maiorino MI, Bellastella G and Esposito K:
Diabetes and sexual dysfunction: Current perspectives. Diabetes
Metab Syndr Obes. 7:95–105. 2014.PubMed/NCBI
|