Introduction
Ischemic stroke is an extremely mortal
cerebrovascular disease which has a high recurrence rate (~26%),
and usually results in disability, thus becoming a severe health
problem worldwide (1). Despite
continued advances in therapeutic techniques, the mechanism of
cerebral ischemia-reperfusion (I/R) injury is still complex
(2); therefore, a clear
understanding of the pathophysiology is warranted.
Neuroinflammation and oxidative stress emerge as
important traits to be considered in the pathophysiology of
ischemic stroke (3).
Malondialdehyde (MDA) is an oxidative stress marker, which is also
a product of lipid peroxidation reaction (4). During ischemia physiological
condition, few free radicals are present, but at the stage of
recovery, known as reperfusion, the blood supply of the tissue
triggers the ‘explosion’ of oxygen free radicals, the antioxidant
enzymes, such as superoxide dismutase (SOD), catalase (CAT),
glutathione (GSH), are involved in the defensive system for
protecting against oxidative stress (5,6).
Txk tyrosine kinases (Txk), also known as
tyrosine-protein kinases Txk or protein-tyrosine kinase 4 is one of
the Tec family of tyrosine kinases that have unique N-termini
followed by Src homology 3 (SH3) and Src homology 2 (SH2) protein
interaction domains and a tyrosine kinase catalytic domain
(7). Txk expression is enhanced in
rheumatoid arthritis, where Th1-dominant immunity occurs (8). Modulation of Txk expression by gene
transfer or similar modality may lead to the correction of aberrant
immunity (9). As an
immune-associated protein, Txk has been studied little in the field
of stroke.
The nuclear factor (NF)-κB signaling pathway
associated with severe immune deficiencies, whereas dysregulated
activation of this pathway contributes to the pathogenesis of
various autoimmune and inflammatory diseases (10). NF-κB signaling pathway is reported
to be involved in neurodegeneration and oxidative stress in
ischemia/reperfusion (I/R) injury (11). However, the mechanism of Txk and
NF-κB signaling pathway in oxidative stress after I/R are still not
fully clarified.
The present study focused on the role of Txk in I/R
injury and Txk knockdown was used to decrease neurological deficit
and oxidative stress in vivo and vitro. Furthermore,
NF-κB overexpression was conducted to activate NF-κB expression,
which reversed the progress after Txk regulation. The present
findings indicated that Txk potentially mediates neurological
deficit and oxidative stress through the NF-κB signaling pathway in
I/R injury. These results may provide a new strategy to recede and
recover I/R injury.
Materials and methods
Animals
A total of 300 (age, 6–8 weeks; mean age, 7.2 weeks)
healthy male adult Sprague-Dawley rats (weighing 250–300 g) were
supplied by Liaoning Changsheng Biotechnology Co., Ltd., which were
housed in a temperature and light control environment. All animals
used in this experiment were cared for in strict accordance with
the Guide for the Care and Use of Laboratory Animals (12). All experimental procedures were
cared for in strict accordance with the Animal Ethics Committee of
Dongyang People's Hospital (permit no. AEWC-20191210A).
Middle cerebral artery occlusion
(MCAO) rat model establishment and experimental design
Cerebral I/R was obtained through the MCAO procedure
as previously described (13). In
brief, the experimental rats were anesthetized with pentobarbital
sodium (30 mg/kg, i.p.) until the rats were irresponsive to touch
and placed on a heating pad to maintain body temperature. The left
common and external carotid arteries were then proximally exposed
and ligated after a ventral midline neck incision. A 5-0 nylon
monofilament (Beijing Cinontech Co. Ltd.), with the blunt end
coated with poly-L-lysine, was inserted into the internal carotid
artery and forwarded into the middle cerebral artery origin until a
slight resistance was met. Meanwhile, the regional cerebral blood
flow was decreased to <20% of the baseline level. Reperfusion
was achieved after 1 h of occlusion by slowly withdrawing the nylon
monofilament and the blood flow was recovered to 75% of baseline.
Sham animals received the same operation without MCAO. All animals
were sacrificed after 48 h upon reperfusion (13).
Then, the subjects were randomly divided into six
groups (n=6 in each group): (i) Sham group, rats that underwent
surgical procedures but had no ischemia; (ii) MCAO group, rats with
60 min of ischemia followed by 6–48 h reperfusion; (iii) Txk-shRNA
group, rats receiving intra-cerebroventricular injection of
lentivirus-sh-Txk one week before MCAO; (iv) control shRNA group,
rats receiving intra-cerebroventricular injection of lentivirus
expressing negative control; (v) NF-κB-oeRNA group, rats receiving
intra-cerebroventricular injection of lentivirus-oe-NF-κB one week
before MCAO; (vi) control oeRNA group, rats receiving
intra-cerebroventricular injection of lentivirus expressing
negative control. Rats were treated according to American College
of Laboratory Animal Medicine guidelines (14). The investigators observed the rats
daily to ensure animal welfare and to determine the humane
endpoints, which included movement, apathy and severe weight loss
for 1 h frequently. The rats were sacrificed when they exhibited
rapid weight loss (>20%) or were nonmobile and showed signs of
deteriorating health, such as hunching, dehydration and labored
breathing.
Rats were sacrificed via heart perfusion with 0.9%
saline solution. The rats were anesthetized by pentobarbital sodium
(30 mg/kg; intraperitoneal), an upper abdominal incision was made,
and the heart was exposed. Then, 0.9% saline solution was injected
into the apex of heart before cutting open the right auricle and
pumped into the systemic circulation until the heartbeat
stopped.
Isolation and culture of primary
cortical neurons
The primary cortical neurons extracted from
embryonic cerebrums of 90 Sprague-Dawley rats (16–18 days) were
used in the present study as described previously (15,16).
The cell suspensions were seeded on six-well plates (concentration:
1.5×105 cells per well) and neurobasal medium containing
2% B27 (Gibco; Thermo Fisher Scientific, Inc.) was used for culture
under 5% CO2 in a 37°C incubator, which was supplemented
with glutamine (2 mM) and penicillin/streptomycin (50 U/ml)
(12). All experimental procedures
were cared for in strict accordance with the Animal Ethics
Committee of Dongyang People's Hospital (permit no.
AEWC-20191210A).
Oxygen and glucose
deprivation/reperfusion (OGD/R) model establishment
For the OGD/R model establishment, cells were
incubated at 37°C with Earles Balanced Salt Solution
(Sigma-Aldrich; Merck KGaA) without glucose in a 1%
oxygen-supplying incubator for 6 h. The cells were then transferred
into an incubator at 37°C at normal conditions for another 1–12 h
(17). The grouping was similar to
that with the rats in vivo (17).
Reagents and antibodies
2,3,5-triphenyltetrazolium chloride (TTC) was
purchased from Sigma Aldrich (Merck KGaA). Lipid peroxidation MDA
assay kit (cat. no. S0131; Beyotime Institute of Biotechnology),
GSH assay kit (cat. no. S0053; Beyotime Institute of
Biotechnology), GSH peroxidase (GSH-PX) assay kit (cat. no. S0058;
Beyotime Institute of Biotechnology), CAT assay kit (cat. no.
S0051; Beyotime Institute of Biotechnology), SOD assay kit (cat.
no. S0109; Beyotime Institute of Biotechnology) and cytochrome C
activity assay kit (cat. no. K257-100; Biovision, California, USA)
were also obtained.
Anti-Txk (cat. no. PA5-98222; Thermo Fisher
Scientific, Inc.), anti-NF-κB (cat. no. BM3940; Boster Biological
Technology), anti-interleukin (IL)-1β (cat. no. ab23437; Abcam),
anti-IL-18 (cat. no. ab71495; Abcam), anti-tumor necrosis factor
(TNF)-α (cat. no. ab1793; Abcam) and anti-β-actin (cat. no.
M01263-2; Boster Biological Technology) primary antibodies,
followed by secondary antibodies conjugated to horseradish
peroxidase anti-rabbit IgG (H+L) (cat. no. AS014; ABclonal Biotech
Co., Ltd.) and anti-mouse IgG (H+L) (cat. no. AS003; ABclonal
Biotech Co., Ltd.) were used for western blotting or
immunofluorescence (IF).
Txk knockdown and NF-κB
overexpression
The Txk knockdown lentivirus (Txk-shRNA), NF-κB
overexpression lentivirus (NF-κB-oeRNA) and control lentivirus were
designed and chemically synthesized by GenePharma Corporation. The
lentivirus vector were stored at −80°C accordingly.
To knockdown Txk in vivo, rats were
anesthetized and placed in a Kopf stereotactic frame, as previously
described (18). In brief, a burr
hole was bored in the pericranium 0.9 mm lateral to the sagittal
suture and 1.9 mm posterior to the coronal suture. A 10-ml
microinjection pump (WPI, Inc.) was inserted 3.5 mm deeper into the
cortex stereotactically. A 5-ml viral suspension consisting of
1×109 genomic copies of Txk-shRNA lentivirus, was
ipsilaterally injected into the right lateral cerebral ventricle at
a rate of 0.2 ml/min. The needle was taken out after 15 min of
injection. To knockdown Txk, cells were subjected to OGD/R
treatment, as previously described (19). The NF-κB overexpression lentivirus
transfection was performed ahead of Txk-shRNA induction, using the
aforementioned method. The sequences of Txk and NF-κB lentivirus
are shown in Tables I and II. Negative controls for sh-Txk-1201,
1446, 1742 And control shTxk were used.
 | Table I.Sequences of Txk. |
Table I.
Sequences of Txk.
| Name | Sequence |
|---|
| sh-Txk-1201 |
5′-ATGAAGAGCTTCCAGAAAAA-3′ |
| sh-Txk-1442 |
5′-CAATCCTCACTGGTGGAAGG-3′ |
| sh-Txk-1746 |
5′-GAAAGACACGCCTTTCAATC-3′ |
| Negative
control |
5′-AGGTTTTCAGAACAGGAAAA-3′ |
| control-shTxk |
5′-TCTTCACCATATGGGGTCAC-3′ |
 | Table II.Sequences of NF-κB. |
Table II.
Sequences of NF-κB.
| Name | Sequence |
|---|
| oe-NF-κB-481 |
5′-GGGCTTACCCATGTCTGGAG-3′ |
| oe-NF-κB-846 |
5′-TGACAGCAAGCTTCCCAAAC-3′ |
| oe-NF-κB-1261 |
5′-ACAGGTCAAGTTCTTTGAGA-3′ |
| Negative
control |
5′-TGAGACTGAGGCTCTGGCTT-3′ |
|
control-oeNF-κB |
5′-TGCTGCATTTCAAATCGATC-3′ |
Western blotting
RIPA buffer (cat. no. P0013K; Beyotime Instituteof
Biotechnology) containing protease inhibitor was used to extract
total protein from the cells and the tissue, and the supernatant
was collected following centrifugation (12,000/rpm). A total of 40
µg protein from each brain sample using the bicinchoninic acid
(cat. no. P0012; Beyotime Institute of Biotechnology) method was
subjected to 12% SDS-polyacrylamide gel electrophoresis and
transferred to nitrocellulose membrane. In brief, after removing
the combs, the target protein and marker were added and the gel was
run at 80V, and then the sample was separated to the bottom of the
gel at 120V. The target protein and marker were then
electrotransferred to a PVDF membrane at 250 mA for 1 h and blocked
with 5% skim milk for 1 h at 37°C. Subsequently, the membranes were
incubated overnight in primary antibody (Txk, 1:2,000; NF-κB,
1:3,000; IL-1β, 1:1,000; IL-18, 1:1,000; TNF-α, 1:2,000; β-actin,
1:5,000) at 4°C. Then, the membrane was washed with 10X TBS-0.1%
Tween 20 (TBST) three times and incubated with the secondary
antibody (1:5,000) for 1 h at 37°C. The membrane was rinsed three
times again with TBST and developed with developing liquid
(horseradish peroxidase and alkaline phosphatase; cat. no. P0019;
Beyotime Institute of Biotechnology). All data were detected with
the ChemiDoc™ Touch Imaging System and analyzed with the Image lab
3.0 software (Bio-Rad Laboratories, Inc.).
IF staining
The expression level of Txk was detected by IF
staining as previously described (13). In brief, paraffin-embedded tissue
fraction (5-mm thickness) was dewaxed with xylene, then dehydrated
using graded concentrations of alcohol, and incubated with 3%
H2O2 inhibitor, endogenous peroxidase. After
blocking in 10% goat serum for 10 min at room temperature, the
tissues were incubated with the primary antibody of Txk (1:100) in
blocking solution overnight at 4°C. Then, the slides were washed in
PBS and HRP-labeled anti-rabbit IgG was applied for 30 min at 37°C,
followed by a PBS wash. Finally, images were acquired with a Nikon
EclipseNi inverted microscope (400× magnification; TE2000; Nikon
Corporation).
Acridine orange (AO)/propidium iodide
(PI) staining
AO/PI staining was conducted as previously reported
(20). The apoptotic ratio was
counted and shown as the ratio of cells with positive ethidium
bromide staining.
TUNEL staining
Tissue fraction was determined by TUNEL assay. The
TUNEL assay was performed according to the manufacturers
instruction manual (cat. no. C1091; Beyotime Institute of
Biotechnology). Images were captured by fluorescence microscopy at
×400 magnification.
Infarct volume evaluation
Animals were anesthetized and sacrificed. The whole
brain was then rapidly removed, frozen for 15 min at −20°C in an
adult rat brain matrix (RWD Life Science) and then cut into 3
coronal sections. Cryosections were stained with 2% TTC for 15 min
at room temperature in the dark, followed by fixation with 4%
paraformaldehyde at 4°C for 48 h.
Neurological deficit score assessment
(Longa Score)
The neurological score was evaluated 24 h after
reperfusion using a 5-point rating scale: Point 0, no deficit;
point 1, failure to extend the left forepaw; point 2, decreased
grip strength of the left forepaw; point 3, circling to left by
pulling the tail; point 4, spontaneous circling; and point 5, no
movement (21).
Brain water content assay
Rats were decapitated under deep anesthesia and
brains were immediately removed. Each brain was divided into two
halves, infarction and contralateral. The wet weight (WW) was
weighed on an electronic balance. Brain tissue was dried in the
oven at 100°C for 24 h to determine the dry weight (DW). Brain
water content was calculated as [(WW-DW)/WW] ×100%.
Cell viability and LDH assays
The viability of neurons was measured by MTT assay.
Briefly, the neurons (2,000 cells/well) were cultured in 96-well
culture plates for 24 h. Then, MTT (5 mg/ml) was added into each
well at 37°C for 4 h. After that, MTT was removed and 150 µl DMSO
was added into the well to solubilize purple formazan. Then,
absorbance at 570 nm was read using a microplate reader (PLUS 384;
Molecular Devices, LLC). LDH cytotoxicity detection kit (cat. no.
C0017; Beyotime Institute of Biotechnology) was used as previously
described (22).
Oxidative stress parameters and
mitochondrial dysfunction detection
For detecting oxidative stress parameters in rat
ischemic hemispheres tissue or neurons, lipid peroxidation MDA,
GSH, GSH-PX, CAT, SOD and cytochrome C were purchased and operated
according to the manufacturers instructions.
Statistical analysis
Data are all expressed as mean ± standard. Data
statistical analysis was performed using GraphPad Prism 6.0
(GraphPad Software, Inc.). Differences were analyzed using unpaired
t-test, one-way ANOVA and multiple comparisons were analyzed using
Tukeys test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression of Txk at different time
points and its approximate localization in cells after I/R or OGD/R
injury
The expression of Txk at different time points after
MCAO or OGD/R and its approximate localization was examined by
western blotting and IF (Fig. 1).
The Txk expression peaked at 24 h after MCAO (P<0.05), compared
with the sham, and MCAO 6, 12, and 48-h groups (Fig. 1A and B). In addition, after OGD/R
stimulation, the Txk expression peaked at 6 and 12 h (P<0.05),
compared with the control group, and OGD/R 1 and 2-h groups
(Fig. 1C and D). Thus, 24 h after
MCAO and 6 h after OGD/R was used for the I/R model for the
follow-up experiments. The approximate localization of Txk is shown
in Fig. 1E and F, the
Txk/DAPI-positive cells or fluorescence intensity were increased
after MCAO and OGD/R, and the Txk molecules gradually entered the
nucleus (Fig. 1G and H).
 | Figure 1.Western blotting and
immunofluorescence results. (A) Western blot analysis of Txk
expression in sham and MCAO 6-, 12-, 24- and 48-h groups. (B)
Quantification of Txk in different groups. (C) Western blot
analysis of Txk expression in control and OGD/R 1-, 2-, 6- and 12-h
neurons. (D) Quantification of Txk in different neurons. (E)
Immunofluorescence assay of Txk in sham and MCAO rats.
Magnification, ×400) (F) Immunofluorescence assay of Txk in control
and OGD/R neurons. Magnification, ×1000 (G) Txk-positive cells in
sham and MCAO rats and (H) fluorescence intensity in control and
OGD/R neurons. Protein level was normalized to β-actin. *P<0.05,
MCAO 24 h vs. sham, MCAO 6, 12 and 48 h groups; OGD/R 6 h vs.
control, OGD/R 1 and 2 h groups; MCAO vs. sham; OGD/R vs. control;
n=6 per group. All data are represented as mean ± standard error.
Txk, Txk tyrosine kinase; MCAO, middle cerebral artery occlusion;
OGD/R, oxygen and glucose deprivation/reperfusion. |
Txk knockdown contributes to the
neuroprotection against acute stroke attack in rats
In order to knockdown Txk expression, Txk-shRNA was
used, and the expression of Txk was decreased after shRNA
administration (Fig. S1). To
investigate the potential function of Txk on cerebral I/R injury in
rats, the impact of Txk on brain infarct volume, neurological score
and brain water content was examined (Fig. 2A-D). The Txk-shRNA group showed a
significantly decreased brain infarct volume, neurological score
and brain water content compared with the MCAO group (P<0.05).
Apoptosis was also detected in Fig.
2E, and the TUNEL-positive cells were significantly decreased
in the Txk-shRNA group (P<0.05) compared with the MCAO group
(Fig. 2F).
 | Figure 2.(A) 2,3,5-Triphenyltetrazolium
chloride staining, (B) brain infarct volume assay, (C) neurological
score assay, (D) brain water content, (E) immunofluorescence assay
of TUNEL (magnification, ×400) and (F) TUNEL assay of sham, MCAO,
Txk-shRNA and control-shRNA groups. *P<0.05, MCAO vs. sham
group; #P<0.05, Txk-shRNA vs. MCAO group; n=6 per
group. Txk, Txk tyrosine kinase; MCAO, middle cerebral artery
occlusion; shRNA, short hairpin RNA. |
Txk knockdown decreases oxidative
stress after I/R injury in rats
As Txk knockdown contributed to the neuroprotection
against acute stroke attack, the associated oxidative stress and
mitochondrial dysfunction parameters were also observed. The level
of MDA and cytochrome C, an indirect indicator of mitochondrial
function (20) in the MCAO group
was obviously increased compared with the sham group (P<0.05);
GSH, GSH-PX, CAT and SOD showed contrasting results. MDA and
cytochrome C levels were decreased in the Txk-shRNA group
(P<0.05) compared with the MCAO group (Fig. 3A-F).
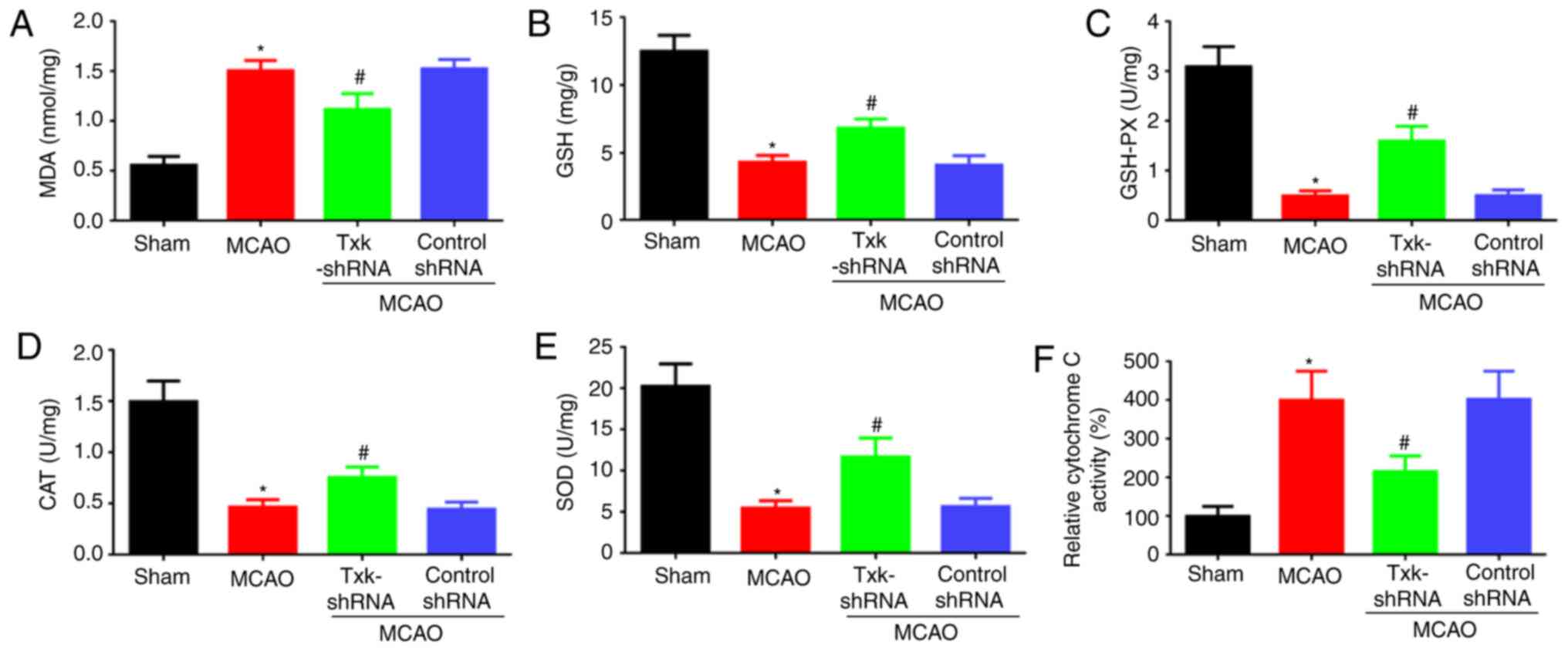 | Figure 3.(A) MDA, (B) GSH, (C) GSH-PX, (D)
CAT, (E) SOD and (F) cytochrome C activity assays of sham, MCAO,
Txk-shRNA and control-shRNA groups. *P<0.05, MCAO vs. sham
group; #P<0.05, Txk-shRNA vs. MCAO group; n=6 per
group. MCAO, middle cerebral artery occlusion; MDA,
malondialdehyde; GSH, glutathione; GSH-PX, GSH peroxidase; CAT,
catalase; SOD, superoxide dismutase; shRNA, short hairpin RNA. |
Txk knockdown contributes to the
neuroprotection after OGD/R stimulation
Since the potential function of Txk on cerebral I/R
injury in rats was investigated, this process was also studied
in vitro. The AO/PI assay is shown in Fig. 4A, and the AO/PI-positive cells were
significantly decreased in the Txk-shRNA group (P<0.05) compared
with the OGD/R group (Fig. 4B). To
investigate the apoptotic function of Txk after OGD/R, TUNEL
staining was conducted in neurons. The TUNEL-positive cells were
significantly decreased in the Txk-shRNA group (P<0.05) compared
with the OGD/R group (Fig. 4C and
D). In order to obtain more direct evidence, cellular viability
and LDH release assays were used to assess OGD/R-induced injury in
neurons. The cellular viability was significantly enhanced in the
Txk-shRNA group (P<0.05) compared with the OGD/R group (Fig. 4E), and the LDH release showed the
opposite result (Fig. 4F).
 | Figure 4.(A) Immunofluorescence assay of AO/PI
(magnification, ×400), (B) AO/PI-positive cells, (C)
immunofluorescence assay of TUNEL (magnification, ×400), (D)
TUNEL-positive cells (E) cellular viability and (F) LDH release
assays of control, OGD/R, Txk-shRNA and control-shRNA groups.
*P<0.05, OGD/R vs. control group; #P<0.05,
Txk-shRNA vs. OGD/R group; n=6 per group. AO/PI, acridine
orange/propidium iodide; Txk, Txk tyrosine kinase; MCAO, middle
cerebral artery occlusion; OGD/R, oxygen and glucose
deprivation/reperfusion; shRNA, short hairpin RNA. |
Txk knockdown decreases oxidative
stress after OGD/R stimulation
Since the potential function of Txk on oxidative
stress and mitochondrial dysfunction was investigated in rats, this
process was also studied in vitro. The level of MDA and
cytochrome C in the OGD/R group was notably increased compared with
the control group (P<0.05); GSH, GSH-PX, CAT and SOD showed the
contrary results. MDA and cytochrome C levels were decreased in the
Txk-shRNA group (P<0.05), compared with the OGD/R group
(Fig. 5A-F).
 | Figure 5.(A) MDA, (B) GSH, (C) GSH-PX, (D)
CAT, (E) SOD and (F) cytochrome C activity assays of control,
OGD/R, Txk-shRNA and control-shRNA groups. *P<0.05, OGD/R vs.
control group; #P<0.05, Txk-shRNA vs. OGD/R group;
n=6 per group. MDA, malondialdehyde; GSH, glutathione; GSH-PX, GSH
peroxidase; CAT, catalase; SOD, superoxide dismutase; Txk, Txk
tyrosine kinase; shRNA, short hairpin RNA; OGD/R, oxygen and
glucose deprivation/reperfusion. |
Txk regulates neurological deficit and
oxidative stress after I/R via NF-κB signaling pathway
In order to investigate Txk-regulated neurological
deficit and oxidative stress after I/R via NF-κB signaling pathway,
a method for increasing the NF-κB level was attempted: NF-κB
overexpression lentivirus (NF-κB-oeRNA). NF-κB-oeRNA was used to
enhance NF-κB expression, and the associated protein, neurological
deficit and oxidative stress were then observed in vivo and
vitro (Fig. 6). As shown in
Fig. 6A and B in vivo,
compared with the MCAO group, the NF-κB, TNF-α, IL-1β and IL-18
levels in the Txk-shRNA group was decreased (P<0.05). However,
in the Txk-shRNA+NF-κB-oeRNA group, the NF-κB, TNF-α, IL-1β and
IL-18 levels were increased (P<0.05) compared with the Txk-shRNA
group. The in vitro results are shown in Fig. 6C and D. The neurological deficit
score, oxidative stress and mitochondrial dysfunction parameters
in vivo (Fig. 6E-G) and
in vitro (Fig. 6H) were also
detected, which showed the identical tendency with the western blot
analysis.
 | Figure 6.(A) Western blot analysis and (B)
quantification of NF-κB, TNF-α, IL-1β and IL-18 expression in sham,
MCAO, Txk-shRNA, control shRNA, Txk-shRNA+NF-κB-oeRNA and
Txk-shRNA+control oeRNA groups in vivo. (C) Western blot
analysis and (D) quantification of NF-κB, TNF-α, IL-1β and IL-18
expression in control, OGD/R, Txk-shRNA, control shRNA,
Txk-shRNA+NF-κB-oeRNA and Txk-shRNA+control oeRNA groups in
vitro. (E) Neurological score assay, (F) MDA, GSH, GSH-PX, CAT
and SOD assays and (G) cytochrome C activity assay in vivo.
(H) MDA, GSH, GSH-PX, CAT, SOD and cytochrome C activity assays
in vitro. Protein level was normalized to β-actin.
*P<0.05, MCAO or OGD/R vs. sham or control group;
#P<0.05, Txk-shRNA vs. MCAO or OGD/R group;
^P<0.05, Txk-shRNA+NF-κB-oeRNA vs. Txk-shRNA group;
n=6 per group. NF-κB, nuclear factor-κB; TNF-α, tumor necrosis
factor-α; IL, interleukin; MCAO, middle cerebral artery occlusion;
OGD/R, oxygen and glucose deprivation/reperfusion; MDA,
malondialdehyde; GSH, glutathione; GSH-PX, GSH peroxidase; CAT,
catalase; SOD, superoxide dismutase; shRNA, short hairpin RNA; oe,
overexpression. |
Discussion
Ischemic stroke is characterized by a decrease in
cerebral blood flow and deprivation of both oxygen and glucose,
which are required to maintain the metabolic demands of the brain
(23–24). Focal cerebral ischemia models, such
as MCAO and neuron OGD, resemble human ischemic stroke (25,26).
Oxidative stress has been associated with different
pathological processes such as neurotoxicity, neuroinflammation,
ischemic stroke and neurodegenerative diseases (27,28).
During I/R injury, the excessive production of reactive oxygen
species, reactive nitrogen species, excitatory amino acid toxicity
and inflammatory reaction are implicated in neuronal damage
(29).
Txk, a member of the Tec family, whose catalytic
activity was essential for its function to enhance Th1 cell
function (30), suggested that Txk
maybe belong to an immune-associated protein. Txk had been reported
that it may be associated with breast cancer, schizophrenia,
rheumatoid arthritis and diabetes (8,31–33).
The Txk expression level was enhanced in rheumatoid arthritis,
where Th1-dominant immunity occurs (8). The modulation of Txk expression by
gene transfer or similar modality may lead to the correction of
aberrant immunity (9). However, Txk
as an inflammation-associated factor had been poorly studied in
neuroscience-associated studies. To address these issues, the Txk
expression level was detected after MCAO and OGD/R by western
blotting and IF staining. The results showed the Txk expression
level was increased after I/R injury. Besides, the Txk molecules
gradually entered the nucleus for expression; this suggests that
Txk may play a biological role after entering the nucleus in
ischemic stroke. This may also indirectly prove that Txk lacks PH
domain and has a nuclear translocation signal sequence, which is
responsible for the nuclear translocation (34).
In order to detect the function of Txk after MCAO, a
Txk knockdown lentivirus was used to block the expression level of
Txk. In the present study, Txk-shRNA decreased the brain infarct
volume, neurological deficit score, brain water content and
apoptosis level, which suggested that blocking Txk could decrease
infarction and neuron apoptosis, and improve neurological function
and encephaledema. Furthermore, Txk-shRNA receded oxidative stress
injury and mitochondrial dysfunction through detecting MDA, GSH,
CAT, SOD and cytochrome C level, an indirect indicator for
mitochondrial function (23).
Txk-shRNA decreased oxidative stress injury and mitochondrial
dysfunction; this may be due to the association between Txk and
inflammation (30).
Furthermore, to verify the function of Txk in
neurons, the Txk knockdown lentivirus was used to decrease Txk
expression after OGD/R stimulation. The results showed that Txk
knockdown could decrease apoptosis level, LDH release, oxidative
stress and increase cellular viability; it exhibited the same
tendency with the experiments in vivo.
NF-κB is activated in cerebral vascular endothelial
cells after cerebral ischemia, which triggers a dramatically
increasing production of inflammatory cytokines, leading to an
inflammatory cascade reaction and aggravating brain damage
(35). NF-κB inhibition played a
key role in reversing ischemic stroke pathologies (36). However, whether the role of Txk
after ischemic stroke was regulated by NF-κB signaling pathway has
not been reported yet. However, in the present study, the
anti-NF-κB from Boster was NF-κB-P65, the other subtype IKBα was
not detected, and the other inflammation signaling pathways or
target inflammatory cells are still unclear; further studies are
required to explore this further.
In the present study, NF-κB overexpression
lentivirus was used to enhance NF-κB expression level, and it was
observed whether the process could be reversed after Txk knockdown.
The results showed that the neurological deficit score, oxidative
stress and mitochondrial dysfunction parameters in vivo and
in vitro was reversed after NF-κB regulation. These results
suggest that the regulation of neurological deficit and oxidative
stress by Txk was possibly mediated by NF-κB signaling pathway. The
present findings indicated that Txk knockdown significantly
regulated apoptosis, neurological deficit and oxidative stress
after I/R by entering the nucleus and is modulated through NF-κB
signaling pathway. Taken together, these findings may provide a new
strategy to decrease neurological deficit and oxidative stress
after I/R injury.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors contributions
All authors contributed to the study conception and
design. Material preparation, data collection and analysis were
performed by QX and JW. The first draft of the manuscript was
written by QX and all authors commented on previous versions of the
manuscript. QX and JW confirm the authenticity of all the raw data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All animals were cared for in strict accordance with
the Guide for the Care and Use of Laboratory Animals (NIH
Publication No. 85-23, revised 1996), and the experimental design
was approved (approval no. 20200416) by the Ethics Committee of
Dongyang People's Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kim JH, Nagy Á, Putzu A, Belletti A,
Biondi-Zoccai G, Likhvantsev VV, Yavorovskiy AG and Landoni G:
Therapeutic hypothermia in Critically Ill patients: A systematic
review and meta-analysis of high quality randomized trials. Crit
Care Med. 48:1047–1054. 2020.PubMed/NCBI
|
|
2
|
Wang J, Mao J, Wang R, Li S, Wu B and Yuan
Y: Kaempferol protects against cerebral ischemia reperfusion injury
through intervening oxidative and inflammatory stress induced
apoptosis. Front Pharmacol. 11:4242020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Boese AC, Lee JP and Hamblin MH:
Neurovascular protection by peroxisome proliferator-activated
receptor alpha in ischemic stroke. Exp Neurol. 331:1133232020.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ren Z, Zhang R, Li Y, Li Y, Yang Z and
Yang H: Ferulic acid exerts neuroprotective effects against
cerebral ischemia/reperfusion-induced injury via antioxidant and
anti-apoptotic mechanisms in vitro and in vivo. Int J
Mol Med. 40:1444–1456. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Granger DN and Kvietys PR: Reperfusion
injury and reactive oxygen species: The evolution of a concept.
Redox Biol. 6:524–551. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Espinos C, Galindo MI, García-Gimeno MA,
Ibáñez-Cabellos JS, Martínez-Rubio D, Millán JM, Rodrigo R, Sanz P,
Seco-Cervera M, Sevilla T, et al: Oxidative stress, a crossroad
between rare diseases and neurodegeneration. Antioxidants (Basel).
9:3132020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Takesono A, Finkelstein L and Schwartzberg
P: Beyond calcium: Mew signaling pathways for Tec family kinases. J
Cell Sci. 115:3039–3048. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Freudenberg J, Lee AT, Siminovitch KA,
Amos CI, Ballard D, Li W and Gregersen PK: Locus category based
analysis of a large genome-wide association study of rheumatoid
arthritis. Hum Mol Genet. 19:3863–3872. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mihara S and Suzuki N: Role of Txk, a
member of the Tec family of tyrosine kinases, in
immune-inflammatory diseases. Int Rev Immunol. 26:333–348. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Q, Lenardo MJ and Baltimore D: 30
years of NF-κB: A blossoming of relevance to human pathobiology.
Cell. 168:37–57. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ali A, Shah FA, Zeb A, Malik I, Alvi AM,
Alkury LT, Rashid S, Hussain I, Ullah N, Khan AU, et al: NF-κB
inhibitors attenuate MCAO induced neurodegeneration and oxidative
stress-a reprofiling approach. Front Mol Neurosci. 13:332020.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bayne K: Revised guide for the care and
use of laboratory animals available. American Physiological
Society. Physiologist. 39:199, 208–211. 1996.PubMed/NCBI
|
|
13
|
He Q, Li Z, Wang Y, Hou Y, Li L and Zhao
J: Resveratrol alleviates cerebral ischemia/reperfusion injury in
rats by inhibiting NLRP3 inflammasome activation through
Sirt1-dependent autophagy induction. Int Immunopharmacol.
50:208–215. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kyha D: Williams. Laboratory Animal
Welfare. Quarterly Rev Biol. 92:802014.
|
|
15
|
Xu SY, Wu YM, Ji Z, Gao XY and Pan SY: A
modified technique for culturing primary fetal rat cortical
neurons. J Biomed Biotechnol. 2012:8039302012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun B, Ou H, Ren F, Huan Y, Zhong T, Gao M
and Cai H: Propofol inhibited autophagy through
Ca2+)/CaMKKβ/AMPK/mTOR pathway in OGD/R-induced neuron
injury. Mol Med. 24:582018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xia P, Pan Y, Zhang F, Wang N, Wang E, Guo
Q and Ye Z: Pioglitazone confers neuroprotection against
ischemia-induced pyroptosis due to its inhibitory effects on
HMGB-1/RAGE and Rac1/ROS pathway by activating PPAR. Cell Physiol
Biochem. 45:2351–2368. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang S, Zhou Y, Yang B, Li L, Yu S, Chen
Y, Zhu J and Zhao Y: C1q/tumor necrosis factor-related protein-3
attenuates brain injury after intracerebral hemorrhage via
AMPK-dependent pathway in rat. Front Cell Neurosci. 10:2372016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xia P, Zhang F, Yuan Y, Chen C, Huang Y,
Li L, Wang E, Guo Q and Ye Z: ALDH 2 conferred neuroprotection on
cerebral ischemic injury by alleviating mitochondria-related
apoptosis through JNK/caspase-3 signing pathway. Int J Biol Sci.
16:1303–1323. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ye Z, Li Q, Guo Q, Xiong Y, Guo D, Yang H
and Shu Y: Ketamine induces hippocampal apoptosis through a
mechanism associated with the caspase-1 dependent pyroptosis.
Neuropharmacology. 128:63–75. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang P, Zhang J, Guo F, Wang S, Zhang Y,
Li D, Xu H and Yang H: Lipopolysaccharide worsens the prognosis of
experimental cerebral ischemia via interferon gamma-induced protein
10 recruit in the acute stage. BMC Neurosci. 20:642019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jiang J, Dai J and Cui H: Vitexin reverses
the autophagy dysfunction to attenuate MCAO-induced cerebral
ischemic stroke via mTOR/Ulk1 pathway. Biomed Pharmacother.
99:583–590. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bock FJ and Tait SWG: Mitochondria as
multifaceted regulators of cell death. Nat Rev Mol Cell Biol.
21:85–100. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Deng Y, Chen D, Wang L, Gao F, Jin B, Lv
H, Zhang G, Sun X, Liu L, Mo D, et al: Silencing of long noncoding
RNA Nespas aggravates microglial cell death and neuroinflammation
in ischemic stroke. Stroke. 50:1850–1858. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shah FA, Li T, Kury LTA, Zeb A, Khatoon S,
Liu G, Yang X, Liu F, Yao H, Khan AU, et al: Pathological
comparisons of the hippocampal changes in the transient and
permanent middle cerebral artery occlusion rat models. Front
Neurol. 10:11782019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jia Y, Cui R, Wang C, Feng Y, Li Z, Tong
Y, Qu K, Liu C and Zhang J: Metformin protects against intestinal
ischemia-reperfusion injury and cell pyroptosis via
TXNIP-NLRP3-GSDMD pathway. Redox Biol. 32:1015342020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lewerenz J, Ates G, Methner A, Conrad M
and Maher P: Oxytosis/Ferroptosis-(Re-) emerging roles for
oxidative stress-dependent non-apoptotic cell death in diseases of
the central nervous system. Front Neurosci. 12:2142018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Masaldan S, Bush AI, Devos D, Rolland AS
and Moreau C: Striking while the iron is hot: Iron metabolism and
ferroptosis in neurodegeneration. Free Radic Biol Med. 133:221–233.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li Z, Yulei J, Yaqing J, Jinmin Z, Xinyong
L, Jing G and Min L: Protective effects of tetramethylpyrazine
analogue Z-11 on cerebral ischemia reperfusion injury. Eur J
Pharmacol. 844:156–164. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Takeba Y, Nagafuchi H, Takeno M,
Kashiwakura J and Suzuki N: Txk, a member of nonreceptor tyrosine
kinase of Tec family, acts as a Th1 cell-specific transcription
factor and regulates IFN-gamma gene transcription. J Immunol.
168:2365–2370. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang Y, Wester L, He J, Geiger T,
Moerkens M, Siddappa R, Helmijr JA, Timmermans MM, Look MP, van
Deurzen CHM, et al: IGF1R signaling drives antiestrogen resistance
through PAK2/PIX activation in luminal breast cancer. Oncogene.
37:1869–1884. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu J, Chen J, Ehrlich S, Walton E, White
T, Perrone-Bizzozero N, Bustillo J, Turner JA and Calhoun VD:
Methylation patterns in whole blood correlate with symptoms in
schizophrenia patients. Schizophr Bull. 40:769–776. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Iwai LK, Benoist C, Mathis D and White FM:
Quantitative phosphoproteomic analysis of T cell receptor signaling
in diabetes prone and resistant mice. J Proteome Res. 9:3135–3145.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Haire RN, Ohta Y, Lewis JE, Fu SM, Kroisel
P and Litman GW: TXK, a novel human tyrosine kinase expressed in T
cells shares sequence identity with Tec family kinases and maps to
4p12. Hum Mol Genet. 3:897–901. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shi SS, Yang WZ, Chen Y, Chen JP and Tu
XK: Propofol reduces inflammatory reaction and ischemic brain
damage in cerebral ischemia in rats. Neurochem Res. 39:793–799.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sivandzade F, Prasad S, Bhalerao A and
Cucullo L: NRF2 and NF-B interplay in cerebrovascular and
neurodegenerative disorders: Molecular mechanisms and possible
therapeutic approaches. Redox Biol. 21:1010592019. View Article : Google Scholar : PubMed/NCBI
|




















