Introduction
Leukemia is a heterogeneous group of hematological
cancer types and is the most common type of childhood malignancy,
accounting for ≤30% of all childhood malignancies (1). Leukemia is classified into four types:
Acute myeloid leukemia (AML), chronic myeloid leukemia (CML), acute
lymphoblastic leukemia and chronic lymphocytic leukemia (2). Cytarabine is a key chemotherapy drug
for leukemia treatment. However, preventing the side effects of
chemotherapy drugs and their ability to induce multidrug resistance
phenotypes remains challenging (3).
Emerging cancer treatment strategies focus on reducing drug
toxicity and multidrug resistance phenotypes (4). Curcumin is a yellow spice and phenolic
compound derived from the plant Curcuma longa, and previous
studies have reported that it is a natural phytochemical with the
potential to overcome drug resistance (5–7).
Another study also observed curcumin induced apoptosis in
cytarabine-resistant HL60 cells (4).
Branched-chain amino acids (BCAAs) are essential
amino acids (8). For example, to
achieve rapid proliferation, cancer cells must obtain BCAAs via the
circulation or from surrounding tissues (9). A retrospective metabolomic study
reported that elevated plasma BCAA levels were associated with a
>2 fold increased risk of pancreatic cancer (10). In addition, amino acid levels in
hepatocellular carcinoma, gastric cancer and colon cancer tissues
are typically higher compared with those in the respective
non-tumorous tissues (11).
Reprogrammed cellular metabolism is a common characteristic of
various cancer types (12–14). However, whether metabolic changes
directly regulate cancer development and progression remains poorly
understood.
BCAA transaminase 1 (BCAT1) is a cytosolic
aminotransferase for BCAAs and is aberrantly activated in several
types of cancer, including esophageal squamous cell carcinoma
(15), gastric cancer (16), breast cancer (17), hepatocellular carcinoma (18) and myeloid leukemia (19). BCAT1 is upregulated during the
progression of CML and promotes BCAA production in leukemia cells
via the amination of branched-chain keto acids. Furthermore,
blocking the expression or activity of BCAT1 can induce cell
differentiation and impair the propagation of blast crisis CML
(19). Another study indicated that
AML with high levels of BCAT1 exhibited a DNA hypermethylation
phenotype similar to cases carrying a mutant isocitrate
dehydrogenase (IDHmut), in which the ten-eleven translocation-2
(TET2) protein is inhibited by the oncometabolite
2-hydroxyglutarate (20). Moreover,
high levels of BCAT1 are closely associated with shorter overall
survival in IDH wild-type (wt)/TET2wt but not in IDHmut or TET2mut
AML (20).
It has been shown that BCAT1 is a key regulator of
intracellular α-ketoglutarate (α-KG) levels in various types of
tumor cells, and changes in intracellular α-KG levels have a major
effect on AML cell biology (20,21).
BCAT1 knockdown in leukemia cells causes α-KG accumulation,
resulting in EGL-9 family hypoxia inducible factor 1-mediated
hypoxia-inducible factor-1α protein degradation (20). This results in defects in growth and
survival of leukemia cell lines, as well as the abrogation of
leukemia-initiating potential. By contrast, BCAT1 overexpression in
leukemia cells reduces intracellular α-KG levels and leads to DNA
hypermethylation by altering TET activity (20). However, this transamination reaction
is reported to be reversible. BCAT1 catalyzes the transamination of
plasma BCKAs to generate BCAAs in order to maintain nutrient
sensing via the mTOR complex 1 (mTORC1), thereby maintaining
proliferation signals in leukemia cells (22).
BCAAs also have crucial allosteric regulation and
signal transduction effects. Among these effects, leucine-induced
mTOR pathway regulation is the most widely discussed. It has been
shown that BCAT1 blockade significantly reduced mTORC1 activity in
K562 and MCF-7 cells (17,19). Moreover, cell proliferation and
colony formation assays revealed that rapamycin neutralizes the
promotive effects of BCAT1 on the cell proliferation rate and
colony formation capacity of cancer cells, suggesting that mTOR
activity contributes to BCAT1 function in tumor progression
(17,23). The mTOR pathway is the catalytic
subunit of two distinct multiprotein complexes, namely mTORC1 and
mTORC2, and it is a critical integrator of growth factor-activating
and nutrient-sensing pathways to modulate various cell functions,
including survival, proliferation, differentiation, autophagy,
apoptosis and metabolism (24,25).
Moreover, up to 80% of human cancer types involve mTORC1 signal
dysregulation (26).
Previous studies have reported that curcumin can
regulate several molecules in cell signal transduction pathways,
including mTOR (27,28). The anticancer effects of curcumin
are reflected in its ability to induce growth arrest and apoptosis
in various premalignant and malignant cells (29). However, to the best of the authors'
knowledge, studies have not investigated the regulatory effect of
curcumin on BCAT1. To fill this gap in the literature, the present
study investigated whether curcumin induces apoptosis by regulating
mTOR and BCAT1 signaling in cytarabine-resistant myeloid cells.
Materials and methods
Cell culture and drug treatment
Kasumi-1, KG-1 and HL60 are three common myeloid
leukemia cell lines and were purchased from ATCC. Kasumi-1 cells
were cultured with RPMI 1640 medium (cat. no. A1049101; Gibco;
Thermo Fisher Scientific, Inc.) with 20% FBS (Thermo Fisher
Scientific, Inc.). KG-1 cells were cultured with Iscove's modified
Dulbecco's medium (cat. no. SH30228.02; HyClone; Cytiva) with 20%
FBS. HL60 and resistant (R)-HL60 cells were cultured with RPMI 1640
medium supplemented with 10% FBS. The R-HL60 cell line, which was
established in the Yu-Hsin Tseng's laboratory (Department of
Pediatrics, Kaohsiung Medical University Hospital), had a
cytarabine resistance level >1,000 times that of the parent HL60
cells (4). All cells are cultured
at an incubator with 5% CO2 and temperature of 37°C.
Curcumin, tetrahydrocurcumin, cytarabine and PP242
were purchased from Sigma-Aldrich (Merck KGaA). The BCATc inhibitor
2 was purchased from Cayman Chemical Company. Stock solutions of
curcumin (50 mM), tetrahydrocurcumin (100 mM), PP242 (10 mM) and
BCATc inhibitor 2 (80 mM) were dissolved in DMSO, and stock
solution of cytarabine (400 mM) was dissolved in ddH2O.
The working concentration and durations of drug treatment were 0–50
µM curcumin for 2–48 h, 0–100 µM tetrahydrocurcumin for 24 h, 0–400
µM cytarabine for 24 h, 10 µM PP242 for 24 h and 80 µM BCATc
inhibitor 2 for 24–48 h. The process of drug treatment was
performed in a 37°C incubator with 5% CO2.
Patients and samples
Patients were recruited from the inpatients
department at Kaohsiung Medical University Hospital between
December 2017 and December 2018. The present study was approved by
the Institutional Review Board of Kaohsiung Medical University
Hospital [approval no. KMUHIRB-SV(I)-20170038]. Written informed
consents of patients were obtained from each participant. Bone
marrow samples were obtained from three patients with relapsed
cytarabine-resistant myeloid leukemia. The characteristic gene
alterations of three patients included FLT3 internal tandem
duplication (FLT3-ITD) and nucleophosmin 1cooperating mutations,
Breakpoint Cluster Region Protein (BCR)-Abelson Tyrosine-Protein
Kinase 1 (ABL) mutations (BCR and ABL genes break off and switch
places to form a fusion protein) and FLT3-ITD mutations. Patients
were 9-year-old male, 17-year-old female and 7-year-old male. The
mononuclear cells were isolated by centrifuging at 1,200 × g for 20
min at room temperature using the Ficoll-Paque method (GE
Healthcare) (30). The percentage
of malignant blasts in bone marrow was the diagnostic basis and
treatment outcome assessment for patients with AML (31). Complete remission, partial remission
and relapse disease were defined as <5, 5–20 and >20%
malignant blasts in bone marrow, respectively. The percentages of
malignant blasts in the three bone marrow samples collected in this
study were 48.6, 21.1 and 22.1%, respectively (data collected from
clinical medical records).
RNA isolation and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was isolated using a TRIzol®
total RNA extraction kit (Thermo Fisher Scientific, Inc.), and cDNA
was synthesized using a Maxima First Strand cDNA Synthesis kit
(Thermo Fisher Scientific, Inc.). The reaction steps of RT were:
Incubation 10 min at 25°C, followed by 15 min at 50°C, and
termination of the reaction by heating 5 min at 85°C. Amplification
reactions of qPCR was set up in 10 µl reaction volumes containing
amplification primers and Fast SYBR Green Master mix (Thermo Fisher
Scientific, Inc.) and detected by an ABI 7500 system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). Initial denaturation
temperature was increased to 95°C for 10 min, following by 40
cycles of denaturation at 95°C for 15 sec, annealing at 60°C for 60
sec and extension at 72°C for 15 sec. GAPDH expression was used as
the internal control and was quantified using the 2−ΔΔCq
method (32). The primer sequences
were as follows: BCAT1 forward, 5′-TTCAACTCGTGATACACCAA-3′ and
reverse, 5′-ATTCCTGTGCTAGAGAGCAT-3′; and GAPDH forward,
5′-CTGGGCTACACTGAGCACC-3′ and reverse,
5′-AAGTGGTCGTTGAGGGCAATG-3′.
Western blotting
R-HL60 cells were lysed using RIPA lysis buffer with
the protease inhibitor and phosphatase inhibitor (Thermo Fisher
Scientific, Inc.). The samples were centrifuged at 13,000 × g for
15 min at 4°C, and the supernatant proteins were then collected for
western blotting. The protein concentrations were determined using
a Pierce BCA protein assay kit (Thermo Fisher Scientific, Inc.).
Protein (20 µg per well) was loaded on 4–12% Bolt Bis-Tris Plus
gels (Invitrogen; Thermo Fisher Scientific, Inc.) and transferred
to PVDF membranes. The membranes were blocked with 5% BSA
(Sigma-Aldrich; Merck KGaA) containing 0.1% Tween-20 for 1 h at
room temperature and then incubated overnight with primary
antibodies against human phosphorylated (p)-mTOR (ser2448; 1:1,000;
Arigo Biolaboratories; cat. no. ARG40666), total (t)-mTOR (1:1,000;
Arigo Biolaboratories; cat. no. ARG57640), BCAT1 (1:3,000; Cell
Signaling Technology, Inc.; cat. no. 12822), poly (ADP-ribose)
polymerase 1 (PARP 1; 1:2,000; Abcam; cat. no. ab32138), cleaved
(c)-PARP 1 (1:15,000; Abcam; cat. no. ab32064) and GAPDH (1:30,000;
Ambion; Thermo Fisher Scientific, Inc.; cat. no. AM4300) at 4°C.
The primary antibodies were washed in phosphate-buffered saline
plus 0.1% Tween-20, then the blots were incubated with HRP-linked
secondary antibodies (1:10,000; Cytiva; cat. no. NA9310) for 1 h at
room temperature. Bands were visualized using an ECL assay kit
(Thermo Fisher Scientific, Inc.). X-ray film was used for
chemiluminescence detection. The densitometry of quantify protein
bands from western blot films were analyzed using ImageJ 1.52t
software (National Institutes of Health).
α-KG quantification assay
In total, 2×106 R-HL60 cells were
harvested for each assay. Cells were washed with cold PBS and lysed
with 100 µl lysis buffer. The deproteinization step was performed
using a ReadiUs TCA Deproteinization Sample Preparation kit (AAT
Bioquest, Inc.). Subsequently, α-KG levels were determined using
the α-KG quantitation assay kit (AAT Bioquest, Inc.), according to
the manufacturer's instructions.
Statistical analysis
Statistical analyses were performed using GraphPad
Prism 5.0 (GraphPad Software, Inc.). The paired t-tests and one-way
ANOVA were used to determine the differences between the
experimental and control groups and Bonferroni was used as a post
hoc test following ANOVA. Error bars presented herein represent the
mean ± SEM from triple replicates. P<0.05 was considered to
indicate a statistically significant difference in all comparisons
of the experimental group with the vehicle control group
(H2O for cytarabine, DMSO for curcumin,
tetrahydrocurcumin, PP242 and BCATc inhibitor 2).
Results
Curcumin inhibits the mRNA expression
levels of BCAT1 in myeloid cell lines
The human myeloid leukemia cell lines Kasumi-1
(Fig. 1A), KG-1 (Fig. 1B) and HL60 (Fig. 1C) were treated with different
concentrations of curcumin for 24 h. The results demonstrated that
curcumin effectively reduced the mRNA expression levels of BCAT1 in
a dose-dependent manner.
Curcumin inhibits the mRNA expression
levels of BCAT1 in cytarabine-resistant myeloid leukemia cells
R-HL60, a cytarabine-resistant HL60 cell line, was
treated with 0–50 µM curcumin, 0–100 µM tetrahydrocurcumin and
0–400 µM cytarabine for 24 h. The results indicated that curcumin
(Fig. 2A), but not
tetrahydrocurcumin (Fig. 2B) or
cytarabine (Fig. 2C), effectively
decreased the mRNA expression levels of BCAT1. The cytotoxicity of
cytarabine in mononuclear cells isolated from the bone marrow of
three patient-derived with AML was measured using an XTT assay. The
IC50 values of cytarabine were 169, 749 and >1,600
µM, respectively (data not shown). Curcumin also reduced the mRNA
expression levels of BCAT1 in these mononuclear cells (Fig. 2D).
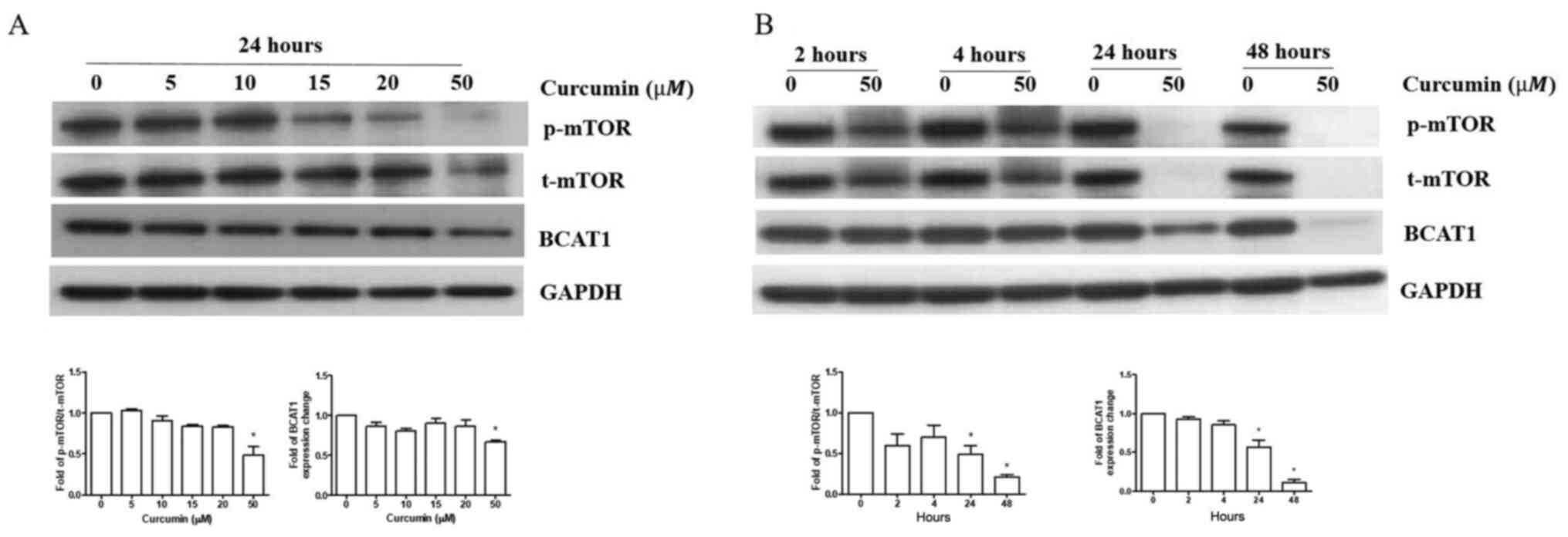
Curcumin inhibits BCAT1 protein
expression and mTOR signaling in R-HL60 cells
R-HL60 cells were treated with 0–50 µM curcumin for
24 h. The results indicated that only 50 µM curcumin treatment
effectively reduced the ratio of p-mTOR/t-mTOR and BCAT1 protein
expression compared with the vehicle control group. (Fig. 3A), so this concentration was chosen
to treat cells for different time periods. The results indicated
that curcumin reduced the ratio of p-mTOR/t-mTOR and BCAT1 protein
expression in 24–48 h (Fig.
3B).
BCAT1 and mTOR pathways modulate each
other and regulate apoptosis in R-HL60 cells
R-HL60 cells were treated with 10 µM PP242 for 24 h
or 80 µM BCATc inhibitor 2 for 48 h to inhibit the mTOR or BCAT1
pathway, respectively. The results demonstrated that the inhibition
of mTOR signaling by PP242 reduced the expression levels of the
BCAT1 and PARP1 proteins, and induced c-PARP1 protein expression
(Fig. 4A). Moreover, inhibition of
BCAT1 signaling through use of the BCATc inhibitor 2 decreased the
ratio of p-mTOR/mTOR and PARP1 protein expression levels and
induced c-PARP1 protein expression (Fig. 4B).
 | Figure 4.mTOR and BCAT1 pathways can modulate
each other and regulate apoptosis. R-HL60 cells were treated with
(A) 10 µM PP242 for 24 h or (B) 80 µM BCATc inhibitor 2 for 48 h,
and p-mTOR, t-mTOR, PARP1, c-PARP1 and BCAT1 protein expression
levels were detected using western blotting. Both PP242 and BCATc
inhibitor 2 significantly decreased the ratio of p-mTOR/t-mTOR,
PARP1 and BCAT1 expression and significantly induced c-PARP1
expression. The data were compared with the vehicle control group
(DMSO) and represented the mean ± SEM of three independent
experiments with triple replicates per experiment. Paired t-tests
was used to determine the differences between the experimental and
control groups. *P<0.05 the experimental group vs. the vehicle
control group. BCATi, BCATc inhibitor 2; BCAT, branched-chain amino
acid transaminase; R-, resistant; p-, phosphorylated; t-, total;
c-, cleaved; PARP 1, poly (ADP-ribose) polymerase 1. |
Curcumin, PP242 and BCATc inhibitor 2
inhibit α-KG levels in R-HL60 cells
R-HL60 cells were treated with 50 µM curcumin, 10 µM
PP242 or 80 µM BCATc inhibitor 2 for 24 h. The results indicated
that curcumin, PP242 and BCATc inhibitor 2 effectively decreased
the levels of α-KG (Fig. 5).
A schematic diagram of curcumin
regulating cell apoptosis through the BCAT1 and mTOR pathways
A schematic of the possible mechanism via which
curcumin regulates apoptosis by regulating the BCAT1 and mTOR
pathways in R-HL60 cells is given as Fig. 6.
Discussion
Resistance to chemotherapy is a major reason for
treatment failure. Our previous study reported that curcumin can
induce the apoptosis in R-HL60 cells (4). The present study further investigated
the mechanism of apoptosis, which may be helpful for the treatment
of cytarabine-resistant leukemia. The current results demonstrated
that curcumin induced apoptosis by inhibiting the BCAT1 and mTOR
pathways, and the two pathways exhibited crosstalk in R-HL60
cells.
Previous studies have revealed that high levels of
BCAT1 are closely associated with shorter overall survival in
IDHwtTET2wt but not in IDHmut or TET2mut AML (20,21).
The patients recruited in the current study are AML with
IDHwtTET2wt, so BCAT1 activation served a key role in the
development of these tumors. The results of the current study
indicated that curcumin treatment reduced the expression level of
BCAT1, which suggested that curcumin can interfere with the
development of cancer cells through BCAT1 signaling. To the best of
the authors' knowledge, the present study was the first to
investigate the mechanism underlying curcumin's apoptotic effects
by inhibiting BCAT1 expression. Tetrahydrocurcumin is a major
curcumin metabolite, and thus, it was also examined whether
tetrahydrocurcumin regulates BCAT1 expression. Curcumin and
tetrahydrocurcumin are known to induce cytarabine-resistant HL60
cell death via distinct pathways (apoptosis and autophagy,
respectively) (4). Therefore, the
current results demonstrating that tetrahydrocurcumin differed from
curcumin in its effect on BCAT1 expression were reasonable. In
addition, R-HL60 cells are resistant to cytarabine treatment, and
therefore, cytarabine treatment can be used as a negative control
group for curcumin treatment to induce apoptosis via the mTOR and
BCAT1 pathways.
Previous studies have reported that abnormal
expression or functional activity in mTOR leads to the occurrence,
progression and drug resistance of various types of tumors
(33,34). Moreover, curcumin acts as an
antitumor agent that inhibits various signaling pathways,
especially mTOR (6). Studies have
also revealed that curcumin inhibits the proliferation of cancer
cells via PARP1 cleavage (35,36).
PARP1 is a nuclear enzyme, and its upregulation has been observed
in various primary human cancer cell lines. PARP1 is cleaved into
fragments during apoptosis, and c-PARP1 has become a useful marker
of apoptosis (6). The ability of
mTOR and BCAT1 to regulate cell apoptosis has been previously
reported (37,38); however, to the best of the authors'
knowledge, the present study was the first to indicate that
curcumin can inhibit BCAT1 protein expression.
BCAT1 blockade reduces mTORC1 activity; however, to
the best of the authors' knowledge, this is the first report
indicating that the mTOR and BCAT1 pathways can modulate each
other. Drugs targeting mTORC1 have been used to treat various types
of malignant tumors; however, the feedback caused by long-term
inhibition of mTOR results in the necessity of additional cycles to
compensate for factors that promote survival (37,39).
Previous studies have highlighted that by targeting the downstream
components of mTOR, the problems associated with the feedback
mechanism can be bypassed, thereby avoiding efficiency limitations,
limiting the toxicity caused by complete mTOR blockade and avoiding
the targeting of other kinases (25,40).
This indicates that curcumin may have a stronger apoptotic effect
on R-HL60 cells than mTOR inhibitors by blocking both the BCAT1 and
mTOR pathways.
Studies have reported that histone modification was
critical for the activation of BCAT1. For example, histone H3
lysine 9 (H3K9) demethylation promotes BCAT1 upregulation, thereby
inducing tyrosine kinase inhibitor resistance-mediated BCAT1
activation in lung cancer cells (41). Curcumin is known to increase histone
deacetylase 2 expression, reduced the levels of H3/H4 acetylation
and increased H3K9 methylation in the promoter region of IL-8,
monocyte chemoattractant protein-1 and macrophage inflammatory
protein 2α genes (42). Moreover,
an in vitro study revealed that curcumin inhibited the
acetylation of H3K9, as well as reversed the upregulation of
caspase activity and downregulation of Bcl-2 in alcohol-induced
apoptosis in cardiac cells (43).
Although, to the best of the authors' knowledge, no research has
yet investigated the regulatory effect of curcumin on BCAT1, it can
be suggested that curcumin may inhibit BCAT1 activation by
regulating the methylation and/or acetylation of H3K9. In addition,
mTOR can regulate histone methylation and demethylation. For
example, mTORC1 phosphorylates the H3K9 demethylase jumonji domain
containing 1C (JMJD1C) in a nutrient-dependent manner. The p-JMJD1C
then interacts with the transcription factor USF-1 to demethylate
H3K9me2 at genes promoting lipogenesis in the liver (44,45).
However, whether mTOR induces BCAT1 expression by demethylating
H3K9me2 and whether curcumin inhibits the expression level of BCAT1
by regulating methylation and acetylation of H3K9 require further
investigation.
Although BCAT1 activity reportedly restricts α-KG
levels, this transamination reaction is also considered reversible.
BCAT1 catalyzes the transamination of plasma BCKAs to generate
BCAAs, and BCAT1 maintains nutrient sensing via mTORC1 to sustain
proliferative signaling in leukemia cells (20). Moreover, BCAAs or α-KG
supplementation reverse the colony forming ability of BCAT1
knockdown cells (19). The present
results indicated that curcumin, PP242 and BCATc inhibitor 2
significantly reduced α-KG levels. This finding suggests that
curcumin reduces the level of α-KG by inhibiting the BCAT1 and mTOR
pathways, ultimately leading to cancer cell death.
The collection of clinical samples must be
coordinated with the immediate cell culture tests in the
laboratory, so it is relatively difficult to obtain data. How to
collect more experimental data of clinical samples and to study
whether curcumin inhibits the expression level of BCAT1 by
regulating histone methylation and acetylation requires further
investigations. In conclusion, curcumin regulates the mTOR pathway;
however, the present study, to the best of the authors' knowledge,
revealed for the first time that curcumin inhibited BCAT1
expression and cell apoptosis via the simultaneous regulation of
the mTOR and BCAT1 pathways. This discovery may broaden the
possible treatment options for leukemia.
Acknowledgements
Not applicable.
Funding
This study was supported by a grant from the
Ministry of Science and Technology (grant nos.
MOST108-2635-B-037-005 and MOST109-2314-B-037-103-MY3) and
Kaohsiung Medical University Hospital (grant nos. KMUH106-M620 and
KMUH108-8R45).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YHT, RCY, SSC, TMS, YHS and PCL made substantial
contributions to conception and design, or acquisition of data, or
analysis and interpretation of data, and confirm the authenticity
of all the raw data. YHT, SSC, TMS and YHS been involved in
drafting the manuscript or revising it critically for important
intellectual content. All authors have read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
This study was approved by the Institutional Review
Board of Kaohsiung Medical University Hospital [approval no.
KMUHIRB-SV(I)-20170038]. Written informed consents of patients were
obtained from each participant between 2017–2018.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Madhusoodhan PP, Carroll WL and Bhatla T:
Progress and prospects in pediatric leukemia. Curr Probl Pediatr
Adolesc Health Care. 46:229–241. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lyengar V and Shimanovsky A: Leukemia.
StatPearls StatPearls Publishing Copyright © 2021. StatPearls
Publishing LLC.; Treasure Island, FL: 2021
|
|
3
|
Liu N, Wang C, Wang L, Gao L, Cheng H,
Tang G, Hu X and Wang J: Valproic acid enhances the antileukemic
effect of cytarabine by triggering cell apoptosis. Int J Mol Med.
37:1686–1696. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tseng YH, Chiou SS, Weng JP and Lin PC:
Curcumin and tetrahydrocurcumin induce cell death in
Ara-C-resistant acute myeloid leukemia. Phytother Res.
33:1199–1207. 2019. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee TY and Tseng YH: The potential of
phytochemicals in oral cancer prevention and therapy: A review of
the evidence. Biomolecules. 10:11502020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kouhpeikar H, Butler AE, Bamian F, Barreto
GE, Majeed M and Sahebkar A: Curcumin as a therapeutic agent in
leukemia. J Cell Physiol. 234:12404–12414. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Song X, Zhang M, Dai E and Luo Y:
Molecular targets of curcumin in breast cancer (Review). Mol Med
Rep. 19:23–29. 2019.PubMed/NCBI
|
|
8
|
Holeček M: Branched-chain amino acids in
health and disease: Metabolism, alterations in blood plasma, and as
supplements. Nutr Metab (Lond). 15:332018. View Article : Google Scholar
|
|
9
|
Neinast M, Murashige D and Arany Z:
Branched chain amino acids. Annu Rev Physiol. 81:139–164. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mayers JR, Wu C, Clish CB, Kraft P,
Torrence ME, Fiske BP, Yuan C, Bao Y, Townsend MK, Tworoger SS, et
al: Elevation of circulating branched-chain amino acids is an early
event in human pancreatic adenocarcinoma development. Nat Med.
20:1193–1198. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Watanabe A, Higashi T, Sakata T and
Nagashima H: Serum amino acid levels in patients with
hepatocellular carcinoma. Cancer. 54:1875–1882. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li Z and Zhang H: Reprogramming of
glucose, fatty acid and amino acid metabolism for cancer
progression. Cell Mol Life Sci. 73:377–392. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun H, Zhou Y, Skaro MF, Wu Y, Qu Z, Mao
F, Zhao S and Xu Y: Metabolic reprogramming in cancer is induced to
increase proton production. Cancer Res. 80:1143–1155. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
DeBerardinis RJ and Chandel NS:
Fundamentals of cancer metabolism. Sci Adv. 2:e16002002016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zeng B, Zhang X, Zhao J, Wei Z, Zhu H, Fu
M, Zou D, Feng Y, Luo H and Lei Y: The role of
DNMT1/hsa-miR-124-3p/BCAT1 pathway in regulating growth and
invasion of esophageal squamous cell carcinoma. BMC Cancer.
19:6092019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu Y, Yu W, Yang T, Zhang M, Liang C, Cai
X and Shao Q: Overexpression of BCAT1 is a prognostic marker in
gastric cancer. Human Pathol. 75:41–46. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang L and Han J: Branched-chain amino
acid transaminase 1 (BCAT1) promotes the growth of breast cancer
cells through improving mTOR-mediated mitochondrial biogenesis and
function. Biochem Biophys Res Commun. 486:224–231. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zheng YH, Hu WJ, Chen BC, Grahn TH, Zhao
YR, Bao HL, Zhu YF and Zhang QY: BCAT1, a key prognostic predictor
of hepatocellular carcinoma, promotes cell proliferation and
induces chemoresistance to cisplatin. Liver Int. 36:1836–1847.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hattori A, Tsunoda M, Konuma T, Kobayashi
M, Nagy T, Glushka J, Tayyari F, McSkimming D, Kannan N, Tojo A, et
al: Cancer progression by reprogrammed BCAA metabolism in myeloid
leukaemia. Nature. 545:500–504. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Raffel S, Falcone M, Kneisel N, Hansson J,
Wang W, Lutz C, Bullinger L, Poschet G, Nonnenmacher Y, Barnert A,
et al: BCAT1 restricts αKG levels in AML stem cells leading to
IDHmut-like DNA hypermethylation. Nature. 551:384–388. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tönjes M, Barbus S, Park YJ, Wang W,
Schlotter M, Lindroth AM, Pleier SV, Bai AHC, Karra D, Piro RM, et
al: BCAT1 promotes cell proliferation through amino acid catabolism
in gliomas carrying wild-type IDH1. Nat Med. 19:901–908. 2013.
View Article : Google Scholar
|
|
22
|
Fox DB and Alvarez JV:
Epithelial-to-mesenchymal transition activates Bcat1 expression to
promote recurrent tumor growth. bioRxiv. Dec 9–2020.(Epub ahead of
print). doi: 10.1101/2020.12.08.416479.
|
|
23
|
Gu Z, Liu Y, Cai F, Patrick M, Zmajkovic
J, Cao H, Zhang Y, Tasdogan A, Chen M, Qi L, et al: Loss of EZH2
reprograms BCAA metabolism to drive leukemic transformation. Cancer
Discov. 9:1228–1247. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chiarini F, Evangelisti C, McCubrey JA and
Martelli AM: Current treatment strategies for inhibiting mTOR in
cancer. Trends Pharmacol Sci. 36:124–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cargnello M, Tcherkezian J and Roux PP:
The expanding role of mTOR in cancer cell growth and proliferation.
Mutagenesis. 30:169–176. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Villar VH, Nguyen TL, Delcroix V, Terés S,
Bouchecareilh M, Salin B, Bodineau C, Vacher P, Priault M,
Soubeyran P and Durán RV: mTORC1 inhibition in cancer cells
protects from glutaminolysis-mediated apoptosis during nutrient
limitation. Nat Commun. 8:141242017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tamaddoni A, Mohammadi E, Sedaghat F,
Qujeq D and As'Habi A: The anticancer effects of curcumin via
targeting the mammalian target of rapamycin complex 1(mTORC1)
signaling pathway. Pharmacol Res. 156:1047982020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tan HK, Moad AI and Tan ML: The mTOR
signalling pathway in cancer and the potential mTOR inhibitory
activities of natural phytochemicals. Asian Pac J Cancer Prev.
15:6463–6475. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Patel SS, Acharya A, Ray RS, Agrawal R,
Raghuwanshi R and Jain P: Cellular and molecular mechanisms of
curcumin in prevention and treatment of disease. Crit Rev Food Sci
Nutr. 60:887–939. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pösel C, Möller K, Fröhlich W, Schulz I,
Boltze J and Wagner DC: Density gradient centrifugation compromises
bone marrow mononuclear cell yield. PLoS One. 7:e502932012.
View Article : Google Scholar
|
|
31
|
Arber DA and Erba HP: Diagnosis and
treatment of patients with acute myeloid leukemia with
myelodysplasia-related changes (AML-MRC). Am J Clin Pathol.
154:731–741. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Su PF and Song SQ: Regulation of mTOR by
miR-107 to facilitate glioma cell apoptosis and to enhance
cisplatin sensitivity. Eur Rev Med Pharmacol Sci. 22:6864–6872.
2018.PubMed/NCBI
|
|
34
|
Murugan AK: mTOR: Role in cancer,
metastasis and drug resistance. Semin Cancer Biol. 59:92–111. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Watson JL, Hill R, Lee PW, Giacomantonio
CA and Hoskin DW: Curcumin induces apoptosis in HCT-116 human colon
cancer cells in a p21-independent manner. Exp Mol Pathol.
84:230–233. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mishra D, Singh S and Narayan G: Curcumin
induces apoptosis in Pre-B acute lymphoblastic leukemia cell lines
via PARP-1 cleavage. Asian Pac J Cancer Prev. 17:3865–3869.
2016.PubMed/NCBI
|
|
37
|
Zou Z, Tao T, Li H and Zhu X: mTOR
signaling pathway and mTOR inhibitors in cancer: Progress and
challenges. Cell Biosci. 10:312020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Eden A and Benvenisty N: Involvement of
branched-chain amino acid aminotransferase (Bcat1/Eca39) in
apoptosis. FEBS Lett. 457:255–261. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rozengurt E, Soares HP and Sinnet-Smith J:
Suppression of feedback loops mediated by PI3K/mTOR induces
multiple overactivation of compensatory pathways: An unintended
consequence leading to drug resistance. Mol Cancer Ther.
13:2477–2488. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tian T, Li X and Zhang J: mTOR signaling
in cancer and mTOR inhibitors in solid tumor targeting therapy. Int
J Mol Sci. 20:7552019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang Y, Zhang J, Ren S, Sun D, Huang HY,
Wang H, Jin Y, Li F, Zheng C, Yang L, et al: Branched-chain amino
acid metabolic reprogramming orchestrates drug resistance to EGFR
tyrosine kinase inhibitors. Cell Rep. 28:512–525.e6. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gan L, Li C, Wang J and Guo X: Curcumin
modulates the effect of histone modification on the expression of
chemokines by type II alveolar epithelial cells in a rat COPD
model. Int J Chron Obstruct Pulmon Dis. 11:2765–2773. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yan X, Pan B, Lv T, Liu L, Zhu J, Shen W,
Huang X and Tian J: Inhibition of histone acetylation by curcumin
reduces alcohol-induced fetal cardiac apoptosis. J Biomed Sci.
24:12017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Laribee RN and Weisman R: Nuclear
functions of TOR: Impact on transcription and the epigenome. Genes
(Basel). 11:6412020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Viscarra JA, Wang Y, Nguyen HP, Choi YG
and Sul HS: Histone demethylase JMJD1C is phosphorylated by mTOR to
activate de novo lipogenesis. Nat Commun. 11:7962020. View Article : Google Scholar : PubMed/NCBI
|