Introduction
Ischemic stroke is a major risk factor for human
health and causes cerebral injury, disability and even mortality
worldwide (1). It is estimated that
ischemic stroke accounts for 70% of all types of strokes (2,3).
Ischemia reperfusion (I/R) mainly contributes to the repair and
functional recovery of damaged tissues and organs. However,
occasionally, I/R aggravates the dysfunction of tissues and organs
and promotes structural damage, which is known as I/R injury
(4,5). Cerebral I/R leads to mitochondrial
dysfunction, inflammation, excessive release of reactive oxygen
species (ROS), massive glutamate excitotoxicity and cell death, and
finally irreversible damage to the brain (6). The inflammatory response and apoptosis
are the major causes of neuronal cell injury after cerebral I/R
(7). Currently, the clinical
therapeutic strategies for ischemic stroke remain unsatisfactory.
Thus, it is necessary to investigate the molecular mechanisms
underlying the inflammatory response and neuronal apoptosis for the
treatment of I/R injury.
Long non-coding RNAs (lncRNAs), which are >200
nucleotides in length, lack complete open reading frames and are
incapable of coding proteins (8,9). An
increasing number of studies have revealed that lncRNAs are
involved in various physiological and pathological processes of
diseases (10–12). Previous studies have verified that
the lncRNA X inactivate-specific transcript (XIST) participates in
the progression of several diseases. For instance, XIST promoted
cisplatin resistance in human lung adenocarcinoma cells by
modulating the let-7i/BAG cochaperone 1 axis (13). XIST also facilitated the
proliferation of human fibroblasts and murine lung fibroblasts, as
well as extracellular matrix protein expression by sponging
microRNA (miRNA/miR)-139 (14).
Moreover, XIST triggered neuropathic pain by binding with miR-544
to activate STAT3 in a rat model of chronic constriction injury
(15). Nevertheless, the molecular
roles and the regulatory mechanisms of XIST in cerebral I/R injury
have not been fully elucidated.
The present study aimed to investigate the molecular
mechanism of XIST in cerebral I/R injury by establishing an I/R
mouse model in vivo and an oxygen and glucose
deprivation/reperfusion (OGD/R)-treated Neuro-2a (N2a) cellular
model in vitro. The findings of the present study may shed
new light on the treatment of cerebral I/R injury.
Materials and methods
Experimental animals and middle
cerebral artery occlusion (MCAO) model
All animal experiments were performed with the
approval and in accordance with standard principles approved by the
Institutional Animal Care and Use Committee of China Resources
& WISCO General Hospital (approval no. 2020-005; Hubei, China).
The Experimental Animal Center of the Chinese Academy of Medical
Sciences (Beijing, China) provided 90 male C57BL/6J mice (age, 8–10
weeks; body weight, 22–30 g) for the current experiments. The mice
were maintained at 25°C in 40–60% humidity on a 12 h light/dark
cycle with free access to food and water. All mice were divided
into seven groups: i) Sham + Adeno-associated virus (AAV)-short
hairpin RNA (sh)-negative control (NC) group (n=20); ii) I/R+
AAV-sh-NC group (n=20); iii) I/R + AAV-sh-XIST group (n=10); iv)
I/R + AAV-sh-FOXO3 group (n=10); v) sham + AAV-NC mimic group
(n=10); vi) I/R + AAV-NC mimic group (n=10); and vii) I/R +
AAV-miR-27a-3p mimic group (n=10).
In all I/R groups, mice were subjected to MCAO.
After anesthesia with pentobarbital sodium (45 mg/kg), a
monofilament nylon suture was inserted into the internal carotid
artery through the external carotid artery, followed by occluding
the origin of the MCA. The occlusion continued for 2 h, and the
suture was slowly removed to allow blood flow and induce
reperfusion. The mice in the sham group were treated in the same
way, but without occlusion of the MCA. Mice that died or failed to
show an 80% reduction in cerebral blood flow after occlusion were
excluded from the following experiments. A total of 24 h after
reperfusion, measurements, including Longa's scores, cerebral blood
flow and infarct volume, were conducted. The experiments were
performed for ~26 h, with 2 h for occlusion and 24 h for
reperfusion. The health and behavior of rats were monitored every 6
h during reperfusion. Animals (n=90) were immediately euthanized
when they showed signs of weight loss >20%, dehydration or were
non-ambulate, using intraperitoneal injection of 45 mg/kg
pentobarbital sodium followed by cervical dislocation. Mouse death
(n=90) was confirmed by bilateral thoracotomy and the subsequent
absence of pupillary response, respiratory movement and heartbeat.
When the I/R model was successfully established, the brain tissues
were instantly collected and used for detection of the RNA
expression.
AAV delivery
AAVs (serotype 9; at a titer of 3.5×1012
vg/ml) carrying short hairpin RNA (sh)-negative control (NC),
sh-XIST, sh-FOXO3, NC mimic and miR-27a-3p mimic were constructed
by Shanghai GeneChem Co., Ltd. AAV-sh-NC served as the control for
AAV-sh-XIST and AAV-sh-FOXO3, and the AAV-NC mimic served as the
control for the AAV-miR-27a-3p mimic. A total of 14 days before
establishment of the MCAO model, 3.5×1010 v.g./mouse
were stereotactically injected into the ipsilateral lateral
ventricle (coordinated from bregma) of the mice using a 10-µl
Hamilton syringe and the needle was maintained for 10 min before
retraction.
Evaluation of Longa's scores
After cerebral I/R injury, the neurological deficit
scores of the mice were evaluated. Mice that underwent MCAO were
allowed to rest for 24 h before the evaluation. Neurological scores
were assessed at 24, 48 and 72 h after MCAO by technicians blinded
to the treatment groups. Longa neurological examination was divided
into five grades as previously described (16): i) 0, normal, without neurological
deficits; ii) 1, the limb could not completely extend to the left,
with mild neurological deficits; iii) 2, movement made in a 0, with
moderate neurological deficits; iv) 3, left descent, with severe
neurological deficits; v) 4, unable to walk and loss of
consciousness; and vi) 5, death.
Measurement of infarct volume
The brain tissue was sectioned, fixed with 4%
paraformaldehyde at room temperature for 24 h, embedded in paraffin
and then cut into coronal sections. Infarct volume was measured on
five slices of 3-mm coronal sections from each brain. A 2%
2,3,5-triphenyltetrazolium chloride (TTC; cat. no. T8877;
Sigma-Aldrich; Merck KGaA) solution was incubated with the slices
for 20 min at 37°C. Stained images were captured with a camera, and
the infarct area was measured with ImageJ version 6.0 software
(National Institutes of Health). For minimization of the effect of
brain edema, the infarct volume was calculated with the following
formula: 100% × (contralateral hemisphere volume-non-infarct
ipsilateral hemisphere volume)/contralateral hemisphere volume.
Measurement of cerebral blood
flow
For the measurement of the cerebral blood flow of
mice, a laser Doppler flowmetry was used as previously described
(17). Briefly, the head of the
mouse was fixed using a stereotaxic device. Then, an incision was
made in the skin to cover the calvarium to expose the bregma.
Finally, the laser Doppler flow probe was placed to dynamically
measure cerebral blood flow for 2 h. Cerebral blood flow decreasing
to ≥20% of baseline during occlusion indicated successful
establishment of the cerebral I/R injury mouse model.
Bioinformatics analysis
miRNAs with potential binding sites for XIST were
predicted using the online software program DIANA (lncBase v.2;
http://carolina.imis.athena-innovation.gr/diana_tools/web/index.php?r=lncbasev2%2Findex-predicted).
miR-27a-3p was predicted to possess a binding site with XIST. The
target genes of miR-27a-3p were identified using the TargetScan
database (version 7.2; http://www.targetscan.org/). miR-27a-3p was revealed
to bind with the 3′UTR of FOXO3.
N2a cell culture and OGD/R
treatment
OGD/R-treated N2a cell models can be used as in
vitro models of cerebral ischemia/reperfusion (18–20).
The mouse neuroblastoma cell line N2a was obtained from the
American Type Culture Collection, and was cultured in DMEM (Gibco;
Thermo Fisher Scientific, Inc.) containing 10% FBS (Gibco; Thermo
Fisher Scientific, Inc.), 1% penicillin/streptomycin (Gibco; Thermo
Fisher Scientific, Inc.) at 37°C with 5% CO2.
To mimic in vitro I/R injury conditions, N2a
cells were transferred to glucose-free DMEM and incubated for 2 h
in a hypoxic atmosphere containing 5% CO2, 1%
O2 and 94% N2. Finally, N2a cells were
collected after reoxygenation in normal culture medium and
maintained at 37°C for 24 h under normoxic conditions (5%
CO2) for further experiments.
Cell transfection
shRNAs against XIST and FOXO3, as well as the
corresponding sh-NC, were synthesized by Sangon Biotech Co., Ltd.
miR-27a-3p mimics and inhibitors, as well as their corresponding
NCs (NC mimics, NC inhibitor; at a concentration of 5 µM),
pcDNA3.1/FOXO3, pcDNA3.1 and pcDNA3.1/XIST were purchased from
Shanghai GenePharma Co., Ltd. Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) was used for
transfection of the aforementioned oligonucleotides (40 mM) or
plasmids (2 µg) into N2a cells at room temperature for 6 h and
cells were then incubated for another 24 h before subsequent
experiments. The sequences of sh-XIST, sh-NC, sh-FOXO3, miR-27a-3p
mimics and inhibitor, NC mimics, NC inhibitor, pcDNA3.1/FOXO3,
pcDNA3.1 and pcDNA3.1/XIST are provided in Tables I–III. The cells were harvested after 48 h,
and reverse transcription-quantitative (RT-q)PCR was used to
measure the expression levels of XIST, FOXO3 and miR-27a-3p. GAPDH
and U6 served as the internal controls.
 | Table I.Relative sequences of shRNAs. |
Table I.
Relative sequences of shRNAs.
| shRNAs | Sequences
(5′→3′) |
|---|
| sh-XIST |
ACCTTCTGCGTGTTCATTATT |
| sh-NC |
ATCTTAATGTTTTCGGCTCCT |
| sh-FOXO3 |
TTTCATCGAGTTCCCGACTCG |
| sh-NC |
GTCTTAATCCGTGGTTCCCCA |
 | Table III.Relative sequences of
pcDNA3.1/RNAs. |
Table III.
Relative sequences of
pcDNA3.1/RNAs.
| pcDNA3.1/RNAs | Sequences
(5′→3′) |
|---|
| pcDNA3.1/XIST |
gagttggctgttttccccgccgccccctgcaccttcgtttaactttagtgatttcttccgtcgtacacttttcattttagatcgttaccctcccggtgtgacttccttgcattatccgtgattttggggtcttactattctcttctttttgcgatttaggagctttctcgccatattttgaccgatcttctatattccgcctttgtttaactcatttttgtatctggtcttttttttatgtcctccgctcatctggcttctatgggtcgttcttggcgcgtctctcttgatttccttgtctttgttgtgacgctggaccatctttcgtctttactggtgcaagttagattgttttctttcttctacgagtctcctcctttctctgcaagttttttttcttattttttcggtctctggttacttacgtcttgtttttcttgtgtttactttttcgttgctttgattttttgcgttcccattcctttgtaccttattggtgttgtagggcttttctatttggttggttattttcctcttatagatttcgcctggttatttgtcttctttttatattcttgattatcttttttttcttctgttttcttccggaggttgttcttttcctctctgtatgtcgccttgtcatttttttttcgagcattttgctctccagttgtcccagtgttttcacctctttttttttgtagccggttatattgctatttatcagacgtattcgtctttttttgttggaattttctattcgtccgttgtttgatttttgcttgtgataattttttttttttacttttgcctgctttcttcgtgtcctcttgttcctagtattctgagttttcatctggtggtccgtccgcttgcctttacctcttgtcctcc |
| pcDNA3.1 |
gtggtagggcctagctcgagggaaccctaccagctgttgcgcctcgctgacagcgtcgtcctgtctattcgagccgcgggacagctctcccgcgtggtgttcattcccgtgtgactagtgggccgtgtaccgcctaacgccggtggcagcgcacgttccagaccgcagcggctttcacgaggcaaccgcatgtgtagactaaacagcttcagtatcagccccgggtccactttcggtgattgtcagacgcgtgctctctcgaagcaagaacttaggagggctcagggggccgctccattgtgcaacgactatcgcgttgtccttcgtcgctggcgccgtggtgaatacacatccgacccgctatctgtgtaggtaaatcaccgggtgggtgcctccggcggcgacccgtaggtcgcactctaacttattagtaggaactcgtgaagggtgtccacgttatgaaacttagtcccctacagcgcgcgtctagcgaccccgcgatggggacattgagagctggctcgagatattcgcccgccaagggtccggagaaggcttggtgctggagtggagaagtgaaccgaagattttcgggggtccgaagccgtgtggtatcgaagtacctagttagtctcgtgcgtttccgttgatgagcaagcaagccccatcgggacccgatcgtaacacagtgttgccgtgacgttgggagccctgattagccggtttactgccatcgcagggtcgtgctaggtgggctccagctgcacgggtcgtcgctatctgccctgccggtcg |
| pcDNA3.1/FOXO3 |
agtttcattactatcgcgacaacctaacgatttgcattcatctggggttggacactgccccggcctaaagaactgaccacgactacacatggtagattcgccctccacctcattctcgtacgacacacacattcaccacatgtttgagacgtgccacgcctcacattctccttaccaagtgttgggccccccggttactgtcaatataactgagtctgtcaccatatcgtgggcgagttcaccgtccttacaacgtgcatatcgaatagcggttttagcctcgcagcagtgcattcttttagcgaccagtgtgcatagtggggtgcggctataattacacaatgacaatttaattaggcccgtggatgtctaggctgtatccattttcttctcgacttcgtcagcgctctgggacacacttctttgatcca |
RNA extraction and RT-qPCR
TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) was used to extract total RNA from brain
tissues and N2a cells. A NanoDrop™ 2000 spectrophotometer (Thermo
Fisher Scientific, Inc.) was used to detect the RNA concentrations.
Then, 1 µg RNA was reverse transcribed into cDNA using a
PrimeScript™ RT kit (cat. no. RR037A; Takara Bio, Inc.) for lncRNA
and mRNA, or the TaqMan™ miRNA RT kit (cat. no. 4366596; Applied
Biosystems; Thermo Fisher Scientific, Inc.) for miRNA, according to
the manufacturer's instructions. RT-qPCR was conducted to amplify
XIST, miR-27a-3p and FOXO3 using a SYBR-Green Real-Time PCR kit
(Takara Bio, Inc.) under the following thermocycling conditions:
Initial denaturation at 95°C for 30 sec, followed by 35 cycles of
denaturation at 95°C for 5 sec, annealing at 55°C for 20 sec and at
72°C for 20 sec, and final extension at 72°C for 3 min. The
relative expression levels of XIST, miR-27a-3p and FOXO3 were
calculated using the 2−ΔΔCq method (21) with GAPDH as an internal control for
XIST and FOXO3, and U6 as an endogenous control for miR-27a-3p. The
primer sequences were as follows: XIST forward (F),
5′-GGTTCTGTCAAGATACTTTCCT-3′ and reverse (R),
5′-CAATGAAGAGCTTGACGTG-3′; miR-27a-3p F,
5′-TTCACAGTGGCTAAGTTCCGC-3′ and R, 5′-CTCTACAGCTATATTGCCAGCCAC-3′;
FOXO3 F, 5′-ATCTACGAGTGGATGGTGC-3′ and R,
5′-CCGGATGGAGTTCTTCCAG-3′; GAPDH F, 5′-GATCATCAGCAATGCCTCC-3′ and
R, 5′-TCCACGATACCAAAGTTGTC-3′; and U6 F,
5′-CAATACAGAGAAAGTTAGCACG-3′ and R, 5′-AATGCTTCAAAGAGTTGTGC-3′.
Flow cytometry
N2a cell apoptosis was detected via flow cytometry
using an Annexin V/PI double staining kit (BD Biosciences)
according to the manufacturer's instructions. N2a cells were
collected and then washed twice with ice-cold PBS. Next, the cells
were resuspended in binding buffer and stained with Annexin V-FITC
and PI at room temperature for 15 min in the dark. Finally, flow
cytometry was performed with a Coulter® EPICS XL
instrument (Beckman Coulter, Inc.). The data of flow cytometry were
analyzed using Flowing version 2.5.1 software (Turku Bioscience)
and Origin version 8 software (OriginLab). The apoptosis rate was
calculated as the percentage of early apoptotic cells to total
cells.
Western blot analysis
Western blot analysis was repeated three times in
this study. Total proteins from brain tissues and N2a cells were
extracted using RIPA lysis buffer (Beyotime Institute of
Biotechnology) with protease inhibitor [Roche Diagnostics
(Shanghai) Co., Ltd.]. The protein concentration was determined
using a BCA kit (Beyotime Institute of Biotechnology). Then, the
proteins (30 µg per lane) were separated by 10% SDS-PAGE and
further transferred onto PVDF membranes. After the membranes were
blocked with 5% skimmed milk for 2 h at room temperature, they were
cultured with anti-FOXO3 (cat. no. ab70315; 1:2,000; Abcam) and
anti-GAPDH (cat. no. ab9485; 1:2,500; Abcam) antibodies overnight
at 4°C. Then, the membrane was incubated with a goat anti-rabbit
HRP-conjugated secondary antibody (cat. no. ab205718; 1:2,000;
Abcam) at room temperature for 1 h. The signals were visualized
using a chemiluminescence imaging system and chemiluminescence
detection reagents (Bio-Rad Laboratories, Inc.) with GAPDH as the
internal control. The Quantity One software (4.5.0 basic; Bio-Rad
Laboratories, Inc.) was used for the semi-quantification of protein
expression.
RNA immunoprecipitation (RIP)
assay
The RIP assay was conducted using a Magna
RNA-binding protein immunoprecipitation kit (EMD Millipore)
following the manufacturer's instructions. N2a cells
(2×107) after indicated transfection were cross-linked
with 0.75% formaldehyde. Chromatin was then sheared by sonication
at 16,000 × g for 10 min at 4°C. N2a cells were lysed with RIPA
lysis buffer (cat. no. R0278; Sigma-Aldrich; Merck KGaA) for 5 min
on ice and then centrifuged at 16,000 × g at 4°C for 10 min.
Briefly, N2a cells anti-argonaute2 (Ago2; cat. no. 2897S; 1:1,000;
Cell Signaling Technology, Inc.) and anti-IgG (cat. no. 14678-1-AP;
1:1,000; ProteinTech Group, Inc.) conjugated with magnetic beads
(cat. no. 88802; Pierce; Thermo Fisher Scientific, Inc.) were mixed
with the cell lysate and RIP buffer. Isolation of
immunoprecipitated RNAs was performed with proteinase K (0.5 mg/ml,
EMD Millipore) for 30 min at 55°C, and the enrichment of purified
RNAs was assessed by RT-qPCR, which was performed as mentioned
above.
Luciferase reporter assay
The sequences of XIST or FOXO3 were inserted into
the luciferase reporter vector pmirGLO (Promega Corporation) to
construct pmirGLO-XIST-wild-type (WT) or pmirGLO-FOXO3-WT
reporters. The reporters of pmirGLO-XIST-mutant (MUT) or
pmirGLO-FOXO3-MUT were generated by site-directed mutagenesis.
Lipofectamine 2000 was used to transfect these plasmids (1 µg) with
miR-27a-3p mimic or inhibitor and NC mimic and NC inhibitor (50 nM)
into N2a cells (2×107). After transfection for 48 h, the
relative luciferase activities were detected using the Luciferase
Reporter Assay System (Promega Corporation). The relative
luciferase activity was calculated as the ratio of firefly
luciferase activity to Renilla luciferase activity.
ELISA
Caspase-3 activity was detected using an ELISA kit
(cat. no. MOEB0497; Dakewe Biotech Co., Ltd.) according to the
manufacturer's instructions. The optical density values of the
reaction product were assessed at 450 nm.
ROS production assay
The transfected N2a cells were stained with 10 µM
DCFH-DA (Beyotime Institute of Biotechnology) in a 37°C bath for 30
min. The ROS activity was analyzed via ImageJ version 6.0 software
(National Institutes of Health) and imaged using a Nikon
fluorescence microscope (magnification, ×100). In total, five wells
were randomly selected for each treatment group.
Statistical analysis
Statistical analysis was performed with GraphPad
Prism 5 software (GraphPad Software, Inc.). The quantitative data
are presented as the mean ± SD. Statistical significance between
two different groups was evaluated using an unpaired Student's
t-tests, while differences among >2 groups were determined
according to one-way ANOVA followed by Tukey's post hoc test. All
assays were carried out at ≥3 times with triplicate samples.
P<0.05 was considered to indicate a statistically significant
difference.
Results
XIST expression is upregulated in mice
with cerebral I/R and in OGD/R-treated N2a cells
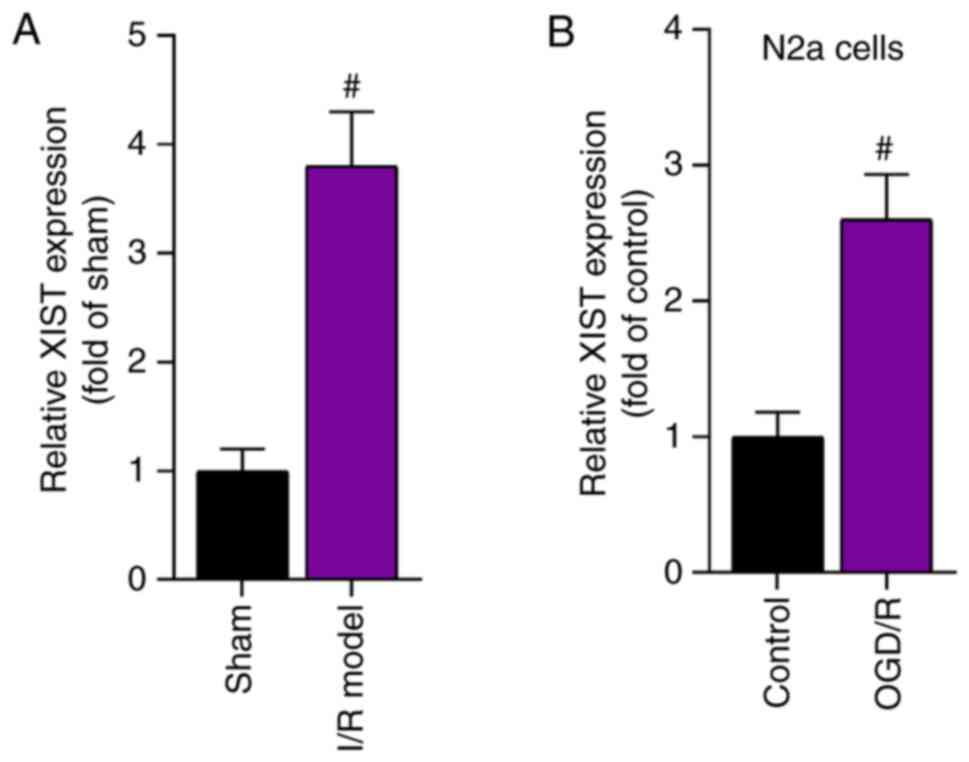
The current study first determined the expression
level of XIST in the I/R mouse model and in OGD/R-treated N2a cells
using RT-qPCR. The results demonstrated that XIST expression was
significantly higher in the cerebral I/R model group compared with
that in the sham group (Fig. 1A).
Moreover, the expression level of XIST in the OGD/R-treated N2a
cells was significantly elevated compared with that in the control
group (Fig. 1B). Taken together,
the expression level of XIST was upregulated in a cerebral I/R
mouse model and in an OGD/R-induced cell model.
Knockdown of XIST inhibits I/R-induced
cerebral injury
Whether XIST could influence I/R-induced cerebral
injury was investigated. First, the knockdown efficiency of sh-XIST
in the brain tissues of the I/R-treated mice was verified (Fig. 2A). Subsequently, TTC staining
revealed that the brain infarct volume was significantly increased
by I/R treatment; however, the infarct volume was reduced by
delivery of AAV-sh-XIST to the brain tissues of the I/R-treated
mice (Fig. 2B and C). Moreover, the
mice with cerebral I/R developed neurological abnormalities and
neurological deficits, and the knockdown of XIST reduced the
neurological deficit score in the I/R-treated mice (Fig. 2D).
 | Figure 2.Knockdown of XIST inhibits cerebral
injury in I/R-treated mice. (A) Knockdown efficiency of XIST in the
brain tissues of I/R-treated mice was assessed via RT-qPCR.
ФP<0.05 vs. sham + AAV-sh-NC group;
#P<0.05 vs. I/R + AAV-sh-NC group. The following
three groups were analyzed: Sham + AAV-sh-NC, I/R + AAV-sh-NC and
I/R + AAV-sh-XIST. (B) TTC staining images and (C) quantification
of the brain infarct volume in the three groups.
#P<0.05 vs. I/R + AAV-sh-NC group. (D) The
neurological deficit score was evaluated in the three groups.
#P<0.05 vs. I/R + AAV-sh-NC group. (E) RT-qPCR and
(F) western blotting were used to detect the mRNA and protein
expression levels of Bcl-2 and Bax in the three groups.
ФP<0.05 vs. sham + AAV-sh-NC group;
#P<0.05 vs. I/R + AAV-sh-NC group. NC, negative
control; sh, short hairpin RNA; AAV, Adeno-associated virus;
RT-qPCR, reverse transcription-quantitative PCR; I/R,
ischemia/reperfusion; XIST, X inactivate-specific transcript; TTC,
2,3,5-triphenyltetrazolium chloride. |
Cell apoptosis is an important index of cerebral
injury; thus, the mRNA and protein expression levels of cell
apoptosis-associated genes (Bax and Bcl-2) were detected. On the
basis of the RT-qPCR and western blotting data, it was found that
the mRNA and protein expression levels of Bax were elevated in the
I/R + AVV-sh-NC group compared with the sham + AVV-sh-NC group, and
were decreased by XIST knockdown in the mice with cerebral I/R,
while the mRNA and protein expression levels of Bcl-2 were reduced
in the I/R + AVV-sh-NC group compared with the sham + AVV-sh-NC
group, and elevated in the I/R + AVV-sh-XIST group compared with
the I/R + AVV-sh-NC group (Fig. 2E and
F). In summary, XIST knockdown inhibited cerebral injury
induced by I/R.
XIST interacts with miR-27a-3p
Furthermore, the current study aimed to identify the
regulatory mechanisms underlying XIST in cerebral I/R. Given that
lncRNAs can function as competitive endogenous RNAs (ceRNAs) by
competitively binding with miRNAs to regulate cerebral injury
(22,23), we hypothesized that XIST serves as a
ceRNA in I/R-induced brain injury. Bioinformatics analysis
indicated that XIST can bind with miR-27a-3p, and the putative
binding sites for XIST and miR-27a-3p are presented in Fig. 3A.
 | Figure 3.XIST directly interacts with
miR-27a-3p. (A) The DIANA database predicted the binding sites
between XIST and miR-27a-3p. (B) RT-qPCR results demonstrated the
expression level of miR-27a-3p after transfection of miR-27a-3p
mimic and miR-27a-3p inhibitor. (C) Luciferase reporter assays
revealed the relative luciferase activity of XIST.
#P<0.05 vs. NC mimic group; ФP<0.05 vs.
NC inhibitor group. (D) RNA immunoprecipitation assays showed the
relative enrichment of XIST and miR-27a-3p. #P<0.05
vs. anti-IgG group. (E) RT-qPCR determined the overexpression and
knockdown efficiency of XIST in N2a cells. (F) RT-qPCR identified
the expression level of miR-27a-3p in the N2a cells with
overexpressed and silenced XIST. #P<0.05 vs. pcDNA
group; ФP<0.05 vs. sh-NC group. (G) RT-qPCR results
of the expression level of XIST in N2a cells after miR-27a-3p
overexpression or knockdown. #P<0.05 vs. NC mimic
group; ФP<0.05 vs. NC inhibitor group. RT-qPCR
results of the expression level of miR-27a-3p in the brain tissues
of an (H) I/R injury mouse model and (I) N2a cells.
#P<0.05 vs. sham group in panel H;
#P<0.05 vs. control group in panel I. RT-qPCR,
reverse transcription-quantitative PCR; I/R, ischemia/reperfusion;
XIST, X inactivate-specific transcript; NC, negative control; sh,
short hairpin RNA; miR, microRNA; WT, wild-type; MUT, mutant; Ago2,
argonaute2; OGD/R, oxygen and glucose deprivation/reperfusion; N2a,
Neuro-2a. |
Subsequently, the overexpression or knockdown
efficiency of miR-27a-3p was verified. The RT-qPCR results
demonstrated that miR-27a-3p expression was increased by the
miR-27a-3p mimic and was decreased by the miR-27a-3p inhibitor
(Fig. 3B). Luciferase reporter
analysis revealed that overexpression of miR-27a-3p decreased the
luciferase activity of the pmirGLO-XIST-WT plasmid, while knockdown
of miR-27a-3p increased the luciferase activity of the
pmirGLO-XIST-WT plasmid. However, neither miR-27a-3p overexpression
or miR-27a-3p knockdown influenced the luciferase activity of the
pmirGLO-XIST-Mut plasmid (Fig. 3C),
which suggested that XIST bound to miR-27a-3p at the predicted
sites. The interaction between XIST and miR-27a-3p was also using
by RIP assays, which identified that XIST and miR-27a-3p were
abundantly enriched in the Ago2-precipitated products compared with
those in the IgG group (Fig.
3D).
Next, RT-qPCR results revealed that XIST was
effectively overexpressed or knocked down in N2a cells (Fig. 3E). Overexpression of XIST resulted
in a significant reduction in miR-27a-3p expression, while
knockdown of XIST caused a significant increase in miR-27a-3p
expression (Fig. 3F). Moreover, the
expression level of XIST was reduced by miR-27a-3p overexpression
and was enhanced by miR-27a-3p knockdown (Fig. 3G). Therefore, the expression level
of miR-27a-3p was further assessed in an I/R-induced mouse model
and N2a cells via RT-qPCR. The results demonstrated that miR-27a-3p
expression was downregulated in the brain tissues of the
I/R-treated mice and in the OGD/R-treated N2a cells (Fig. 3H and I). Overall, these results
suggested that XIST interacted with miR-27a-3p.
Overexpression of miR-27a-3p
suppresses I/R-induced cerebral injury
As the interaction of XIST and miR-27a-3p was
previously confirmed, the function of miR-27a-3p in cerebral I/R
was further investigated. The overexpression efficiency of
miR-27a-3p in the brain tissues of the I/R-treated mice was
confirmed via RT-qPCR (Fig. 4A).
TTC staining revealed that the brain infarct volume of the
I/R-treated mice was reduced by miR-27a-3p overexpression (Fig. 4B and C). Moreover, miR-27a-3p
overexpression reduced the neurological deficit score in the
I/R-treated mice (Fig. 4D).
To detect the effect of miR-27a-3p on apoptosis, the
mRNA and protein expression levels of Bax and Bcl-2 were detected.
The RT-qPCR results demonstrated that the Bax mRNA and protein
expression levels were decreased, while those of Bcl-2 were
elevated by the overexpression of miR-27a-3p in the brain tissues
of the I/R-treated mice compared with the I/R + AAV-NC mimic group
(Fig. 4E and F). In summary,
overexpression of miR-27a-3p suppressed I/R-induced cerebral
injury.
XIST regulates the apoptosis and ROS
production of N2a cells by binding with miR-27a-3p
Whether XIST regulated the apoptosis and ROS
production of N2a cells via interaction with miR-37a-3p was
evaluated. Flow cytometry results indicated that OGD/R induced the
apoptosis of N2a cells, while knockdown of XIST decreased cell
apoptosis in the OGD/R-treated N2a cells. Furthermore, the
repressive effects of XIST knockdown on cell apoptosis were
reversed by co-transfection with the miR-37a-3p inhibitor (Fig. 5A and B). It was found that knockdown
of XIST decreased the mRNA and protein expression levels of Bax and
increased those of Bcl-2 in the OGD/R-treated N2a cells, while
knockdown of miR-27a-3p counteracted these effects (Fig. 5C-E). The ELISA results demonstrated
that the relative caspase-3 activity and ROS production were
enhanced in the supernatant of the OGD/R-treated N2a cells.
Moreover, XIST knockdown decreased caspase-3 activity and ROS
production compared with the OGD/R group, while knockdown of
miR-27a-3p neutralized these inhibitory effects of XIST (Fig. 5F and G). Collectively, it was
suggested that XIST promoted the apoptosis and ROS production of
N2a cells by binding with miR-27a-3p.
 | Figure 5.XIST regulates the apoptosis of N2a
cells by binding with miR-27a-3p. (A) Flow cytometry was used to
measure N2a cell apoptosis, and (B) the percentage of cell
apoptosis was quantified. *P<0.05 vs. control group;
#P<0.05 vs. OGD/R group; ФP<0.05 vs.
OGD/R + sh-XIST group. (C) Reverse transcription-quantitative PCR
and (D) western blot analysis of the mRNA and (E) protein
expression levels of Bax and Bcl-2 in N2a cells. ELISA results of
the relative (F) caspase-3 activity. (G) ROS production changes in
the OGD/R-treated N2a cells. The ROS staining images in the four
indicated groups for panel G. Scale bar, 100 µm. *P<0.05 vs.
control group; #P<0.05 vs. OGD/R group;
ФP<0.05 vs. OGD/R + sh-XIST group. I/R,
ischemia/reperfusion; NC, negative control; miR, microRNA; OGD/R,
oxygen and glucose deprivation/reperfusion; N2a, Neuro-2a; sh,
short hairpin RNA; XIST, X inactivate-specific transcript; ROS,
reactive oxygen species. |
FOXO3 acts as a downstream target of
miR-27a-3p
Subsequently, the downstream targets of miR-27a-3p
were investigated. Bioinformatics analysis indicated that FOXO3 had
binding sites with miR-27a-3p (Fig.
6A). The transfection efficiency of pcDNA3.1-XIST was verified
via RT-qPCR in N2a cells (Fig. 6B).
Then, pmirGLO-FOXO3-WT luciferase reporter plasmids were
constructed by inserting the WT or MUT 3′untranslated region of
FOXO3 into pmirGLO plasmids. A luciferase reporter assay revealed
that the luciferase activity of pmirGLO-FOXO3-WT was reduced by the
overexpression of miR-27a-3p, but was increased by the
overexpression of XIST, while the luciferase activity of
pmirGLO-FOXO3-MUT showed no evident changes (Fig. 6C). Then, the overexpression
efficiency of FOXO3 was confirmed via RT-qPCR in N2a cells
transfected with pcDNA3.1-FOXO3 (Fig.
6D). According to RT-qPCR and western blot analyses results,
the mRNA and protein expression levels of FOXO3 were downregulated
by miR-27a-3p overexpression and were further enhanced by XIST
overexpression (Fig. 6E and F).
 | Figure 6.FOXO3 acts as a downstream target of
miR-27a-3p. (A) Bioinformatics analysis indicated that FOXO3 had
binding sites for miR-27a-3p. (B) The overexpression efficiency of
XIST was detected using RT-qPCR in N2a cells. #P<0.05
vs. pcDNA3.1 group. (C) A luciferase reporter assay revealed the
luciferase activity of FOXO3. #P<0.05 vs. NC mimic
group; ФP<0.05 vs. miR-27a-3p mimic group. (D)
Transfection efficiency of FOXO3 was examined via RT-qPCR in N2a
cells transfected with pcDNA3.1-FOXO3. #P<0.05 vs.
pcDNA3.1 group. (E) RT-qPCR and (F) western blot analysis results
demonstrated the mRNA and protein expression level changes of FOXO3
in N2a cells. #P<0.05 vs. NC mimic group;
ФP<0.05 vs. miR-27a-3p mimic group. RT-qPCR was used
to measure the expression of FOXO3 in the (G) brain tissues of the
I/R-treated mice and the (H) OGD/R-treated N2a cells.
#P<0.05 vs. sham group in panel G;
#P<0.05 vs. control group in panel H. N2a, Neuro-2a;
XIST, X inactivate-specific transcript; I/R, ischemia/reperfusion;
NC, negative control; miRNA/miR, microRNA; OGD/R, oxygen and
glucose deprivation/reperfusion; RT-qPCR, reverse
transcription-quantitative PCR; WT, wild-type; MUT, mutant; UTR,
untranslated region. |
Next, the expression level of FOXO3 in the
I/R-induced mouse model and the OGD/R-treated N2a cells was
assessed. The results demonstrated that the expression level of
FOXO3 was significantly increased in the I/R-induced mouse model
and the OGD/R-treated N2a cells (Fig.
6G and H). In summary, it was identified that miR-27a-3p
directly bound with FOXO3.
Knockdown of FOXO3 alleviates cerebral
I/R injury
The function of FOXO3 in cerebral injury after I/R
was investigated. First, the knockdown efficiency of FOXO3 in the
brain tissues of the I/R-treated mice was verified (Fig. 7A). It was identified that FOXO3
knockdown significantly reduced the brain infarct volume in the
I/R-treated mice compared with the I/R + AAV-sh-NC group (Fig. 7B and C). Consistently, the
neurological deficit score was decreased by the knockdown of FOXO3
in the I/R-treated mice (Fig. 7D).
In addition, FOXO3 knockdown decreased the mRNA and protein
expression levels of Bax and enhanced those of Bcl-2 in these mice
(Fig. 7E and F). In conclusion, the
knockdown of FOXO3 alleviated I/R-induced cerebral injury.
Discussion
Recent studies have revealed that lncRNAs are novel
biomarkers in the progression of cerebral I/R injury (24,25).
YY1 transcription factor-induced growth arrest specific 5 promoted
6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 transcription
to increase neuronal glycolysis, and thus, aggravated cerebral I/R
injury (26). Moreover, small
nucleolar RNA host gene (SNHG) 12 protected neuronal cells from
cerebral I/R injury (27), while
SNHG14 accelerated the inflammatory response triggered by cerebral
I/R injury by binding with miR-136-5p to regulate Rho associated
coiled-coil containing protein kinase (28).
XIST has been reported to serve a role in human lung
adenocarcinoma (13), pulmonary
fibrosis (14) and neuropathic pain
(15), but the exact function of
XIST in cerebral I/R injury remains unknown. The present study
identified that XIST expression was upregulated in the brain
tissues of an I/R mouse model and in OGD/R-induced N2a cells.
Furthermore, it was found that XIST knockdown inhibited brain
injury by suppressing apoptosis and ROS production.
lncRNAs can serve as ceRNAs to regulate mRNA
expression by competitively binding with shared miRNAs in brain
injury. For example, AK038897 functions as a ceRNA against
miR-26a-5p to upregulate death associated protein kinase 1 and
aggravate cerebral I/R injury (29). It has also been shown that
myocardial infarction associated transcript competed with high
mobility group box 1 to bind with miR-204-5p, and thereby,
regulated cerebral microvascular endothelial cell injury after
cerebral ischemia (30).
Furthermore, SNHG6 modulated neuronal cell apoptosis by modulating
miR-181c-5p/Bcl-2-like protein 11 signaling in ischemic stroke
(31). Using bioinformatics
analysis and mechanistic assays, miR-27a-3p was revealed to bind
with XIST in the present study. It has been verified that
miR-27a-3p suppresses the progression of occlusive bronchiolitis
(31), and upregulation of
miR-27a-3p inhibits the inflammatory response in spinal cord injury
by targeting Toll-like receptor 4 (32). The present study demonstrated that
miR-27a-3p was downregulated in the brain tissues of an I/R mouse
model and in OGD/R-treated N2a cells. In addition, overexpression
of miR-27a-3p attenuated brain injury induced by I/R in mice. It
was found that apoptosis and ROS production in N2a cells were
suppressed by the overexpression of miR-27a-3p. Moreover, the
rescue assays demonstrated that XIST facilitated cerebral I/R
injury by downregulating miR-27a-3p.
In the current study, FOXO3 was confirmed to be a
downstream target of miR-27a-3p. FOXO3 was previously reported to
regulate pulmonary fibrosis (33)
and inflammation in mice with necrotizing colitis (34). The present results indicated that
FOXO3 expression was negatively regulated by miR-27a-3p and was
positively regulated by XIST. Furthermore, FOXO3 expression was
significantly upregulated in the brain tissues of the I/R-treated
mice and in the OGD/R-induced N2a cells. More importantly, FOXO3
knockdown alleviated I/R-induced injury, as well as suppressed the
apoptosis and ROS production of N2a cells.
In conclusion, the present study demonstrated that
XIST promoted cerebral I/R injury by binding with miR-27a-3p to
upregulate FOXO3, which may further the understanding of the
pathogenesis of cerebral I/R injury.
Acknowledgements
Not applicable.
Funding
This study was supported by Key Project Fund for
Clinical Research of Wuhan Health and Family Planning Commission
(grant no. WX15B22), Young Talents Project Fund of Hubei Municipal
Health Commission (grant no. WJ2019H168) and Wuhan Young and
Middle-Aged Medical Backbone Talents Training Project Fund [Wuhan
Health and Family Planning Commission (2017); no. 51].
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HZ and XW conceived and designed the experiments.
HZ, JX, QH, LX, HC and MC performed the experiments and constructed
the table and figures. HZ, JX, QH and XW provided the reagents,
materials and analysis tools. HZ and XW wrote the paper. HZ, JX,
QH, LX and XW revised the manuscript. All authors read and approved
the final version of the manuscript. All authors confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
The study protocols of all animal experiments were
approved and performed in accordance with standard principles
approved by the Institutional Animal Care and Use Committee of
China Resources & WISCO General Hospital (Hubei, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ou J, Kou L, Liang L and Tang C: MiR-375
attenuates injury of cerebral ischemia/reperfusion via targetting
Ctgf. Biosci Rep. 37:BSR201712422017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Henriksson KM, Farahmand B, Åsberg S,
Edvardsson N and Terént A: Comparison of cardiovascular risk
factors and survival in patients with ischemic or hemorrhagic
stroke. Int J Stroke. 7:276–281. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ma J, Shui S, Han X, Guo D, Li T and Yan
L: microRNA-200a silencing protects neural stem cells against
cerebral ischemia/reperfusion injury. PLoS One. 12:e01721782017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dorweiler B, Pruefer D, Andrasi TB, Maksan
SM, Schmiedt W, Neufang A and Vahl CF: Ischemia-reperfusion injury:
Pathophysiology and clinical implications. Eur J Trauma Emerg Surg.
33:600–612. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pan J, Konstas AA, Bateman B, Ortolano GA
and Pile-Spellman J: Reperfusion injury following cerebral
ischemia: Pathophysiology, MR imaging, and potential therapies.
Neuroradiology. 49:93–102. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schrepfer E and Scorrano L: Mitofusins,
from mitochondria to metabolism. Mol Cell. 61:683–694. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Khoshnam SE, Winlow W, Farzaneh M, Farbood
Y and Moghaddam HF: Pathogenic mechanisms following ischemic
stroke. Neurol Sci. 38:1167–1186. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ma L, Bajic VB and Zhang Z: On the
classification of long non-coding RNAs. RNA Biol. 10:925–933. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kapranov P, Cheng J, Dike S, Nix DA,
Duttagupta R, Willingham AT, Stadler PF, Hertel J, Hackermüller J,
Hofacker IL, et al: RNA maps reveal new RNA classes and a possible
function for pervasive transcription. Science. 316:1484–1488. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ge Y, Wang J, Wu D, Zhou Y, Qiu S, Chen J,
Zhu X, Xiang X, Li H and Zhang D: lncRNA NR_038323 Suppresses renal
fibrosis in diabetic nephropathy by targeting the miR-324-3p/DUSP1
axis. Mol Ther Nucleic Acids. 17:741–753. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang G, Li S, Lu J, Ge Y, Wang Q, Ma G,
Zhao Q, Wu D, Gong W, Du M, et al: LncRNA MT1JP functions as a
ceRNA in regulating FBXW7 through competitively binding to
miR-92a-3p in gastric cancer. Mol Cancer. 17:872018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li D, Zhang J, Li X, Chen Y, Yu F and Liu
Q: Insights into lncRNAs in Alzheimer's disease mechanisms. RNA
Biol. Jul 14–2020.(Epub ahead of print). doi:
10.1080/15476286.2020.1788848. View Article : Google Scholar
|
|
13
|
Sun J, Pan LM, Chen LB and Wang Y: LncRNA
XIST promotes human lung adenocarcinoma cells to cisplatin
resistance via let-7i/BAG-1 axis. Cell Cycle. 16:2100–2107. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang Y, Liang Y, Luo J, Nie J, Yin H, Chen
Q, Dong J, Zhu J, Xia J and Shuai W: XIST/miR-139 axis regulates
bleomycin (BLM)-induced extracellular matrix (ECM) and pulmonary
fibrosis through β-catenin. Oncotarget. 8:65359–65369. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jin H, Du XJ, Zhao Y and Xia DL:
XIST/miR-544 axis induces neuropathic pain by activating STAT3 in a
rat model. J Cell Physiol. 233:5847–5855. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ansari S, Azari H, Caldwell KJ, Regenhardt
RW, Hedna VS, Waters MF, Hoh BL and Mecca AP: Endothelin-1 induced
middle cerebral artery occlusion model for ischemic stroke with
laser Doppler flowmetry guidance in rat. J Vis Exp. 500142013.doi:
10.3791/50014. PubMed/NCBI
|
|
18
|
Hu X, Xiang Z, Zhang W, Yu Z, Xin X, Zhang
R, Deng Y and Yuan Q: Protective effect of DLX6-AS1 silencing
against cerebral ischemia/reperfusion induced impairments. Aging
(Albany NY). 12:23096–23113. 2020.PubMed/NCBI
|
|
19
|
Lu Y, Han Y, He J, Zhou B, Fang P and Li
X: LncRNA FOXD3-AS1 knockdown protects against cerebral
ischemia/reperfusion injury via miR-765/BCL2L13 axis. Biomed
Pharmacother. 132:1107782020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cao Y, Gao W, Tang H, Wang T and You C:
Long Non-coding RNA TALNEC2 Aggravates Cerebral
Ischemia/Reperfusion Injury via Acting as a competing endogenous
RNAs for miR-650 to target apoptotic peptidase activating factor 1.
Neuroscience. 458:64–76. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gao N, Tang H, Gao L, Tu GL, Luo H and Xia
Y: LncRNA H19 aggravates cerebral ischemia/reperfusion injury by
functioning as a ceRNA for miR-19a-3p to Target PTEN. Neuroscience.
437:117–129. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shan W, Chen W, Zhao X, Pei A, Chen M, Yu
Y, Zheng Y and Zhu S: Long noncoding RNA TUG1 contributes to
cerebral ischaemia/reperfusion injury by sponging mir-145 to
up-regulate AQP4 expression. J Cell Mol Med. 24:250–259. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ghafouri-Fard S, Shoorei H and Taheri M:
Non-coding RNAs participate in the ischemia-reperfusion injury.
Biomed Pharmacother. 129:1104192020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liang J, Wang Q, Li JQ, Guo T and Yu D:
Long non-coding RNA MEG3 promotes cerebral ischemia-reperfusion
injury through increasing pyroptosis by targeting miR-485/AIM2
axis. Exp Neurol. 325:1131392020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang XC, Gu AP, Zheng CY, Li YB, Liang
HF, Wang HJ, Tang XL, Bai XX and Cai J: YY1/LncRNA GAS5 complex
aggravates cerebral ischemia/reperfusion injury through enhancing
neuronal glycolysis. Neuropharmacology. 158:1076822019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yao X, Yao R, Huang F and Yi J: LncRNA
SNHG12 as a potent autophagy inducer exerts neuroprotective effects
against cerebral ischemia/reperfusion injury. Biochem Biophys Res
Commun. 514:490–496. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhong Y, Yu C and Qin W: LncRNA SNHG14
promotes inflammatory response induced by cerebral
ischemia/reperfusion injury through regulating miR-136-5p /ROCK1.
Cancer Gene Ther. 26:234–247. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wei R, Zhang L, Hu W, Wu J and Zhang W:
Long non-coding RNA AK038897 aggravates cerebral
ischemia/reperfusion injury via acting as a ceRNA for miR-26a-5p to
target DAPK1. Exp Neurol. 314:100–110. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Deng W, Fan C, Shen R, Wu Y, Du R and Teng
J: Long noncoding MIAT acting as a ceRNA to sponge microRNA-204-5p
to participate in cerebral microvascular endothelial cell injury
after cerebral ischemia through regulating HMGB1. J Cell Physiol.
235:4571–4586. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang X, Liu Z, Shu Q, Yuan S, Xing Z and
Song J: LncRNA SNHG6 functions as a ceRNA to regulate neuronal cell
apoptosis by modulating miR-181c-5p/BIM signalling in ischaemic
stroke. J Cell Mol Med. 23:6120–6130. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang P, Li LQ, Zhang D and Shen Y:
Over-expressed miR-27a-3p inhibits inflammatory response to spinal
cord injury by decreasing TLR4. Eur Rev Med Pharmacol Sci.
22:5416–5423. 2018.PubMed/NCBI
|
|
33
|
Qian W, Cai X, Qian Q, Wang D and Zhang L:
Angelica sinensis polysaccharide suppresses epithelial-mesenchymal
transition and pulmonary fibrosis via a DANCR/AUF-1/FOXO3
regulatory axis. Aging Dis. 11:17–30. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yin Y, Wang J, Zhao X, Wu X, Zou H, Qin Z
and Cao J: Overexpressed FOXO3 improves inflammatory status in mice
by affecting NLRP3-mediated cell coronation in necrotizing colitis
mice. Biomed Pharmacother. 125:1098672020. View Article : Google Scholar : PubMed/NCBI
|