Introduction
The cleft lip is a very common congenital oral and
maxillofacial malformation, often accompanied by cleft palate and
alveolar cleft. Although surgical repair techniques are
continuously being improved, numerous patients still experience
inevitable secondary scar formation after surgery. In recent years,
with the development of prenatal diagnosis and treatment technology
(1), intrauterine surgery has made
it possible to correct developmental deformities, such as a cleft
lip.
The concept of scarless healing was first proposed
by Burrington (2) in 1971. It was
later observed that fetal skin wounds that occur during early
pregnancy can heal quickly and restore intact skin barrier
functions. In contrast, fetal skin damage that occurs in the third
trimester of pregnancy can result in the formation of scar tissue
similar to that of an adult (3).
Therefore, the different manifestations of scarless healing of
mammalian fetal wounds are related to the gestational age of the
fetus (4). Dang et al
(5) and Longaker et al
(6) demonstrated that this
transition period from scarless healing to scar formation occurred
between day 16.5 of gestational age (GA) and GA18.5 in rats and
mice, which have a gestation period of ~21.5 days. Lorenz et
al (7) and Cass et al
(8) suggested that when 1–2 mm
incisions are inflicted on fetal rats, the transition period of
scarless healing to healing with scar formation was still between
16.5 (GA16.5) and 18.5 days (GA18.5).
This phenotypic difference in fetal wounds has
inspired further examination of the specific underlying mechanisms.
Initially, it was hypothesized that the reason for early scar
repair was that the fetus developed in amniotic fluid, which is
rich in growth factors and extracellular matrix (ECM) components
(9,10). Previous studies typically utilized
large animal models to study the presence of scars following repair
(11,12). However, only a few studies have
reported the use of a fetal rat cleft lip wound model to establish
the effectiveness of surgical repair at different gestational ages.
Moreover, due to the short gestation period of rats, the
experimental cycle can be shortened, and the experiment can
therefore be repeated.
Given the importance of this process, the present
study, screened out several specific markers of early fetal
scarless repair. The present study aimed to gain insight into the
occurrence and mechanisms of scarless repair, and to identify new
clinical targets for the prevention and treatment of scars.
Materials and methods
Animals
A total of 36 SPF-grade adult Sprague-Dawley (SD)
rats (female; mean weight, 250 g; age, 12 weeks) were obtained from
the Third Xiangya Hospital of Central South University Animal
Experiment Center (Hunan, China) and divided into two groups that
received surgery once their fetuses reached GA16.5 or GA18.5,
respectively (n=18 each). The following housing conditions were
implemented: A temperature between 25±2°C, relative humidity of
55±15%, ventilation rate of 10–20 times per hour, time-controlled
artificial lighting (12-h day-night cycle) and ad libitum
access to food and water. The experiments were supervised
throughout and were performed in accordance with animal
experimentation ethics.
Preliminary study on different repair
modes applicable to fetal rats with artificial cleft lip
wounds
Fetal rats located away from the uterine horn were
selected to prevent subsequent abortion, as described previously
(13). In the current study, rats
were anesthetized with 30 mg/kg pentobarbital sodium
intraperitoneally before surgery. A wedge-shaped cleft-like defect
was created on the upper-left lip of the fetal rats. The
upper-right lip did not receive any treatment and was used as a
control condition. The fetal rats were then returned to the uterus.
Fetal rats from the GA16.5 and GA18.5 groups were then removed
three days post-surgery as previously described (4) (i.e., at GA19.5 or GA21.5,
respectively). All fetuses and rats were euthanized using carbon
dioxide (30% volume displaced/min). Death was confirmed using
cervical dislocation. A total of three fetal rats were obtained
from each pregnant rat, for a total of 54 fetal rats from both
GA16.5 and GA18.5 groups, and the survival rate was calculated.
Tissue samples from the surgical site on the upper-left lip and
asymmetrical sections from the upper-right lip were collected from
the fetal rats for histological examination, including hematoxylin
and eosin (H&E) staining, Masson's Trichrome staining and
type-I collagen immunohistochemical staining as previously
described (14–16).
The upper-left lip tissue samples from the GA16.5
group were defined as group 1, whereas the upper-right lip tissue
samples from the GA16.5 group were defined as group 2. In addition,
the upper-left lip tissue samples from the GA18.5 group were
defined as group 3, whereas the upper-right lip tissue samples from
the GA18.5 group were defined as group 4. Each subgroup included 27
samples. Protein expression was compared between group 1 and 2,
group 3 and 4, as well as group 3 and 1. Label-free quantification
PRM was performed as previously described (17) and was used to detect the
differentially expressed proteins among the different groups.
MaxQuant 1.5.6 (https://www.maxquant.org) and Perseus 1.4 (https://www.maxquant.org/perseus/) were used to
analyze the results of label-free quantification PRM: Volcano plots
were generated for differentially expressed proteins: Y-axis,
-log10(P-value); x-axis: log2(ratio). The points distributed
outside the two vertical borders and above the horizontal border
represented the proteins with significant differences; proteins
with at least a 1.5-fold change in expression and P<0.05 were
considered significant. Subsequently, bioinformatics analysis,
including GO and KEGG pathway analysis, was performed to identify
differentially expressed proteins (18).
Experimental verification of tissue
repair proteins in fetal rats with artificial cleft lip wounds
The mRNA levels of the differentially expressed
molecules were assessed using reverse transcription-quantitative
(RT-q) PCR, as previously described (19). Differentially expressed levels of
proteins were detected by label-free quantification PRM as
previously described (17).
Statistical analysis
GraphPad Prism 8.0 (GraphPad Software, Inc.) and
SPSS 22.0 (IBM Corp.) were used to perform calculations and carry
out statistical analysis. Student's t-test was used to compare
differences between two groups. The experimental data from each
group were analyzed for congruence of variance before the t-test
were applied. The FDR values were within 0.01 in the comparisons.
Mixed ANOVA followed by Sidak's post hoc test was used to analyze
the differences between multiple groups. P<0.05 was considered
to indicate a statistically significant difference.
Results
Gross observation
All fetal rats were observed before delivery. The
nasolabial cleft was first observed before surgery and images were
captured to facilitate the observation of changes in the fetal rats
from the GA16.5 and GA18.5 groups. We observed the same area again
72 h post-surgery to identify differences. The cuneiform tissue of
the upper-left lip was removed by microsurgery to create a cleft
lip wound. The changes in the fetal rats were observed
macroscopically. In the GA16.5 group, the upper-left cleft lip
wound completely healed 72 h after surgery (i.e., GA19.5) and the
continuity of the upper lip tissue was restored. Only a slight
depression was observed in the surgical area. The upper-left lip
tissue was nearly symmetrical with that of the right side. However,
in the GA18.5 group, the cleft lip wound was not completely healed
72 h after surgery (i.e., GA21.5); a clear scar was observed in the
surgical area, and the upper lip was asymmetrical on both sides due
to wound contracture (Fig. 1).
Histological analysis
In the GA16.5 group 72 h after surgery, the tissue
of the upper-left lip wound demonstrated complete regeneration when
observed under the microscope (Figs.
2–4). The results of H&E
staining demonstrated complete epithelialization of the upper-left
lip, and the structure of new follicles was detected under the
epidermis. Compared with the normal skin of the upper-right lip, a
slight depression in the cleft part of the upper-left lip and
thickening of the skin was noted, whereas inflammatory cell
infiltration and neovascularization were not apparent (Fig. 2). Masson's Trichrome staining
revealed collagen fibers under the epidermis, demonstrating a fine
reticular and emerging follicular structure (Fig. 3). Immunohistochemical analysis
indicated no obvious difference in the amount of type-I collagen in
the upper-left cleft lip area and the rest of the upper lip
(Fig. 4).
In the GA18.5 group, the position of the wound was
easily identified by a distinct scar on the upper-left lip. H&E
staining demonstrated that partial epithelialization occurred in
the upper-left cleft lip area. Compared with the normal skin of the
upper-right lip, the upper-left lip displayed a clear scar, new
capillary formation around the wound and increased fibroblast
proliferation and ECM volume, whereas structural components of hair
follicles were not observed under the epidermis (Fig. 5). Masson's Trichrome staining
demonstrated the absence of new follicular structure and the
presence of dense collagen fibers under the epidermis (Fig. 6). Immunohistochemical analysis in
the upper-left cleft lip wound demonstrated an increase in type-I
collagen expression and fiber density, as well as a more compact
structure and absence of adnexal skin (Fig. 7), compared with normal upper lip
tissue.
Immunohistochemical analysis of cell proliferation
markers was also carried out. Compared with GA16.5 fetal rats, the
expression of Ki67 and CD31 slightly increased in the GA18.5 group
following surgery. By contrast, the expression of CK10 decreased in
the GA18.5 group, compared with the GA16.5 group (Fig. 8).
RT-qPCR analysis of inflammatory
factors
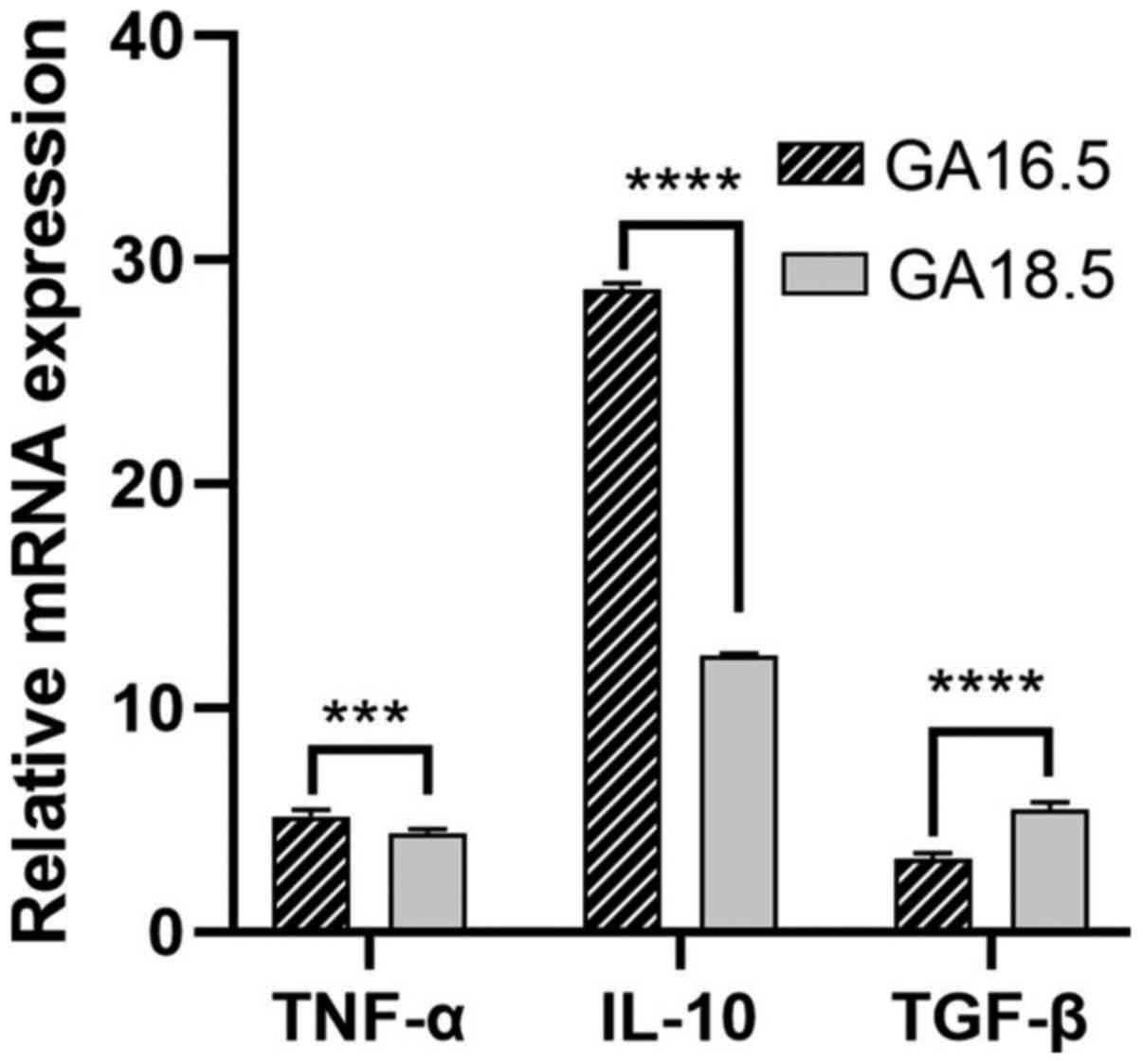
The relative mRNA expression levels of the
pro-inflammatory factors TNF-α, IL-10 and TGF-β were evaluated in
the two groups of fetal rats. The mRNA levels of TNF-α and IL-10
were significantly higher in GA18.5 rats, compared with GA16.5
rats. Furthermore, the mRNA expression levels of TGF-β were
significantly reduced in the GA18.5 group (Fig. 9).
Protein identification and
differential protein screening
Compared with group 1, 57 differentially expressed
proteins were identified in group 2, of which 37 were upregulated
and 20 were downregulated. A comparison of groups 3 and 4 revealed
312 differentially expressed proteins, of which 171 were
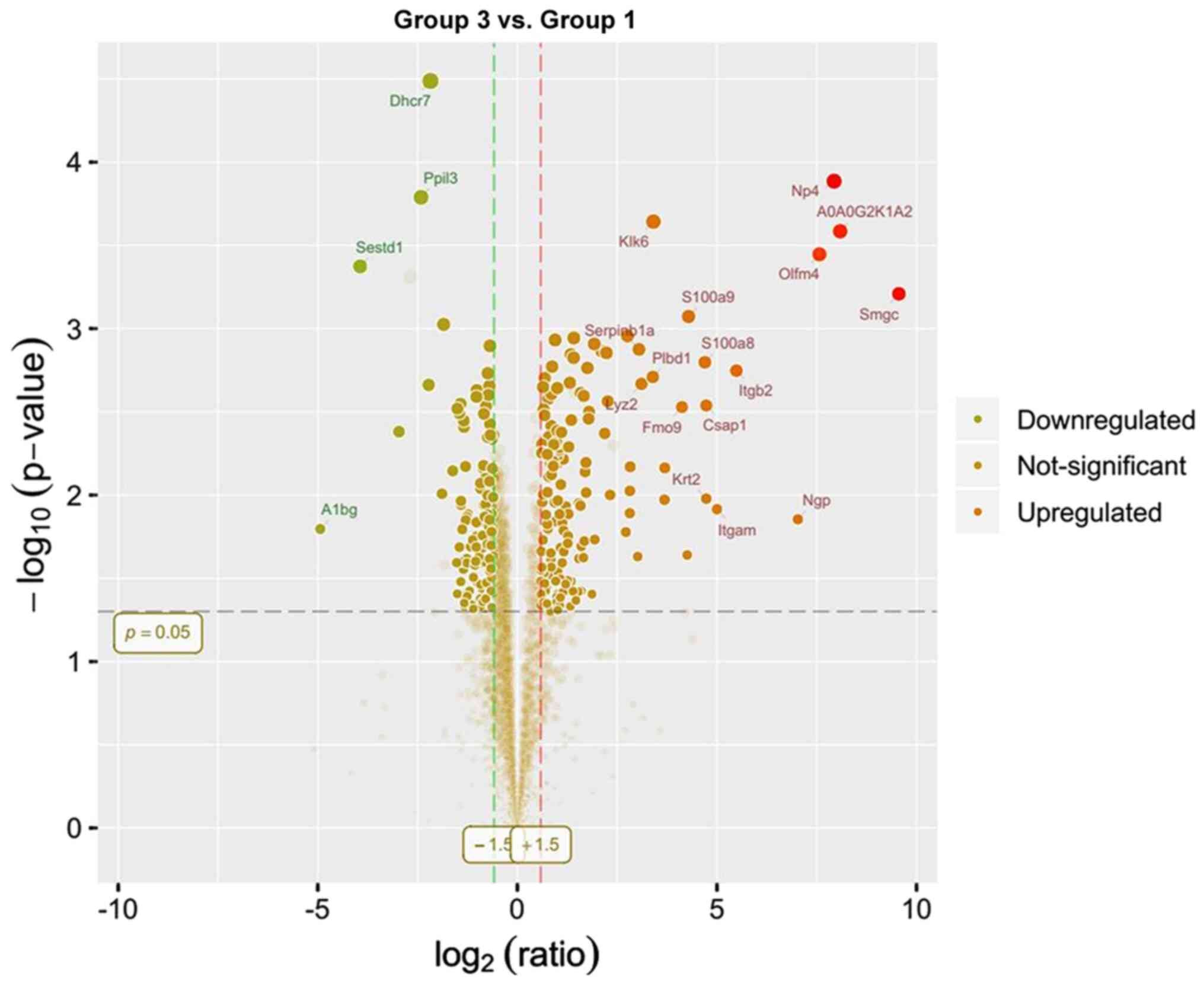
upregulated and 141 were downregulated. Lastly, compared with group
1,289 differentially expressed proteins were identified in group 3,
of which 151 were upregulated and 138 were downregulated. Only 50
differentially expressed proteins and their multiple variations
were upregulated or downregulated between all groups (Tables I–IV). The distribution of the
differentially expressed proteins among the selected samples is
presented as volcano plots (Figs.
10,11,12).
 | Table I.Comparison of differentially
expressed protein numbers between samples. |
Table I.
Comparison of differentially
expressed protein numbers between samples.
| Sample | Differentially
expressed proteins, n | Upregulated
proteins, n | Downregulated
protein, n |
|---|
| Group 1 vs. Group
2 | 57 | 37 | 20 |
| Group 3 vs. Group
4 | 312 | 171 | 141 |
| Group 3 vs. Group
1 | 312 | 151 | 138 |
 | Table IV.Differential protein expression in
group 3 and group 1. |
Table IV.
Differential protein expression in
group 3 and group 1.
| Protein ID | Gene name | Protein name | P-value | Fold change |
|---|
| Q6jhy3 | Smgc | Submandibular gland
protein c precursor | 0.001 | 753.286 |
| D3zge2 | Mpo | Myeloperoxidase
precursor | 0.000 | 271.832 |
| Q62714 | Np4 | Neutrophil
antibiotic peptide np-4 | 0.000 | 244.647 |
| D3zmi6 | Olfm4 | Olfactomedin-4
precursor | 0.000 | 189.639 |
| D3zy96 | Ngp | Neutrophilic
granule protein precursor | 0.014 | 130.459 |
| B2ryb8 | Itgb2 | Integrin beta 2
precursor | 0.002 | 44.874 |
| G3v8l7 | Itgam | Integrin alpha-m
precursor | 0.012 | 32.024 |
| Q6ig02 | Krt2 | Keratin, type ii
cytoskeletal 2 epidermal | 0.011 | 26.577 |
| Q63015 | Csap1 | Common salivary
protein 1 precursor | 0.003 | 26.571 |
| P50115 | S100a8 | S100 calcium
binding protein a8 | 0.002 | 25.933 |
| P50116 | S100a9 | S100 calcium
binding protein a9 | 0.001 | 19.538 |
| Q9jkb7 | Gda | Guanine
deaminase | 0.023 | 19.076 |
| D3zd07 | Fmo9 | Flavin containing
monooxygenase 9 pseudogene | 0.003 | 17.428 |
| Q5u1y2 | Rac2 | Ras-related c3
botulinum toxin substrate 2 | 0.011 | 12.859 |
| O54854 | Klk6 | Kallikrein-6
precursor | 0.000 | 10.614 |
| Q5u2v4 | Plbd1 | Phospholipase
b-like 1 | 0.002 | 10.508 |
| Q6pdv1 | Lyz1 | Lysozyme c-1
precursor | 0.002 | 8.631 |
| Q4g075 | Serpinb1a | Leukocyte elastase
inhibitor a | 0.001 | 8.260 |
| Q6ldz3 | Ptprc | Receptor-type
tyrosine-protein phosphatase c | 0.023 | 8.077 |
| Q9wuq4 | Slpi | Secretory leukocyte
peptidase inhibitor precursor | 0.007 | 7.093 |
| Q9erl1 | Cybb | Cytochrome b-245,
beta polypeptide | 0.009 | 7.056 |
| E0a3n4 | Serpina3n | Serine protease
inhibitor a3n | 0.013 | 7.049 |
| G3v6k1 | Tcn2 | Transcobalamin-2
precursor | 0.017 | 6.577 |
| P14669 | Anxa3 | Annexin a3 | 0.010 | 5.005 |
| P22985 | Xdh | Xanthine
dehydrogenase/oxidase | 0.003 | 4.802 |
| Q91zn1 | Coro1a | Coronin-1a | 0.001 | 4.694 |
| P05982 | Nqo1 | Nad(p)h quinone
dehydrogenase 1 | 0.001 | 4.334 |
| P23640 | Rab27a | Ras-related protein
rab-27a | 0.019 | 3.830 |
| Q6ifu9 | Krt16 | Keratin, type i
cytoskeletal 16 | 0.001 | 3.797 |
| Q62894 | Ecm1 | Extracellular
matrix protein 1 | 0.039 | 3.655 |
| Q5×i38 | Lcp1 | Plastin-2 | 0.003 | 3.469 |
| P07150 | Anxa1 | Annexin a1 | 0.003 | 3.446 |
| Q78zr5 | Hopx | Homeodomain-only
protein | 0.002 | 3.376 |
| P01015 | Agt | Angiotensinogen
angiotensin-1 angiotensin-2 angiotensin-3 | 0.010 | 3.300 |
| Q6axy8 | Dhrs1 |
Dehydrogenase/reductase sdr family member
1 | 0.006 | 3.287 |
| Q91w30 | Akr1b8 | Aldose
reductase-related protein 2 | 0.007 | 3.244 |
| P32755 | Hpd |
4-hydroxyphenylpyruvate dioxygenase | 0.024 | 3.149 |
| Q499n7 | Ptpn6 | Tyrosine-protein
phosphatase non-receptor type 6 | 0.038 | 3.025 |
| G3v755 | Sprr1a | Cornifin-a | 0.002 | 3.006 |
| Q5×fv4 | Fabp4 | Fatty acid-binding
protein, adipocyte | 0.024 | 2.910 |
| B1wbv8 | Pld4 | Phospholipase
d4 | 0.011 | 2.909 |
| D3zpf9 | Serpinb12 | Serpin b12 | 0.038 | 2.880 |
| Q4qqv6 | Lsp1 | Lymphocyte specific
1 | 0.001 | 2.665 |
| P29524 | Serpinb2 | Plasminogen
activator inhibitor 2 type a | 0.001 | 2.653 |
| O55162 | Lypd3 | Ly6/plaur
domain-containing protein 3 | 0.004 | 2.551 |
| D4a5u3 | Tgm3 | Protein-glutamine
gamma-glutamyltransferase e protein | 0.033 | 2.547 |
| D3zsh7 | Col17a1 | Collagen
alpha-1(xvii) chain | 0.002 | 2.485 |
| D3zjk2 | Serpinb3a | Protein
serpinb3a | 0.038 | 2.445 |
| Q6ie17 | Stfa2l2 | Stefin-3 | 0.005 | 2.439 |
| Q5u206 | Calml3 | Calmodulin-like
protein 3 | 0.013 | 2.429 |
| Q4v885 | Colec12 | Collectin-12 | 0.017 | 0.547 |
| D3zqi1 | Gpx7 | Glutathione
peroxidase 7 precursor | 0.036 | 0.541 |
| D3z9m5 | Fkbp7 | Peptidyl-prolyl
cis-trans isomerase fkbp7 precursor | 0.014 | 0.530 |
| O88201 | Clec11a | C-type lectin
domain family 11 member a | 0.047 | 0.529 |
| D3zrd3 | Pde6d | Retinal rod
rhodopsin-sensitive cgmp 3′,5′-cyclic phosphodiesterase subunit
delta | 0.009 | 0.528 |
| P21807 | Prph | Peripherin | 0.008 | 0.527 |
| G3v6m4 | Capn6 | Calpain-6 | 0.024 | 0.514 |
| D3zg88 | Sssca1 | Sjogren
syndrome/scleroderma autoantigen 1 homolog | 0.034 | 0.502 |
| Q2eja0 | Yap1 | Yorkie homolog | 0.002 | 0.494 |
| Q3b7u1 | Maged2 | Melanoma-associated
antigen d2 | 0.003 | 0.492 |
| O35276 | Nrp2 | Neuropilin-2 | 0.015 | 0.491 |
| D3zun5 | Pofut2 | Gdp-fucose protein
o-fucosyltransferase 2 precursor | 0.018 | 0.490 |
| P70583; d4a6v3 | Dut | Deoxyuridine
5′-triphosphate nucleotidohydrolase | 0.021 | 0.489 |
| P19527 | Nefl | Neurofilament light
polypeptide | 0.026 | 0.479 |
| M0r649 | Exoc4 | Exocyst complex
component 4 | 0.031 | 0.466 |
| Q99pd6 | Tgfb1i1 | Transforming growth
factor beta-1-induced transcript 1 protein | 0.048 | 0.466 |
| P54001 | P4ha1 | Prolyl
4-hydroxylase subunit alpha-1 | 0.019 | 0.461 |
| D3zt07 | Sept5 | Septin-5 | 0.046 | 0.452 |
| P12839; g3v7s2 | Nefm | Neurofilament
medium polypeptide | 0.020 | 0.444 |
| B5df50 | Galnt2 | Polypeptide
n-acetylgalactosaminyltransferase 2 | 0.038 | 0.436 |
| D3zuq0 | Rilpl1 | Rilp-like protein
1 | 0.041 | 0.418 |
| D4a8h3 | Uba6 | Ubiquitin-like
modifier-activating enzyme 6 | 0.024 | 0.415 |
| D4a9u4 | Eln | Elastin | 0.041 | 0.409 |
| D4ad75 | Dpy19l1 | Protein dpy-19
homolog 1 | 0.014 | 0.408 |
| Q6p7d4 | Cyp20a1 | Cytochrome p450
20a1 | 0.007 | 0.406 |
| Q5×i28 | Raver1 | Ribonucleoprotein
ptb-binding 1 | 0.045 | 0.398 |
| P09117 | Aldoc |
Fructose-bisphosphate aldolase c | 0.004 | 0.396 |
| D3zct5 | Pald1 | Paladin | 0.004 | 0.395 |
| A1l1k3 | Anapc5 | Anaphase-promoting
complex subunit 5 | 0.028 | 0.392 |
| P62966 | Crabp1 | Cellular retinoic
acid-binding protein 1 | 0.016 | 0.384 |
| Q569b7 | Rwdd4 | Rwd
domain-containing protein 4 | 0.040 | 0.384 |
| Q5hze4 | Mri1 |
Methylthioribose-1-phosphate
isomerase | 0.011 | 0.376 |
| F1lqz3 | Kif3a | Kinesin family
member 3a | 0.011 | 0.376 |
| O88752 | Hbe1 | Hemoglobin, epsilon
1 | 0.033 | 0.375 |
| Q5u1z0 | Rab3gap2 | Rab3
gtpase-activating protein non-catalytic subunit | 0.003 | 0.373 |
| A1a5r1 | Rbfox1 | Fox-1 homolog
c | 0.033 | 0.366 |
| D4a845 | Rpa3 | Replication protein
a 14 kda subunit | 0.021 | 0.366 |
| D3zwc6 | Sntb1 |
Beta-1-syntrophin | 0.003 | 0.365 |
| G3v8m1 | Pold1 | Dna polymerase
delta catalytic subunit | 0.003 | 0.353 |
| P23565 | Ina |
Alpha-internexin | 0.039 | 0.352 |
| Q4klk9 | Ssu72 | Rna polymerase ii
subunit a c-terminal domain phosphatase ssu72 | 0.025 | 0.349 |
| F1mah6 | Cdh11 | Cadherin 11 | 0.007 | 0.326 |
| Q6ayg3 | Prune | Prune homolog
(drosophila) (ec:3.6.1.1) | 0.001 | 0.278 |
| P04638 | Apoa2 | Apolipoprotein
a-ii | 0.010 | 0.270 |
| Q9z2z8 | Dhcr7 |
7-dehydrocholesterol reductase | 0.000 | 0.221 |
| Q10758 | Krt8 | Keratin, type ii
cytoskeletal 8 | 0.002 | 0.214 |
| Q812d3 | Ppil3 | Peptidyl-prolyl
cis-trans isomerase-like 3 | 0.000 | 0.188 |
| G3v8r3 | Hbz | Hemoglobin,
zeta | 0.004 | 0.128 |
| B5dfl9 | Sestd1 | Sec14 and spectrin
domains 1 | 0.000 | 0.065 |
| Q9eph1 | A1bg |
Alpha-1b-glycoprotein | 0.016 | 0.033 |
Bioinformatics analysis
Gene ontology (GO) enrichment analysis was performed
on the differentially expressed proteins, and their properties were
generally described as biological process (BP), molecular function
(MF) or cellular component (CC). The first 10 GO enrichment results
from each group are displayed in Fig.
13. The results demonstrated that 73, 542 and 376
differentially expressed proteins were significantly enriched
between groups 1 and 2, 3 and 4 and 3 and 1, respectively. The
results of the Kyoto Encyclopedia of Genes and Genomes (KEGG)
pathway enrichment analysis identified the possible pathway related
to the differentially expressed proteins between groups (Fig. 14).
In addition, the interaction network of the
differentially expressed proteins that regulate wound repair were
analyzed. Examples of the interaction networks of the
differentially expressed proteins involved in wound repair are as
follows: i) Smad4, Tgf1i1, Ptpn6 and Hmox1 in group 1 and 2; ii)
S100a9, Fgg, Anxa1, Fgb, Plg and S100a8 in group 3 and 4; and iii)
CD36, S100a9, S100a8, Cd9Fgg, Anxa1, Fgb, Plg and S100a8 in group 3
and 1 (Fig. 15).
RT-qPCR analysis of possible target
protein in cleft lip repair
RNA was extracted from tissue samples with
TRIzol® reagent and the quality was checked using gel
electrophoresis. Relative mRNA levels were analyzed using RT-qPCR
(Fig. 16). The relative mRNA
expression levels of Smad4 were significantly higher in group 2,
compared with group 1 (P<0.05). Moreover, the relative mRNA
expression levels of Fabp5 were significantly lower in groups 4 and
1, compared with group 3 (P<0.05). Additionally, the relative
mRNA expression levels of S100a4 were significantly lower in group
4, compared with group 3 (P<0.05). S100a8 and S100a9 were
significantly higher in group 3, compared with in groups 1 and 4
(P<0.05).
 | Figure 16.RT-qPCR analysis of Smad4, Fabp5,
S100a4, S100a8 and S100a9. RT-qPCR detection and amplification of
(A) Smad4, (B) Fabp5, (C) S100a4, (D) S100a8 and (E) S100a9. The
dissolution curves and relative mRNA expression levels are shown
for each target. RT-qPCR, reverse transcription-quantitative PCR;
Fabp5, fatty acid binding protein 5; Smad4, Smad family member 4;
S100, S100 calcium binding protein. Group 1, upper-left lip of
fetus at 72 h after modeling in GA16.5 rats; Group 2, upper-right
lip of fetus at 72 h after modeling in GA16.5 rats; Group 3,
upper-left lip of fetus at 72 h after modeling in GA18.5 rats;
Group 4, upper-right lip of fetus at 72 h after modeling in GA18.5
rats. *P<0.05; **P<0.01. GA, gestational age. |
Immunofluorescence results
Immunofluorescence staining of Smad4, Fabp5, S100a4,
S100a8 and S100a9 was performed on samples from both the GA16.5 and
GA18.5 groups 72 h post-surgery. The expression levels of all five
proteins increased in GA18.5 compared to GA16.5, and the
differences were statistically significant (P<0.05; Fig. 17).
 | Figure 17.Immunofluorescence analysis Smad4,
Fabp5, S100a4, S100a8 and S100a9. (A and B) Smad4 staining of upper
lip tissue from (A) the GA16.5 group and (B) the GA18.5 group. (C
and D) Fabp5 staining of upper lip tissue from (C) the GA16.5 group
and (D) the GA18.5 group. (E and F) S100a4 staining of upper lip
tissue from (F) the GA16.5 group and (F) the GA18.5 group. (G and
H) S100a8 staining of upper lip tissue from (G) the GA16.5 group
and (H) the GA18.5 group. (I and J) S100a9 staining of upper lip
tissue from (I) the GA16.5 group and (J) the GA18.5 group. (K)
Relative IOD values for Smad4, Fabp5, S100a4, S100a8 and S100a9
staining. *P<0.05; **P<0.01; ***P>0.001. Fabp5, fatty acid
binding protein 5; Smad4, Smad family member 4; S100, S100 calcium
binding protein; IOD, integral optical density; GA, gestational
age. |
PRM analysis of differential protein
expression
The differences in multiple variations of Smad4
expression were compared between groups 1 and 2. The panel reaction
monitoring calculated this difference as 0.557, indicating
downregulation in group 1 compared with in group 2 (P=0.043)
(Table II). In contrast, no
statistically significant differences were observed between groups
3 and 4. The difference in multiple variations of Fabp5 between
groups 3 and 4 was calculated as 2.91, indicating upregulation in
group 3 compared with in group 4 (P=0.024) (Table III). Additionally, the expression
levels of Fabp5 were upregulated (P=0.01) in group 3 compared with
in group 1; however, the difference between the variations present
in groups 1 and 2 was not statistically significant. The difference
in the multiple variations of S100a4 and S100a8 between groups 3
and 4 was calculated as 2.897 and 92.828, respectively, indicating
an upregulation of the expression levels of both proteins in group
3 (P=0.001 and P=0.002, respectively) (Table III). Furthermore, the difference
in the multiple variations of S100a8 between groups 3 and 1 was
25.933, which indicates upregulation in group 3 (P=0.002) (Table IV). However, the differences were
not statistically significant between groups 1 and 2. The
difference in the multiple variations of S100a9 was 30.191 and
19.538 between groups 3 and 4 and groups 3 and 1, respectively,
suggesting upregulation in group 3 (P=0.0004 and P=0.001,
respectively) (Tables III and
IV). In contrast, the difference
in the multiple variations of S100a9 between groups 1 and 2 was not
statistically significant (Tables
II–IV).
 | Table II.Differential protein expression in
group 1 and group 2. |
Table II.
Differential protein expression in
group 1 and group 2.
| Protein ID | Gene name | Protein name | P-value | Fold-change |
|---|
| G3V8R3 | Hbz | Hemoglobin,
zeta | 0.004 | 7.788 |
| B2RYS8 | Ndufb8 | NADH
dehydrogenase | 0.000 | 5.024 |
| Q920P6 | Ada | Adenosine
deaminase | 0.038 | 4.831 |
| O88752 | Hbe1 | Hemoglobin, epsilon
1 | 0.003 | 4.801 |
| Q499N7 | Ptpn6 | Tyrosine-protein
phosphatase non-receptor type 6 | 0.003 | 4.700 |
| Q4FZU2 | Krt6a | Keratin 6A | 0.001 | 4.044 |
| P06762 | Hmox1 | Heme oxygenase
1 | 0.011 | 3.902 |
| Q6IFU9 | Krt16 | Keratin, type I
cytoskeletal 16 | 0.006 | 3.613 |
| Q99PD6 | Tgfb1i1 | Transforming growth
factor beta-1-induced transcript 1 protein | 0.004 | 3.590 |
| Q6P7S1 | Asah1 | Acid
ceramidase | 0.029 | 3.167 |
| Q63066 | Hbg1 | Hemoglobin, gamma
A | 0.002 | 3.082 |
| Q10758 | Krt8 | Keratin, type II
cytoskeletal 8 | 0.004 | 2.901 |
| Q6AYQ4 | Tmem109 | Transmembrane
protein 109 | 0.048 | 2.710 |
| Q9Z2Q7 | Stx8 | Syntaxin-8 | 0.035 | 2.660 |
| G3V9M8 | Fam50a | Protein fam50a | 0.034 | 2.515 |
| M0R9Y3 | Nup43 | Nucleoporin 43 | 0.005 | 2.461 |
| B2GVB9 | Fermt3 | Fermitin family
homolog 3 | 0.018 | 2.425 |
| G3V8H | Olfml3 | Olfactomedin-like
protein 3 precursor | 0.045 | 2.383 |
| D4A531 | Polr2i | Rna polymerase ii
subunit i | 0.049 | 2.290 |
| Q68FS1 | Nubp2 | Cytosolic Fe-S
cluster assembly factor | 0.028 | 2.221 |
| D3ZLS5 | Hectd1 | Hect domain e3
ubiquitin protein ligase 1 | 0.033 | 2.112 |
| D4A0M2 | Nxn | Nucleoredoxin | 0.033 | 2.056 |
| Q6IE17 | Stfa2l2 | Stefin-3 | 0.007 | 1.969 |
| P27139 | Ca2 | Carbonic anhydrase
2 | 0.032 | 1.957 |
| D3ZF44 | LOC684499 | Protein
LOC684499 | 0.015 | 1.940 |
| Q6LDZ3 | Ptprc | Receptor-type
tyrosine-protein phosphatase C | 0.007 | 1.878 |
| Q5XI38 | Lcp1 | Plastin-2 | 0.018 | 1.843 |
| Q5PPG2 | Lgmn | Legumain
precursor | 0.015 | 1.820 |
| P06765 | Pf4 | Platelet factor
4 | 0.050 | 1.708 |
| Q9R1T3 | Ctsz | Cathepsin Z | 0.011 | 1.707 |
| Q5U1Y2 | Rac2 | Ras-related C3
botulinum toxin substrate 2 | 0.021 | 1.669 |
| Q5U2V4 | Plbd1 | Phospholipase
B-like 1 | 0.028 | 1.630 |
| Q9EPX0 | Hspb8 | Heat shock protein
beta-8 | 0.005 | 1.603 |
| O35532 | Msmo1 | Methylsterol
monooxygenase 1 | 0.037 | 1.592 |
| Q91ZN1 | Coro1a | Coronin-1A | 0.013 | 1.586 |
| O88201 | Clec11a | C-type lectin
domain family 11 member A | 0.025 | 1.547 |
| Q5U329 | Slc4a1 | Band 3 anion
transport protein | 0.027 | 1.512 |
| Q496Z5 | Prph | Peripherin | 0.041 | 0.626 |
| P19527 | Nefl | Neurofilament light
polypeptide | 0.040 | 0.608 |
| Q9ESI7 | Dcx | Neuronal migration
protein doublecortin | 0.003 | 0.597 |
| Q6AY98 | Ube2e2 | Ubiquitin
conjugating enzyme e2 e2 | 0.046 | 0.577 |
| Q7TSX7 | Nr3c1;gr | Glucocorticoid
receptor | 0.026 | 0.560 |
| O70437 | Smad4 | Mothers against
decapentaplegic homolog 4 | 0.043 | 0.557 |
| F1M754 | Map4k4 | Mitogen-activated
protein kinase kinase kinase kinase 4 | 0.022 | 0.526 |
| D4A2Z8 | Dhx36 | Probable
ATP-dependent RNA helicase DHX36 | 0.009 | 0.522 |
| P31430 | Dpep1 | Dipeptidase 1 | 0.010 | 0.513 |
| Q6AXY8 | Dhrs1 |
Dehydrogenase/reductase SDR family member
1 | 0.019 | 0.495 |
| D4A414 | Cox15 | COX15 homolog | 0.031 | 0.476 |
| D4ABV5 | Calm1 | Calmodulin 1 | 0.012 | 0.473 |
| D3ZRN3 | Actbl2 | Beta-actin-like
protein 2 | 0.048 | 0.413 |
| Q8CGS4 | Chmp3 | Charged
multivesicular body protein 3 | 0.022 | 0.410 |
| D3ZHA7 | Myl6b | Myosin light chain
6b | 0.011 | 0.390 |
| P70541 | Eif2b3 | Translation
initiation factor eif-2B subunit gamma | 0.002 | 0.324 |
| D3ZX50 | Krtap11-1 | Uncharacterized
protein | 0.037 | 0.287 |
| D3ZD07 | Fmo9 | Flavin containing
monooxygenase 9 pseudogene | 0.007 | 0.277 |
| Q6IFX1 | Krt24 | Keratin, type I
cytoskeletal 24 | 0.012 | 0.050 |
| Q6IG02 | Krt2 | Keratin, type II
cytoskeletal 2 | 0.008 | 0.021 |
 | Table III.Differential protein expression in
group 3 and group 4. |
Table III.
Differential protein expression in
group 3 and group 4.
| Protein ID | Gene name | Protein name | P-value | Fold change |
|---|
| D3ZGE2 | Mpo |
Myeloperoxidase | 0.000 | 377.923 |
| Q62714 | Np4 | Neutrophil
antibiotic peptide NP-4 | 0.000 | 231.771 |
| D3ZY96 | Ngp | Neutrophilic
granule protein precursor | 0.010 | 226.724 |
| P50115 | S100a8 | S100 Calcium
Binding Protein A8 | 0.001 | 92.828 |
| Q7TP54 | Fam65b | Protein FAM65B | 0.000 | 77.718 |
| D3ZMI6 | Olfm4 | Olfactomedin-4
precursor | 0.001 | 63.833 |
| D4A081 | Setdb1 | Histone-lysine
N-methyltransferase SETDB1 | 0.000 | 47.032 |
| Q9JI30 | Itgam | Integrin alpha-M
precursor | 0.011 | 39.489 |
| B2RYB8 | Itgb2 | Integrin beta 2
precursor | 0.003 | 34.443 |
| P50116 | S100a9 | S100 Calcium
Binding Protein A9 | 0.000 | 30.191 |
| Q920P6 | Ada | Adenosine
deaminase | 0.028 | 27.433 |
| Q499N7 | Ptpn6 | Tyrosine-protein
phosphatase non-receptor type 6 | 0.003 | 23.157 |
| Q9ERL1 | Cybb | Cytochrome b-245,
beta polypeptide | 0.002 | 21.484 |
| Q9JKB7 | Gda | Guanine
deaminase | 0.019 | 20.202 |
| Q5U1Y2 | Rac2 | Ras-related C3
botulinum toxin substrate 2 | 0.007 | 19.291 |
| Q6IFU9 | Krt16 | Keratin, type I
cytoskeletal 16 | 0.001 | 13.524 |
| O54854 | Klk6 | Kallikrein-6
precursor | 0.000 | 11.605 |
| B2GVB9 | Fermt3 | Fermitin family
homolog 3 | 0.012 | 11.176 |
| Q5PQW8 | Gbp2 | Interferon-induced
guanylate-binding protein 2 | 0.019 | 10.854 |
| Q6LDZ3 | Ptprc | Receptor-type
tyrosine-protein phosphatase C | 0.015 | 10.626 |
| Q4G075 | Serpinb1a | Leukocyte elastase
inhibitor A | 0.001 | 9.051 |
| Q6PDV1 | Lyz1 | Lysozyme C-1
precursor | 0.002 | 9.049 |
| Q6IE17 | Stfa2l2 | Stefin-3 | 0.000 | 8.930 |
| Q5U2V4 | Plbd1 | Phospholipase
B-like 1 | 0.003 | 8.669 |
| Q91ZN1 | Coro1a | Coronin-1A | 0.001 | 8.199 |
| P14669 | Anxa3 | Annexin A3 | 0.008 | 8.100 |
| Q9R0D6 | Tcn2 | Transcobalamin-2
precursor | 0.014 | 7.286 |
| Q4QQV6 | Lsp1 | Lymphocyte specific
1 | 0.004 | 6.785 |
| P06768 | Rbp2 | Retinol-binding
protein 2 | 0.006 | 6.051 |
| Q5XI38 | Lcp1 | Plastin-2 | 0.001 | 5.841 |
| Q91W30 | Akr1b8 | Aldo-Keto Reductase
Family 1 Member B8 | 0.001 | 5.492 |
| Q63015 | Csap1 | Common salivary
protein 1 precursor | 0.001 | 5.454 |
| P31720 | C1qa | Complement C1q
subcomponent subunit A | 0.008 | 5.339 |
| G3V904 | Pld4 | Phospholipase
D4 | 0.005 | 4.857 |
| D4ADD7 | Glrx5 |
Glutaredoxin-related protein 5 | 0.002 | 4.782 |
| P22985 | Xdh | Xanthine
dehydrogenase/oxidase | 0.003 | 4.221 |
| P06866 | Hp | Haptoglobin
Haptoglobin alpha chain Haptoglobin beta chain | 0.002 | 3.945 |
| B2RYS9 | Trmt112 | Uncharacterized
protein | 0.016 | 3.827 |
| P23640 | Rab27a | Ras-related protein
Rab-27A | 0.017 | 3.774 |
| P06762 | Hmox1 | Heme oxygenase
1 | 0.008 | 3.769 |
| Q9WUQ4 | Slpi | Secretory leukocyte
peptidase inhibitor precursor | 0.015 | 3.710 |
| P07150 | Anxa1 | Annexin A1 | 0.003 | 3.449 |
| D3ZX79 | Ly6g6c | Lymphocyte antigen
6 complex G6C precursor | 0.018 | 3.236 |
| O88752 | Hbe1 | Hemoglobin, epsilon
1 | 0.029 | 3.211 |
| Q9R1T3 | Ctsz | Cathepsin Z | 0.002 | 3.195 |
| D3ZJH9 | Me2 | NAD-dependent malic
enzyme, mitochondrial | 0.034 | 2.926 |
| P05942 | S100a4 | S100 Calcium
Binding Protein A4 | 0.002 | 2.897 |
| Q5XJW6 | Cfh | Complement factor H
precursor | 0.008 | 2.891 |
| O54892 | Hk2 | Hexokinase-2 | 0.007 | 2.876 |
| Q6P7D4 | Cyp20a1 | Cytochrome P450
20A1 | 0.013 | 0.490 |
| D3ZWC6 | Sntb1 |
beta-1-syntrophin | 0.025 | 0.490 |
| Q62997 | Gfra1 | GDNF family
receptor alpha-1 | 0.043 | 0.488 |
| P02600 | Myl1 | Myosin light chain
1/3 | 0.011 | 0.482 |
| O35878 | Hspb2 | Heat shock protein
beta-2 | 0.005 | 0.481 |
| P17209 | Myl4 | Myosin light chain
4 | 0.004 | 0.475 |
| D4A8H3 | Uba6 | Ubiquitin-like
modifier-activating enzyme 6 | 0.031 | 0.471 |
| A1L1K3 | Anapc5 | Anaphase-promoting
complex subunit 5 | 0.046 | 0.470 |
| D3ZTW9 | Exog | Nuclease EXOG | 0.028 | 0.467 |
| D4A3D2 | Smyd1 | SET and MYND
domain-containing protein 1 | 0.004 | 0.465 |
| P04466 | Mylpf | Myosin regulatory
light chain 2 | 0.009 | 0.464 |
| P12847 | Myh3 | Myosin-3 | 0.007 | 0.461 |
| P13413 | Tnni1 | Troponin I | 0.001 | 0.460 |
| D4A4Y2 | Hsd17b14 |
17-beta-hydroxysteroid dehydrogenase
14 | 0.033 | 0.455 |
| P23928 | Cryab | Alpha-crystallin B
chain | 0.020 | 0.454 |
| Q7TNB2 | Tnnt1 | Troponin T | 0.002 | 0.451 |
| D3ZCD7 | Tp53rk | TP53-regulating
kinase | 0.004 | 0.450 |
| P00564 | Ckm | Creatine kinase
M-type | 0.037 | 0.450 |
| Q80W59 | Hrc | Sarcoplasmic
reticulum histidine-rich calcium-binding protein precursor | 0.019 | 0.445 |
| P50463 | Csrp3 | Cysteine and
glycine-rich protein 3 | 0.013 | 0.444 |
| Q5XIG1 | Ldb3 | Ldb3 protein | 0.017 | 0.443 |
| D3ZUB7 | Anapc4 | Anaphase-promoting
complex subunit 4 | 0.030 | 0.442 |
| Q64578 | Atp2a1 | ATPase, Ca++
transporting, cardiac muscle, fast twitch 1 | 0.032 | 0.442 |
| Q6P792 | Fhl1 | Four and a half LIM
domains protein 1 | 0.013 | 0.431 |
| Q8K4F2 | Alox15b | Arachidonate
15-lipoxygenase B | 0.025 | 0.428 |
| M0RBL8 | Tceal6 | Protein
LOC679974 | 0.003 | 0.427 |
| P51868 | Casq2 | Calsequestrin-2
precursor | 0.008 | 0.425 |
| B4F789 | Apobec2 | Probable
C->U-editing enzyme APOBEC-2 | 0.008 | 0.421 |
| P16290 | Pgam2 | Phosphoglycerate
mutase 2 | 0.014 | 0.418 |
| Q9Z2J4 | Nexn | Nexilin | 0.002 | 0.412 |
| Q9QYU4 | Crym |
Thiomorpholine-carboxylate
dehydrogenase | 0.017 | 0.411 |
| D3ZUQ0 | Rilpl1 | RILP-like protein
1 | 0.006 | 0.409 |
| D4A2H6 | Rbfox3 | Fox-1 homolog
C | 0.037 | 0.408 |
| D3ZVM5 | Hspa12b | Heat shock 70 kDa
protein 12B | 0.038 | 0.406 |
| O54747 | Pold1 | DNA polymerase
delta catalytic subunit | 0.001 | 0.403 |
| P52481 | Cap2 | Adenylyl
cyclase-associated protein 2 | 0.007 | 0.396 |
| Q63544 | Sncg |
Gamma-synuclein | 0.004 | 0.381 |
| Q496Z5 | Prph | Peripherin | 0.001 | 0.376 |
| P07483 | Fabp3 | Fatty acid-binding
protein, heart | 0.011 | 0.357 |
| P23565 | Ina |
Alpha-internexin | 0.005 | 0.332 |
| D4ADS4 | Mgst3 | Microsomal
glutathione S-transferase 3 | 0.024 | 0.328 |
| P19527 | Nefl | Neurofilament light
polypeptide | 0.006 | 0.326 |
| P12839 | Nefm | Neurofilament
medium polypeptide | 0.004 | 0.326 |
| B2RZ77 | Dpt | Dermatopontin
precursor | 0.024 | 0.320 |
| Q6AYG3 | Prune | Prune homolog | 0.017 | 0.320 |
| G3V7K1 | Myom2 | Myomesin 2 | 0.025 | 0.299 |
| G3V6V5 | Atp1b4 | Protein ATP1B4 | 0.005 | 0.272 |
| Q9Z2Z8 | Dhcr7 |
7-dehydrocholesterol reductase | 0.000 | 0.270 |
| P19633 | Casq1 |
Calsequestrin-1 | 0.021 | 0.201 |
| D3ZX18 | Myoz2 | Myozenin-2 | 0.001 | 0.198 |
| Q812D3 | Ppil3 | Peptidyl-prolyl
cis-trans isomerase-like 3 | 0.000 | 0.179 |
Discussion
In recent decades, various animal models of
congenital cleft lip have been successfully established through
surgical induction (4,20). It has been suggested that
intrauterine cleft lip repair can effectively improve this defect
and reduce the impact of scars on normal facial development after
birth. Thus, it also provides a new way for the effective repair of
congenital cleft lips. In the present study, pregnant SD rats were
used to establish a fetal rat model of cleft lip wound at two time
points, GA16.5 and GA18.5. The different pregnancy models were
induced by using two different repair methods of a cleft lip wound
of the fetus (21,22). The exact gestational age is
particularly important for the results of the repair of cleft lip
in the fetal rats. Thus, the use of a rat model provides an added
advantage in that the exact time of conception can be replicated,
thereby minimizing differences between groups.
The present findings confirmed the hypothesis that
fetal rat defects can be regenerated during early pregnancy without
scar formation (23). It was also
demonstrated that fetal rat defects could not be completely
regenerated in late pregnancy and resulted in scarring (24). Furthermore, the expression of
pro-inflammatory factors was different between the two groups.
However, these observations were only made at one time point (72 h)
after constructing cleft lip wounds in fetal rats. Future studies
are needed to examine samples collected at different time points
following surgery. Another shortcoming of this study entails the
lack of comparison between the cleft lip wound repairs of fetal
rats at different ages, such as the fetus in the early stages of
pregnancy, or in newborn and/or adult rats. Label-free quantitative
proteomics were used to examine proteins that play important roles
in the postoperative repair process of fetal cleft lip. Protein
expression was examined in four groups of samples. In addition,
bioinformatics analysis was conducted to identify potential
biological markers, providing a theoretical reference and
methodological basis for the examination of relevant mechanisms
underlying fetal intrauterine scar repair. However, further studies
are required to determine whether any one protein or several
proteins, plays a key role in wound healing.
Smad4 belongs to the family of Smad proteins and is
a common mediator in the signal transduction processes of the TGF-β
family (25). TGF-β expression can
lead to fibroblast proliferation and ECM deposition (26,27).
The present findings indicated that the mRNA and protein expression
levels of Smad4 were downregulated in the scar-free repair
group.
Furthermore, the mRNA and protein expression levels
of Fabp5 were upregulated in the scar formation group. Therefore,
it may be hypothesized that Fabp5 could be involved in the fibrosis
of the fetal cleft lip wound, which may be mediated by the TGF-β
signaling pathway (28–30).
S100a4 is a member of the S100 calcium-binding
protein family, and its expression is associated with various
non-neoplastic diseases, such as chronic obstructive pulmonary
disease and cardiac hypertrophy (31–35).
The present study demonstrated that the mRNA and protein expression
levels of S100a4 were upregulated in the scar formation group,
which may be associated with scar repair of fetal rat cleft lip
wounds.
S100a8 is also a member of the S100 calcium-binding
protein family (36–42). mRNA and protein expression levels of
S100a8 were significantly upregulated in the scar repair group in
the present study, indicating a potential role for S100a8 in the
process of fetal cleft lip wound healing.
Current reports frequently associate S100a9, a
member of the calcium-binding protein family S100, with infectious
diseases, immune diseases and tumors, such as non-small cell lung
adenocarcinoma (43–45). mRNA and protein expression levels of
S100a9 were significantly upregulated in the scar formation group.
Therefore, we speculated that S100a9 may play an important role in
the process of fetal wound healing. However, whether the reduced
expression levels of Fabp5, S100a4, S100a8 and S100a9 in the third
trimester of pregnancy would reduce or worsen scar formation
remains unclear. Further functional testing and regulatory studies
are required to confirm the role of these five differentially
expressed proteins in fetal wound repair.
The cleft lip is a very common congenital condition
that often leaves life-long scarring. The present study identified
five differentially expressed proteins, namely Smad4, Fabp5,
S100a4, S100a8 and S100a9, that may be potential biomarkers of the
scarless repair process in fetal rat cleft lip wounds. These
findings may facilitate the discovery of new clinical targets for
the prevention and treatment of scars. However, the role of these
proteins in fetal wound repair and potential underlying mechanisms
require further examination.
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant from the
National Science and Basic Resources Survey Special Foundation,
China (grant no. 2017FY101204), the Technology Innovation Guide
Program of Hunan Province, China (grant no. 2017SK50124), the
Science and Technology Major Project of Hunan Province, China
(grant no. 2019SK1010) and the Science and Technology Major Project
of Hunan Province, China (grant no. 2019SK1015).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YY, FH and JZho conceived and designed research. YY
and FH performed animal experiments and staining. YY, HL, PJ, FH,
JY, KG, SH and JZha performed PCR and label-free quantification
PRM. YY, FH, JC, JY, ZC, AW and JZha analyzed data. YY and FH
prepared figures. YY drafted the manuscript. FH and JZho edited and
revised the manuscript. YY, FH, HL AW, PJ and JZho approved the
final version of the manuscript. FH and JZho confirmed the
authenticity of all of the raw data. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
This study followed the regulations stipulated by
the People's Republic of China regarding the Management of
Experimental Animals and was approved by The Animal Experiment
Management and Medical Ethics Sub-committee of The Third Xiangya
Hospital of Central South University, Hunan, China (approval no.
2014-S168).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
VanKoevering KK, Morrison RJ, Prabhu SP,
Torres MF, Mychaliska GB, Treadwell MC, Hollister SJ and Green GE:
Antenatal three-dimensional printing of aberrant facial anatomy.
Pediatrics. 136:e1382–e1385. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Burrington JD: Wound healing in the fetal
lamb. J Pediatr Surg. 6:523–528. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Beanes SR, Hu FY, Soo C, Dang CM, Urata M,
Ting K, Atkinson JB, Benhaim P, Hedrick MH and Lorenz HP: Confocal
microscopic analysis of scarless repair in the fetal rat: Defining
the transition. Plast Reconstr Surg. 109:160–170. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Walmsley GG, Hu MS, Hong WX, Maan ZN,
Lorenz HP and Longaker MT: A mouse fetal skin model of scarless
wound repair. J Vis Exp. 16:522972015.
|
|
5
|
Dang CM, Beanes SR, Lee H, Zhang X, Soo C
and Ting K: Scarless fetal wounds are associated with an increased
matrix metalloproteinase-to-tissue-derived inhibitor of
metalloproteinase ratio. Plast Reconstr Surg. 111:2273–2285. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Longaker MT, Whitby DJ, Adzick NS,
Crombleholme TM, Langer JC, Duncan BW, Bradley SM, Stern R,
Ferguson MW and Harrison MR: Studies in fetal wound healing, VI.
Second and early third trimester fetal wounds demonstrate rapid
collagen deposition without scar formation. J Pediatr Surg.
25:63–69. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lorenz HP, Whitby DJ, Longaker MT and
Adzick NS: Fetal wound healing. The ontogeny of scar formation in
the non-human primate. Ann Surg. 217:391–396. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cass D, Bullard KM, Sylvester KG, Yang EY,
Longaker MT and Adzick NS: Wound size and gestational age modulate
scar formation in fetal wound repair. J Pediatr Surg. 32:411–415.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Longaker MT and Adzick NS: The biology of
fetal wound healing: A review. Plast Reconstr Surg. 87:788–798.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Armstrong JR and Ferguson MW: Ontogeny of
the skin and the transition from scar-free to scarring phenotype
during wound healing in the pouch young of a marsupial, Monodelphis
domestica. Dev Biol. 169:242–260. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stern M, Dodson TB, Longaker MT, Lorenz
HP, Harrison MR and Kaban LB: Fetal cleft lip repair in lambs:
Histologic characteristics of the healing wound. Int J Oral
Maxillofac Surg. 22:371–374. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Longaker MT, Dodson TB and Kaban LB: A
rabbit model for fetal cleft lip repair. J Oral Maxillofac Surg.
48:714–719. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Oberg KC, Evans ML, Nguyen T, Peckham NH,
Kirsch WM and Hardesty RA: Intrauterine repair of surgically
created defects in mice (lip incision model) with a microclip:
Preamble to endoscopic intrauterine surgery. Cleft Palate Craniofac
J. 32:129–137. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hu F, Yan Y, Wang CW, Liu Y, Wang JJ, Zhou
F, Zeng QH, Zhou X, Chen J, Wang AJ and Zhou JD: Article effect and
mechanism of ganoderma lucidum polysaccharides on human fibroblasts
and skin wound healing in mice. Chin J Integr Med. 25:203–209.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xue YN, Yan Y, Chen ZZ, Chen J, Tang FJ,
Xie HQ, Tang SJ, Cao K, Zhou X, Wang AJ and Zhou JD: LncRNA TUG1
regulates FGF1 to enhance endothelial differentiation of
adipose-derived stem cells by sponging miR-143. J Cell Biochem.
120:19087–19097. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guo H, Chen T, Liang Z, Fan L, Shen Y and
Zhou D: iTRAQ and PRM-based comparative proteomic profiling in
gills of white shrimp Litopenaeus vannamei under copper stress.
Chemosphere. 263:1282702021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen PS, Li YP and Ni HF: Morphology and
evaluation of renal fibrosis. Adv Exp Med Biol. 1165:17–36. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Luo Y, Yan Y, Zhang S and Li Z:
Computational approach to investigating key GO terms and KEGG
pathways associated with CNV. Biomed Res Int. 2018:84068572018.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dong X, Landford WN, Hart J, Risolino M,
Kaymakcalan O, Jin J, Toyoda Y, Ferretti E, Selleri L and Spector
JA: Toward microsurgical correction of cleft lip ex utero through
restoration of craniofacial developmental programs. Plast Reconstr
Surg. 140:75–85. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stelnicki EJ, Lee S, Hoffman W, Lopoo J,
Foster R, Harrison MR and Longaker MT: A long-term,
controlled-outcome analysis of in utero versus neonatal cleft lip
repair using an ovine model. Plast Reconstr Surg. 104:607–615.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Harling TR, Stelnicki EJ, Hedrick MH and
Longaker MT: In utero models of craniofacial surgery. World J Surg.
27:108–116. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mast BA, Haynes JH, Krummel TM, Diegelmann
RF and Cohen IK: In vivo degradation of fetal wound hyaluronic acid
results in increased fibroplasia, collagen deposition, and
neovascularization. Plast Reconstr Surg. 89:503–509. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Frantz FW, Diegelmann RF, Mast BA and
Cohen IK: Biology of fetal wound healing: Collagen biosynthesis
during dermal repair. J Pediatr Surg. 27:945–949. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wilgus TA: Regenerative healing in fetal
skin: A review of the literature. Ostomy Wound Manage. 53:16–33.
2007.PubMed/NCBI
|
|
25
|
Hu HH, Chen DQ, Wang YN, Feng YL, Cao G,
Vaziri ND and Zhao YY: New insights into TGF-beta/Smad signaling in
tissue fibrosis. Chem Biol Interac. 292:76–83. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Honardoust D, Ding J, Varkey M, Shankowsky
HA and Tredget EE: Deep dermal fibroblasts refractory to migration
and decorin-induced apoptosis contribute to hypertrophic scarring.
J Burn Care Res. 33:668–677. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu C, Jiang J, Boye A, Jiang Y and Yang Y:
Compound Astragalus and Salvia miltiorrhiza extract suppresses
rabbits' hypertrophic scar by modulating the TGF-β/Smad signal.
Dermatology. 229:363–368. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Furuhashi M, Ogura M, Matsumoto M, Yuda S,
Muranaka A, Kawamukai M, Omori A, Tanaka M, Moniwa N, Ohnishi H, et
al: Serum FABP5 concentration is a potential biomarker for residual
risk of atherosclerosis in relation to cholesterol efflux from
macrophages. Sci Rep. 7:2172017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yeung DC, Wang Y, Xu A, Cheung SC, Wat NM,
Fong DY, Fong CH, Chau MT, Sham PC and Lam KS: Epidermal
fatty-acid-binding protein: A new circulating biomarker associated
with cardio-metabolic risk factors and carotid atherosclerosis. Eur
Heart J. 29:2156–2163. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Song J, Zhang H, Wang Z, Xu W, Zhong L,
Cao J, Yang J, Tian Y, Yu D, Ji J, et al: The role of FABP5 in
radiation-induced human skin fibrosis. Radiat Res. 189:177–186.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fei F, Qu J, Li C, Wang X, Li Y and Zhang
S: Role of metastasis-induced protein S100A4 in human non-tumor
pathophysiologies. Cell Biosci. 7:642017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Grotterød I, Maelandsmo GM and Boye K:
Signal transduction mechanisms involved in S100A4-induced
activation of the transcription factor NF-kappaB. BMC Cancer.
10:2412010. View Article : Google Scholar
|
|
33
|
Schneider M, Kostin S, Strøm CC, Aplin M,
Lyngbaek S, Theilade J, Grigorian M, Andersen CB, Lukanidin E,
Lerche Hansen J and Sheikh SP: S100A4 is upregulated in injured
myocardium and promotes growth and survival of cardiac myocytes.
Cardiovasc Res. 75:40–50. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tomcik M, Palumbo-Zerr K, Zerr P, Avouac
J, Dees C, Sumova B, Distler A, Beyer C, Cerezo LA, Becvar R, et
al: S100A4 amplifies TGF-β-induced fibroblast activation in
systemic sclerosis. Ann Rheum Dis. 74:1748–1755. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhao YX, Ho CK, Xie Y, Chen YH, Li HZ,
Zhang GY and Li QF: Calcimycin suppresses S100A4 expression and
inhibits the stimulatory effect of transforming growth factor β1 on
Keloid fibroblasts. Ann Plast Surg. 81:163–169. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Donato R: Intracellular and extracellular
roles of S100 proteins. Microsc Res Tech. 60:540–551. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lin H, Andersen GR and Yatime L: Crystal
structure of human S100A8 in complex with zinc and calcium. BMC
Struct Biol. 16:82016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bouzidi F and Doussiere J: Binding of
arachidonic acid to myeloid-related proteins (S100A8/A9) enhances
phagocytic NADPH oxidase activation. Biochem Biophys Res Commun.
325:1060–1065. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gebhardt C, Németh J, Angel P and Hess J:
S100A8 and S100A9 in inflammation and cancer. Biochem Pharmacol.
72:1622–1631. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Basso D, Bozzato D, Padoan A, Moz S,
Zambon CF, Fogar P, Greco E, Scorzeto M, Simonato F, Navaglia F, et
al: Inflammation and pancreatic cancer: Molecular and functional
interactions between S100A8, S100A9, NT-S100A8 and TGFβ1. Cell
Commun Signal. 26:12–20. 2014.PubMed/NCBI
|
|
41
|
Shabani F, Farasat A, Mahdavi M and Gheibi
N: Calprotectin (S100A8/S100A9): A key protein between inflammation
and cancer. Inflamm Res. 67:801–812. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yaundong L, Dongyan W, Lijun H and Zhibo
X: Effects of downregulation of S100A8 protein expression on cell
cycle and apoptosis of fibroblasts derived from hypertrophic scars.
Aesthet Surg J. 34:160–167. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hessian PA, Edgeworth J and Hogg N: MRP-8
and MRP-14, two abundant Ca (2+)-binding proteins of neutrophils
and monocytes. J Leukoc Biol. 53:197–204. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Markowitz J and Carson WE III: Review of
S100A9 biology and its role in cancer. Biochim Biophys Acta.
1835:100–109. 2013.PubMed/NCBI
|
|
45
|
Zhong A, Xu W, Zhao J, Xie P, Jia S, Sun
J, Galiano RD, Mustoe TA and Hong SJ: S100A8 and S100A9 are induced
by decreased hydration in the epidermis and promote fibroblast
activation and fibrosis in the dermis. Am J Pathol. 186:109–122.
2016. View Article : Google Scholar : PubMed/NCBI
|