Introduction
Osteoarthritis (OA), a degenerative disease of
joints, is the most common cause of musculoskeletal disability
(1,2). Various factors, such as age, genetics,
adiposis and sex, are involved in the development of osteoarthritis
(3). Destroyed cartilage structure
and loss of function are the central hallmarks of OA (4). Although studies have revealed
potential mechanisms involved in the progression of OA, its
pathological etiology is unknown. Moreover, there are no effective
therapeutic options that can prevent disease progression (5,6). OA is
associated with degradation of matrix cartilage and chondrocytes,
which are the only type of cell in articular cartilage (7). Extracellular matrix (ECM) molecules
are involved in maintaining the structure and function of regular
cartilage, whose components include proteoglycans and collagen,
produced by chondrocytes (8).
Articular cartilage degradation is initiated by several
inflammatory cytokines (IL-6, TNF-α, prostaglandin 2) and reactive
oxygen species (ROS) that induce oxidative stress (9). Excessive chondrocyte apoptosis and
senescence occur due to ROS accumulation (10). Therefore, inhibiting chondrocyte
apoptosis and senescence is a potential strategy for preventing
articular cartilage degradation in OA.
Endoplasmic reticulum (ER), which is the first site
in the secretory pathway, synthesize and fold proteins (11). ER regulate the balance between
pathological and physiological conditions (12–14).
The ER stress (ERS) response enhances cell survival by decreasing
the load of unfolded proteins. However, cells undergo apoptosis
when ERS is overwhelming (15).
ERS-associated apoptosis is an important signaling pathway during
apoptosis (16). It has been
demonstrated that ERS signaling in this pathway promotes repair of
the unfolded protein response (UPR) of the ER (17). UPR serves a critical role in cell
growth regulation, differentiation and apoptosis (18). ERS has been shown to stimulate the
C/EBP-homologous protein (CHOP) pathway while activating
glucose-regulated protein 78 (GRP78) (19). The Bcl-2 protein family acts against
apoptosis by inhibiting apoptotic mediators, such as Bax and
Cytochrome c (20). Studies have
reported that ER and mitochondrial pathways regulate apoptosis
during rat intervertebral disc degeneration (21,22).
These findings show the importance of ER and mitochondria in
chondrocytes.
There are seven known members of mammalian Sirtuins
(SIRTs). Among them, SIRT1 regulates multiple functions in
metabolic syndrome, oxidative stress, inflammation and aging
(23–25). Li et al (26) reported that sulforaphane (SFN) can
downregulate ER-induced apoptosis protein expression levels in a
rat model of hypoxia/reoxygenation injury by activating SIRT1.
Activated SIRT1 inhibits oxidative damage and inflammation
(26) and is therefore a
therapeutic target for OA via regulating mitochondrial biogenesis
(27). Feng et al (28) reported that curcumin exerts a
protective effect against OA by activating SIRT1.
SFN (a type of dietary isothiocyanate) is primarily
found in cruciferous vegetables (29). It exhibits various pharmacological
properties, including anti-cancer, anti-inflammatory and
anti-oxidative stress (30). SFN
exerts a protective effect during post-ischemic cardiac injury
(31). Moreover, it exerts
cardioprotective effects by activating SIRT1 and suppressing ER
stress (32). Davidson et al
(33) reported that SFN inhibits
inflammation via the NF-κB signaling pathway in OA mice. Moreover,
they found that SFN suppresses IL-1/NF-κB and Wnt3a/T cell
factor/lymphoid enhancer factor signaling while enhancing
TGFβ/SMAD2/3 and bone morphogenetic protein 6/SMAD1/5/8 signaling
in IL-1/oncostatin M-induced chondrocytes (34). However, its anti-apoptotic effects
during OA have not been established. The aim of the present study
was to elucidate the role of SFN in apoptosis, including its
specific mechanisms of action, in
H2O2-treated chondrocytes. The effect of SFN
on cartilage degeneration was investigated in a mouse model of
OA.
Materials and methods
Reagents and antibodies
SFN (purity ≥98%), Safranin O and toluidine blue
stain were obtained from Beyotime Institute of Biotechnology;
collagenase type II, H2O2 and DMSO were
bought from Sigma-Aldrich (Merck KGaA). BCA protein assay kit was
obtained from Beyotime Institute of Biotechnology; primary
antibodies against GRP78, CHOP and GAPDH were obtained from Wuhan
Sanying Biotechnology; primary antibodies against Bax, Bcl-2,
Cleaved caspase-3 and SIRT1 were purchased from Cell Signaling
Technology, Inc.; In Situ Cell Death Detection kit was obtained
from Roche Diagnostics; secondary antibodies (cat. no. SA00001-14;
Wuhan Sanying Biotechnology), including Goat Anti-Rabbit IgG (cat.
no. B900210; Wuhan Sanying Biotechnology) and Alexa
Fluor® 488-labeled goat anti-rabbit IgG (H+L) secondary
antibody (cat. no. SA00009-3; Wuhan Sanying Biotechnology), were
obtained from Jackson ImmunoResearch Laboratories, Inc.;
Cell-Counting Kit-8 (CCK-8) was purchased from Dojindo Molecular
Technologies, Inc. DMEM-F12 was purchased from Gibco (Thermo Fisher
Scientific, Inc.).
Primary chondrocyte isolation and
culture
A total of 10 C57BL/6 mice (5 males and 5 females;
age, 10 days; weight, 4 g; the Animal Center of the Chinese Academy
of Sciences) were euthanized by 100 mg/kg sodium pentobarbital. All
animal procedures were performed in accordance with the Guidelines
for Care and Use of Laboratory Animals of Wenzhou Medical
University (35) and experiments
were approved by the Animal Ethics Committee of Wenzhou Medical
University. The knee cartilage of mice were collected carefully
under aseptic conditions using a dissecting microscope, and tissue
was treated with 2 mg/ml (0.1%) collagenase II for 4 h at 37°C. The
digested tissue was centrifuged (800 × g) for 5 min at 37°C. Then,
chondrocytes (5×104 cells/cm2) were seeded
into culture flasks. Cells were cultured in DMEM/F12 supplemented
with 10% fetal bovine serum and 1% antibiotics
(penicillin/streptomycin) and incubated in an atmosphere of 5%
CO2 at 37°C. The culture medium was replaced every 2–3
days. When 80–90% confluency was attained, cells were fused and
0.25% trypsin-EDTA solution was used to subculture the
chondrocytes. In order to avoid phenotypic loss, chondrocytes from
the first and second channels were used.
Cell viability assay
CCK-8 assay was used to evaluate chondrocyte
viability according to the manufacturer's instructions. First,
chondrocytes were incubated in 96-well plates (8,000 cells/well)
for 24 h. Then, they were treated with a concentration gradient
(0.0, 12.5, 25.0, 50.0, 100.0 and 200.0 µM) of SFN for 24 h and 48
h. Finally, 10 mol/l CCK-8 solution was added to each well for 2 h
at 37°C, after which optical density was spectrophotometrically
measured at 450 nm (Thermo Fisherr Scientific, Inc.).
Intracellular ROS production
assay
Intracellular ROS levels were detected using the
HDCFDA Probe Assay kit (Beyotime Institute of Biotechnology),
according to the manufacturer's instructions. Intracellular ROS
generation was measured at 485 nm (excitation) and 535 nm
(emission) using a microplate reader (Bio-Rad Laboratories,
Inc.).
Western blot analysis
In order to obtain total proteins from chondrocytes,
RIPA lysis buffer (Beyotime Institute of Biotechnology) was added
to 1 mM PMSF and kept on ice for 10 min, after which the mixture
was centrifuged at 1,000 × g for 15 min at 4°C. BCA protein
detection kit was used to estimate protein concentration. Then, 40
ng protein was separated by SDS-PAGE (10%) and transferred to
polyvinylidene fluoride membrane. Membranes were blocked using 5%
skimmed milk for 2 h at 37°C and detected overnight at 4°C with the
following antibodies: CHOP (1:1,000; cat. no. 15204-1-AP; Wuhan
Sanying Biotechnology), GRP78 (1:1,000; cat. no. 11587-1-AP; Wuhan
Sanying Biotechnology), Bcl-2 (1:1,000; cat. no. 15071; Cell
Signaling Technology, Inc.), Bax (1:1,000; cat. no. 5023; Cell
Signaling Technology, Inc.), Cleaved caspase-3 (1:1,000; cat. no.
9661; Cell Signaling Technology, Inc.), SIRT1 (1:500; cat. no.
2493; Cell Signaling Technology, Inc.) and GAPDH (1:1,000; cat. no.
10494-1-AP; Wuhan Sanying Biotechnology).
The membrane was washed using TBS-Tween-20 (TBST; 5%
Tween-20) and incubated with the corresponding secondary antibodies
(1:1,000; cat. no. SA00001-14; Wuhan Sanying Biotechnology) for 2 h
at room temperature. After being washed three times using TBST, the
blots were visualized using electrochemiluminescence plus reagent
(Invitrogen; Thermo Fisherr Scientific, Inc.). Image Lab 3.0
software (Bio-Rad Laboratories, Inc.) was used to measure the
intensity of each band.
TUNEL assay
Apoptotic chondrocytes were measured by TUNEL
staining using an In Situ Cell Death Detection kit, according to
the manufacturer's instructions. The chondrocytes
(1×105) were seeded in a 6-well plate and treated with
SFN (50 µM) or H2O2 (50 µM) for 24 h at 37°C,
fixed in 4% paraformaldehyde for 45 min at 37°C, incubated with
0.5% Triton X-100 for 15 min at 37°C and washed using PBS for 5
min. Finally, cells were stained using the In Situ Cell
Death Detection kit for 60 min at 65°C, after which the nuclei were
stained with DAPI for 1 min at 37°C (Beijing Solarbio Science &
Technology Co., Ltd.). In total, 25 fields of each slide were
randomly selected and images were observed using a fluorescence
microscope (magnification, ×100; scale bar, 50 µm; Olympus
Corporation).
Small interfering (si)RNA transfection
for 24 h
Double-stranded siRNA for mouse SIRT1 gene silencing
was purchased from Abcam. The SIRT1 siRNA sequence was as follows:
5′-UUGGGAUUCACGCAUAGGAGCACUG-3′. Cells (1×105) were
seeded into a 6-well plate, incubated for 24 h at 37°C and
transfected with negative control [non-specific non-targeting siRNA
(scramble); Abcam] or SIRT1 siRNA duplexes for 36 h at 50 nM using
Lipofectamine® 2000 siRNA transfection reagent (Thermo
Fisherr Scientific, Inc.), according to the manufacturer's
instructions. The chondrocytes were serum-starved overnight
followed by incubation at 37°C with SFN (50 µM) for 24 h. After 24
h, the interference efficiency of the siRNA was evaluated by
reverse transcription-quantitative PCR (RT-qPCR).
RT-qPCR
Total RNA was extracted from cells using
TRIzol® reagent (Invitrogen; Thermo Fisherr Scientific,
Inc.). The concentration and purity of RNA was evaluated by
spectrometry at 260 and 280 nm. The synthesis of cDNA was performed
using the PrimeScript™ RT reagent kit [cat. no. 2312, Hangzhou
Multisciences (Lianke) Biotech Co., Ltd.]. qPCR was performed using
SYBR Green Real-Time PCR Master mix (Thermo Fisherr Scientific,
Inc.) according to the manufacturer's protocol. The amplification
conditions were as follows: SIRT1, 45 sec at 94°C followed by 30
cycles of 30 sec at 94°C, 30 sec at 52°C and 60 sec at 72°C; GAPDH,
45 sec at 94°C followed by 30 cycles of 30 sec at 94°C, 30 sec at
58°C and 60 sec at 72°C. Primers sequences, designed by
Primer-Express V3.0 (Thermo Fisherr Scientific, Inc.), were as
follows: SIRT1 forward, 5′-CTCTGAAAGTGAGACCAGTAGC-3′ and reverse,
5′-TGTAGATGAGGCAAAGGTTCC-3′ (product, 213 bp); and GAPDH forward,
5′-TCTTGCTCAGTGTCCTTGC-3′ and reverse, 5′-CTTTGTCAAGCTCATTTCCTGG-3′
(product, 457 bp). Relative gene expression was analyzed using the
2−ΔΔCq method (36).
Immunofluorescence
Cells were rinsed using PBS and fixed in 4%
paraformaldehyde for 15 min at 37°C after which they were washed
three times using PBS. Then, they were treated with 0.1% Triton
X-100 diluted in PBS for 15 min at room temperature. Next,
chondrocytes were blocked using 10% goat serum (cat. no. SL038;
Beijing Solarbio Science & Technology Co., Ltd.; dissolved in
PBS) for 4 h at 37°C and incubated at 4°C overnight in the presence
of primary antibodies against collagen II (1:300; cat. no.
28459-1-AP; Wuhan Sanying Biotechnology). Following incubation,
cells were exposed to Alexa Fluor® 594-labelled
conjugated secondary antibodies (1:400; cat. no. IC1051T; R&D
Systems, Inc.) for 1.5 h. Finally, they were exposed to DAPI (10
µg/ml; 37°C) (Beyotime Institute of Biotechnology) for 1 min and
observed using a fluorescence microscope (magnification, ×200;
scale bar, 20 µm; Olympus Corporation). Fluorescence intensity was
assessed using ImageJ software 6.0 (National Institutes of
Health).
Immunohistochemical assay
The paraffin-embedded sections (6 µm) were
deparaffinized in xylene for 15 min at 37°C and rehydrated by
incubation in series of graded ethanol (100, 95, 85, 75 and 0%) for
5 min at 37°C and endogenous peroxidase. Then, sections were
treated with 3% (v/v) hydrogen peroxide for 10 min at 37°C. Antigen
retrieval of the sections was performed using 0.4% pepsin (Sangon
Biotech Co., Ltd.) in 5 mM HCl at 37°C for 20 min. Then, sections
were incubated in the presence of 10% BSA (cat. no. ST025; Beyotime
Institute of Biotechnology) for 45 min at room temperature, after
which they were treated with primary antibodies against SIRT1
(1:500; cat. no. 2493; Cell Signaling Technology, Inc.) overnight
at 4°C. Finally, they were treated with horseradish
peroxidase-conjugated secondary antibodies (1:1,000; cat. no.
SA00001-14; Wuhan Sanying Biotechnology) for 3 h at 4°C. Following
incubation at room temperature for 1 h, the slices were rinsed (5
min, 37°C), stained using a Metal Enhanced DAB Substrate kit (2
min, 37°C; cat. no. DA1015; Beijing Solarbio Science &
Technology Co., Ltd.), dehydrated (5 min, 37°C), mounted and
examined under a microscope (magnification, ×40; CX41; Olympus
Corporation). Quantitative analysis was performed using Image-Pro
Plus 6.0 software (Media Cybernetics, Inc.).
Animals
A total of 45 C57BL/6 male wild-type mice (age, 10
weeks; weight, 40 g) were obtained from the Animal Center of the
Chinese Academy of Sciences (Shanghai, China). The protocol for
animal care and use conformed to The Guide for the Care and Use of
Laboratory Animals of the National Institutes of Health published
by the National Institutes of Health (NIH Publication No. 85-23,
revised 1996) (37) and was
approved by the Animal Care and Use Committee of Wenzhou Medical
University. Experimental OA mice were established by
destabilization of the medial meniscus (DMM) as previously
described (38). Mice were housed
under 12-h light/dark cycles and constant temperature (21–23°C) and
humidity (50–60%). Water and food were provided ad libitum.
Anesthesia was performed via intraperitoneal injection of 2% (w/v)
pentobarbital (40 mg/kg). Faint breathing, myasthenia, lack of
independent reaction, cyanosis or coma were considered to indicate
that mice were close to death; mice were euthanized by cervical
dislocation. The joint capsule was carved followed by transection
of the medial meniscotibial ligament of the right knee using
microsurgical scissors. During surgery, the lateral meniscotibial
ligament was always protected. As a control, the medial
meniscotibial ligament was not transected while arthrotomy was
performed in the left knee. Mice were randomly separated into three
groups (n=15/group): Control (sham-operated), DMM and DMM + SFN
[mice were intraperitoneally administered with SFN at 20 mg/kg body
weight (39,40)]. Parameters indicating the condition
of mice were observed daily, including fur brightness, food and
water intake, defecation and behavior. Furthermore, body weight was
measured each week.
Histopathologic analysis
The extent of cartilage degeneration and synovitis
in stained sections was assessed using light microscopy with the
Osteoarthritis Research Society International (OARSI) scoring
system, as previously described (41).
Statistical analysis
The data are presented as mean ± SD (n≥3).
Statistical analysis was performed using GraphPad Prism version 5.0
software (GraphPad Software, Inc.). Comparisons between groups were
performed using one-way ANOVA followed by Tukey's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Identification of mouse
chondrocytes
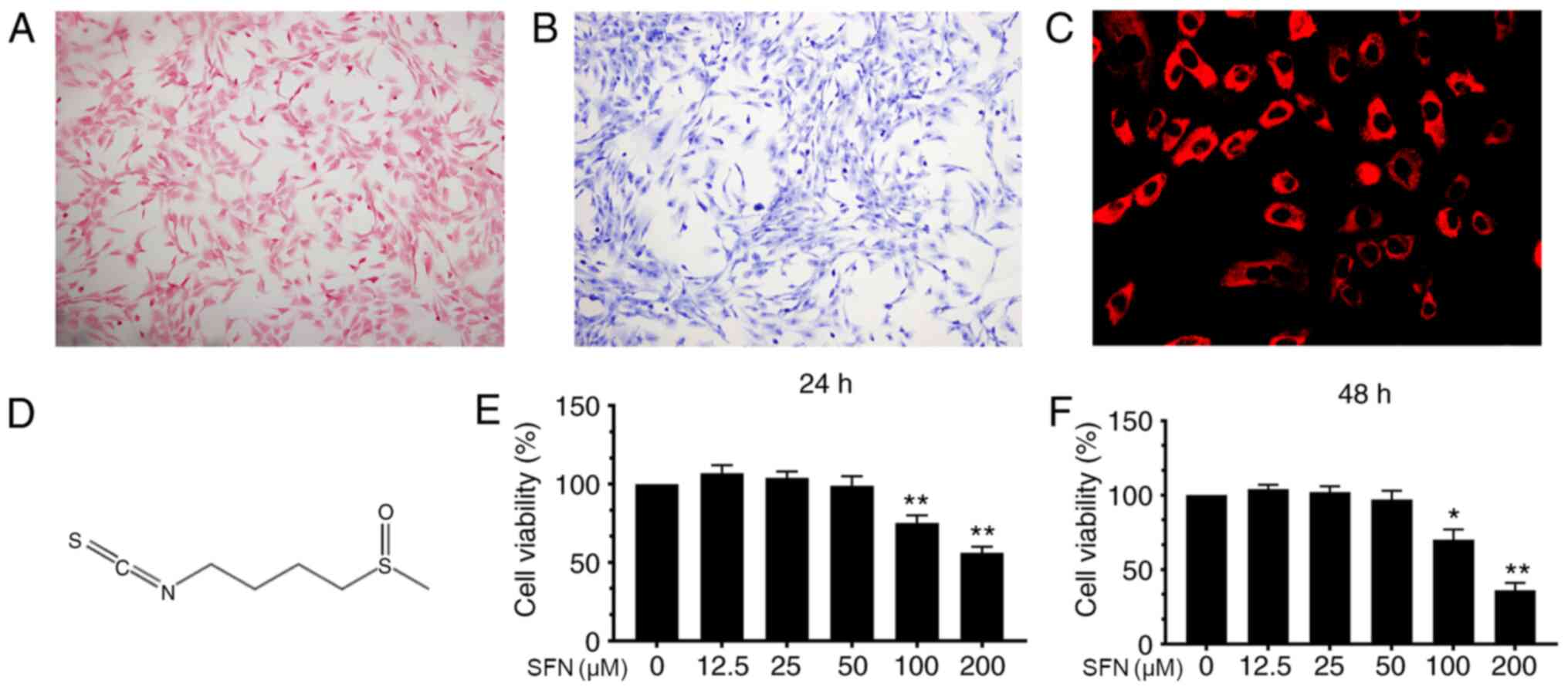
Safranin O and toluidine blue staining were used to
characterize mouse chondrocytes. Chondrocytes were stained red by
Safranin O and the cytoplasm was stained purple by toluidine blue
(Fig. 1A and B). In addition,
collagen II in chondrocyte cytoplasm was stained red by
immunofluorescence with no positive staining observed in the
nucleus (Fig. 1C). These results
imply that the cells isolated from mouse articular cartilage were
chondrocytes.
Cytotoxicity of SFN on mouse
chondrocytes
The chemical structure of SFN is shown in Fig. 1D. In order to determine the
cytotoxic effects of SFN, CCK-8 assay was performed. Cells were
incubated with increasing concentrations (0.0, 12.5, 25.0, 50.0,
100.0 and 200.0 µM) of SFN for 24 and 48 h. SFN exhibited cytotoxic
effects at ≥100 µM after 24 or 48 h but not <100 µM (Fig. 1E and F). Therefore, 50 µM was
selected for use in subsequent experiments.
Effects of SFN on apoptosis in
H2O2-treated chondrocytes
In order to determine the anti-apoptotic effect of
SFN on H2O2-induced chondrocytes, the
expression levels of Bax, Cleaved caspase-3 and Bcl-2 were
evaluated by western blot analysis. H2O2
elevated levels of Bax and Cleaved caspase-3, whereas Bcl-2 levels
were downregulated (Fig. 2A-D). SFN
downregulated levels of Bax and Cleaved caspase-3. Moreover, SFN
significantly activated SIRT1 (Fig. 2A
and E). These data indicate that SFN exerted an anti-apoptotic
effect in mouse chondrocytes.
SFN alleviates ERS and apoptosis of
H2O2-treated chondrocytes
In order to determine whether ERS in chondrocytes
was associated with the cytoprotective effects of SFN on cartilage,
cells were treated with H2O2, a typical ERS
inducer. Expression levels of ERS-associated factors GRP78 and CHOP
were measured. Western blotting assay exhibited increased
expression levels of these proteins in response to
H2O2; these effects were stopped by SFN
(Fig. 3A-C). Moreover, apoptosis of
cells in the H2O2 group was high compared
with the control group. SFN treatment decreased cell apoptosis
(Fig. 3D and E). Furthermore,
H2O2 induced ROS production, which was
inhibited by SFN (Fig. 3F).
SFN pretreatment enhances SIRT1
activity in H2O2-treated chondrocytes
In order to confirm the aforementioned results,
SIRT1 siRNA was used to detect the potential mechanism of SFN on
H2O2-stimulated chondrocytes. The mRNA
expression levels of SIRT1 were significantly lower in
SIRT1-silenced cells than in control cells (Fig. 4F). SIRT1 levels were elevated in the
H2O2 + SFN group but suppressed in the SIRT1
siRNA transfection group (Fig. 4A and
E). Moreover, Bcl-2 levels were suppressed in the SIRT1 siRNA
transfection group whereas the expression levels of Bax and Cleaved
caspase-3 increased significantly (Fig.
4A-D). These results demonstrated the anti-apoptotic effect of
SFN, which was mediated by activation of SIRT1.
SFN ameliorates OA development in a
DMM mouse model
The chondroprotective effect of SFN on OA was
investigated in vivo. A DMM mouse model was established,
followed by intraperitoneal injection of 20 mg/kg SFN dissolved in
DMSO every 2 days for 8 weeks. Safranin O staining was used to
evaluate the effect of SFN on the development of OA. Compared with
the sham group, the surface of the cartilage was notably destroyed
in the DMM group (Fig. 5A). The
degradation level of the cartilage matrix in the SFN group was
lower than the OA but higher than the sham group. The OARSI scores
in the SFN group were lower than those of the DMM group (Fig. 5B). Moreover, synovitis score in the
DMM group was higher than in the sham group; SFN reversed this
effect (Fig. 5A and C). In order to
investigate the effect of SFN on SIRT1 expression in vivo,
immunohistochemical staining for SIRT1 was performed (Fig. 5D and E). Compared with the DMM
group, significantly more SIRT1-positive chondrocytes were detected
in the DMM + SFN group. These results imply that SFN exhibited
chondrocyte-protective effects during the progression of OA.
Discussion
OA is a chronic joint degradation disease that is
characterized by long-term pain and joint limitation (42). Oral or topical non-steroidal
anti-inflammatory drugs (such as ibuprofen and diclofenac sodium)
are the primary therapeutic options for OA but only relieve
clinical symptoms (3). These drugs
are also associated with side effects, such as heart attack and
stroke (43). Nonsteroidal
anti-inflammatory drugs can only delay the progression of OA and
surgery is the recommended option (44). Therefore, an agent that prevents OA
progression, accompanied by fewer side effects, would be a
potential therapeutic option for OA. Previous studies aimed to
evaluate the efficacy of anti-apoptotic compounds, which exhibit
few side effects (45,46). Biochemical and biomechanical factors
(such as ROS) induce chondrocyte apoptosis, as well as an imbalance
between catabolism and anabolism of the ECM (47). Pharmacological administration of
compounds (such as vitamin C and Glutathione) with the ability to
regulate genetic mechanisms involved in apoptosis exhibit
beneficial effects against OA development in vitro and in
vivo (48). Therefore, the
mechanism involved in chondrocyte apoptosis may be a potential
therapeutic target for OA.
SFN, an isothiocyanate found in cruciferous
vegetables, has been shown to exhibit anti-anxiety,
sedative-hypnotic and anti-depression effects (49,50).
It is effective against oxidative-induced cardiomyocyte damage
(51). Moreover, SFN has been shown
to be effective against ERS in different models of cell injury,
including chick yolk sac membrane and chorioallantoic membrane
models (52). It inhibits ERS by
suppressing the apoptosis of cells in type 1 diabetes mellitus
(53). The anti-apoptotic effect of
SFN was also found to be present in human hepatocytes (54). Wang et al (52) reported that SFN ameliorates
ethanol-suppressed embryonic angiogenesis by alleviating excessive
ROS production and ERS. Moreover, Li et al (26) documented that SFN suppresses
expression levels of ERS-associated apoptosis proteins by
activating SIRT1. The present study determined whether SFN
ameliorates chondrocyte apoptosis and delays OA progression.
H2O2, a key pro-apoptosis factor, was used to
induce mitochondrial dysfunction, and upregulated the expression
levels of caspase family proteins (55). The effect of SFN on ERS was
evaluated in OA mouse chondrocytes. ER are found in the cytoplasm
of all eukaryotic cells (56). ERS
is involved in the development of OA (57). In the present study, the levels of
ERS-associated apoptosis proteins (GRP78 and CHOP), Bax and Cleaved
caspase-3 were elevated in chondrocytes following
H2O2 treatment. However, pretreatment with
SFN downregulated GRP78, CHOP, Bax and Cleaved caspase-3 levels and
elevated Bcl-2 levels compared with the H2O2
group. These findings imply that SFN exerted chondroprotective
effects against H2O2-induced apoptosis by
decreasing ERS-dependent apoptosis.
SIRT1 exerts anti-apoptotic effects in OA (58). Certain drugs, such as melatonin,
curcumin and sildenafil, have been shown to exhibit
chondroprotective effects in OA by modulating the SIRT1 signaling
pathway (59,60). The present study evaluated the
effect of SIRT1 pathway signaling in SFN-induced chondroprotection.
Compared with the H2O2 group, SIRT1 levels
were significantly elevated in the SFN-pretreatment group.
SIRT1 siRNA was used to detect the potential
mechanisms of SFN in H2O2-stimulated
apoptotic chondrocytes. Consistent with previous studies, RNA
interference against SIRT1 suppressed SFN-mediated inhibition of
the anti-apoptosis effect (61,62).
Expression levels of SIRT1 were suppressed whereas levels of
Cleaved caspase-3 were significantly elevated in the SIRT1 siRNA
transfection group. These findings suggested that SIRT1 activation
and ERS are associated with H2O2-induced
apoptosis. A DMM mouse model was used to investigate the
chondroprotective effects of SFN in vivo. Histological
staining showed that SFN significantly alleviated disease
progression in mice. This study has certain limitations; for
example, flow cytometry analysis revealed the anti-apoptosis effect
of SFN on OA, which needs to be further studied in future.
SFN protected chondrocytes against
H2O2-induced apoptosis via the SIRT1
signaling pathway. Furthermore, SFN was shown to significantly
relieve disease progression in a DMM mouse model. Therefore, SFN
has a potential value in the prevention and treatment of OA.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Basic Research
on the Application of Suzhou Science and Technology Bureau (grant
no. SYS201626), Key Technologies of People's Livelihood Technology
of Suzhou Science and Technology Bureau (grant no. SS201764),
Natural Science Foundation for Colleges and Universities in Jiangsu
Province (grant no. 16KJB320009), Postgraduate Research &
Practice Innovation Program of Jiangsu Province (grant no.
SJCX17_0658), The Second Affiliated Hospital of Soochow University
Grant (grant no. XKQ2015003) and Orthopedics Department and
Postgraduate Research & Practice Innovation Program of Jiangsu
Province (grant no. KYCX18_ 2530).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MC and LH wrote the manuscript. YL and LL designed
and supervised the study. MC, LH, YL and QD performed the
experiments. MC, YL and LL analyzed and interpreted the
experimental data. All the authors discussed the results and
commented on the manuscript. MC LH, YL and QD confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The protocol for animal care and use conformed to
The Guide for the Care and Use of Laboratory Animals of the
National Institutes of Health and was approved by the Animal Care
and Use Committee of Wenzhou Medical University (ethics approval
no. wydw2019-0377).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Xing D, Xu Y, Liu Q, Ke Y, Wang B, Li Z
and Lin J: Osteoarthritis and all-cause mortality in worldwide
populations: Grading the evidence from a meta-analysis. Sci Rep.
6:243932016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hunter DJ and Felson DT: Osteoarthritis.
BMJ. 332:639–642. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lane NE, Shidara K and Wise BL:
Osteoarthritis year in review 2016: Clinical. Osteoarthritis
Cartilage. 25:209–215. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Reginster JY: The prevalence and burden of
arthritis. Rheumatology (Oxford). 41 (Suppl 1):S3–S6. 2002.
View Article : Google Scholar
|
|
5
|
Mobasheri A: The future of osteoarthritis
therapeutics: Emerging biological therapy. Curr Rheumatol Rep.
15:3852013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Portal-Núñez S, Esbrit P, Alcaraz MJ and
Largo R: Oxidative stress, autophagy, epigenetic changes and
regulation by miRNAs as potential therapeutic targets in
osteoarthritis. Biochem Pharmacol. 108:1–10. 2016. View Article : Google Scholar
|
|
7
|
Xia H, Cao D and Yang F, Yang W, Li W, Liu
P, Wang S and Yang F: Jiawei Yanghe decoction ameliorates cartilage
degradation in vitro and vivo via Wnt/β-catenin signaling pathway.
Biomed Pharmacother. 122:1097082020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dai M, Sui B, Xue Y, Liu X and Sun J:
Cartilage repair in degenerative osteoarthritis mediated by squid
type II collagen via immunomodulating activation of M2 macrophages,
inhibiting apoptosis and hypertrophy of chondrocytes. Biomaterials.
180:91–103. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee AS, Ellman MB, Yan D, Kroin JS, Cole
BJ, van Wijnen AJ and Im HJ: A current review of molecular
mechanisms regarding osteoarthritis and pain. Gene. 527:440–447.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Musumeci G, Castrogiovanni P, Trovato FM,
Weinberg AM, Al-Wasiyah MK, Alqahtani MH and Mobasheri A:
Biomarkers of chondrocyte apoptosis and autophagy in
osteoarthritis. Int J Mol Sci. 16:20560–20575. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bondeson J, Wainwright S, Hughes C and
Caterson B: The regulation of the ADAMTS4 and ADAMTS5 aggrecanases
in osteoarthritis: A review. Clin Exp Rheumatol. 26:139–145.
2008.PubMed/NCBI
|
|
12
|
Sharif M, Whitehouse A, Sharman P, Perry M
and Adams M: Increased apoptosis in human osteoarthritic cartilage
corresponds to reduced cell density and expression of caspase-3.
Arthritis Rheum. 50:507–515. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Claudio N, Dalet A, Gatti E and Pierre P:
Mapping the crossroads of immune activation and cellular stress
response pathways. EMBO J. 32:1214–1224. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Feng K, Chen Z, Pengcheng L, Zhang S and
Wang X: Quercetin attenuates oxidative stress-induced apoptosis via
SIRT1/AMPK-mediated inhibition of ER stress in rat chondrocytes and
prevents the progression of osteoarthritis in a rat model. J Cell
Physiol. 234:18192–18205. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bernales S, Papa FR and Walter P:
Intracellular signaling by the unfolded protein response. Annu Rev
Cell Dev Biol. 22:487–508. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wictome M, Henderson I, Lee AG and East
JM: Mechanism of inhibition of the calcium pump of sarcoplasmic
reticulum by thapsigargin. Biochem J. 283:525–529. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hetz C: The unfolded protein response:
Controlling cell fate decisions under ER stress and beyond. Nat Rev
Mol Cell Biol. 13:89–102. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang L, Xie H and Liu H: Endoplasmic
reticulum stress, diabetes mellitus, and tissue injury. Curr
Protein Pept Sci. 15:812–818. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen D, Wang Y and Chin ER: Activation of
the endoplasmic reticulum stress response in skeletal muscle of
G93A*SOD1 amyotrophic lateral sclerosis mice. Front Cell Neurosci.
9:1702015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rasmussen ML, Kline LA, Park KP, Ortolano
NA, Romero-Morales AI, Anthony CC, Beckermann KE and Gama V: A
non-apoptotic function of MCL-1 in promoting pluripotency and
modulating mitochondrial dynamics in stem cells. Stem Cell Reports.
10:684–692. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao CQ, Zhang YH, Jiang SD, Jiang LS and
Dai LY: Both endoplasmic reticulum and mitochondria are involved in
disc cell apoptosis and intervertebral disc degeneration in rats.
Age (Dordr). 32:161–177. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lin J, Zhuge J, Zheng X, Wu Y, Zhang Z, Xu
T, Meftah Z, Xu H, Wu Y, Tian N, et al: Urolithin A-induced
mitophagy suppresses apoptosis and attenuates intervertebral disc
degeneration via the AMPK signaling pathway. Free Radic Biol Med.
150:109–119. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shah SA, Khan M, Jo MH, Jo MG, Amin FU and
Kim MO: Melatonin stimulates the SIRT1/Nrf2 signaling pathway
counteracting lipopolysaccharide (LPS)-induced oxidative stress to
rescue postnatal rat brain. CNS Neurosci Ther. 23:33–44. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Morris BJ: Seven sirtuins for seven deadly
diseases of aging. Free Radic Biol Med. 56:133–171. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rajendran R, Garva R, Krstic-Demonacos M
and Demonacos C: Sirtuins: Molecular traffic lights in the
crossroad of oxidative stress, chromatin remodeling, and
transcription. J Biomed Biotechnol. 2011:3682762011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li YP, Wang SL, Liu B, Tang L, Kuang RR,
Wang XB, Zhao C, Song XD, Cao XM, Wu X, et al: Sulforaphane
prevents rat cardiomyocytes from hypoxia/reoxygenation injury in
vitro via activating SIRT1 and subsequently inhibiting ER stress.
Acta Pharmacol Sin. 37:344–353. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Katto J, Engel N, Abbas W, Herbein G and
Mahlknecht U: Transcription factor NFκB regulates the expression of
the histone deacetylase SIRT1. Clin Epigenetics. 5:112013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Feng K, Ge Y, Chen Z, Li X, Liu Z, Li X,
Li H, Tang T, Yang F and Wang X: Curcumin inhibits the PERK-eIF2
α-CHOP pathway through promoting SIRT1 expression in oxidative
stress-induced rat chondrocytes and ameliorates osteoarthritis
progression in a rat model. Oxid Med Cell Longev. 2019:85743862019.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Danilov CA, Chandrasekaran K, Racz J,
Soane L, Zielke C and Fiskum G: Sulforaphane protects astrocytes
against oxidative stress and delayed death caused by oxygen and
glucose deprivation. Glia. 57:645–656. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nguyen B, Luong L, Naase H, Vives M, Jakaj
G, Finch J, Boyle J, Mulholland JW, Kwak JH, Pyo S, et al:
Sulforaphane pretreatment prevents systemic inflammation and renal
injury in response to cardiopulmonary bypass. J Thorac Cardiovasc
Surg. 148:690–697. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Forster T, Rausch V, Zhang Y, Isayev O,
Heilmann K, Schoensiegel F, Liu L, Nessling M, Richter K, Labsch S,
et al: Sulforaphane counteracts aggressiveness of pancreatic cancer
driven by dysregulated Cx43-mediated gap junctional intercellular
communication. Oncotarget. 5:1621–1634. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ho JN, Yoon HG, Park CS, Kim S, Jun W,
Choue R and Lee J: Isothiocyanates ameliorate the symptom of heart
dysfunction and mortality in a murine AIDS model by inhibiting
apoptosis in the left ventricle. J Med Food. 15:781–787. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Davidson RK, Jupp O, de Ferrars R, Kay CD,
Culley KL, Norton R, Driscoll C, Vincent TL, Donell ST, Bao Y and
Clark IM: Sulforaphane represses matrix-degrading proteases and
protects cartilage from destruction in vitro and in vivo. Arthritis
Rheum. 65:3130–3140. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Davidson RK, Green J, Gardner S, Bao Y,
Cassidy A and Clark IM: Identifying chondroprotective diet-derived
bioactives and investigating their synergism. Sci Rep. 8:171732018.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zheng G, Zhan Y, Tang Q, Chen T, Zheng F,
Wang H, Wang J, Wu D, Li X, Zhou Y, et al: Monascin inhibits IL-1β
induced catabolism in mouse chondrocytes and ameliorates murine
osteoarthritis. Food Funct. 9:1454–1464. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
The Guide for the Care and Use of
Laboratory Animals of the National Institutes of Health published
by the National Institutes of Health. NIH Publication No. 85-23,
revised 1996.
|
|
38
|
Glasson SS, Blanchet TJ and Morris EA: The
surgical destabilization of the medial meniscus (DMM) model of
osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage.
15:1061–1069. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yoo IH, Kim MJ, Kim J, Sung JJ, Park ST
and Ahn SW: The anti-inflammatory effect of sulforaphane in mice
with experimental autoimmune encephalomyelitis. J Korean Med Sci.
34:e1972019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Huo L, Su Y, Xu G, Zhai L and Zhao J:
Sulforaphane protects the male reproductive system of mice from
obesity-induced damage: Involvement of oxidative stress and
autophagy. Int J Environ Res Public Health. 16:37592019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Glasson SS, Chambers MG, Van Den Berg WB
and Little CB: The OARSI histopathology initiative-recommendations
for histological assessments of osteoarthritis in the mouse.
Osteoarthritis Cartilage. 18 (Suppl 3):S17–S23. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
French HP, Galvin R, Horgan NF and Kenny
RA: Prevalence and burden of osteoarthritis amongst older people in
Ireland: Findings from The Irish LongituDinal Study on Ageing
(TILDA). Eur J Public Health. 26:192–198. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Glyn-Jones S, Palmer AJ, Agricola R, Price
AJ, Vincent TL, Weinans H and Carr AJ: Osteoarthritis. Lancet.
386:376–387. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Johnson VL and Hunter DJ: The epidemiology
of osteoarthritis. Best Pract Res Clin Rheumatol. 28:5–15. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Asada S, Fukuda K, Nishisaka F, Matsukawa
M and Hamanisi C: Hydrogen peroxide induces apoptosis of
chondrocytes; involvement of calcium ion and extracellular
signal-regulated protein kinase. Inflamm Res. 50:19–23. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kim EN, Lee HS and Jeong GS:
Cudratricusxanthone O Inhibits H2O2-Induced
cell damage by activating Nrf2/HO-1 pathway in human chondrocytes.
Antioxidants (Basel). 9:7882020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sutipornpalangkul W, Morales NP and
Harnroongroj T: Free radicals in primary knee osteoarthritis. J Med
Assoc Thai. 92 (Suppl 6):S268–S274. 2009.PubMed/NCBI
|
|
48
|
Hadjigogos K: The role of free radicals in
the pathogenesis of rheumatoid arthritis. Panminerva Med. 45:7–13.
2003.PubMed/NCBI
|
|
49
|
Russo M, Spagnuolo C, Russo GL,
Skalicka-Woźniak K, Daglia M, Sobarzo-Sánchez E, Nabavi SF and
Nabavi SM: Nrf2 targeting by sulforaphane: A potential therapy for
cancer treatment. Crit Rev Food Sci Nutr. 58:1391–1405. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Sita G, Hrelia P, Graziosi A and Morroni
F: Sulforaphane from cruciferous vegetables: Recent advances to
improve glioblastoma treatment. Nutrients. 10:17552018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Corssac GB, Campos-Carraro C, Hickmann A,
da Rosa Araujo AS, Fernandes RO and Belló-Klein A: Sulforaphane
effects on oxidative stress parameters in culture of adult
cardiomyocytes. Biomed Pharmacother. 104:165–171. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wang G, Nie JH, Bao Y and Yang X:
Sulforaphane rescues ethanol-suppressed angiogenesis through
oxidative and endoplasmic reticulum stress in chick embryos. J
Agric Food Chem. 66:9522–9533. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Pu D, Zhao Y, Chen J, Sun Y, Lv A, Zhu S,
Luo C, Zhao K and Xiao Q: Protective effects of sulforaphane on
cognitive impairments and AD-like lesions in diabetic mice are
associated with the upregulation of Nrf2 transcription activity.
Neuroscience. 381:35–45. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Tubbs E, Axelsson AS, Vial G, Wollheim CB,
Rieusset J and Rosengren AH: Sulforaphane improves disrupted
ER-mitochondria interactions and suppresses exaggerated hepatic
glucose production. Mol Cell Endocrinol. 461:205–214. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Chen Z, Yuan Q, Xu G, Chen H, Lei H and Su
J: Effects of quercetin on proliferation and
H2O2-induced apoptosis of intestinal porcine
enterocyte cells. Molecules. 23:20122018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Chandrika BB, Yang C, Ou Y, Feng X, Muhoza
D, Holmes AF, Theus S, Deshmukh S, Haun RS and Kaushal GP:
Endoplasmic reticulum stress-induced autophagy provides
cytoprotection from chemical hypoxia and oxidant injury and
ameliorates renal ischemia-reperfusion injury. PLoS One.
10:e01400252015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Tang Q, Zheng G, Feng Z, Chen Y, Lou Y,
Wang C, Zhang X, Zhang Y, Xu H, Shang P and Liu H: Trehalose
ameliorates oxidative stress-mediated mitochondrial dysfunction and
ER stress via selective autophagy stimulation and autophagic flux
restoration in osteoarthritis development. Cell Death Dis.
8:e30812017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Deng Z, Li Y, Liu H, Xiao S, Li L, Tian J,
Cheng C, Zhang G and Zhang F: The role of sirtuin 1 and its
activator, resveratrol in osteoarthritis. Biosci Rep.
39:BSR201901892019. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Guo JY, Li F, Wen YB, Cui HX, Guo ML,
Zhang L, Zhang YF, Guo YJ and Guo YX: Melatonin inhibits
Sirt1-dependent NAMPT and NFAT5 signaling in chondrocytes to
attenuate osteoarthritis. Oncotarget. 8:55967–55983. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Hu A, Liu HB, Mlynski R, Plontke S, Zhang
JF, Dai WJ, Duan JL, Fan JP, Zheng HL, Xu WH, et al: Therapeutic
ultrasound potentiates the anti-nociceptive and anti-inflammatory
effects of curcumin to postoperative pain via Sirt1/NF-κB signaling
pathway. Am J Transl Res. 10:3099–3110, eCollection.
2018.PubMed/NCBI
|
|
61
|
Li T, Pang Q, Liu Y, Bai M, Peng Y and
Zhang Z: Sulforaphane protects human umbilical vein endothelial
cells from oxidative stress via the miR-34a/SIRT1 axis by
upregulating nuclear factor erythroid-2-related factor 2. Exp Ther
Med. 21:1862021. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Sun X, Mi L, Liu J, Song L, Chung LF and
Gan N: Sulforaphane prevents microcystin-LR-induced oxidative
damage and apoptosis in BALB/c mice. Toxicol Appl Pharmacol.
255:9–17. 2011. View Article : Google Scholar : PubMed/NCBI
|