Introduction
The oxygen utilization rate of the retina is ~9.7 ml
O2/100 ml tissue/min, which is 2–3 times that of the
brain (1). In order to maintain the
high metabolic requirements required for normal vision, the retina
is the most oxygen-dependent part of the human body (2). Retinal hypoxia, when retinal blood
circulation is not sufficient for meeting the metabolic needs of
the retina, serves an important role in the development of retinal
artery and vein occlusion, diabetic retinopathy and age-related
macular degeneration (3,4). Hypoxia-inducible transcription
factor-1 (HIF-1) is an important factor involved in the hypoxic
reactions that affect several biological functions, including
angiogenesis, cell proliferation and inflammation (5,6).
Retinal pigment epithelium (RPE) cells form a
monolayer of cells that are located between the photoreceptors and
Bruch membrane-choroid complex. They act as an external barrier to
the blood-retina system. RPE cells regulate delivery of nutrients
and oxygen to the retina, and also remove metabolic waste from
photoreceptor cells (7,8). As RPE cells are adjacent to the
choroidal capillaries, they are susceptible to ischemia or hypoxia
(9). The tissues formed by the RPE
cells have also been reported to be the most metabolically active
of all tissues in the human body, and are extremely sensitive to
any changes in oxygen tension (10). The functional properties of RPE
cells have been extensively studied under appropriate culture
conditions in vitro (11). A
previous study showed that in hypoxic RPE cells, HIF-1α expression
is stable and may lead to the production of several angiogenic
factors, including vascular endothelial growth factor (VEGF)
(12).
Cryptotanshinone (CT), an active component of
Salvia miltiorrhiza, exerts a protective effect against
several diseases, such as ischemia, atherosclerosis and Alzheimer's
disease, without any notable side effects (13). CT has several pharmacological
effects, including anti-oxidant, anti-inflammatory and
anti-angiogenic effects (14).
Moreover, Feng et al (15)
showed that combined treatment with CT and albendazole
significantly improved ganglion cell injury and reduced optic nerve
demyelination caused by infection by Angiostrongylus
cantonensis. Jian et al (16) found that, as one of the components
of Fufang Xueshuantong capsules, CT reduced the retinal
damage induced by streptozotocin in rats. Considering that there
have been no studies assessing the effects of CT on the retinal
cells under hypoxic conditions to the best of our knowledge, in the
present study, the potential protective effects of CT on RPE cells
in the presence of cobalt chloride (CoCl2)-induced
chemical hypoxia was assessed.
Materials and methods
Reagents and antibodies
CT (cat. no. SC8640) was purchased from Beijing
Solarbio Science & Technology Co., Ltd. CoCl2 (cat.
no. C8661) was purchased from Sigma-Aldrich; Merck KGaA. DMEM/F-12
(cat. no. 11330032) and FBS (cat. no. 16140071) were purchased from
Gibco; Thermo Fisher Scientific, Inc. Streptomycin-penicillin (cat.
no. C0222) and an Annexin V-FITC Apoptosis Detection kit (cat. no.
C1062M) were purchased from Beyotime Institute of Biotechnology.
Primers were synthesized by Genewiz, Inc. The Cell Counting Kit-8
(CCK-8) assay was purchased from Dojindo Molecular Technologies,
Inc. An AxyPrep Multisource Total RNA Miniprep kit (cat. no.
AP-MN-MS-RNA-50) was purchased from Axygen; Corning, Inc. The Prime
Script RT Master Mix (cat. no. RR036A-1) and TB Green Premix Ex Taq
(Perfect Real Time; cat. no. RR420) were purchased from Takara Bio,
Inc.
The following antibodies were used in the present
study: VEGF rabbit PolyAb (cat. no. 19003-1-AP; ProteinTech Group,
Inc.), HIF1-α rabbit PolyAb (cat. no. 20960-1-AP; ProteinTech
Group, Inc.), Bcl-2 rabbit PolyAb (cat. no. AB112; Beyotime
Institute of Biotechnology), Bax (D2E11) rabbit mAb (cat. no. 5023;
Cell Signaling Technology, Inc.), β-actin rabbit mAb (cat. no.
AB0035; Abways Technology), and the secondary horseradish
peroxidase-conjugated goat anti-rabbit IgG PolyAb (cat. no. SE134;
Beijing Solarbio Science & Technology Co., Ltd.).
Cell culture
Human RPE cells (ARPE-19) were obtained from The
Cell Bank of the Chinese Academy of Sciences (Shanghai, China). The
cells were grown in DMEM/F-12 supplemented with 10% FBS and 1%
streptomycin-penicillin, and cultured in a humidified incubator
with 5% CO2 at 37°C.
Preparation of CT stock solution
A 10 mM stock solution was prepared by dissolving CT
in DMSO, and further diluted to 5, 10 and 20 µM using serum-free
medium.
Cell viability
Cell viability was assessed using a CCK-8 assay. A
total of 5×103 cells/well were seeded in 96-well plates.
Cells were grown to 70–80% confluence, and then treated with
CoCl2 (200, 400, 600 or 800 µM) or CT (5, 10 or 20 µM).
After determining the concentration of CoCl2 needed for
the subsequent experiments, cells were exposed to 5, 10 or 20 µM CT
with or without 600 µM CoCl2. The negative control cells
(NC) were treated with serum-free DMEM/F12. After 24 h of
incubation, the morphology of cells was imaged using a light
microscope (magnification, ×200) (Olympus Corporation), then medium
was aspirated from the cells, and then a mixture containing 10 µl
CCK-8 and 100 µl serum-free medium was added to the cells and
cultured for a further 2 h. Subsequently, the absorbance of each
well was measured using a Multiskan FC plate reader (Thermo Fisher
Scientific, Inc.) at a wavelength of 450 nm (17).
Cell apoptosis
Apoptosis of ARPE-19 was detected using an Annexin
V-FITC Apoptosis Detection kit. Briefly, cells were exposed to CT
(5, 10 or 20 µM) with 600 µM CoCl2 for 24 h, then cells
were harvested, washed once with PBS, and stained with Annexin
V-FITC and PI at room temperature for 30 min. The apoptotic rate
was detected on a Beckman Coulter FC500 flow cytometer (Beckman
Coulter, Inc.), and the proportion of early and late apoptotic
cells were further analyzed using FlowJo version 7.6.3 (FlowJo LLC)
(18,19).
Reverse transcription-quantitative
(RT-qPCR)
Cells were treated with CT (5, 10 or 20 µM) with or
without 600 µM CoCl2 for 12 h. The expression of a
target protein is usually expressed later than that of its mRNA
(20). When referring to the
previous references (21,22), 12 h was selected to carry out the
experiments to study the mRNA expression of these cytokines. Total
RNA from ARPE-19 cells was extracted using an AxyPrep Multisource
Total RNA Miniprep kit according to the manufacturer's protocol.
The RNA concentration was measured using a BioDrop µLITE PC
spectrophotometer (BioDrop). Prime Script RT Master Mix was used to
reverse transcribe the RNA into cDNA; the RT reaction conditions
were 37°C for 15 min, followed by 85°C for 5 sec. qPCR was
performed using a CFX96 Real-Time system (Bio-Rad Laboratories,
Inc.), and the thermocycling conditions were: Initial denaturation
at 95°C for 30 sec, followed by 40 cycles of 95°C for 5 sec and
60°C for 30 sec. qPCR was performed using TB Green PCR Master Mix.
mRNA expression levels were calculated using the 2−∆∆Cq
method (23). The sequences of the
primers used are listed in Table
I.
 | Table I.Sequences of the primers used in the
present study. |
Table I.
Sequences of the primers used in the
present study.
| Gene name | Sequence,
5′-3′ |
|---|
| VEGFA |
|
| Forward |
AGGGCAGAATCATCACGAAGT |
| Reverse |
AGGGTCTCGATTGGATGGCA |
| HIF-1α |
|
| Forward |
ATCCATGTGACCATGAGGAAATG |
| Reverse |
TCGGCTAGTTAGGGTACACTTC |
| TNF-α |
|
| Forward |
CCTCTCTCTAATCAGCCCTCTG |
| Reverse |
GAGGACCTGGGAGTAGATGAG |
| IL-1β |
|
| Forward |
AGCTACGAATCTCCGACCAC |
| Reverse |
CGTTATCCCATGTGTCGAAGAA |
| IL-6 |
|
| Forward |
CCTGAACCTTCCAAAGATGGC |
| Reverse |
TTCACCAGGCAAGTCTCCTCA |
| β-actin |
|
| Forward |
CATGTACGTTGCTATCCAGGC |
| Reverse |
CTCCTTAATGTCACGCACGAT |
Western blotting
ARPE-19 cells were lysed in lysis buffer for 30 min,
then the cells were centrifuged at 12,000 × g for 10 min at 4°C,
and the supernatant, which contained the total protein, was
collected. The protein was loaded on a 10% SDS-gel, resolved using
SDS-PAGE, and transferred to a PVDF membrane. Membranes were
blocked using 5% non-fat milk, followed by incubation with one of
the following antibodies at 4°C overnight: Anti-β-actin (1:1,000),
anti-Bcl-2 (1:800), anti-VEGF (1:1,000), anti-Bax (1:1,000) or
anti-HIF-1α (1:500). After washing with PBS-Tween, the membranes
were incubated with the horseradish peroxidase-conjugated goat
anti-rabbit IgG secondary antibody (1:5,000) at room temperature
for 45 min. Finally, enhanced chemiluminescence reagent was mixed
in equal proportions and used to visualize the signals.
Densitometry analysis was performed using ImageJ version 2.0.0
(National Institutes of Health).
Statistical analysis
Data are presented as the mean ± standard error of
the mean of at least three repeats. Differences between groups were
compared using an unpaired Student's t-test or a one-way ANOVA
followed by a post hoc Tukey's test. Statistical analysis was
performed using GraphPad Prism version 8.0 (GraphPad Software,
Inc.). P<0.05 was considered to indicate a statistically
significant difference.
Results
CT inhibits CoCl2-induced
cytotoxicity in ARPE-19 cells
First, whether CoCl2 (200, 400, 600 or
800 µM) or CT (5, 10 or 20 µM) treatment induced cytotoxicity in
ARPE-19 cells was determined. In the range of CoCl2
concentrations used, cell viability decreased gradually in a
dose-dependent manner. As the concentration that inhibited the cell
viability of ARPE-19 cells by ~60% was 600 µM CoCl2
(Fig. 1A), in all subsequent
experiments, this concentration was used. Although CT did not
affect the cell viability when 5, 10 or 20 µM was used (Fig. 1B), CT treatment did exert a
protective effect on cell viability against
CoCl2-induced hypoxia (Fig.
1C and D).
CT inhibits CoCl2-induced
apoptosis of ARPE-19 cells by regulating Bax and Bcl-2
expression
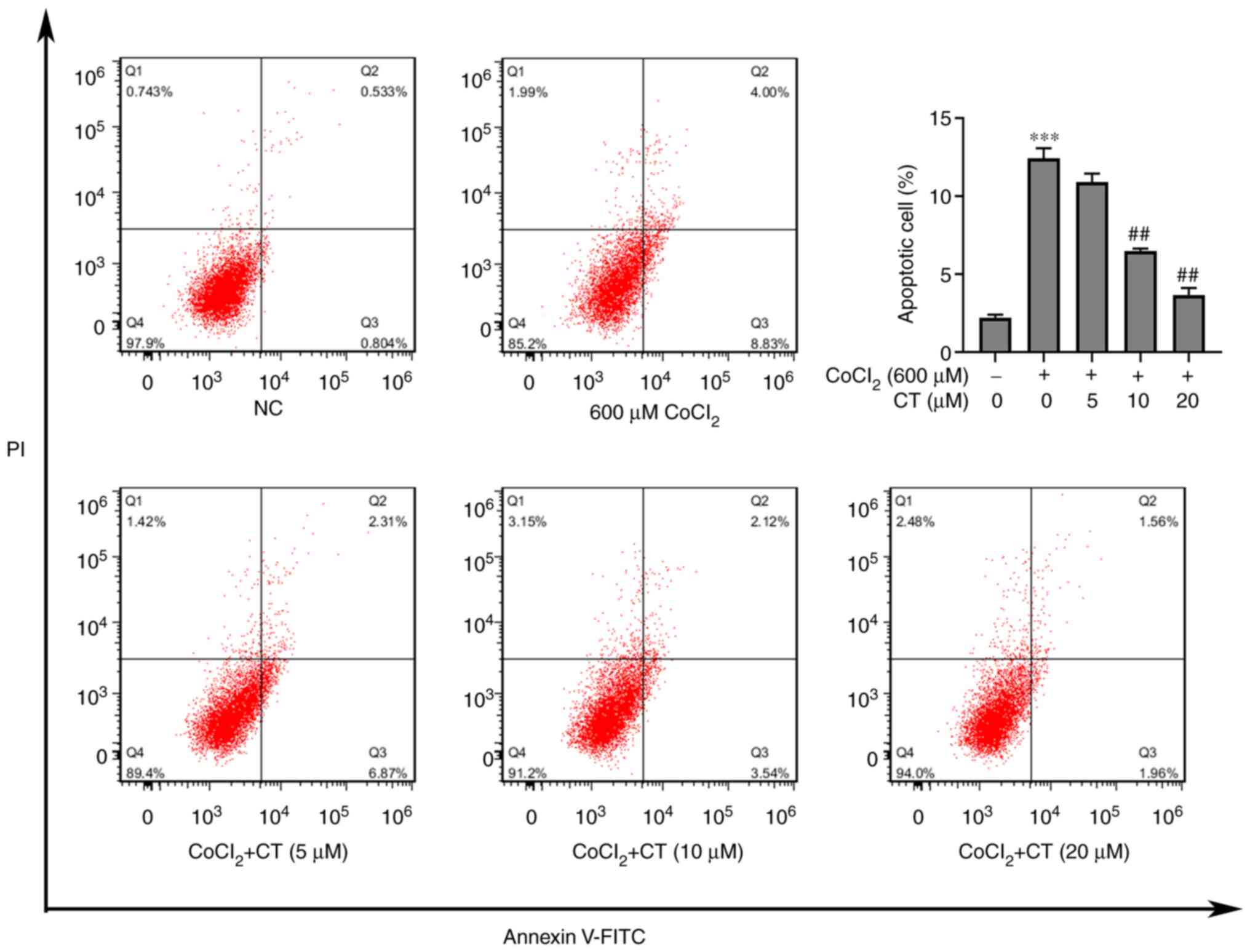
Since CT could protect against
CoCl2-induced cytotoxicity, the effect of CT on
apoptosis of ARPE-19 cells treated with CoCl2 was
assessed. Flow cytometry analysis showed that CT (10 or 20 µM)
inhibited apoptosis of ARPE-19 cells induced by CoCl2
(Fig. 2).
Furthermore, western blotting showed that Bax
expression was increased and Bcl-2 expression was decreased
following treatment with CoCl2 when compared with the NC
group (Fig. 3). In contrast,
treatment with CT reversed these trends (Fig. 3). These results indicated that CT
(10 and 20 µM) could inhibit CoCl2-mediated apoptosis,
at least partly through regulation of Bax and Bcl-2 expression. The
current study also examined the effects of CT (5, 10 or 20 µM)
alone on RPE cell apoptosis, as shown in Figs. S1 and S2, under normal conditions. It was found
there were no statistically significant differences among them.
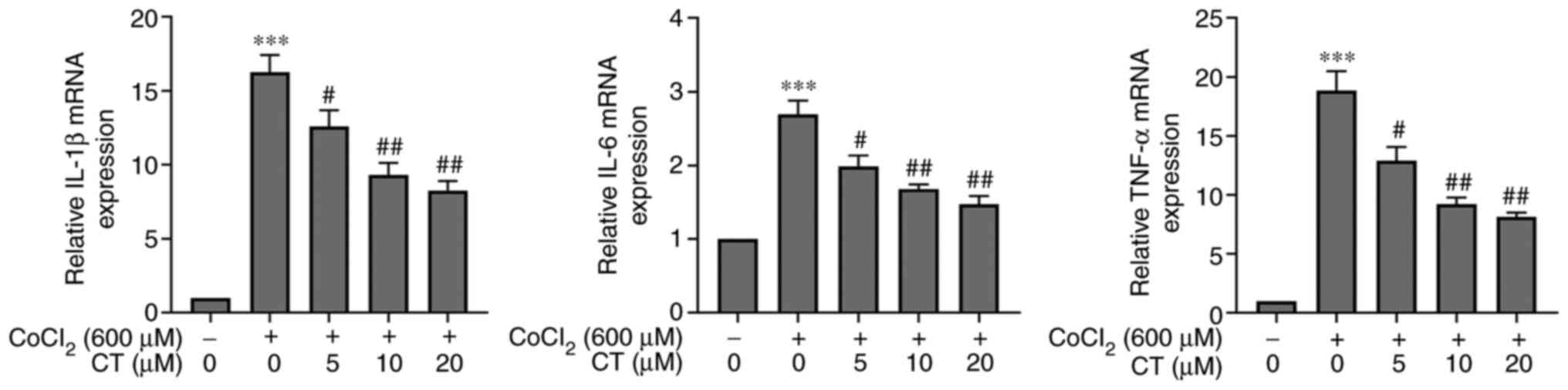
CT regulates inflammatory factors in
hypoxic ARPE-19 cells
It has been previously shown that CT exerts an
anti-inflammatory effect on different diseases (13). The mRNA levels of TNF-α, IL-1β and
IL-6 in ARPE-19 cells treated with CoCl2 were
significantly increased compared with the NC group, and CT (5, 10
and 20 µM) treatment decreased the mRNA expression levels of these
inflammatory factors in the CoCl2 treated cells
(Fig. 4). Furthermore, the
transcriptional levels of these inflammatory factors were not
statistically significant when RPE cells were treated with CT (5,
10 or 20 µM) alone (Fig. S3).
CT reduces VEGF expression in ARPE-19
cells under CoCl2-induced hypoxic conditions
Since hypoxia is a major inducer of angiogenesis
(24), the anti-angiogenic effects
of CT under hypoxic conditions were assessed. Hypoxic conditions
induced by CoCl2 significantly increased the expression
of VEGF and HIF-1α in ARPE-19 cells, both at the mRNA and protein
level (Fig. 5). In contrast, CT
treatment inhibited the CoCl2-induced increase of HIF-1α and VEGF
expression (Fig. 5). However, under
normal conditions, CT (5, 10 or 20 µM) alone treatment did not
affect the gene and protein expression levels of HIF-1α and VEGF in
RPE cells (Figs. S2 and S3).
 | Figure 5.CT reduces VEGF expression in ARPE-19
cells following CoCl2-induced hypoxia. The mRNA
expression levels of (A) HIF-1α and (B) VEGF in ARPE-19 cells were
detected after treatment with different concentrations of CT (5,
10, 20 M) and CoCl2 (600 M) for 12 h, and the (C-E)
protein expression levels were detected after 24 h. Data are
presented as the mean ± the standard error of the mean.
#P<0.05, ##P<0.01,
###P<0.001 vs. 600 µM CoCl2 alone;
**P<0.01, ***P<0.001 vs. NC. NC, negative control cells
treated in serum-free DMEM/F12; CT, cryptotanshinone; VEGF,
vascular endothelial growth factor; HIF-1α, hypoxia-inducible
transcription factor-1α; CoCl2, cobalt chloride. |
Discussion
In the present study, a model of hypoxia in RPE
cells using CoCl2 was established, and it was shown that
CT could protect RPE cells in the following three ways: i) CT
exhibited an anti-apoptotic effect by regulating the expression of
Bax and Bcl-2; ii) CT served an anti-inflammatory effect by
reducing the transcriptional levels of the inflammatory factors
TNF-α, IL-6 and IL-1β; and iii) CT inhibited the expression of
HIF-1α and VEGF, which may inhibit the formation of new blood
vessels.
HIF-1 is widely recognized as a major regulator of
the response to hypoxia. HIF-1 is a transcription factor composed
of HIF-1α and HIF-1β (25). HIF-1α
is hydroxylated by prolyl hydroxylase under conditions of
sufficient oxygen, leading to its degradation. However, under
hypoxic conditions, the HIF-1α protein is stabilized and
accumulates due to inhibition of prolyl hydroxylase (26). The hydroxylation of prolyl
hydroxylase requires molecular oxygen, and cobalt can replace the
ferrous ions bound to the active site, causing the inactivation of
hydroxylase, thereby stabilizing the HIF-1α protein, and this has
been widely used to simulate hypoxia in vitro (27).
Following treatment with CoCl2, the
apoptotic rate of RPE cells was increased as confirmed by Annexin
V-FITC and PI double staining, and the addition of CT to cells
reduced the apoptotic rates of cells. Next, the mechanism
underlying the effects of CT were determined. Cytochrome c
is an initiator of apoptosis, and is primarily associated with the
Bcl-2 family of proteins, such as Bax and Bcl-2. Bax promotes the
release of cytochrome c from the mitochondria, thereby
activating a series of downstream caspase reactions leading to
apoptosis, whereas Bcl-2 prevents the release of cytochrome C by
maintaining the integrity of the mitochondrial membrane (26,28).
Elevated Bax and decreased Bcl-2 levels leads to
initiation of apoptosis (29). In
the present study, compared with the NC group, Bax protein levels
were increased and Bcl-2 protein levels were decreased in the
CoCl2 group. CT inhibited the CoCl2-induced
apoptosis, suggesting that CT exerted an anti-apoptotic effect by
regulating Bax and Bcl-2 protein expression levels in the hypoxic
RPE cells. However, whether CT affects the release of
caspase-activated enzymes requires further experimental study. Zhu
et al (30) found that in a
rat model of stroke, CT exerted an anti-apoptotic effect by
increasing the levels of Bcl-2 in the cerebral cortex and
peripheral blood. However, Kim et al (31) found that in non-small cell lung
cancer cells, CT increased caspase-3 and Bax expression levels and
inhibited Bcl-2, thereby promoting the activation of apoptosis and
reducing cell proliferation. These differences in effects mediated
by CT may be attributed to the use of different cell lines, and the
dysregulation of several signaling pathways in cancer cell lines
compared with normal healthy cells.
In addition, hypoxia is also related to
inflammation. Hypoxia can cause inflammation and tissue damage in
certain diseases, such as rheumatoid arthritis and stroke (10). Just as hypoxia can trigger an
inflammatory response, hypoxia often occurs at the site of
inflammation. It has also been reported that under hypoxic
conditions, HIF-1α and HIF-1β are bound together and translocate to
the nucleus to activate the inflammatory cascade by promoting
transcription of pro-inflammatory genes (32,33).
CoCl2 may increase HIF-1α expression, and subsequently
affect activation of various inflammation-associated transcription
factors, such as NF-κB, which in-turn increases the expression of
pro-inflammatory cytokines, including TNF-α, IL-6 and NO in the
retina (34,35). Since hypoxia and inflammation are
the primary causes of several eye diseases (36), and CT has been reported to exert an
anti-inflammatory effect (37), the
mRNA levels of the inflammatory factors, IL-6, IL-1β and TNF-α,
under hypoxic conditions were explored, and whether CT exerted
anti-inflammatory effect was assessed.
The results showed that hypoxia was positively
related to inflammation, as the expression levels of the
inflammatory factors, IL-6, IL-1β and TNF-α, were elevated when the
cells were treated with CoCl2, suggesting that hypoxia
could promote the transcription of inflammatory cytokines.
Treatment with CT reduced the levels of these inflammatory factors,
thus exerting an anti-inflammatory effect on hypoxic RPE cells.
Zhang et al (38) reported
that CT not only inhibited the secretion of IL-1, IL-8 and TNF-α in
CT26 cells, but also inhibited the expression of IL-6, TNF-α and
pro-IL-1 in vivo.
HIF-1α can upregulate the expression of several
genes that encode proteins related to angiogenesis, such as VEGF,
which serves a critical role in retinal angiogenesis (12). Studies have reported that patients
with various eye diseases related to angiogenesis have
significantly elevated levels of VEGF protein in the aqueous fluid
or vitreous body (39,40). Currently available anti-VEGF drugs,
such as bevacizumab, ranibizumab and abribercept, which are used
for treatment of neovascular eye diseases, have notable side
effects following repeated high dose administration, limiting their
applicability (41). It has been
reported that regulation of the HIF pathway may be more suitable
for the management of neovascular eye diseases than drugs that only
target VEGF (42).
In the present study, the data showed that both
HIF-1α mRNA and protein levels were increased, and this increased
VEGF production in RPE cells under hypoxic conditions induced by
CoCl2. It is generally hypothesized that HIF-1α mRNA
expression is unaffected when the oxygen concentration is altered
(43,44). However, Semenza (45) found an increase in HIF-1α mRNA
expression levels when they exposed animals to prolonged or
intermittent hypoxic conditions. In fact, there are a variety of
factors caused by hypoxia that could affect the mRNA levels of
HIF-1α (43). Hypoxia-induced
activation of NF-κB can bind to the HIF-1α promoter and lead to a
rapid increase in HIF-1α transcription (46). The elevated expression of the
inflammatory factors IL-1 and TNF-α induced by hypoxia also
increases the mRNA expression levels of HIF-1α (47). Additionally, it has been previously
shown that CoCl2-induced hypoxia increases the mRNA
expression levels of HIF-1α (48).
For example, Oh et al (49)
found that HIF-1α mRNA levels were increased in
CoCl2-induced hypoxic RPE cells.
The results of the present study showed that the
mRNA and protein expression levels of HIF-1α were increased in the
CoCl2 treated groups, suggesting that hypoxia promoted
HIF-1α expression. Further experiments showed that CT treatment
protected RPE cells against the CoCl2-induced hypoxia by
reducing HIF-1α mRNA and protein expression levels, suggesting that
CT could inhibit HIF-1α protein accumu2lation and transcriptional
activity in hypoxic RPE cells. Thus, the effects of CT on HIF-1α
protein stability may be related to the inhibition of nuclear
translocation of HIF-1α. It is well established that HIF-1α
primarily exerts its effects through nuclear translocation
(50). Under hypoxic conditions,
HIF-1α is translocated to the nucleus and further activates
transcription of several factors (27). Zhang et al (38) reported that CT could reduce the
nuclear levels and increase the cytosolic levels of HIF-1α, which
may have an effect on the stability of the HIF-1α protein.
In the present study, the decreased expression of
VEGF following CT treatment may partially be due to the inhibition
of HIF-1α expression, limiting binding of HIF-1α to the VEGF
promoter region (51). This may
partially reduce the formation of new blood vessels caused by
hypoxia, thus playing a therapeutic role in wet age-related macular
degeneration, which is characterized by aberrant angiogenesis
(52). CT was also shown to exert a
similar anti-angiogenic effect in bovine aortic endothelial cells
(53) and human umbilical vein
endothelial cells (54).
The present study has some limitations. Although CT
exerted a protective effect on cytotoxicity, apoptosis and
inflammation of RPE cells induced by CoCl2, the
underlying molecular mechanisms require further study. Another
limitation of this study was the lack of in vivo
experiments.
In conclusion, hypoxia is closely associated with a
variety of ophthalmic diseases. Neurodegenerative glaucoma is
associated with fluctuations in oxygen levels, and hypoxia has been
used as a model for studying multiple neurodegenerative diseases in
animals (55). Diabetic retinopathy
and retinopathy of prematurity are proliferative retinopathies that
are characterized by retinal blood vessel ischemia, resulting in
hypoxia (56). HIF-1α may directly
increase angiogenesis and inflammation, both of which are involved
in the progression of age-related macular degeneration, and studies
have shown that specific targeting of HIF has emerged as an
attractive strategy for the treatment of neovascular age-related
macular degeneration (57,58).
To the best of our knowledge, the present study is
the first study to show that CT can protect RPE cells against
hypoxia through its anti-inflammatory, anti-apoptotic and anti-VEGF
effects, and CT did not exert any notable cytotoxic effects on the
RPE cells at the doses used. The beneficial pharmacological effects
and the apparent lack of notable side effects of CT highlight it as
a novel therapeutic option for the treatment of hypoxic eye
diseases.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation in China (grant nos. 81671641 and
81970830), Jiangsu Provincial Medical Youth Talent (grant no.
QNRC2016718), Jiangsu Provincial Medical Innovation Team (grant no.
CXTDA2017039) and the Soochow Scholar Project of Soochow University
(grant no. R5122001).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
PL and GL conceived the study and revised the
manuscript. YG and WL performed the experiments and were
responsible for the draft manuscript. YG and XL analyzed data and
organized the figures. YG, WL and XL confirm the authenticity of
all the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Anderson B Jr and Saltzman HA: Retinal
oxygen utilization measured by hyperbaric blackout. Arch
Ophthalmol. 72:792–795. 1964. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Peet DJ, Kittipassorn T, Wood JP, Chidlow
G and Casson RJ: HIF signalling: The eyes have it. Exp Cell Res.
356:136–140. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
dell'Omo R, Semeraro F, Bamonte G,
Cifariello F, Romano MR and Costagliola C: Vitreous mediators in
retinal hypoxic diseases. Mediators Inflamm. 2013:9353012013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Arjamaa O, Nikinmaa M, Salminen A and
Kaarniranta K: Regulatory role of HIF-1alpha in the pathogenesis of
age-related macular degeneration (AMD). Ageing Res Rev. 8:349–358.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Engin A: Adipose tissue hypoxia in obesity
and its impact on preadipocytes and macrophages: Hypoxia
hypothesis. Adv Exp Med Biol. 960:305–326. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang Z, Yan J, Chang Y, ShiDu Yan S and
Shi H: Hypoxia inducible factor-1 as a target for neurodegenerative
diseases. Curr Med Chem. 18:4335–4343. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Terao R, Honjo M and Aihara M:
Apolipoprotein M Inhibits Angiogenic and inflammatory response by
sphingosine 1-phosphate on retinal pigment epithelium cells. Int J
Mol Sci. 19:1122017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tong Y and Wang S: Not all stressors are
equal: Mechanism of stressors on RPE cell degeneration. Front Cell
Dev Biol. 8:5910672020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cheng Z, Yao W, Zheng J, Ding W, Wang Y,
Zhang T, Zhu L and Zhou F: A derivative of betulinic acid protects
human Retinal Pigment Epithelial (RPE) cells from cobalt
chloride-induced acute hypoxic stress. Exp Eye Res. 180:92–101.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Arjamaa O, Aaltonen V, Piippo N, Csont T,
Petrovski G, Kaarniranta K and Kauppinen A: Hypoxia and
inflammation in the release of VEGF and interleukins from human
retinal pigment epithelial cells. Graefes Arch Clin Exp Ophthalmol.
255:1757–1762. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Du Y, Yang X, Gong Q, Xu Z, Cheng Y and Su
G: Inhibitor of growth 4 affects hypoxia-induced migration and
angiogenesis regulation in retinal pigment epithelial cells. J Cell
Physiol. Jan 22–2019.(Epub ahead of print). View Article : Google Scholar
|
|
12
|
Zhu J, Wang YS, Zhang J, Zhao W, Yang XM,
Li X, Jiang TS and Yao LB: Focal adhesion kinase signaling pathway
participates in the formation of choroidal neovascularization and
regulates the proliferation and migration of choroidal
microvascular endothelial cells by acting through HIF-1 and VEGF
expression in RPE cells. Exp Eye Res. 88:910–918. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu YH, Wu YR, Li B and Yan ZY:
Cryptotanshinone: A review of its pharmacology activities and
molecular mechanisms. Fitoterapia. 145:1046332020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu X, Wu L, Zhou X, Zhou N, Zhuang Q, Yang
J, Dai J, Wang H, Chen S and Mao W: Cryptotanshinone inhibits
VEGF-induced angiogenesis by targeting the VEGFR2 signaling
pathway. Microvasc Res. 111:25–31. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Feng F, Feng Y, Liu Z, Li WH, Wang WC, Wu
ZD and Lv Z: Effects of albendazole combined with TSII-A (a Chinese
herb compound) on optic neuritis caused by Angiostrongylus
cantonensis in BALB/c mice. Parasit Vectors. 8:6062015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jian W, Yu S, Tang M, Duan H and Huang J:
A combination of the main constituents of Fufang Xueshuantong
Capsules shows protective effects against streptozotocin-induced
retinal lesions in rats. J Ethnopharmacol. 182:50–56. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dang Y, Wu W, Xu Y, Mu Y, Xu K, Wu H, Zhu
Y and Zhang C: Effects of low-level laser irradiation on
proliferation and functional protein expression in human RPE cells.
Lasers Med Sci. 30:2295–2302. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang X, Xu J, Ju S, Ni H, Zhu J and Wang
H: Livin gene plays a role in drug resistance of colon cancer
cells. Clin Biochem. 43:655–660. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yin X, Zhang B, Chen L, Xia W, Liu G, Zhu
X, Ren C, Liu W and Lu P: Essential contribution of macrophage Tie2
signalling in a murine model of laser-induced choroidal
neovascularization. Sci Rep. 10:96132020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schwanhäusser B, Busse D, Li N, Dittmar G,
Schuchhardt J, Wolf J, Chen W and Selbach M: Global quantification
of mammalian gene expression control. Nature. 473:337–342. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen Z, Liu G, Xiao Y and Lu P:
Adrenomedullin22-52 suppresses high-glucose-induced migration,
proliferation, and tube formation of human retinal endothelial
cells. Mol Vis. 20:259–269. 2014.PubMed/NCBI
|
|
22
|
Hwang N, Kwon MY, Woo JM and Chung SW:
Oxidative stress-induced Pentraxin 3 expression human retinal
pigment epithelial cells is involved in the pathogenesis of
age-related macular degeneration. Int J Mol Sci. 20:60282019.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta C(T)) method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Calvani M, Rapisarda A, Uranchimeg B,
Shoemaker RH and Melillo G: Hypoxic induction of an
HIF-1alpha-dependent bFGF autocrine loop drives angiogenesis in
human endothelial cells. Blood. 107:2705–2712. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang Z, Yao L, Yang J, Wang Z and Du G:
PI3K/Akt and HIF-1 signaling pathway in hypoxia-ischemia (Review).
Mol Med Rep. 18:3547–3554. 2018.PubMed/NCBI
|
|
26
|
Li T and Wang L: Riparsaponin isolated
from Homonoia riparia Lour induces apoptosis of oral cancer cells.
Oncol Lett. 14:6841–6846. 2017.PubMed/NCBI
|
|
27
|
Rosen R, Vagaggini T, Chen Y and Hu DN:
Zeaxanthin inhibits hypoxia-induced VEGF secretion by RPE cells
through decreased protein levels of hypoxia-inducible factors-1α.
Biomed Res Int. 2015:6873862015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li Z, Xu X, Huang Y, Ding L, Wang Z, Yu G,
Xu D, Li W and Tong D: Swainsonine activates mitochondria-mediated
apoptotic pathway in human lung cancer A549 cells and retards the
growth of lung cancer xenografts. Int J Biol Sci. 8:394–405. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang C, Zhao Y and Zeng B: Enhanced
chemosensitivity by simultaneously inhibiting cell cycle
progression and promoting apoptosis of drug-resistant osteosarcoma
MG63/DXR cells by targeting Cyclin D1 and Bcl-2. Cancer Biomark.
12:155–167. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhu W, Qiu W and Lu A: Cryptotanshinone
exhibits therapeutical effects on cerebral stroke through the
PI3K/AKT-eNOS signaling pathway. Mol Med Rep. 16:9361–9366. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim SA, Kang OH and Kwon DY:
Cryptotanshinone induces cell cycle arrest and apoptosis of NSCLC
cells through the PI3K/Akt/GSK-3β pathway. Int J Mol Sci.
19:27392018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Palazon A, Goldrath AW, Nizet V and
Johnson RS: HIF transcription factors, inflammation, and immunity.
Immunity. 41:518–528. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Taylor CT, Doherty G, Fallon PG and
Cummins EP: Hypoxia-dependent regulation of inflammatory pathways
in immune cells. J Clin Invest. 126:3716–3724. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shweta, Mishra KP, Chanda S, Singh SB and
Ganju L: A comparative immunological analysis of CoCl2
treated cells with in vitro hypoxic exposure. Biometals.
28:175–185. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Peng X, Li C, Yu W, Liu S, Cong Y, Fan G
and Qi S: Propofol attenuates Hypoxia-induced inflammation in BV2
microglia by inhibiting oxidative stress and NF-κB/Hif-1α
signaling. Biomed Res Int. 2020:89787042020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gahlaut N, Suarez S, Uddin MI, Gordon AY,
Evans SM and Jayagopal A: Nanoengineering of therapeutics for
retinal vascular disease. Eur J Pharm Biopharm. 95:323–330. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang N, Dong X, Shi D, Li N and Zhang Q:
Cryptotanshinone ameliorates placental oxidative stress and
inflammation in mice with gestational diabetes mellitus. Arch Pharm
Res. 43:755–764. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang L, Chen C, Duanmu J, Wu Y, Tao J,
Yang A, Yin X, Xiong B, Gu J, Li C and Liu Z: Cryptotanshinone
inhibits the growth and invasion of colon cancer by suppressing
inflammation and tumor angiogenesis through modulating MMP/TIMP
system, PI3K/Akt/mTOR signaling and HIF-1α nuclear translocation.
Int Immunopharmacol. 65:429–437. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Funatsu H, Yamashita H, Noma H, Mimura T,
Yamashita T and Hori S: Increased levels of vascular endothelial
growth factor and interleukin-6 in the aqueous humor of diabetics
with macular edema. Am J Ophthalmol. 133:70–77. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Katsura Y, Okano T, Noritake M, Kosano H,
Nishigori H, Kado S and Matsuoka T: Hepatocyte growth factor in
vitreous fluid of patients with proliferative diabetic retinopathy
and other retinal disorders. Diabetes Care. 21:1759–1763. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Vadlapatla RK, Vadlapudi AD, Pal D,
Mukherji M and Mitra AK: Ritonavir inhibits HIF-1α-mediated VEGF
expression in retinal pigment epithelial cells in vitro. Eye
(Lond). 28:93–101. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Alzhrani RM, Alhadidi Q, Bachu RD, Shah Z,
Dey S and Boddu SH: Tanshinone IIA inhibits VEGF secretion and
HIF-1α expression in cultured human retinal pigment epithelial
cells under hypoxia. Curr Eye Res. 42:1667–1673. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Frede S, Berchner-Pfannschmidt U and
Fandrey J: Regulation of hypoxia-inducible factors during
inflammation. Methods Enzymol. 435:405–419. 2007.PubMed/NCBI
|
|
44
|
Kwasek K, Rimoldi S, Cattaneo AG, Parker
T, Dabrowski K and Terova G: The expression of hypoxia-inducible
factor-1α gene is not affected by low-oxygen conditions in yellow
perch (Perca flavescens) juveniles. Fish Physiol Biochem.
43:849–862. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Semenza GL: Surviving ischemia: Adaptive
responses mediated by hypoxia-inducible factor 1. J Clin Invest.
106:809–812. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Belaiba RS, Bonello S, Zähringer C,
Schmidt S, Hess J, Kietzmann T and Görlach A: Hypoxia up-regulates
hypoxia-inducible factor-1alpha transcription by involving
phosphatidylinositol 3-kinase and nuclear factor kappaB in
pulmonary artery smooth muscle cells. Mol Biol Cell. 18:4691–4697.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Frede S, Freitag P, Otto T, Heilmaier C
and Fandrey J: The proinflammatory cytokine interleukin 1beta and
hypoxia cooperatively induce the expression of adrenomedullin in
ovarian carcinoma cells through hypoxia inducible factor 1
activation. Cancer Res. 65:4690–4697. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yang G, Xu S, Peng L, Li H, Zhao Y and Hu
Y: The hypoxia-mimetic agent CoCl2 induces chemotherapy
resistance in LOVO colorectal cancer cells. Mol Med Rep.
13:2583–2589. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Oh JH, Oh J, Togloom A, Kim SW and Huh K:
Effects of ginkgo biloba extract on cultured human retinal pigment
epithelial cells under chemical hypoxia. Curr Eye Res.
38:1072–1082. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Li HS, Zhou YN, Li L, Li SF, Long D, Chen
XL, Zhang JB, Feng L and Li YP: HIF-1α protects against oxidative
stress by directly targeting mitochondria. Redox Biol.
25:1011092019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lee HJ, Jung DB, Sohn EJ, Kim HH, Park MN,
Lew JH, Lee SG, Kim B and Kim SH: Inhibition of hypoxia inducible
factor alpha and astrocyte-elevated gene-1 mediates
cryptotanshinone exerted antitumor activity in hypoxic PC-3 cells.
Evid Based Complement Alternat Med. 2012:3909572012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zimna A and Kurpisz M: Hypoxia-inducible
factor-1 in physiological and pathophysiological angiogenesis:
Applications and therapies. Biomed Res Int. 2015:5494122015.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hur JM, Shim JS, Jung HJ and Kwon HJ:
Cryptotanshinone but not tanshinone IIA inhibits angiogenesis in
vitro. Exp Mol Med. 37:133–137. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Gong Y, Li Y, Lu Y, Li L, Abdolmaleky H,
Blackburn GL and Zhou JR: Bioactive tanshinones in Salvia
miltiorrhiza inhibit the growth of prostate cancer cells in vitro
and in mice. Int J Cancer. 129:1042–1052. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Vohra R, Dalgaard LM, Vibaek J, Langbøl
MA, Bergersen LH, Olsen NV, Hassel B, Chaudhry FA and Kolko M:
Potential metabolic markers in glaucoma and their regulation in
response to hypoxia. Acta Ophthalmol. 97:567–576. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Aouiss A, Anka Idrissi D, Kabine M and
Zaid Y: Update of inflammatory proliferative retinopathy: Ischemia,
hypoxia and angiogenesis. Curr Res Transl Med. 67:62–71. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Mammadzada P, Corredoira PM and André H:
The role of hypoxia-inducible factors in neovascular age-related
macular degeneration: A gene therapy perspective. Cell Mol Life
Sci. 77:819–833. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Lee D, Miwa Y, Kunimi H, Ibuki M, Shoda C,
Nakai A and Kurihara T: HIF inhibition therapy in ocular diseases.
Keio J Med. Apr 10–2021.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|