Introduction
Acute pancreatitis (AP) is a type of inflammatory
disease, which results from the dysregulation of pancreatic enzymes
in the pancreas (1,2). AP is very common, has a considerably
high mortality rate and can cause systemic complications (3). The mortality rate of AP has increased
from 15 to 30% (4), while the
combination of AP with sepsis increases the mortality rate to
>50% (5). Although significant
efforts have been made to prevent and treat AP (6), no specific treatment methods are
currently available. The development of the inflammatory response
in pancreatic acinar cells correlates with the progression of AP
(7). Therefore, inhibition of the
inflammation in pancreatic acinar cells is crucial for the
treatment of AP.
Emodin (1,3,8-Trihydroxy-6-methylanthra-quinone) is
a natural product, which originates from Rheum palmatum.
Previous studies have reported that emodin exhibits
anti-inflammatory effects (8,9). In
addition, it has been revealed that emodin can alleviate the
symptoms of AP (10). However, the
underlying mechanism by which emodin regulates the progression of
AP remains unclear.
Long non-coding RNAs (lncRNAs) can play important
roles in the development of multiple diseases (11,12).
Moreover, lncRNAs participate in the prevention of AP. For example,
Zhu et al (13) demonstrated
that lncRNA maternally expressed 3 alleviated caerulein-induced
inflammatory injury in human pancreatic cells. In addition, lncRNA
cancer susceptibility 2 has been reported to be involved in the
progression of AP (14).
Furthermore, the expression levels of lncRNA taurine upregulated 1
(TUG1) are upregulated in pancreatic tissues (15). However, the role of TUG1 in the
development of AP is yet to be fully elucidated.
Exosomes are a type of extracellular vesicles, which
can be secreted by cells and promote the transfer of molecules to
cells (16,17). In addition, exosomes play key roles
in the progression of multiple diseases (18,19). A
previous report indicated that exosomes can regulate the
progression of AP (20). However,
the underlying mechanism requires further investigation.
It has been reported that dysregulation of immune
responses may lead to the progression of AP (21,22).
Guo et al (23) highlighted
that the T helper 17 (Th17) cell/regulatory T cell (Treg) imbalance
was involved in the development of AP and that it may be correlated
with its severity and prognosis (24). Therefore, Treg cells play a key role
in mediating the progression of AP. In addition, it has been shown
that TUG1 regulates Treg cell differentiation (25). Based on this evidence, the present
study aimed to explore the association between emodin and Treg
cells in AP.
In the current study, the effects of emodin on AR42J
cells cotreated with caerulein and lipopolysaccharide (LPS) were
investigated. The present study aimed to provide a novel treatment
strategy for AP.
Materials and methods
Cell culture
Rat pancreatic acinar cell lines (AR42J) were
purchased from Tongpai (Shanghai) Biotechnology Co., Ltd. and
maintained in DMEM (Thermo Fisher Scientific, Inc.) containing 10%
FBS (26), 1% penicillin and 1%
streptomycin (Thermo Fisher Scientific, Inc.) at 37°C with 5%
CO2. To mimic AP in vitro, AR42J cells were
treated with LPS (10 µg/ml; Beijing Solarbio Science &
Technology Co., Ltd.) and caerulein (100 nM; Beijing Solarbio
Science & Technology Co., Ltd.) for 3 h, as previously
described (27).
Cell transfection
AR42J cells (3×105 cells/well) were
transfected with pcDNA3.1 (negative control; NC; 1 µg/µl) or
pcDNA3.1-TUG1 [1 µg/µl, TUG1 overexpression (OE)] using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) at 37°C for 48 h. pcDNA3.1 and pcDNA3.1-TUG1 were
purchased from Shanghai GenePharma Co., Ltd. After 48 h of
transfection, the transfected cells were used for subsequent
analysis.
Exosome isolation
Briefly, AR42J cells were cotreated with LPS (10
µg/ml) and caerulein (100 nM) at 37°C for 3 h. Then, AR42J cells
(3×105 cells/well) or AR42J cells cotreated with LPS and
caerulein were centrifuged at 300 × g for 15 min, 2,000 × g for 15
min, and 10,000 × g for 30 min at room temperature to collect the
supernatant. The samples were filtered with a 0.22-µm filter and
collected to isolate exosomes via ultracentrifugation at 4°C
(120,000 × g for 70 min). For collection of the pellet, the samples
were centrifuged at 10,000 × g for 1 h at 4°C. Then, the pellet was
resuspended in PBS for further analysis. Meanwhile, the particle
sizes of exosomes were investigated by Nanoparticle Tracking
Analysis (NTA), the structure of exosomes was observed by
transmission electron microscopy (TEM) and the exosome markers were
detected by western blotting. Exosomes isolated from AR42J cells
were the control-exo group; exosomes isolated from AR42J cells
cotreated with LPS and caerulein were the AP-exo group.
Reagents
Emodin was purchased from Beijing Solarbio Science
& Technology Co., Ltd. IL-2 (cat. no. SRP3242) and anti-CD3
(cat. no. SAB4700040)/CD28 (cat. no. SAB4700739) were purchased
from Sigma-Aldrich; Merck KGaA. Caerulein was obtained from
MedChemExpress. Meanwhile, ARJ21 cells were treated with 20 and 40
µM emodin, according to previous references (28,29).
TEM
The exosome pellet was incubated for 5 min and
subsequently immersed in 2% phosphotungstic acid solution for 1
min. The pellet was fixed using 2.5% glutaraldehyde (pH 7.2) at 4°C
overnight. Then, 100 µl suspension was placed on a parafilm sheet
and a copper grid coated with carbon was placed onto the drop for
10 sec and then removed. The grid was then rinsed 10 times with
MiliQ H2O (1 min each) at room temperature.
Subsequently, the grid was laid on a drop of uranyl acetate (pH
7.0; cat. no. 2624; SPI-CHEM) at room temperature for 10 min. After
rinsing with Milli-Q H2O and methylcellulose uranyl (pH
4.0), the grid was incubated at room temperature for 10 min on a
drop of methylcellulose uranyl (pH 4.0, cat. no. M-6385;
Sigma-Aldrich; Merck KGaA). Finally, the grid was dried for 10 min
at room temperature and observed using a transmission electron
microscope (JEOL, Ltd.).
Cell Counting Kit-8 (CCK-8) assay
The viability of AR42J cells (5×103/well)
was determined in each group using the CCK-8 assay (Beyotime
Institute of Biotechnology). In brief, ARJ21 cells were plated
(5×103 cells/well) into 96-well plates and treated for
0, 24, 48 or 72 h at 37°C. Subsequently, cells were incubated with
10 µl CCK-8 reagents for 2 h at 37°C. The optical density values
were measured at 450 nm using a microplate reader to assess cell
viability.
Cell apoptosis analysis
The early + late apoptosis of AR42J cells was
detected by flow cytometry. In brief, AR42J cells (1×104
per well) were trypsinized, washed with PBS and resuspended in
binding buffer. Subsequently, the cells were stained with 5 µl
Annexin V-FITC and PI (BD Biosciences) in the dark at 37°C for 30
min. Flow cytometry (FACScan™; BD Biosciences) was applied to
investigate the apoptotic rate using FlowJo (version 10.6.2; BD
Biosciences).
Western blot analysis
Total protein was isolated from cell lysates using
RIPA buffer (Beyotime Institute of Biotechnology). The protein was
quantified by the BCA protein kit (Thermo Fisher Scientific, Inc.).
Subsequently, the proteins (30 µg/lane) were separated with
SDS-PAGE gel (10%) and separated proteins were subsequently
transferred to PVDF membranes, which were incubated with primary
antibodies overnight at 4°C following blocking with 3% non-fat milk
at room temperature for 1 h. The membranes were incubated with Goat
Anti-Rabbit antibody (HRP-conjugated, 1:5,000; cat. no. ab7090;
Abcam) at room temperature for 1 h, scanned by an Odyssey Imaging
System (Thermo Fisher Scientific, Inc.) and analyzed with ImageJ
software (version 1.8.0; National Institutes of Health). The
primary antibodies used in the present study were as follows:
Anti-CD63 (1:1,000; cat. no. ab134045; Abcam), anti-Calnexin
(1:1,000; cat. no. ab133615; Abcam), anti-IL-10 (1:1,000; cat. no.
ab133575; Abcam), anti-TGF-β (1:1,000; cat. no. ab215715; Abcam)
and anti-β-actin (1:1,000; cat. no. ab8226; Abcam). All the
antibodies used were purchased from Abcam.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA from cells or exosomes was isolated using
the TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) and the exoRNeasy Midi kit (Qiagen, Inc.). The
All-in-One™ First-Strand cDNA Synthesis kit (GeneCopoeia, Inc.) was
used to transcribe total RNA into cDNA according to the
manufacturer's protocol. RT-qPCR was performed using the SYBR™
Green Master Mix (Qiagen, Inc.). qPCR was performed in triplicate
under the following protocol: 2 min at 94°C, followed by 35 cycles
for 30 sec at 94°C and 45 sec at 55°C. The 2−ΔΔCq method
(30) was used to quantify the data
and GAPDH was used for normalization. The primer sequences used
were as follows: TUG1 forward (F), 5′-ATCTAATCAGTAAGCGGA-3′ and
reverse (R), 5′-AAAGCAAGTCAAGACCTC-3′; IL-10 F,
5′-GAAAAATTGAACCACCCGGCA-3′ and R, 5′-TTCCAAGGAGTTGCTCCCGT-3′;
TGF-β F, 5′-CTGCTGACCCCCACTGATAC-3′ and R,
5′-AGCCCTGTATTCCGTCTCCT-3′; and GADPH F, 5′-ACAGCAACAGGGTGGTGGAC-3′
and R, 5′-TTTGAGGGTGCAGCGAACTT-3′.
NTA
The hydrodynamic radius and concentration of
exosomes were detected via NTA, as previously described (31). In brief, a total of ~0.3 ml
supernatant was loaded into the sample chamber of an LM10 Nanosight
unit (Nanosight, Ltd.) and three videos of either 30 or 60 sec were
recorded of each sample. Data analysis was performed using NTA 2.1
software (Nanosight, Ltd.). The paths of unlabeled particles acting
as point scatterers undergoing Brownian motion in a 0.25 ml chamber
through which a 635-nm laser beam is passed were determined from a
video recording, with the mean squared displacement determined for
each possible particle. The diffusion coefficient and
sphere-equivalent hydrodynamic radius were subsequently determined
using the Stokes-Einstein equation.
ELISA
The levels of IL-6 (cat. no. SEA079Ra), IL-1β (cat.
no. SEA563Ra) and TNF-α (cat. no. SEA133Ra) in the serum of mice or
the cell supernatants were detected using ELISA kits (Wuhan USCN
Business Co., Ltd.). The cell supernatants were harvested by
centrifugation (500 × g, 4°C, 10 min). Subsequently, cells were
incubated with a secondary antibody (cat. no. BA1054; Wuhan Boster
Biological Technology, Ltd.) at room temperature for 1 h. Finally,
following incubation with hydrochloric acid at room temperature for
5 min, the absorbance was measured using a microplate reader.
Animal experiments
A male Wistar rat (12-weeks-old, 200 g) was obtained
from The Chinese Academy of Sciences. The rat was housed within a
dedicated specific pathogen-free (SPF) facility (it was raised in
standard cages with 12-h light/dark cycle, a constant temperature
of 23±1°C and a humidity of 50–60%) and had free access to food and
water. The protocols for care and use of laboratory animals were
approved by the Zhejiang Chinese Medical University (Hangzhou,
China). The rat was euthanized using CO2 at a
displacement rate of 30% of the chamber volume/min (CO2
flow rate, 2.5 l/min). Then, peripheral blood mononuclear cells
(PBMCs, 5 ml) were collected. PBMCs were collected as described in
a previous study (32). In brief,
CD4+T cells were isolated from PBMCs, and then
CD4+T cells were treated with 10 ng/ml IL-2 and 2 µg/ml
anti-CD3/CD28 for 72 h in order to induce the activation of Treg
cells (33).
BALB/c mice (n=18, male, 28–35 g; age, 6–8 weeks)
were purchased from Beijing Vital River Laboratory Animal
Technology Co., Ltd. All mice were maintained under SPF conditions.
All procedures performed in this study involving animals were in
accordance with the National Institutes of Health Guide for the
Care and Use of Laboratory Animals (34). The animal experiments were approved
by the Animal Care and Use Committee of The First Affiliated
Hospital of Zhejiang Chinese Medical University (approval no.
20210201003B). To mimic AP in vivo, mice were fasted for 12
h, and a mouse model of AP was constructed according to a previous
method (35). Briefly, mice in the
AP group were injected intraperitoneally with 50 µg/kg caerulein 12
times (0.2 ml/mice, each time interval was 1 h). In addition, mice
in the control group were injected with saline with the same
procedure. Meanwhile, before the second injection and after the
eighth injection of caerulein, emodin (40 mg/kg) was injected
intraperitoneally into mice (AP + emodin group). The mice were
sacrificed 48 h after the last injection of caerulein, and the
tissues and serum were then collected. All mice were euthanized
using CO2 at a displacement rate of 30% of the chamber
volume/min (CO2 flow rate, 2.5 l/min).
To evaluate the effects of emodin in AP mice,
inflammatory factor (IL-1β, IL-6 and TNF-α) contents in serum of
mice were detected with the ELISA kits, as mentioned above.
Hematoxylin and eosin (H&E)
staining
H&E staining was used to observe pancreatic
injury and inflammation as previously described (36). Paraffin-embedded tissues were cut to
4 µm thickness, deparaffinized, rehydrated and stained with H&E
using standard procedures. The slides were then mounted with
BioMount mounting media and observed under a light microscope
(Olympus Corporation).
Isolation of CD4+ T
cells
A total of 2×106 PBMCs/ml were seeded in
6-well cell culture plates. The cells were treated with 50 ng/ml
Phorbol-12-myristate-13-acetate (Beijing Solarbio Science &
Technology Co., Ltd.), Ionomycin (2 µg/ml; Beijing Solarbio Science
& Technology Co., Ltd.) and Brefelin (2 µg/ml; Beijing Solarbio
Science & Technology Co., Ltd.). These reagents were incubated
with the cells at 37°C for 4 h. The CD4+T cell
population was isolated from the cell cultures by immunomagnetic
selection (MidiMACS™ Separator; Thermo Fisher Scientific,
Inc.).
Flow cytometry
To further induce the differentiation of Treg cells,
CD4+T cells (5×103/well) were treated with
anti-CD3 (cat. no. SAB4700040)/anti-CD28 (cat. no. SAB4700739; 2
µg/ml; Sigma-Aldrich; Merck KGaA) and IL-2 (10 ng/ml;
Sigma-Aldrich; Merck KGaA) at 37°C for 72 h. The cell suspension
was collected and the cells were incubated with anti-CD4 (labeled
with FITC; cat. no. 11-0040-82; Thermo Fisher Scientific, Inc.) and
anti-CD25 (labeled with PE; cat. no. MA1-90766; Thermo Fisher
Scientific, Inc.) for 30 min. Subsequently, the cells were
centrifuged at 3,000 × g at 4°C for 10 min, washed and fixed with
4% paraformaldehyde for 20 min at 4°C. The samples were resuspended
and incubated with anti-FOXP3 antibody (labeled with APC; cat. no.
17-5773-82; Thermo Fisher Scientific, Inc.) for 30 min at 4°C.
Finally, the cells were centrifuged at 3,000 × g at 4°C for 10 min,
washed, fixed with 4% paraformaldehyde at 4°C for 10 min and the
ratio of CD4+/CD25+/FOXP3+ T cells
was measured by FACS (FACSLyric™; BD Biosciences). FlowJo (version
10.6.2; BD Biosciences) was used to analyze the data.
Statistical analysis
All data are expressed as the mean ± SEM. The CCK-8
assay was performed five times. Western blotting, ELISA, RT-qPCR
and flow cytometry assays were performed in triplicate. One-way
ANOVA followed by the Tukey's post hoc test were used for
comparisons between ≥3 groups. P<0.05 was considered to indicate
a statistically significant difference.
Results
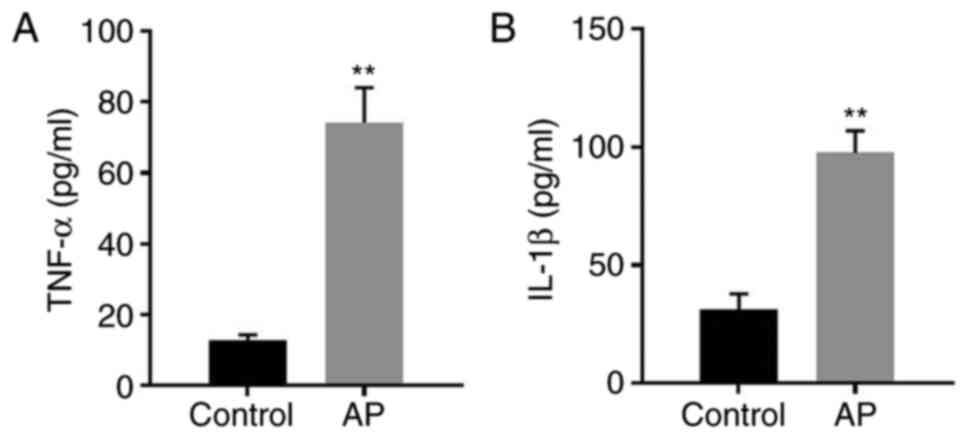
AP in vitro model
To mimic AP in vitro, AR42J cells were
cotreated with caerulein and LPS. The levels of IL-1β and TNF-α in
the supernatants of AR42J cells were significantly increased
following treatment of the cells with caerulein and LPS (Fig. 1A and B). The data suggested that the
in vitro model of AP was successfully established.
lncRNA TUG1 expression is upregulated
in AR42J cells cotreated with caerulein and LPS and in exosomes
derived from caerulein and LPS pretreated AR42J cells
The separation efficiency of exosomes was examined
by TEM. Exosomes demonstrated disc-shaped crescent-shaped and
double-layered membrane structure (Fig.
2A). In addition, the expression levels of CD63 were notably
higher in the AP-exo group than those noted in the control-exo,
while western blotting demonstrated the absence of calnexin
expression in the control-exo and AP-exo groups (Fig. 2B). In addition, rounded particles of
~80–100 nm in diameter were noted following treatment of the cells
with LPS and caerulein (Fig. 2C).
The data suggested that exosomes were successfully separated from
AR42J cells. Furthermore, the expression levels of TUG1 in AR42J
cells and exosomes derived from AR42J cells were significantly
increased following treatment of the cells with caerulein and LPS
(Fig. 2D and E). In summary, the
data indicated that lncRNA TUG1 expression was upregulated in
caerulein and LPS pre-treated AR42J cells and exosomes derived from
AR42J cells cotreated with caerulein and LPS.
Emodin inhibits the expression of
lncRNA TUG1 in cells cotreated with LPS and caerulein and exosomes
derived from the aforementioned cell samples
The effects of emodin on AR42J cell viability were
assessed using the CCK-8 assay. The viability of AR42J cells was
significantly inhibited by caerulein and LPS, while the effects of
caerulein and LPS were reversed following treatment of the cells
with 20 or 40 µM emodin (Fig. 3A).
In addition, the apoptotic rate of AR42J cells cotreated with
caerulein and LPS was significantly inhibited following treatment
of the cells with 20 or 40 µM emodin (Fig. 3B). Moreover, ELISA demonstrated that
the levels of IL-1β and TNF-α in supernatants derived from AR42J
cells were significantly increased following treatment of the cells
with caerulein and LPS, while application of 20 or 40 µM emodin
significantly reversed this effect (Fig. 3C and D). Furthermore, caerulein and
LPS significantly increased the levels of TUG1 in AR42J cells or
exosomes derived from AR42J cells, while their effects were
significantly reversed by emodin treatment of the cells (20 or 40
µM; Fig. 3E and F). Since AR42J
cells were more sensitive to 40 µM emodin, this concentration was
selected for subsequent analysis. Taken together, the data
indicated that emodin inhibited the expression levels of cellular
and exosomal TUG1 in AR42J cells cotreated with caerulein and
LPS.
TUG1 overexpression induces apoptosis
and significantly reverses the effects of emodin on the viability
of AR42J cells, which were pretreated with caerulein and LPS
To investigate the effects of TUG1 on emodin-treated
AR42J cells, cells were transfected with a TUG1 OE plasmid. The
expression levels of TUG1 were upregulated following transfection
of AR42J cells with TUG1 OE (Fig.
4A). Moreover, overexpression of TUG1 caused a significant
reduction in the emodin-induced increase of AR42J cell viability
following treatment with caerulein and LPS (Fig. 4B). The inhibitory effect of emodin
on the induction of apoptosis in AR42J cells cotreated with
caerulein and LPS was significantly reduced following
overexpression of TUG1 (Fig. 4C).
In conclusion, TUG1 overexpression reversed the effects of emodin
on the proliferation of AR42J cells cotreated with caerulein and
LPS by inducing cell apoptosis.
Emodin promotes the differentiation of
Treg cells by inhibition of TUG1 in exosomes derived from AR42J
cells cotreated with caerulein and LPS
In order to confirm whether emodin mediates Treg
cell differentiation via regulation of exosomal TUG1, RT-qPCR was
performed. The expression levels of TUG1 in CD4+T cells
cotreated with anti-CD3/anti-CD28 and IL-2 were significantly
increased by AP-exo (exosomes derived from AR42J cells cotreated
with LPS and caerulein), while the effects of AP-exo were reversed
by emodin treatment (Fig. 5A). In
addition, the ratio of
CD4+/CD25+/FOXP3+ T cells in
CD4+ T cells was significantly increased following their
combined treatment with anti-CD3/anti-CD28 and IL-2, while this
effect was reversed by AP-exo treatment (Fig. 5B and C). Moreover, emodin
significantly inhibited the effects of AP-exo on Treg cell
differentiation (Fig. 5B and C).
The expression levels of IL-10 and TGF-β in CD4+T cells
were notably increased by combined treatment of anti-CD3/anti-CD28
and IL-2, while these effects were partially reversed by AP-exo
treatment (Fig. 5D-F). However,
emodin restored the effects of anti-CD3/anti-CD28 and IL-2
(Fig. 5D-F). In summary, emodin may
promote the differentiation of Treg cells by inhibition of exosomal
lncRNA TUG1 expression.
Emodin significantly inhibits the
progression of AP in vivo
To further confirm the function of emodin in AP, an
in vivo model of AP was established. As indicated in
Fig. 6A, notable injury of the
pancreas tissue and inflammatory infiltration were observed in AP
mice, while emodin markedly reversed this phenomenon. In addition,
AP-induced upregulation of IL-6, IL-1β and TNF-α was significantly
inhibited in the presence of emodin (Fig. 6B). Overall, emodin significantly
inhibited the progression of AP in vivo.
Discussion
A number of agents have been reported to have
anti-inflammatory effects on AP. For instance, Lin et al
(37) found Flos Lonicerae
Japonicae water extract (FLJWE) could inhibit pseudorabies
virus-induced inflammation in RAW264.7 cells. Furthermore, Esmail
et al (38) indicated that
niclosamide could inhibit inflammation in liver fibrosis.
Meanwhile, it has been reported that emodin exhibits
anti-inflammatory effects on AP (10,39).
In the present study, the results indicated that this compound
could inhibit the inflammatory response in AR42J cells cotreated
with LPS and caerulein. Moreover, the present study demonstrated
that emodin promoted Treg cell differentiation in AP by regulation
of TUG1 in exosomes derived from AR42J cells cotreated with
caerulein and LPS. Meanwhile, compared with other anti-inflammatory
agents (for example, FLJWE and niclosamide), emodin has been
confirmed to significantly relieve inflammation of the
gastrointestinal tract (40,41).
Based on the present data and previous reports, the findings
demonstrated the underlying mechanism by which emodin mediated the
progression of AP, suggesting that it could act as an inhibitor of
AP.
lncRNAs are involved in the progression of AP
(1,42). The current study demonstrated that
TUG1 expression was upregulated in AR42J cells cotreated with
caerulein and LPS, suggesting that TUG1 may act as a promoter of
AP. Moreover, TUG1 has been shown to regulate disease progression
by sponging microRNAs (miRNAs/miRs). For example, Tang et al
(43) demonstrated that TUG1
promoted the function of oxidized low-density lipoprotein-treated
human aortic vascular smooth muscle cells by activation of the
miR-141-3p/receptor tyrosine kinase like orphan receptor 2 axis.
Moreover, Pei et al (44)
indicated that TUG1 mediated the proliferation and migration of
ovarian cancer cells by sponging miR-1299. Therefore, the potential
target miRNAs of TUG1 should be investigated in future studies.
Exosomes are known to be secreted by multiple types
of cells and may be associated with the progression of multiple
diseases (45,46). Accumulating evidence has indicated
that exosomes can create an immune suppressive environment by
inducing inflammation (47,48). Wang et al (49) suggested that exosomes from
mesenchymal stem cells that overexpressed Klotho could reverse
apoptosis and NF-kB activation in caerulein-stimulated AR42J cells.
Similarly, the findings suggested that exosomes derived from AR42J
cells caused upregulation of the expression levels of TUG1 in order
to inhibit the immune response during the progression of AP.
Furthermore, Salminen et al (50) highlighted that exosomal vesicles
enhanced immunosuppression during chronic inflammation by
regulation of Treg cell differentiation. The present findings
demonstrated that exosomes derived from AR42J cells cotreated with
LPS and caerulein reduced the number of
CD4+CD25+FOXP3+ T cells, which may
lead to the induction of inflammation. Taken together, the data
indicated that exosomes were important components of the
inflammatory response and acted as important messengers, which
mediated the cross-talk between cells. Therefore, emodin promoted
Treg cell differentiation by regulating the function of exosomes
derived from AR42J cells cotreated with caerulein and LPS.
The current study has the following limitations: i)
The underlying mechanism by which exosomes mediated Treg cell
differentiation was not fully investigated; ii) the ratio of
Th17/Treg cells was not assessed; iii) the mechanism by which
emodin regulated TUG1 expression requires further investigation;
and iv) the expression of FOXP3 in CD4+CD25+
cells and the effect of emodin on FOXP3 expression in terms of
differentiation require further confirmation. Thus, additional
investigations are required in future studies.
In summary, the data of the present study indicated
that emodin inhibited the progression of AP in vitro by
regulation of the expression levels of cellular and exosomal lncRNA
TUG1. Therefore, emodin may be useful as a novel agent for the
treatment of AP.
Acknowledgements
Not applicable.
Funding
This research was supported by the foundation of
State Administration of Traditional Chinese Medicine of Zhejiang
Province (grant no. 2016ZB064) and Medical Health Science and
Technology Project of Zhejiang Provincial Health Commission (grant
no. 2019KY478).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
ZC conceived and supervised the present study. XW
designed the experiments. XW, BH, XT and BW performed the
experiments. ZC and XW confirm the authenticity of all the raw
data. All authors reviewed the results. All authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
All procedures performed in this study involving
animals were in accordance with the National Institutes of Health
Guide for the Care and Use of Laboratory Animals. The animal
experiments were approved by the Animal Care and Use Committee of
The First Affiliated Hospital of Zhejiang Chinese Medical
University (approval no. 20210201003B; Hangzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Song G, Zhou J, Song R, Liu D, Yu W, Xie
W, Ma Z, Gong J, Meng H, Yang T and Song Z: Long noncoding RNA H19
regulates the therapeutic efficacy of mesenchymal stem cells in
rats with severe acute pancreatitis by sponging miR-138-5p and
miR-141-3p. Stem Cell Res Ther. 11:4202020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Numoto I, Tsurusaki M, Oda T, Yagyu Y,
Ishii K and Murakami T: Transcatheter arterial embolization
treatment for bleeding visceral artery pseudoaneurysms in patients
with pancreatitis or following pancreatic surgery. Cancers (Basel).
12:27332020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Barreto SG, Habtezion A, Gukovskaya A,
Lugea A, Jeon C, Yadav D, Hegyi P, Venglovecz V, Sutton R and
Pandol SJ: Critical thresholds: Key to unlocking the door to the
prevention and specific treatments for acute pancreatitis. Gut.
70:194–203. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yu B, Li J, Li N, Zhu Y, Chen Y, He W and
Lu N: Progression to recurrent acute pancreatitis after a first
attack of acute pancreatitis in adults. Pancreatology.
20:1340–1346. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ko J, Skudder-Hill L, Cho J, Bharmal SH
and Petrov MS: The relationship between abdominal fat phenotypes
and insulin resistance in non-obese individuals after acute
pancreatitis. Nutrients. 12:e17912021.
|
|
6
|
Kurihara Y, Maruhashi T, Wada T, Osada M,
Oi M, Yamaoka K and Asari Y: Pancreatitis in a patient with severe
coronavirus disease pneumonia treated with Veno-venous
extracorporeal membrane oxygenation. Intern Med. 59:2903–2906.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang SR, Hsu WH, Wu CY, Shang HS, Liu FC,
Chen A, Hua KF and Ka SM: Accelerated, severe lupus nephritis
benefits from treatment with honokiol by immunoregulation and
differentially regulating NF-κB/NLRP3 inflammasome and sirtuin
1/autophagy axis. FASEB J. 34:13284–13299. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu L, Lin Z, Zheng B, Wang L, Zou J, Wu
S, Jiang Z, Jin Q, Lai X and Lin P: Reduced intellectual ability in
offspring born from preeclamptic mothers: A Prospective cohort
study. Risk Manag Healthc Policy. 13:2037–2046. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guo R, Li Y, Han M, Liu J and Sun Y:
Emodin attenuates acute lung injury in Cecal-ligation and puncture
rats. Int Immunopharmacol. 85:1066262020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gao Z, Sui J, Fan R, Qu W, Dong X and Sun
D: Emodin protects against acute Pancreatitis-Associated lung
injury by inhibiting NLPR3 inflammasome activation via Nrf2/HO-1
signaling. Drug Des Devel Ther. 14:1971–1982. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun Z, Xue S, Zhang M, Xu H, Hu X, Chen S,
Liu Y, Guo M and Cui H: Aberrant NSUN2-mediated m5 C
modification of H19 lncRNA is associated with poor differentiation
of hepatocellular carcinoma. Oncogene. 39:6906–6919. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Szikszai K, Krejcik Z, Klema J, Loudova N,
Hrustincova A, Belickova M, Hruba M, Vesela J, Stranecky V, Kundrat
D, et al: LncRNA profiling reveals that the deregulation of H19,
WT1-AS, TCL6, and LEF1-AS1 Is associated with higher-risk
myelodysplastic syndrome. Cancers (Basel). 12:27262020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu X, Qin X, Wang X, Wang Y, Cao W, Zhang
J and Chen W: Oral cancer cellderived exosomes modulate natural
killer cell activity by regulating the receptors on these cells.
Int J Mol Med. 46:2115–2125. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zeng J, Chen JY, Meng J and Chen Z:
Inflammation and DNA methylation coregulate the CtBP-PCAF-c-MYC
transcriptional complex to activate the expression of a long
non-coding RNA CASC2 in acute pancreatitis. Int J Biol Sci.
16:2116–2130. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu K and Zhang L: Inhibition of
TUG1/miRNA-299-3p axis represses pancreatic cancer malignant
progression via suppression of the notch1 pathway. Dig Dis Sci.
65:1748–1760. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gao Q, Fang X, Chen Y, Li Z and Wang M:
Exosomal lncRNA UCA1 from cancer-associated fibroblasts enhances
chemoresistance in vulvar squamous cell carcinoma cells. J Obstet
Gynaecol Res. 47:73–87. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang X, Gong W, Cao S, Yin J, Zhang J,
Cao J and Shen Y: Comprehensive Analysis of Non-coding RNA profiles
of exosome-like vesicles from the protoscoleces and hydatid cyst
fluid of echinococcus granulosus. Front Cell Infect Microbiol.
10:3162020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang D, Xing N, Yang T, Liu J, Zhao H, He
J, Ai Y and Yang J: Exosomal lncRNA H19 promotes the progression of
hepatocellular carcinoma treated with Propofol via
miR-520a-3p/LIMK1 axis. Cancer Med. 9:7218–7230. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yuan Z, Yang Z, Li W, Wu A, Su Z and Jiang
B: Exosome-mediated transfer of long noncoding RNA HOTAIR regulates
temozolomide resistance by miR-519a-3p/RRM1 axis in glioblastoma.
Cancer Biother Radiopharm. Jul 24–2020.(Epub ahead of print).
View Article : Google Scholar
|
|
20
|
Wu XB, Sun HY, Luo ZL, Cheng L, Duan XM
and Ren JD: Plasma-derived exosomes contribute to
pancreatitis-associated lung injury by triggering NLRP3-dependent
pyroptosis in alveolar macrophages. Biochim Biophys Acta Mol Basis
Dis. 1866:1656852020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang L, Chen S, Zhao Q, Sun Y and Nie H:
The critical role of Bach2 in shaping the balance between
CD4+ T cell subsets in immune-mediated diseases.
Mediators Inflamm. 2019:26097372019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qiao Y, Chen J, Ma C, Liu Y, Li P, Wang Y,
Hou L and Liu Z: Increased KIF15 expression predicts a poor
prognosis in patients with lung adenocarcinoma. Cell Physiol
Biochem. 51:1–10. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guo J, Li Z, Tang D and Zhang J: Th17/Treg
imbalance in patients with severe acute pancreatitis: Attenuated by
high-volume hemofiltration treatment. Medicine (Baltimore).
99:e214912020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang D, Tang M, Zong P, Liu H, Zhang T,
Liu Y and Zhao Y: miRNA-155 regulates the Th17/Treg ratio by
targeting SOCS1 in severe acute pancreatitis. Front Physiol.
9:6862018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
He Y, Li M, Wujisiguleng, Lv B, Huan Y,
Liu B, Wang D, Yu H, Zhang L and Shi Z: Zhenbao Pill reduces Treg
cell proportion in acute spinal cord injury rats by regulating
TUG1/miR-214/HSP27 axis. Biosci Rep. 38:BSR201808952018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shen Y, Xue CJ, You GL and Liu C: miR-9
alleviated the inflammatory response and apoptosis in
caerulein-induced acute pancreatitis by regulating FGF10 and the
NF-κB signaling pathway. Exp Ther Med. 22:7952021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang KK, Yu SS, Li GY, He L and Liang XQ:
miR-135a deficiency inhibits the AR42J cells damage in
cerulein-induced acute pancreatitis through targeting FAM129A.
Pflugers Arch. 471:1519–1527. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu C, Luo Y, Ntim M, Quan W, Li Z, Xu Q,
Jiang L, Zhang J, Shang D, Li L, et al: Effect of emodin on long
non-coding RNA-mRNA networks in rats with severe acute
pancreatitis-induced acute lung injury. J Cell Mol Med.
25:1851–1866. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao JY, Wang JQ, Wu L, Zhang F, Chen ZP,
Li WD, Cai H and Liu X: Emodin attenuates cell injury and
inflammation in pancreatic acinar AR42J cells. J Asian Nat Prod
Res. 21:186–195. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guo Z, Wang X, Yang Y, Chen W, Zhang K,
Teng B, Huang C, Zhao Q and Qiu Z: Hypoxic Tumor-derived exosomal
long noncoding RNA UCA1 promotes angiogenesis via miR-96-5p/AMOTL2
in pancreatic cancer. Mol Ther Nucleic Acids. 22:179–195. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jiang X, Li X, Zheng S, Du G, Ma J, Zhang
L, Wang H and Tian J: Comparison study of different indoleamine-2,3
dioxygenase inhibitors from the perspective of pharmacodynamic
effects. Int J Immunopathol Pharmacol. 34:20587384209505842020.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fiori A, Uhlig S, Kluter H and Bieback K:
Human adipose tissue-derived mesenchymal stromal cells inhibit CD4+
T cell proliferation and induce regulatory T cells as well as CD127
expression on CD4+CD25+ T cells. Cells.
10:582021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang XD, Yu WL and Sun Y: Activation of
AMPK restored impaired autophagy and inhibited inflammation
reaction by up-regulating SIRT1 in acute pancreatitis. Life Sci.
277:1194352021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xiang H, Guo F, Tao X, Zhou Q, Xia S, Deng
D, Li L and Shang D: Pancreatic ductal deletion of S100A9
alleviates acute pancreatitis by targeting VNN1-mediated ROS
release to inhibit NLRP3 activation. Theranostics. 11:4467–4482.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dai J, He Y, Jiang M, Niu M, Li B, Wu Z,
Bao J, Wen L, Wang X and Hu G: Reg4 regulates pancreatic
regeneration following pancreatitis via modulating the Notch
signaling. J Cell Physiol. Apr 26–2021.(Epub ahead of print).
View Article : Google Scholar
|
|
37
|
Lin HW, Lee YJ, Yang DJ, Hsieh MC, Chen
CC, Hsu WL, Chang YY and Liu CW: Anti-inflammatory effects of flos
lonicerae japonicae water extract are regulated by the STAT/NF-κB
pathway and HO-1 expression in Virus-infected RAW264.7 cells. Int J
Med Sci. 18:2285–2293. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Esmail MM, Saeed NM, Michel HE and El-Naga
RN: The ameliorative effect of niclosamide on bile duct ligation
induced liver fibrosis via suppression of NOTCH and Wnt pathways.
Toxicol Lett. 347:23–35. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xu C, Zhang J, Liu J, Li Z, Liu Z, Luo Y,
Xu Q, Wang M, Zhang G, Wang F and Chen H: Proteomic analysis
reveals the protective effects of emodin on severe acute
pancreatitis induced lung injury by inhibiting neutrophil proteases
activity. J Proteomics. 220:1037602020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xia S, Ni Y, Zhou Q, Liu H, Xiang H, Sui H
and Shang D: Emodin attenuates severe acute pancreatitis via
antioxidant and anti-inflammatory activity. Inflammation.
42:2129–2138. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Luo S, Deng X, Liu Q, Pan Z, Zhao Z, Zhou
L and Luo X: Emodin ameliorates ulcerative colitis by the
flagellin-TLR5 dependent pathway in mice. Int Immunopharmacol.
59:269–275. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen X and Song D: LncRNA MEG3
participates in caerulein-induced inflammatory injury in human
pancreatic cells via regulating miR-195-5p/FGFR2 axis and
inactivating NF-κB pathway. Inflammation. 44:160–173. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tang Y, Hu J, Zhong Z, Liu Y and Wang Y:
Long noncoding RNA TUG1 promotes the function in ox-LDL-treated
HA-VSMCs via miR-141-3p/ROR2 axis. Cardiovasc Ther.
2020:67589342020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Pei Y, Li K, Lou X, Wu Y, Dong X, Wang W,
Li N, Zhang D and Cui W: miR1299/NOTCH3/TUG1 feedback loop
contributes to the malignant proliferation of ovarian cancer. Oncol
Rep. 44:438–448. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang C, Zhang C, Xu Y, Li C, Cao Y and Li
P: Exosomes derived from human placenta-derived mesenchymal stem
cells improve neurologic function by promoting angiogenesis after
spinal cord injury. Neurosci Lett. 739:1353992020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Colucci M, Ruggiero B, Gianviti A, Rosado
MM, Carsetti R, Bracaglia C, De Benedetti F, Emma F and Vivarelli
M: IgM on the surface of T cells: A novel biomarker of
pediatric-onset systemic lupus erythematosus. Pediatr Nephrol.
36:909–916. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Matikainen S, Nyman TA and Cypryk W:
Inflammasomes: Exosomal miRNAs loaded for action. J Cell Biol.
219:e2020081302020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Elashiry M, Elashiry MM, Elsayed R,
Rajendran M, Auersvald C, Zeitoun R, Rashid MH, Ara R, Meghil MM,
Liu Y, et al: Dendritic cell derived exosomes loaded with
immunoregulatory cargo reprogram local immune responses and inhibit
degenerative bone disease in vivo. J Extracell Vesicles.
9:17953622020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang N, Ma J, Ren Y, Xiang S and Jia R:
Secreted klotho from exosomes alleviates inflammation and apoptosis
in acute pancreatitis. Am J Transl Res. 11:3375–3383.
2019.PubMed/NCBI
|
|
50
|
Salminen A, Kaarniranta K and Kauppinen A:
Exosomal vesicles enhance immunosuppression in chronic
inflammation: Impact in cellular senescence and the aging process.
Cell Signal. 75:1097712020. View Article : Google Scholar : PubMed/NCBI
|