Introduction
Gastric cancer (GC) is one of the most common types
of cancer with a high mortality and morbidity, and accounts for ~1
million deaths annually worldwide (1). Due to advances in diagnostic and
therapeutic approaches, the incidence and mortality rate of GC in
developed countries have decreased to relatively lower levels. In
the United States, GC is the sixth leading cause of cancer-related
deaths (2). However, GC is often
diagnosed at an advanced stage, which increases the difficulty for
doctors to effectively treat this disease (3). Therefore, it is important to identify
novel treatment methods to improve the life quality of patients
with GC.
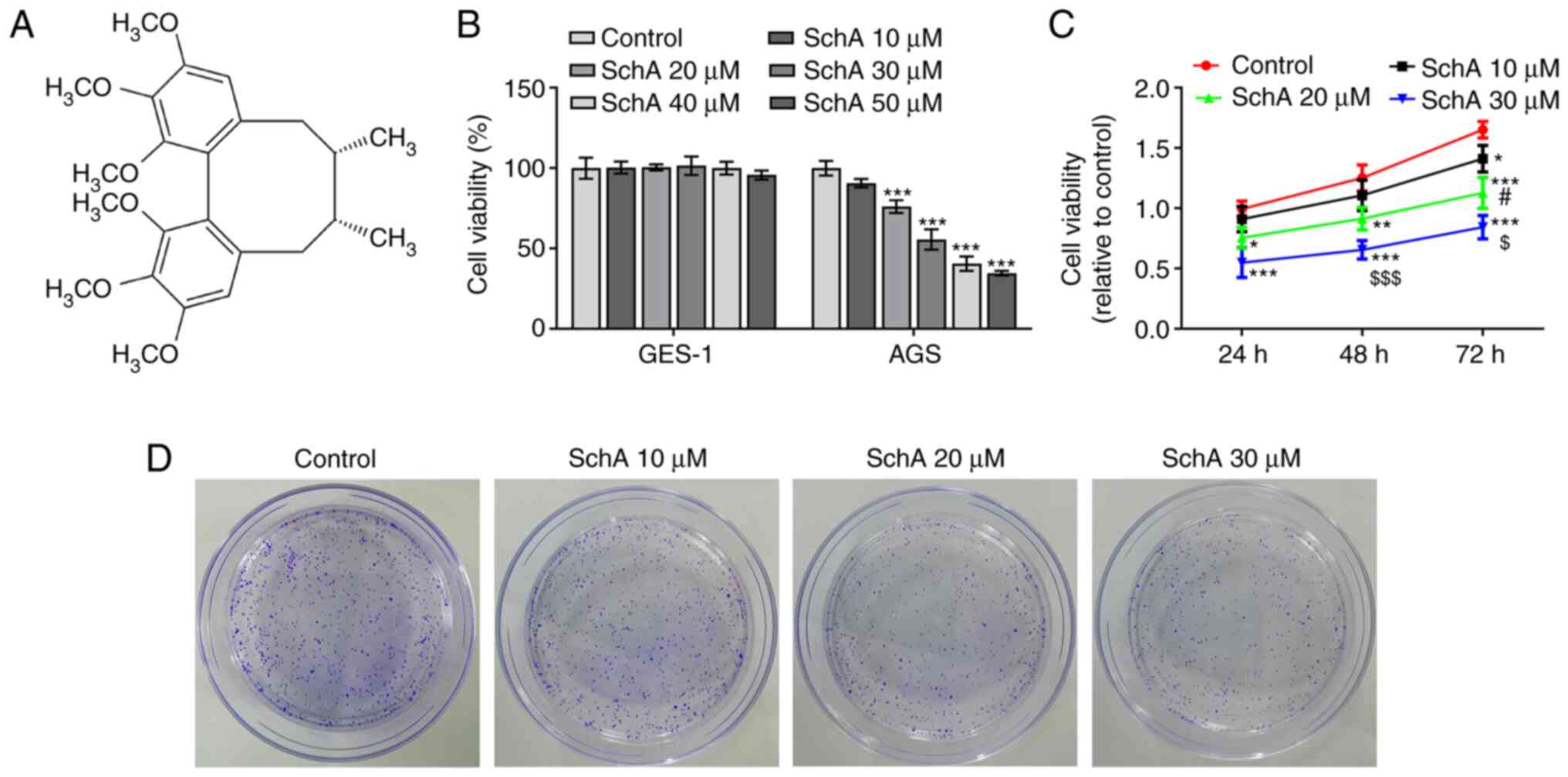
Schizandrin A (SchA; Fig. 1A), isolated from Fructus
schisandra, can exert a variety of therapeutic effects,
including antioxidant, anti-inflammatory and anticancer effects
(4). Accumulating evidence has
demonstrated that SchA can suppress the proliferation, invasion and
migration, and enhance the apoptosis of breast cancer cells by
decreasing the expression levels of microRNA (miR)-155 (5). Additionally, it can promote the cell
cycle arrest, apoptosis and cell death induced by heat shock
transcription factor 1, thereby impeding the proliferation of
colorectal cancer cells (6). SchA
can obstruct the proliferation and invasion of melanoma cells and
the phosphorylation of PI3K/AKT by repressing H19 imprinted
maternally expressed transcript expression (7). In addition, it can modulate the
overactivation of Wnt and activate endoplasmic reticulum (ER)
stress to induce the cell cycle arrest and apoptosis of
triple-negative breast cancer cells (4). It has been shown that inhibition of
IKKβ/NF-κB signaling enhances the efficacy of gefitinib in the
treatment of non-small cell lung cancer (4). Furthermore, a previous study reported
the role of SchA in controlling the malignant behaviors of the
TPC-1 thyroid cancer cell line by decreasing the expression levels
of miR-429 (8). However, to the
best of our knowledge, the effect of SchA on GC has not been
investigated to date.
Based on the hypothesis that SchA may affect the
malignant behaviors of GC cells, SchA was purchased and used to
treat GC cell suspensions. The present study investigated whether
SchA could induce the apoptosis and suppress the proliferation,
invasion and migration of GC cells by activating ER stress.
Materials and methods
Cell culture and drug preparation
SchA (C24H32O6;
cat. no. 61281-38-7) was procured from Shanghai Aladdin Biochemical
Technology Co., Ltd. GES-1 human gastric epithelial cells and the
AGS GC cell line, which were purchased from the American Type
Culture Collection, were incubated in RPMI-1640 medium (Thermo
Fisher Scientific, Inc.) supplemented with 10% FBS (Thermo Fisher
Scientific, Inc.). The cells were cultured at 37°C in an incubator
with 5% CO2.
For the detection of ER stress, the ER stress
inhibitor 4-phenylbutyric acid (4-PBA; Sigma-Aldrich; Merck KGaA)
at a concentration of 7 mM was used to pretreat AGS cells for 4 h
at 37°C, according to a previous study (9), to determine the role of SchA in ER
stress.
Cell counting kit-8 (CCK-8) assay
CCK-8 (MedChemExpress) was used to determine cell
viability, according to the manufacturer's instructions. Briefly,
AGS cells were seeded at a density of 4×103 cells/well
in 96-well plates and cultured. SchA at concentrations of 10, 20,
30, 40 and 50 µM was administered to the cells, as previously
described (4–7,10).
After incubation at 37°C for the corresponding time intervals (24,
48 and 72 h), 10 µl CCK-8 was added to the cells at 37°C for 4 h to
detect cell viability. The optical density at 450 nm was measured
using a microplate reader (Thermo Fisher Scientific, Inc.).
Colony formation assay
AGS cells were seeded onto 6-well plates (500 cells
per well) with or without treatment of SchA at different
concentrations (10, 20 and 30 µM) for 24 h and/or 4-PBA (7 mM,
37°C, 4 h), and cultured at 37°C for 2 weeks. Following fixation
with 3.7% paraformaldehyde at room temperature for 20 min, the cell
colonies were stained with 0.05% crystal violet solution at room
temperature for 30 min. The macroscopic cell group formed by the
continuous proliferation of a single cell in vitro for more
than six generations was regarded as a clone, and cells were
counted manually under an inverted optical microscope
(magnification, ×10; Olympus Corporation).
Wound healing assay
For the evaluation of cell migration, cells were
incubated in 96-well plates at a density of 4×105 cells
per well. A scratch was created in the cell monolayer using a
200-µl pipette tip. After culturing in complete medium at 37°C for
0 and 24 h, the plates and the wound closure were visualized using
a light microscope (magnification, ×100; Nikon Corporation). The
cell migration rate was analyzed using Image-Pro Plus 6.0 software
(Media Cybernetics, Inc.).
Transwell assay
All cells were inoculated at a density of
2×104 cells/well in the upper chamber of Transwell
plates (8-µm pore size; Corning, Inc.). The surface of the upper
chamber was precoated with Matrigel™ (BD Biosciences) at 37°C for 1
h. The FBS-free RPMI-1640 medium was added in the upper chamber,
and 500 µl fresh medium containing 10% FBS was added in the lower
chamber. Cells were incubated at 37°C with 5% CO2 for 24
h, followed by staining using 0.1% crystal violet solution at room
temperature for 20 min. Images were obtained using a light
microscope (magnification, ×100; Nikon Corporation).
Western blotting
Total protein was extracted from cells under
different culture conditions using RIPA lysis buffer
(Sigma-Aldrich; Merck KGaA) according to the manufacturer's
protocol. A BCA assay kit (Beyotime Institute of Biotechnology) was
used for the quantification of total protein. Proteins (30 µg per
lane) were separated via 12% SDS-PAGE and then transferred to PVDF
membranes. After blocking in 5% skimmed milk at room temperature
for 2 h, membranes were incubated with antibodies against MMP-2
(cat. no. ab92536; 1:1,000; Abcam), MMP-9 (cat. no. ab76003;
1:1,000; Abcam), E-cadherin (cat. no. ab40772; 1:10,000; Abcam),
N-cadherin (cat. no. ab76011; 1:5,000; Abcam), vimentin (cat. no.
ab92547; 1:1,000; Abcam), Bcl-2 (cat. no. ab32124; 1:1,000; Abcam),
Bax (cat. no. ab32503; 1:1,000; Abcam), cleaved-caspase 3 (cat. no.
ab32042; 1:500; Abcam), cleaved-poly (ADP-ribose) polymerase (PARP;
cat. no. ab32064; 1:1,000; Abcam), caspase 3 (cat. no. ab184787;
1:2,000; Abcam), PARP (cat. no. ab191217; 1:1,000; Abcam), heat
shock protein family A (Hsp70) member 5 (HSPA5; cat. no. ab108615;
1:1,000; Abcam), phosphorylated (p)-PERK (cat. no. MA5-15033;
1:1,000; Thermo Fisher Scientific, Inc.), p-eukaryotic initiation
factor 2 α (p-eIF2α; cat. no. 3398; 1:1,000; Cell Signaling
Technology, Inc.), CHOP (cat. no. PA5-28956; 1:1,000; Thermo Fisher
Scientific, Inc.), PERK (cat. no. ab79483; 1:1,000; Abcam), eIF2α
(cat. no. 5324; 1:1,000; Cell Signaling Technology, Inc.) and GAPDH
(cat. no. ab9485; 1:2,500; Abcam) overnight at 4°C, and then a Goat
Anti-Rabbit IgG H&L (HRP) secondary antibody (cat. no. ab6721;
1:2,000; Abcam) was added for 1 h at room temperature. An
electrochemical luminescence reagent (MilliporeSigma) was applied
for the visualization of the bands. The grey region of bands was
semi-quantified using ImageJ software (v1.8.0; National Institutes
of Health).
TUNEL assay
Apoptosis was analyzed using a TUNEL Apoptosis
Detection kit (Beyotime Institute of Biotechnology) according to
the manufacturer's protocol. The cells were fixed in 4%
paraformaldehyde at room temperature for 0.5 h and then washed in
PBS twice. After incubation with TUNEL reaction mixture for 1 h at
37°C in the dark, cells were washed with PBS and incubated with a
converter-peroxidase reagent for 30 min. The nuclei were
counterstained with DAPI for 5 min at room temperature in the dark.
Subsequently, diaminobenzidine solution was used to treat the
cells, and five fields of view were randomly selected to capture
cell images using a fluorescence microscope (magnification, ×100;
Nikon Corporation) and analyzed using Image Pro Plus 6.0 software
(Media Cybernetics, Inc.).
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 6.0 (GraphPad Software, Inc.) and SPSS 22.0 (IBM Corp.)
software. Continuous variables are presented as the mean ± SD.
P-values were calculated using unpaired Student's t-test for
comparisons between two groups or one-way ANOVA with Tukey's test
for comparisons among multiple groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
SchA suppresses the viability,
proliferation, invasion and migration of GC cells
To investigate the role of SchA in the functions of
GC cells, GES-1 and AGS cells were treated with different
concentrations of SchA. As shown in Fig. 1B, the viability of GES-1 cells was
not affected by SchA, while increasing concentrations of SchA
gradually reduced the viability of AGS cells. It was further
demonstrated that the viability of AGS cells was decreased in a
dose- and time-dependent manner (Fig.
1C). Next, the effects of SchA on the proliferation, invasion
and migration of GC cells were measured, and the results indicated
that SchA exerted inhibitory effects on the proliferation (Fig. 1D), migration (Fig. 2A) and invasion (Fig. 2B) of AGS cells in a dose-dependent
manner. The expression levels of MMP-2 and MMP-9 were also
decreased by SchA treatment in AGS cells (Fig. 2C). The expression level of
E-cadherin was increased, while the expression levels of N-cadherin
and vimentin were decreased by SchA treatment in AGS cells
(Fig. 2D).
SchA induces the apoptosis of AGS
cells
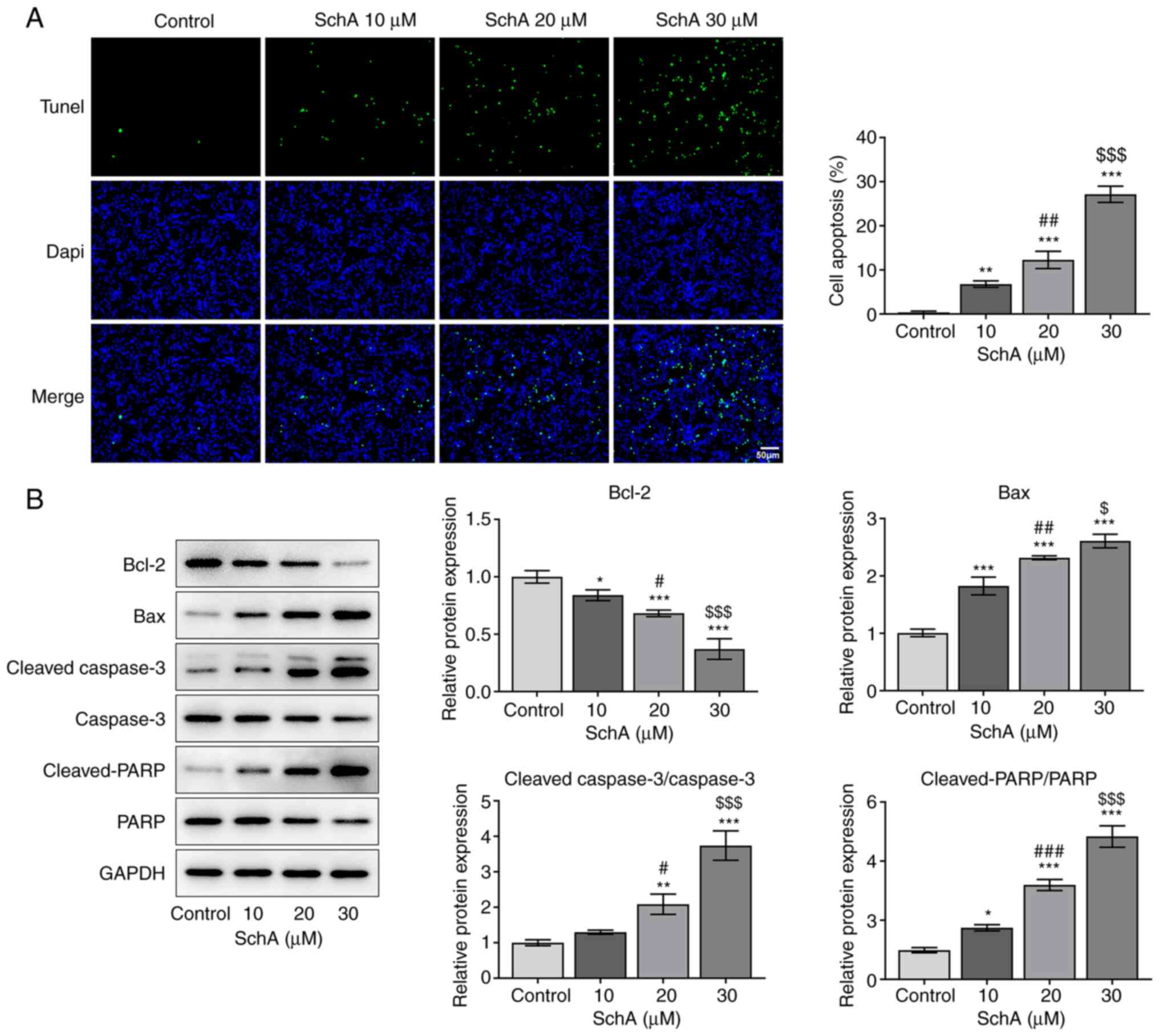
The apoptosis of AGS cells exposed to SchA was
subsequently detected to determine the role of SchA in this
process. Notably, the apoptosis of AGS cells was enhanced by
increasing doses of SchA when compared with the control group
(Fig. 3A). Similarly, the
expression levels of the anti-apoptotic protein Bcl-2 were
downregulated, while those of the pro-apoptotic proteins Bax,
cleaved-caspase 3/caspase 3 and cleaved-PARP/PARP were increased
after SchA treatment in AGS cells (Fig.
3B). However, the expression levels of caspase 3 and PARP
remained unchanged after exposure to 10 µM SchA in AGS cells, while
they were markedly decreased as the doses of SchA increased to 20
and 30 µM. Overall, it was suggested that SchA induced the
apoptosis of AGS cells.
SchA activates ER stress in AGS
cells
Stimulating factors, which can disrupt the
homeostasis of proteins, can induce ER stress in tumor cells
(11). The expression level of
HSPA5, the marker of ER stress, was increased by SchA in AGS cells
(Fig. 4A). Increasing doses of SchA
increased the phosphorylation of eIF2α and PERK, as well as the
expression levels of CHOP, indicating the activation of ER stress
by SchA in AGS cells (Fig. 4B).
Since 30 µM SchA could notably activate ER stress in AGS cells,
this dose was selected for the subsequent experiments.
4-PBA, an ER stress inhibitor,
reverses the anti-proliferative, anti-invasive, anti-migratory and
pro-apoptotic effects of SchA on AGS cells
To further determine whether SchA affects the
functions of AGS cells, the ER stress inhibitor 4-PBA was used to
treat AGS cells. Cell viability was decreased after treatment with
SchA, which was restored by 4-PBA (Fig.
5A). The proliferation, migration and invasion of AGS cells
were suppressed by SchA, while co-treatment with 4-PBA and SchA
alleviated this effect (Fig. 5B-D).
The expression levels of MMP-2 and MMP-9, which are associated with
invasion and cancer angiogenesis (12), were found to be increased by
co-treatment with 4-PBA and SchA compared with those in the SchA
group (Fig. 5E). The expression of
E-cadherin was downregulated, while expression levels of N-cadherin
and vimentin were upregulated in the 4-PBA + SchA group compared
with that in the SchA group (Fig.
5F). The apoptosis of AGS cells was induced by SchA, while
co-treatment of cells with 4-PBA and SchA led to decreased levels
of apoptosis compared with the SchA group (Fig. 6A and B). In addition, the results of
western blotting demonstrated that HSPA5 expression was increased
by SchA treatment, but was reversed by 4-PBA treatment (Fig. 6C). Moreover, the expression levels
of p-PERK, p-eIF2α and CHOP were increased by SchA treatment, while
these levels were decreased by 4-PBA and SchA treatment, suggesting
that 4-PBA inhibited ER stress, which was activated by SchA
(Fig. 6D). These results suggested
the important role of 4-PBA in reversing the anti-proliferative,
anti-invasive, anti-migratory and pro-apoptotic effects of SchA on
AGS cells.
 | Figure 6.4-PBA, an ER stress inhibitor,
reverses the pro-apoptotic role of SchA in AGS cells. (A) The
apoptosis of AGS cells after SchA and 4-PBA exposure. (B) The
expression levels of apoptosis-related proteins after in AGS cells
after SchA and 4-PBA exposure. (C) The expression levels of the
marker of ER stress (HSPA5). (D) The expression levels of markers
related to ER stress. *P<0.05 and
***P<0.001 vs. Control; #P<0.05,
##P<0.01, ###P<0.001 vs. SchA. SchA,
schizandrin A; 4-PBA, 4-phenylbutyric acid; ER, endoplasmic
reticulum; HSPA5, heat shock protein family A (Hsp70) member 5; p-,
phosphorylated; eiF2α, eukaryotic initiation factor 2 α; PARP,
poly(ADP-ribose) polymerase. |
Discussion
GC is one of the most prevalent types of cancer and,
at present, it remains difficult to completely cure (13). Surgical interventions, which are
essential methods for cancer treatment, have increased the overall
survival rates of patients with GC (14). However, the prognosis of GC remains
poor, and the majority of patients with GC are diagnosed at an
advanced stage (15). In recent
years, the active ingredients of traditional Chinese medicine (TCM)
have been demonstrated to serve critical roles in the treatment of
cancer, since the combined use of chemotherapy and TCM can greatly
improve the overall survival of patients with GC (16).
Sch is the fruit of Schisandra chinensis and
is a type of TCM used as a food supplement, as well as for medical
interventions (17). In addition to
its use in the treatment of clinical symptoms, such as fatigue,
cough, dysentery and insomnia, it has been investigated for its
notable antioxidant and antiviral effects in recent years (18). Accumulating evidence has indicated
that the cytotoxicity of SchA can suppress the proliferation of
human cancer cells, thereby inducing their apoptosis and delaying
cancer progression (19). The
present study first revealed that the viability of AGS cells was
affected by increasing doses of SchA, and the results of the colony
formation assay demonstrated the reduced colony-forming ability of
AGS cells upon SchA exposure. Moreover, the migration and invasion
of AGS cells were both decreased after SchA treatment, and SchA at
the concentration of 30 µM exerted the most obvious inhibitory
effects on the migration and invasion of AGS cells. Furthermore,
the apoptosis of AGS cells was significantly induced by SchA, which
was in line with previous findings suggesting the promoting role of
SchA in the apoptosis of human cancer cells (7).
The ER is the organelle where protein handling,
modification and folding occur (20). The homeostasis of the ER is
essential for cell functions and cell fate (20). However, upon stimulation by certain
environmental factors, the homeostasis of the ER cannot be
maintained, thereby triggering ER stress (21). ER stress has been well-documented to
be involved in the pathophysiology of most common diseases,
including metabolic disease, neurodegenerative disease,
inflammatory disease and cancer (22). ER stress, which is associated with
the form of heat shock protein 70-type binding immunoglobulin
protein/HSPA5, activates intraluminal ER sensors, including eIF2α,
PERK and activating transcription factor 6 (23). p-eIF2α suppresses the commencement
of protein synthesis, which reduces the protein loading in the ER
and ameliorates ER stress (24).
PERK serves as a critical signaling protein related to the
evolution of ER stress, and it is considered to block the
aggregation of unfolded proteins in the ER, thereby modulating ER
stress in an adverse feedback pattern (25). CHOP is considered as a key protein
responsible for the modulation of ER stress-mediated cell apoptosis
(26). A previous study reported
that ER stress hyperactivated both PERK and inositol-requiring
transmembrane kinase/endoribonuclease 1α (IRE-1α), contributing to
the entry into the apoptosis pathway (27). In the present study, SchA triggered
the apoptosis of AGS cells and phosphorylation of PERK and IRE-1α.
Therefore, it was hypothesized that SchA may affect the behaviors
of AGS cells via the activation of ER stress. These results
suggested that the phosphorylation of PERK and eIF2α, and elevated
expression levels induced by SchA were reversed by 4-PBA, which
verified this hypothesis.
Studies have shown that the ER stress response was
ubiquitous in tumor tissues, and that it regulates the occurrence
and development of tumors, and participates in tumor invasion and
metastasis (28–31). The degree of ER stress is positively
correlated with the depth of invasion and the degree of metastasis
(32). Li et al (33) revealed that downregulation of
heparinase can reverse ER stress-mediated invasion of breast cancer
cells. Moreover, Liu et al (34) observed that knocking down the
expression of HSPA5, an indicator protein of ER stress, could
significantly reduce the invasive ability of tumor cells by
inhibiting the PI3K/AKT signaling pathway. These studies suggest
that ER stress may be an important inducement for tumor cell
invasion, and could play a key role in tumor metastasis. The
invasion and migration of gastric cancer cells are significantly
enhanced after treatment with tunicamycin (an ER stress inducer),
indicating that ER stress promotes the invasion and migration of
gastric cancer cells (35). Thus,
ER stress may be an important regulatory mechanism of gastric
cancer metastasis. In the present study, 4-PBA reversed the
anti-proliferative, anti-invasive, anti-migratory and pro-apoptotic
effects of SchA on AGS cells. However, a limitation of the present
study is the lack of flow cytometry assays to further confirm the
apoptosis rates. Furthermore, the downstream regulators of SchA in
the regulation of ER stress are still unknown, and thus will be
investigated in the future.
In conclusion, the present study demonstrated that
SchA induced the apoptosis and suppressed the proliferation,
invasion and migration of GC cells by activating ER stress,
providing a theoretical basis for the use of SchA in the treatment
of GC.
Acknowledgements
Not applicable.
Funding
This study was supported by the Interventional study
of Yang Yin Jian Pi San Du Method for advanced gastric cancer
(grant no. YB2017056).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HP designed the present study and wrote the
manuscript. HP, QQ, FW and MG performed the experiments and
analyzed the data. XG conceived and supervised the study and
co-wrote the manuscript. XG and HP confirm the authenticity of all
the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare they have no competing
interests.
References
|
1
|
Shomali N, Mansoori B, Mohammadi A,
Shirafkan N, Ghasabi M and Baradaran B: MiR-146a functions as a
small silent player in gastric cancer. Biomed Pharmacother.
96:238–245. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Howson CP, Hiyama T and Wynder EL: The
decline in gastric cancer: Epidemiology of an unplanned triumph.
Epidemiol Rev. 8:1–27. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Van Cutsem E, Moiseyenko VM, Tjulandin S,
Majlis A, Constenla M, Boni C, Rodrigues A, Fodor M, Chao Y, Voznyi
E, et al: Phase III study of docetaxel and cisplatin plus
fluorouracil compared with cisplatin and fluorouracil as first-line
therapy for advanced gastric cancer: A report of the V325 study
group. J Clin Oncol. 24:4991–4997. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu X, Rajamanicham V, Xu S, Liu Z, Yan T,
Liang G, Guo G, Zhou H and Wang Y: Schisandrin A inhibits triple
negative breast cancer cells by regulating Wnt/ER stress signaling
pathway. Biomed Pharmacother. 115:1089222019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yan H and Guo M: Schizandrin A inhibits
cellular phenotypes of breast cancer cells by repressing miR-155.
IUBMB Life. 72:1640–1648. 2020. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen BC, Tu SL, Zheng BA, Dong QJ, Wan ZA
and Dai QQ: Schizandrin A exhibits potent anticancer activity in
colorectal cancer cells by inhibiting heat shock factor 1. Biosci
Rep. 40:BSR202002032020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bi Y, Fu Y, Wang S, Chen X and Cai X:
Schizandrin A exerts anti-tumor effects on A375 cells by
down-regulating H19. Braz J Med Biol Res. 52:e83852019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ding Q, Li X, Sun Y and Zhang X:
Schizandrin A inhibits proliferation, migration and invasion of
thyroid cancer cell line TPC-1 by down regulation of microRNA-429.
Cancer Biomark. 24:497–508. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ma YY, Di ZM, Cao Q, Xu WS, Bi SX, Yu JS,
Shen YJ, Yu YQ, Shen YX and Feng LJ: Xanthatin induces glioma cell
apoptosis and inhibits tumor growth via activating endoplasmic
reticulum stress-dependent CHOP pathway. Acta Pharmacol Sin.
41:404–414. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xian H, Feng W and Zhang J: Schizandrin A
enhances the efficacy of gefitinib by suppressing IKKβ/NF-κB
signaling in non-small cell lung cancer. Eur J Pharmacol.
855:10–19. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Urra H, Dufey E, Avril T, Chevet E and
Hetz C: Endoplasmic reticulum stress and the hallmarks of cancer.
Trends Cancer. 2:252–262. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Farina P, Tabouret E, Lehmann P, Barrie M,
Petrirena G, Campello C, Boucard C, Graillon T, Girard N and Chinot
O: Relationship between magnetic resonance imaging characteristics
and plasmatic levels of MMP2 and MMP9 in patients with recurrent
high-grade gliomas treated by Bevacizumab and Irinotecan. J
Neurooncol. 132:433–437. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shi Y, Shi H, Zhang B, Yan Y, Han X, Jiang
W, Qian H and Xu W: miR-373 suppresses gastric cancer metastasis by
downregulating vimentin. Mol Med Rep. 17:4027–4034. 2018.PubMed/NCBI
|
|
14
|
Wu D, Zhang P, Ma J, Xu J, Yang L, Xu W,
Que H, Chen M and Xu H: Serum biomarker panels for the diagnosis of
gastric cancer. Cancer Med. 8:1576–1583. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rona KA, Schwameis K, Zehetner J, Samakar
K, Green K, Samaan J, Sandhu K, Bildzukewicz N, Katkhouda N and
Lipham JC: Gastric cancer in the young: An advanced disease with
poor prognostic features. J Surg Oncol. 115:371–375. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu X, Xiu LJ, Jiao JP, Zhao J, Zhao Y, Lu
Y, Shi J, Li YJ, Ye M, Gu YF, et al: Traditional Chinese medicine
integrated with chemotherapy for stage IV non-surgical gastric
cancer: A retrospective clinical analysis. J Integr Med.
15:469–475. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee K, Ahn JH, Lee KT, Jang DS and Choi
JH: Deoxyschizandrin, isolated from schisandra berries, induces
cell cycle arrest in ovarian cancer cells and inhibits the
protumoural activation of tumour-associated macrophages. Nutrients.
10:912018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Panossian A and Wikman G: Pharmacology of
Schisandra chinensis Bail.: An overview of Russian research
and uses in medicine. J Ethnopharmacol. 118:183–212. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li L, Pan Q, Sun M, Lu Q and Hu X:
Dibenzocyclooctadiene lignans: A class of novel inhibitors of
multidrug resistance- associated protein 1. Life Sci. 80:741–748.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen X and Cubillos-Ruiz JR: Endoplasmic
reticulum stress signals in the tumour and its microenvironment.
Nat Rev Cancer. 21:71–88. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bettigole SE and Glimcher LH: Endoplasmic
reticulum stress in immunity. Annu Rev Immunol. 33:107–138. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang S and Kaufman RJ: The impact of the
unfolded protein response on human disease. J Cell Biol.
197:857–867. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bertolotti A, Zhang Y, Hendershot LM,
Harding HP and Ron D: Dynamic interaction of BiP and ER stress
transducers in the unfolded-protein response. Nat Cell Biol.
2:326–332. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mohamed E, Cao Y and Rodriguez PC:
Endoplasmic reticulum stress regulates tumor growth and anti-tumor
immunity: A promising opportunity for cancer immunotherapy. Cancer
Immunol Immunother. 66:1069–1078. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang J, Hu X and Jiang H: ERS-PERK
signaling pathway-mediated Nrf2/ARE-HO-1 axis: A novel therapeutic
target for attenuating myocardial ischemia and reperfusion injury.
Int J Cardiol. 203:779–780. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sozen E, Karademir B and Ozer NK: Basic
mechanisms in endoplasmic reticulum stress and relation to
cardiovascular diseases. Free Radic Biol Med. 78:30–41. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Oakes SA and Papa FR: The role of
endoplasmic reticulum stress in human pathology. Annu Rev Pathol.
10:173–194. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kaira K, Toyoda M, Shimizu A, Shino M,
Sakakura K, Takayasu Y, Takahashi K, Asao T and Chikamatsu K:
Expression of ER stress markers (GRP78/BiP and PERK) in adenoid
cystic carcinoma. Acta Otolaryngol. 136:1–7. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zheng YZ, Cao ZG, Hu X and Shao ZM: The
endoplasmic reticulum stress markers GRP78 and CHOP predict
disease-free survival and responsiveness to chemotherapy in breast
cancer. Breast Cancer Res Treat. 145:349–358. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nagelkerke A, Bussink J, Sweep FC and Span
PN: The unfolded protein response as a target for cancer therapy.
Biochim Biophys Acta. 1846:277–284. 2014.PubMed/NCBI
|
|
31
|
Moon SY, Kim HS, Nho KW, Jang YJ and Lee
SK: Endoplasmic reticulum stress induces epithelial-mesenchymal
transition through autophagy via activation of c-Src kinase.
Nephron Exp Nephrol. 126:127–140. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang M and Kaufman RJ: The impact of the
endoplasmic reticulum protein-folding environment on cancer
development. Nat Rev Cancer. 14:581–597. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li Y, Liu H, Huang YY, Pu LJ, Zhang XD,
Jiang CC and Jiang ZW: Suppression of endoplasmic reticulum
stress-induced invasion and migration of breast cancer cells
through the downregulation of heparanase. Int J Mol Med.
31:1234–1242. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu R, Li X, Gao W, Zhou Y, Wey S, Mitra
SK, Krasnoperov V, Dong D, Liu S, Li D, et al: Monoclonal antibody
against cell surface GRP78 as a novel agent in suppressing PI3K/AKT
signaling, tumor growth, and metastasis. Clin Cancer Res.
19:6802–6811. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dong KN, Huang X, Xing WY, Guo WW and Feng
R: Effect of endoplasmic reticulum stress on gastric cancer cell
migration and invasion. World Chin J Dig. 24:14852016. View Article : Google Scholar
|