Introduction
NSCLC is the most commonly diagnosed cancer and is
also the main cause of cancer-related death worldwide. NSCLC has
three major types: Adenocarcinoma (ADC), squamous cell carcinoma
(SCC) and large cell carcinoma (LCC) (1,2).
Despite advances in surgery, radiotherapy and chemotherapy, the
5-year survival rate of lung cancer is <15%, mainly because
numerous patients are diagnosed at the late stage of the disease
and due to the frequent occurrence of metastasis (3,4). In
recent years, to treat lung cancer, certain genes (e.g., epidermal
growth factor receptor, human epidermal growth factor receptor 2
and ALK receptor tyrosine kinase) have been successfully targeted
and there have been ongoing advances in NSCLC care (5). In addition, the treatment of lung
cancer with immunotherapy has made significant progress; however,
only a few patients can benefit from these treatments (6). Therefore, it has become clear that
additional therapeutic strategies are required to provide an
improved survival benefit for patients with NSCLC. Inhibin βA
(INHBA) is a disulfide-linked homodimer of activin A and is a
member of the transforming growth factor (TGF)-β superfamily
(7). Previous research has focused
on the controversial function of activin A in cancer (8), and both tumor-promoting and
tumor-suppressing roles of activin A have been identified (9–13).
Notably, previous studies have demonstrated the tumor-promoting
effects of INHBA on gastric cancer, urothelial carcinoma and
nasopharyngeal carcinoma, and INHBA expression has been shown to be
correlated with enhanced invasion, proliferation and poor prognosis
(14–16). However, the role of INHBA in NSCLC
pathogenesis remains unclear. Seder et al (17) revealed that in >70% of cases of
lung ADC, INHBA was overexpressed, and a significantly worse
prognosis was observed in patients with high INHBA
transcription compared with those with low INHBA
transcription (17). Wamsley et
al (18) further demonstrated
increased activin expression in primary LCC, SCC and ADC tumors.
These studies suggested that metastasis and invasion of NSCLC may
be regulated by INHBA; however, the potential molecular mechanism
remains unknown.
Previous research has investigated the cross-talk
between TGF-β and Hippo signaling. Varelas et al (19) and Narimatsu et al (20) suggested that tafazzin (TAZ) and
yes-associated protein (YAP) may mediate nuclear Smad signals.
Moreover, Fujii et al (21)
suggested that YAP was critical for TGF-β-mediated induction of a
small subset of target genes dependent on YAP (e.g., Smad2 and
matrix metalloproteinase 7) (21).
Therefore, we hypothesized that INHBA, the TGF-β superfamily
member, may be associated with the Hippo pathway in NSCLC. When the
Hippo signaling pathway is activated, its core components,
mammalian sterile 20-like 1/MOB kinase activator and large tumor
suppressor kinase (LATS)1/2 can regulate the transcriptional
co-activator YAP/TAZ, forming a kinase cascade that culminates in
phosphorylation and inactivation of YAP/TAZ, thereby inhibiting the
development of lung cancer (22–24).
Conversely, certain upstream regulators of the Hippo pathway, such
as Merlin [encoded by the neurofibromin 2 (NF2) gene], FERM
domain-containing 6 (FRMD6) and KIBRA [also known as WW and C2
domain-containing 1 (WWC1)], have also been shown to regulate NSCLC
invasion and metastasis (25).
In view of the potential association of INHBA with
the invasion and metastasis of NSCLC, the present study aimed to
investigate the molecular mechanism underlying the INHBA-mediated
regulation of invasion and metastasis of NSCLC. In addition, the
clinical significance of INHBA in patients with NSCLC was assessed
and the present study aimed to reveal effects of INHBA on
regulation of the Hippo signaling pathway in NSCLC.
Materials and methods
Ethics statement
The Institute Research Medical Ethics Committee of
Sun Yat-sen University Cancer Center (Guangzhou, China) approved
the present study, and the patients involved in this study provided
informed consent (verbal or written) for the retrospective analysis
of tissue samples. All samples were subjected to anonymization.
Clinical samples
Tissue samples were collected from 238 cases of
NSCLC diagnosed at the Sun Yat-sen University Cancer Center between
January 2010 and December 2015. The samples were from 172 men and
66 women (median age, 58 years). The lung tumor histological
criteria (2015) from the World Health Organization (26), were used to classify the samples as
ADC (138 cases), SCC (82 cases) and LCC (18 cases). Differentiation
was assessed as being high in 32 cases, moderate in 94 cases and
poor in 112 cases. Lymph node metastases were present in 133 of the
patients. Cases with lymph node metastases were selected for a
comparison of metastatic nodules with the primary tumor. The
tumor-node-metastasis (TNM) staging system of the International
Union against Cancer (27) was used
for tumor staging. A total of 100 patients had stage I disease and
138 patients had stage II–IV disease. Before surgery, none of the
patients had been treated with radiotherapy or chemotherapy;
however, after surgery, these two treatments were provided as
standard care. For pathological analysis and diagnosis, all
collected samples were fixed with 10% formalin at room temperature
for 24 h, paraffin-embedded, cut into 4-µm thick sections and
stained with hematoxylin and eosin for pathological analysis.
Furthermore, an additional 24 fresh NSCLC and adjacent non-tumor
tissues samples were obtained from 24 patients who underwent
surgical resection for NSCLC at Sun Yat-sen University Cancer
Center between January 2018 and December 2020. The 24 NSCLC samples
were obtained from 17 (70.8%) men and 7 (29.2%) women (mean age,
59.0 years). In total, 14 cases were ADC, 9 cases were SCC and 1
case was LCC. Differentiation was high in 8 cases, moderate in 10
cases and poor in 6 cases. In addition, 10 cases showed lymph node
metastases. All 24 samples were randomly selected from the fresh
sample library diagnosed with NSCLC, which were frozen in liquid
nitrogen immediately after resection and stored at −80°C until
use.
Cell culture
The human lung cancer cell lines A549, H1299 and
H460 were obtained from The Cell Bank of Type Culture Collection of
The Chinese Academy of Sciences in 2019. H157, H520 and normal
human bronchial epithelial (HBE) cells were obtained from the
American Type Culture Collection. RPMI-1640 medium (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% FBS (Biological
Industries), penicillin (100 U/ml), streptomycin (100 µg/ml), 2 mM
glutamine and 10 mM HEPES buffer was used for cell culture at
37°C.
Immunohistochemistry (IHC)
For IHC, a tissue microarray (TMA) consisting of 238
primary NSCLC tissues, 30 paired metastatic nodule samples and 30
adjacent normal lung tissues was constructed. The paraffin-embedded
samples were serially cut into 4-µm sections, de-paraffinized in
xylene, rehydrated through a graded alcohol series, immersed in 3%
hydrogen peroxide at room temperature for 10 min to block
endogenous peroxidase activity and subjected to antigen retrieval
by pressure cooking for 3 min in citrate buffer (pH 6.0).
Subsequently, the slides were incubated with 10% normal goat serum
(Beyotime Institute of Biotechnology) at room temperature for 30
min to reduce non-specific reactivity and then incubated with
anti-YAP antibodies (1:400; cat. no. 14074; Cell Signaling
Technology, Inc.) and anti-INHBA antibodies (1:200; cat. no.
ab97705; Abcam) overnight at 4°C. Slides were then washed twice
with PBS for 5 min, incubated with a secondary antibody (ready to
use; cat. no. GK500705; Envision; Dako; Agilent Technologies, Inc.)
for 1 h at room temperature, washed twice with PBS for 5 min and
developed using 3,3-diaminobenzidine. Finally, the sections were
counterstained with Mayer's hematoxylin for 3 min at room
temperature, dehydrated and mounted with neutral gum. Negative
controls were prepared by replacing the primary antibody with
normal murine IgG (1:200; cat. no. A7028; Beyotime Institute of
Biotechnology). Two experienced pathologists scored all the
sections independently. As described previously (24), the IHC score was obtained by
multiplying the score for the percentage of positively stained
cells (0, <5%; 1, 6–25%; 2, 26–50%; 3, 51–75%; 4, 76–100%) by
the score of the staining intensity (0, negative staining; 1, weak
staining; 2, moderate staining and 3, strong staining) to give a
final score for each section. Sections with a total score of ≥3
were defined as exhibiting positive staining. Nuclear YAP
positivity was scored by determining the percentage of positive
nuclei, regardless of cytoplasmic YAP expression and staining
intensity. All tissues were examined using an Olympus BX53
microscope (Olympus Corporation; magnification, ×400).
Western blotting
Western blotting was performed according to a
previously described method (28).
Cells were lysed using sonication at 20 KHz or RIPA lysis buffer
(Pierce; Thermo Fisher Scientific, Inc.) at 4°C for 20 min, and
proteins were quantified using the Bradford protein assay. A total
of 50 µg protein/lane was separated by SDS-PAGE on a 10%
polyacrylamide gel. The separated proteins were transferred to
polyvinylidene fluoride membranes (MilliporeSigma), which were
blocked with 5% bovine serum albumin (Merck KGaA) at 37°C for 2 h.
The membranes were then incubated overnight at 4°C with the
following antibodies: YAP (1:500; cat. no. 14074), phosphorylated
(p)YAP-S127 (1:500; cat. no. 57706), vimentin (1:1,000; cat. no.
12826), Snail (1:500; cat. no. 3895), GAPDH (1:1,000; cat. no.
5174s) (all from Cell Signaling Technology, Inc.); INHBA (1:500;
cat. no. ab113489), LATS1/2 (1:500; cat. no. ab70565), pLATS1/2
(T1079 + T1041; 1:500; cat. no. ab111344), tubulin (1:1,000; cat.
no. ab6160), Lamin B (1:1,000; cat. no. ab16048), connective tissue
growth factor (CTGF; 1:500; cat. no. ab94939), cysteine-rich
angiogenic inducer 61 (CYR61; 1:500; cat. no. ab228592), WWC1
(1:1,000; cat. no. ab216508), FRMD6 (1:1,000; cat. no. ab110675)
(all from Abcam); E-cadherin (1:500; cat. no. 610181), N-cadherin
(1:1,000; cat. no. 610920) (both from BD Biosciences) and Merlin
(1:100; sc-331; Santa Cruz Biotechnology, Inc.). After incubation
with anti-mouse (1:2,000; cat. no. E030110-01) or anti-rabbit
(1:2,000; cat. no. E030120-01) IgG antibodies (EarthOx Life
Sciences) at 37°C for 2 h, the protein bands were visualized using
enhanced chemiluminescence (Thermo Fisher Scientific, Inc.) and
semi-quantified using a BioImaging Systems platform. Relative
protein expression levels were calculated using GAPDH as the
loading control.
Construction of plasmids and
transfection
Plasmids expressing full-length human INHBA and
Merlin constructs, and empty vectors were all purchased from
Shanghai GeneChem Co., Ltd. Specifically, the coding sequences of
INHBA and Merlin were inserted between the BamHI and
AgeI restriction enzyme sites of the
Ubi-3FLAG-CBh-gcGFP-IRES-puromycin vector to generate the following
overexpression plasmids of these two genes:
Ubi-INHBA-3FLAG-CBh-gcGFP-IRES-puromycin and
Ubi-Merlin-3FLAG-CBh-gcGFP-IRES-puromycin. The small interfering
RNA (siRNA) sequences targeting INHBA and a negative control (NC)
were all purchased from Guangzhou Ruibo Co., Ltd.; the sequences
are shown in Table SI. The NC
siRNA targets no known genes. Lipofectamine® 3000
(Invitrogen; Thermo Fisher Scientific, Inc.) was used to perform
all transfections of lung cancer cells in 6-well plates. The
concentration of plasmids transfected into cells was 1 µg/µl and
that of siRNA was 20 pmol/µl. Briefly, A549 and H1299 cells were
seeded 24 h prior to transfection in 6-well plates
(5×105 cells/well) and were transfected using
Lipofectamine 3000 according to the manufacturer's protocol. After
transfection, cells were cultured at 37°C for 48 h, and then
collected for reverse transcription-quantitative PCR (RT-qPCR) or
western blotting.
RNA extraction and RT-qPCR
RT-qPCR was performed as described previously
(28). TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) was used to extract
total RNA from tissues and cells according to the manufacturer's
protocol. Subsequently, 1 µg total RNA was used to produce cDNA
using a PrimeScript RT Reagent kit (Takara Biotechnology Co., Ltd.)
according to the manufacturer's protocol. The SYBR-Green PCR Master
Mix and StepOne-Plus Real-Time PCR system (both Applied Biosystems;
Thermo Fisher Scientific, Inc.) were used to perform qPCR. The
thermocycling conditions were as follows: 94°C for 2 min, followed
by 40 cycles at 94°C for 15 sec and 60°C for 1 min according to the
manufacturer's protocol. The primers used for qPCR are shown in
Table SII. The mRNA ΔCq values
from each sample were calculated by normalizing to the reference
gene 18S and using the 2−∆∆Cq method (29). Experiments were performed in
triplicate.
In vitro migration and invasion
In vitro invasion and migration assays were
performed as described previously (30). For the migration assay, cells
(3–5×104 cells; 100 µl) were seeded into the upper
chamber of a Transwell plate (8-µm pore size; Corning, Inc.) in 200
µl serum-free media, whereas the lower chamber was filled with 600
µl media supplemented with 20% FBS. For the invasion assay, cells
(3–5×104 cells; 100 µl) in serum-free medium were seeded
into the upper chamber of a Transwell plate, which had been
pre-coated with Matrigel (BD Biosciences) at 4°C overnight and then
37°C for 30 min, and the lower chamber was filled with 600 µl media
supplemented with 20% FBS. After incubation at 37°C for 24 h,
migrated/invaded cells in the lower compartment were washed with
PBS, fixed with 4% paraformaldehyde for 30 min and stained with 1%
crystal violet for 20 min. Both the fixing and staining steps were
conducted at room temperature. For each filter, the numbers of
migrated/invaded cells on the lower surface of the membrane were
counted in five randomly selected fields at ×200 magnification
under a Nikon E200 light microscope (Nikon Corporation). The
experiment was performed three times to calculate the mean number
of cells.
A rescue experiment was performed using Verteporfin
(VP; Selleck Chemicals), a small molecule Hippo pathway inhibitor
that can inhibit the YAP-TEA domain transcription factor
interaction. DMSO was used to dissolve VP, which was added to the
H1299 cells with culture medium at a final concentration of 10 µM
at 37°C for 24 h. Next, H1299 cells were collected for western
blotting, and migration and invasion assays.
Subcellular fractionation
A Nuclear and Cytoplasmic Protein Extraction Kit
(Beyotime Institute of Biotechnology) was used to separate the
nuclear and cytoplasmic fractions of lung cancer cell extracts,
according to the manufacturer's protocol. The proteins in the
extracts were then analyzed using western blotting.
Co-immunoprecipitation
Proteins were extracted from the cells prior to and
following transfection. The cells were collected and incubated with
300 µl RIPA lysis buffer (Beyotime Institute of Biotechnology) with
protease inhibitors for 40 min on ice. After protein concentration
was quantified using a Bradford assay, 6,000 µg each lysate was
added to 2 µg INHBA, Merlin or IgG (cat. no. B900620; ProteinTech
Group, Inc.) antibodies and incubated at 4°C overnight.
Subsequently, 20 µl protein A/G-agarose beads (Santa Cruz
Biotechnology, Inc.) were added and the samples were gently
agitated for 3 h at 4°C. The samples were then centrifuged at 2,500
× g for 5 min at 4°C and washed three times with lysis buffer to
collect the precipitate. Finally, the precipitate was boiled with
40 µl loading buffer at 100°C for 5 min and analyzed using western
blotting as aforementioned.
Statistical analysis
All experiments were repeated more than three times,
and the data are presented as the mean ± SD. All data were analyzed
using SPSS statistical software package version 16.0 (SPSS, Inc.)
and GraphPad Prism 5 software (GraphPad Software, Inc.).
χ2 test was used to analyze the association between
INHBA expression and the clinical characteristics of patients with
NSCLC. Wilcoxon test and Mann-Whitney U tests were used for
non-parametric data as appropriate. The Kaplan-Meier method and
log-rank tests and Cox regression analysis were used to analyze the
association between INHBA expression and OS and DFS in patients
with NSCLC. An unpaired Student's t-test was used to compare the
inter-group difference between two groups. Comparative data among
multiple groups were analyzed using one-way ANOVA followed by
Tukey's test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Clinical relevance of INHBA positivity
in samples of NSCLC
To determine the clinical relevance of INHBA in
NSCLC, the expression levels of INHBA were detected using
immunostaining of a TMA containing 238 NSCLC and 30 normal lung
samples. The IHC scores of INHBA in 238 NSCLC tissues were
significantly higher compared with those in the 30 cases of normal
lung tissues (Fig. 1A). Moreover,
INHBA immunoreactivity was graded as negative or positive. Out of
the 238 cases, INHBA expression was positive in 73.5% (175/238) of
NSCLC tissues, whereas only 13.3% (4/30) of normal lung samples
demonstrated a positive INHBA signal (Table I). Furthermore, 24 NSCLC tissues and
paired adjacent non-tumor tissues were processed for western blot
analysis of INHBA. The protein expression levels of INHBA in NSCLC
samples were significantly higher compared with those in non-tumor
tissues (P<0.001; Fig. 1B and
C). Similar results were observed with regard to the mRNA
expression levels (P<0.01; Fig.
1D). In addition, INHBA expression was detected in 30 pairs of
NSCLC primary tumors and corresponding metastatic nodules using
IHC. The expression levels of INHBA were significantly increased in
metastatic nodules compared with those in the primary tumors
(P=0.003; Fig. 1E and F). Pearson
χ2 tests for clinical association revealed that positive
INHBA expression in NSCLC samples was significantly associated with
advanced tumor stage (TNM stage II–IV; P=0.017) and poor
differentiation (P=0.016) (Table
I).
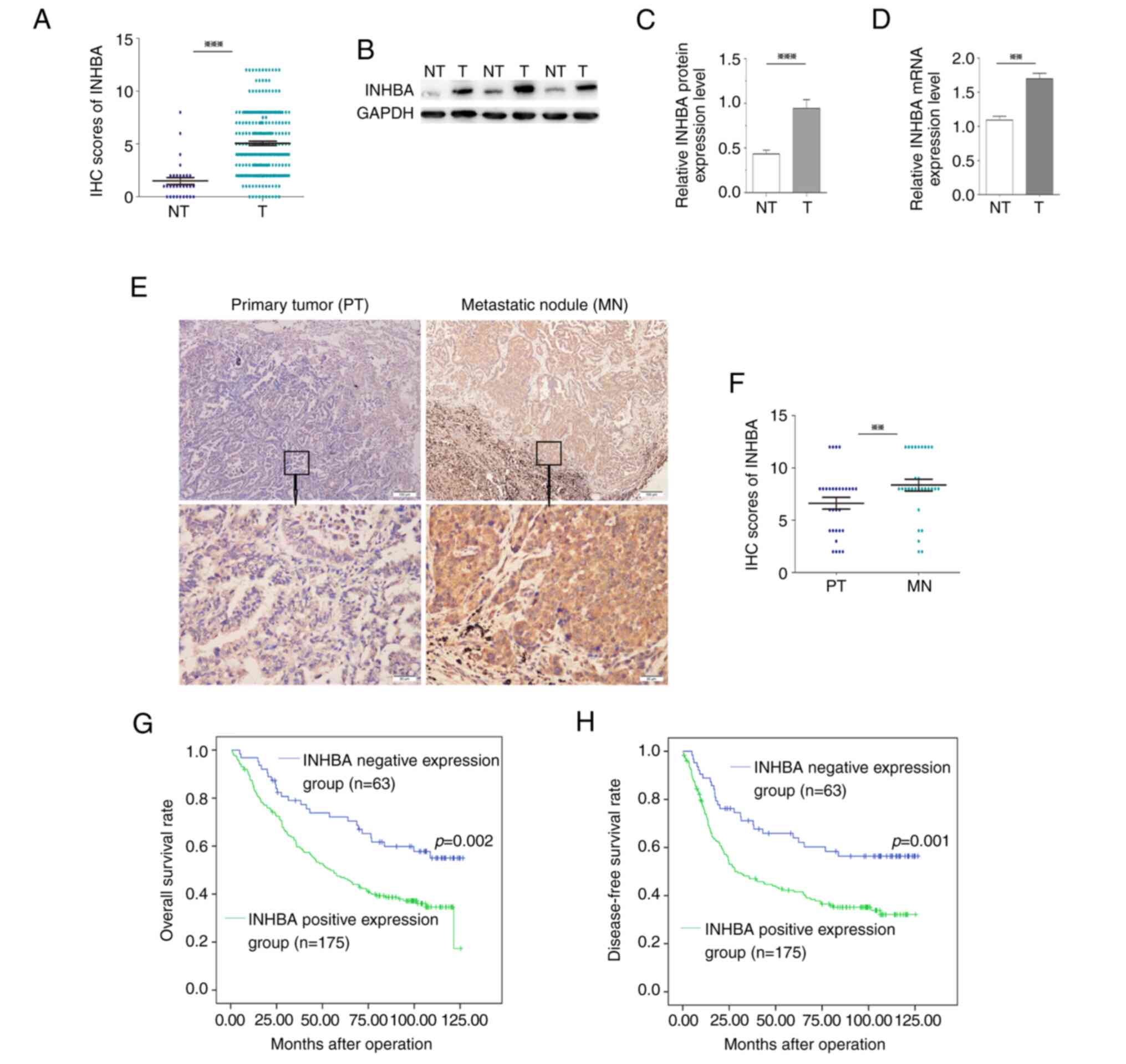 | Figure 1.Clinical significance of INHBA in
non-small cell lung cancer. (A) IHC scores of INHBA in 238 NSCLC
tissues were significantly higher compared with those in 30 cases
of normal lung tissues. (B) Western blotting of INHBA expression
levels in T tissues and in matched, adjacent NT tissues. (C)
Semi-quantification of western blotting of 24 paired tissue
samples. INHBA protein expression levels were significantly higher
in T samples compared with those in NT samples. (D) mRNA expression
levels of INHBA were significantly higher in T samples
compared with those in NT samples. (E) Representative cases showed
that INHBA expression was increased in MN tissues compared with in
paired PT tissues (original magnification, ×40 or ×200). (F) Based
on the scores from immunohistochemistry, the abundance of INHBA was
elevated in MN tissues compared with in PT tissues (n=30). (G)
Kaplan-Meier curve for overall survival in 238 patients. Higher
INHBA expression levels in NSCLC, based on immunohistochemistry
scores, were associated with poorer overall survival. (H)
Kaplan-Meier curve for disease-free survival. Higher INHBA
expression levels in NSCLC were associated with poorer disease-free
survival. **P<0.01, ***P<0.001. INHBA, inhibin βA; IHC,
immunohistochemistry; NSCLC, non-small cell lung cancer; T, tumor;
NT, non-tumor; MN, metastatic nodule; PT, primary tumor. |
 | Table I.Clinical characteristics of patients
(n=238) with non-small cell lung cancer who were positive or
negative for INHBA expression. |
Table I.
Clinical characteristics of patients
(n=238) with non-small cell lung cancer who were positive or
negative for INHBA expression.
|
|
| INHBA |
|
|---|
|
|
|
|
|
|---|
| Characteristic | Number of
patients | Negative | Positive | P-value |
|---|
| Group |
|
|
| 0.001a |
|
Non-tumor | 30 | 26 | 4 |
|
|
Tumor | 238 | 63 | 175 |
|
| Age, years |
|
|
| 0.660 |
|
<60 | 124 | 31 | 93 |
|
|
≥60 | 114 | 32 | 82 |
|
| Sex |
|
|
| 0.871 |
|
Female | 66 | 18 | 48 |
|
|
Male | 172 | 45 | 127 |
|
| Histology |
|
|
| 0.082 |
|
ADC | 138 | 44 | 94 |
|
|
SCC | 82 | 16 | 66 |
|
|
LCC | 18 | 3 | 15 |
|
| Carcinoma
differentiation |
|
|
| 0.016a |
|
Well | 32 | 10 | 22 |
|
|
Moderate | 94 | 33 | 61 |
|
|
Poor | 112 | 20 | 92 |
|
| Venous
infiltration |
|
|
| 0.431 |
|
Absent | 76 | 23 | 53 |
|
|
Present | 162 | 40 | 122 |
|
| TNM stage |
|
|
| 0.017a |
| I | 100 | 35 | 65 |
|
|
II–IV | 138 | 28 | 110 |
|
INHBA positivity is associated with
poor prognosis for patients with NSCLC
To assess the ability of INHBA to predict patient
prognosis in NSCLC, the present study examined whether INHBA
expression was associated with the overall survival (OS) and
disease-free survival (DFS) of patients with NSCLC. OS-based
Kaplan-Meier survival curves were constructed to analyze cases with
positive and negative staining of INHBA among patients with NSCLC.
As shown in Fig. 1G, high INHBA
expression in NSCLC was significantly associated with a shorter OS
(log-rank=9.875; P=0.002); the OS rate decreased from 58.7% in
patients with INHBA-negative NSCLC to 36.6% in patients with
INHBA-positive tumors. Multivariate Cox regression revealed that in
addition to tumor stage (TNM stage II–IV; P=0.001), INHBA
expression was an independent prognostic marker for OS of patients
with NSCLC (P=0.019) (Table
II).
 | Table II.Analysis of overall survival and
disease-free survival using multivariate Cox regression. |
Table II.
Analysis of overall survival and
disease-free survival using multivariate Cox regression.
|
| OS |
| DFS |
|
|---|
|
|
|
|
|
|
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age, years (≥60 vs.
<60) | 1.258 | 0.888–1.782 | 0.197 | 1.196 | 0.843–1.698 | 0.315 |
| Sex (male vs.
female) | 0.881 | 0.598–1.297 | 0.521 | 0.873 | 0.591–1.288 | 0.493 |
| Histology |
|
|
|
|
|
|
| ADC vs.
SCC | 0.502 | 0.278–1.136 | 0.221 | 1.253 | 0.478–3.525 | 0.680 |
| ADC vs.
LCC | 1.601 | 0.889–2.569 | 0.086 | 0.917 | 0.293–2.990 | 0.821 |
| Carcinoma
differentiation |
|
|
|
|
|
|
| Well
vs. moderate | 1.698 | 0.601–4.112 | 0.396 | 0.478 | 0.259–1.632 | 0.102 |
| Well
vs. poor | 0.852 | 0.421–1.698 | 0.395 | 2.459 | 1.125–4.963 | 0.061 |
| Venous infiltration
(present vs. absent) | 1.040 | 0.714–1.515 | 0.836 | 1.035 | 0.711–1.505 | 0.859 |
| TNM stage (I vs.
II–IV) | 2.773 | 1.887–4.074 |
<0.0001a | 2.852 | 1.935–4.203 | 0.001a |
| INHBA (positive vs.
negative) | 1.698 | 1.092–2.639 | 0.019a | 1.196 | 1.098–2.638 | 0.017a |
Kaplan-Meier analysis also demonstrated that INHBA
positivity was associated with shorter DFS (log-rank=10.511;
P=0.001; Fig. 1H). Using
multivariate Cox regression analysis, INHBA was revealed to be an
independent indicator of DFS (P=0.017), in addition to the tumor
stage (TNM stage II–IV; P=0.001) (Table II). These data suggested that INHBA
might be a potential prognostic marker in NSCLC.
Overexpression of INHBA promotes the
invasion of lung cancer cells
The expression of INHBA was significantly higher in
all five NSCLC cell lines compared with HBE cells. To assess the
effects of INHBA on lung cancer invasion, cells expressing low
levels (H1299 cells) and high levels of INHBA (A549 cells) were
selected for further study (Fig. 2A and
B). Western blotting revealed that in H1299 cells, INHBA
overexpression reduced the expression levels of E-cadherin, and
increased the expression levels of N-cadherin, vimentin and Snail
(P=0.02, P=0.01, P=0.03, P=0.001, respectively; Fig. 2C). By contrast, in A549 cells,
siRNA-mediated INHBA knockdown increased E-cadherin
expression levels, and decreased the expression levels of
N-cadherin, vimentin and Snail (P=0.03, P=0.07, P=0.0001, P=0.02,
respectively; Fig. 2D).
Furthermore, the invasive and migratory abilities of H1299 cells
were increased by INHBA overexpression (P=0.0025 for
invasion; P=0.012 for migration; Fig.
2E), whereas in A549 cells, INHBA knockdown decreased
invasive and migratory abilities (P=0.0123 for invasion; P=0.0004
for migration; Fig. 2F).
 | Figure 2.INHBA overexpression promotes
the invasion of lung cancer cells. (A) Western blotting and (B)
semi-quantification of INHBA expression levels in five lung cancer
cell lines and HBE cells. (C) Transient transfection of H1299 cells
with the INHBA plasmid resulted in decreased expression
levels of E-cadherin, and increased expression levels of
N-cadherin, vimentin and Snail. (D) INHBA knockdown
increased the expression levels of E-cadherin, and decreased the
expression levels of N-cadherin, Snail and vimentin in A549 cells.
(E) Cell invasion and migration were enhanced in H1299 cells
transiently transfected with the INHBA plasmid. (F)
Knockdown of INHBA decreased the invasive and migratory
abilities of A549 cells. Original magnification, ×200. *P<0.05,
**P<0.01, ***P<0.001. INHBA, inhibin βA; NC, negative
control; si, small interfering; HBE, human bronchial
epithelial. |
Nuclear YAP protein levels are
associated with INHBA expression in NSCLC tissues
In NSCLC cases, YAP can be found in either the
nucleus or the cytoplasm (31,32);
therefore, the association between INHBA levels and nuclear and
cytoplasmic YAP levels was examined. For the quantitative analysis
of INHBA and YAP levels in 238 NSCLC samples, IHC scores were used.
The results revealed INHBA levels negatively associated with
cytoplasmic YAP levels (P=0.014) and positively associated with
nuclear YAP levels (P=0.045; Fig.
3A-F; Table III).
 | Table III.Association between INHBA expression
and the cytoplasmic and nuclear expression of YAP in NSCLC. |
Table III.
Association between INHBA expression
and the cytoplasmic and nuclear expression of YAP in NSCLC.
|
|
| INHBA
expression |
|
|
|---|
|
|
|
|
|
|
|---|
| Expression | Number of
patients | Negative | Positive | χ2 | P-value |
|---|
| Cytoplasmic YAP
expression |
|
|
|
|
|
|
Negative | 84 | 14 | 70 | 6.411 | 0.014a |
|
Positive | 154 | 49 | 105 |
|
|
| Nuclear YAP
expression |
|
|
|
|
|
|
Negative | 81 | 28 | 53 | 4.136 | 0.045a |
|
Positive | 157 | 35 | 122 |
|
|
INHBA induces YAP nuclear
translocation and activation in lung cancer cells
To assess if YAP was activated by INHBA, the present
study analyzed the phosphorylation status of YAP and LATS1/2, which
are major components of the Hippo pathway. Western blotting
revealed that the protein expression levels of p-YAP (Ser127) and
p-LATS1/2 (T1079 + T1041), and the ratio of p-YAP/YAP and
p-LATS1/2/LATS1/2 were all significantly decreased (P=0.0045,
P=0.0313, P=0.0378 and P=0.0145, respectively) at 48 h in H1299
cells transfected with the INHBA overexpression plasmid,
whereas total YAP and LATS1/2 expression levels were not affected
compared with those in cells transfected with the vector control
plasmid (Fig. 4A). By contrast, in
A549 cells where INHBA expression was knocked down using
siRNA, the protein expression levels of p-YAP and p-LATS1/2, and
the ratio of p-YAP/YAP and p-LATS1/2/LATS1/2 were higher compared
with those in the NC-siRNA-transfected cells (P=0.0306, P=0.0028,
P=0.0217 and P=0.0352, respectively; Fig. 4B). However, total YAP and LATS1/2
levels were unaffected by INHBA siRNA. These results
indicated that INHBA may inhibit Hippo signaling. For YAP
activation, its nuclear localization is the key regulatory
mechanism (22); therefore,
subcellular fractionation was performed to assess the cellular
localization of YAP. Compared with the vector groups, YAP
accumulated in the nucleus to a greater extent than in the
cytoplasm in cells overexpressing INHBA (P=0.014 for
cytoplasmic YAP; P=0.0041 for nuclear YAP; Fig. 4C-G). In addition, overexpression of
INHBA increased transcription of the canonical YAP target
genes CTGF and cysteine rich angiogenic inducer 61
(CYR61) (P=0.0173 for CTGF; P=0.0094 for
CYR61; Fig. 4H and I).
Conversely, knockdown of INHBA decreased the mRNA expression
levels of the two target genes in A549 cells (P=0.0104 for
CTGF; P=0.0057 for CYR61; Fig. 4J and K). Similar results were
observed at the protein expression levels (P=0.0031 and 0.0046 for
CTGF in the INHBA overexpression and knockdown groups,
respectively; P=0.0015 and 0.0313 for CYR61, in the INHBA
overexpression and knockdown groups, respectively; Fig. 4L-Q). These results suggested that in
lung cancer cells, INHBA induced YAP nuclear translocation and
activation by regulating LATS1/2 and then inhibiting Hippo
signaling.
 | Figure 4.INHBA activates YAP and induces its
nuclear translocation in lung cancer cells. (A and B) Western
blotting revealed that the expression levels of pYAP, pLATS1/2, and
the ratio of pYAP/YAP and pLATS1/2/LATS1/2, were decreased in H1299
cells transfected with the INHBA overexpression plasmid and
increased in A549 cells transfected with si-INHBA; total YAP and
LATS1/2 levels were not affected. (C-G) Subcellular fractionation
indicated that, after INHBA overexpression in cytoplasm, YAP
accumulation in the cytoplasmic fraction decreased and YAP
accumulation in the nuclear fraction increased. RT-qPCR indicated
that H1299 cells transiently transfected with the INHBA
plasmid exhibited increased (H) CTGF and (I) CYR61
mRNA expression levels. RT-qPCR demonstrated that A549 cells
transiently transfected with si-INHBA exhibited decreased
(J) CTGF and (K) CYR61 mRNA expression levels. (L-N)
Western blotting indicated that H1299 cells transiently transfected
with the INHBA plasmid exhibited increased CTGF and CYR61
protein expression levels. (O-Q) Western blotting demonstrated that
A549 cells transiently transfected with si-INHBA exhibited
decreased CTGF and CYR61 protein expression levels. *P<0.05,
**P<0.01. INHBA, inhibin βA; YAP, yes-associated protein; si,
small interfering; NC, negative control; LATS, large tumor
suppressor kinase; p, phosphorylated; CTGF, connective tissue
growth factor; CYR61, cysteine rich angiogenic inducer 61; RT-qPCR,
reverse transcription-quantitative PCR. |
INHBA promotes lung cancer cell
invasion by negatively regulating Hippo signaling
The Hippo-YAP pathway is involved in lung cancer
(25). The present study revealed
that in lung cancer cells INHBA overexpression induced YAP
nuclear translocation and inhibited LATS1/2 phosphorylation. Thus,
it was hypothesized that INHBA might function as an upstream
regulator in tumor metastasis via the Hippo-YAP pathway. The
present study further assessed whether INHBA overexpression
could promote NSCLC invasion via activation of Hippo signaling and
attenuating non-phosphorylated YAP nuclear translocation.
Therefore, a rescue experiment was performed using VP, a small
molecule inhibitor that can inhibit the YAP-TEA domain
transcription factor interaction, in H1299 cells to determine its
influence on the restoration of migration and invasion caused by
INHBA overexpression. As expected, after VP treatment, the
effects of INHBA overexpression on the promotion of NSCLC
invasion and migration were significantly reversed in vitro
(P=0.0026 for migration; P=0.0056 for invasion; Fig. 5A and B). Moreover, the increases in
the protein expression levels of N-cadherin, vimentin and Snail,
and the decreases in E-cadherin and pYAP expression, and pYAP/YAP
ratio were also reversed after VP treatment in H1299 cells
(P=0.0125 for N-cadherin, P=0.0024 for vimentin, P=0.0161 for
Snail, P=0.0004 for E-cadherin, P=0.0058 for pYAP and P=0.0028 for
pYAP/YAP, respectively; Fig. 5C).
These findings indicated that increased INHBA expression may
promote NSCLC invasion and epithelial-mesenchymal transition via
inactivation of the Hippo pathway.
 | Figure 5.INHBA promotes lung cancer cell
invasion by negatively regulating Hippo signaling. Rescue
experiments showed that INHBA overexpression-induced
promotion of NSCLC (A) invasion and (B) migration were reversed
following VP treatment in H1299 cells. Original magnification,
×200. (C) Rescue experiments showed that INHBA
overexpression-induced increases in N-cadherin, vimentin and Snail
expression levels, and decreases in E-cadherin, pYAP expression
levels and the pYAP/YAP ratio were also reversed after VP treatment
in H1299 cells. *P<0.05, **P<0.01, ***P<0.001. INHBA,
inhibin βA; YAP, yes-associated protein; p, phosphorylated; VP,
Verteporfin. |
INHBA negatively regulates Hippo
signaling by downregulating Merlin protein expression levels in
lung cancer cells
To determine how INHBA enhances nuclear
translocation of oncogenic YAP, the present study assessed the
upstream regulators of the Hippo-YAP pathway. The present study had
already verified that INHBA could affect the phosphorylation status
of YAP and LATS1/2; therefore, it was further determined whether
the other three important upstream regulators of Hippo-YAP
signaling, Willin/FRMD6, Merlin/NF2 and KIBRA/WWC1, were regulated
by INHBA. Western blotting revealed that in H1299 cells
overexpressing INHBA, at 48 h, only the protein expression
levels of Merlin were significantly decreased (P=0.0049, compared
with cells transfected with the vector control plasmid; Fig. 6A). Merlin suppresses tumorigenesis
by regulating certain pathways, including the mammalian target of
rapamycin and Hippo signaling pathways. Merlin activates the Hippo
pathway, which induces oncogenic YAP1 cytoplasmic retention
(33). To further determine whether
overexpression of INHBA inhibited Hippo signaling and
increased YAP nuclear translocation via the downregulation of
Merlin, a rescue experiment was performed using plasmid-mediated
exogenous overexpression of Merlin. The effectiveness of Merlin
overexpression plasmids was confirmed by western blotting (Fig. 6B). As expected, overexpression of
Merlin significantly reversed the effect of INHBA on
inhibiting the Hippo pathway and activating YAP in vitro in
H1299 cells (P=0.0007 for pYAP, P=0.0124 for pYAP/YAP; Fig. 6C). Notably, INHBA
overexpression only downregulated the protein expression levels of
Merlin, whereas no significant alterations were noted in the mRNA
expression levels (Fig. 6D).
Moreover, INHBA could bind to the Merlin protein (Fig. 6E). These results suggested that
INHBA may downregulate Merlin levels via post-translational
modification. Overall, these results demonstrated that increased
expression of INHBA induced NSCLC invasion via Hippo pathway
inactivation by downregulating Merlin levels, which inhibits
phosphorylation and promotes nuclear translocation of YAP, which
then upregulates CTGF and CYR61 levels (Fig. 7A and B).
 | Figure 7.INHBA promotes lung cancer cell
invasion via inhibiting the Hippo pathway. (A) Protein complex of
Merlin, FRMD6 and WWC1 activates the Hippo pathway, which increases
the phosphorylation of YAP and prevents its translocation and
binding to TEAD in the nucleus. (B) INHBA inhibits the Hippo
pathway by downregulating Merlin expression, which decreases the
phosphorylation of YAP, and promotes its translocation and binding
to TEAD in the nucleus. INHBA, inhibin βA; YAP, yes-associated
protein; WWC1, WW and C2 domain-containing 1; FRMD6, FERM
domain-containing 6; MST, mammalian sterile 20-like 1/MOB kinase;
LATS, large tumor suppressor kinase; p, phosphorylated; TEAD, TEA
domain; CTGF, connective tissue growth factor; Cyr61, cysteine rich
angiogenic inducer 61. |
Discussion
Investigations into the role of INHBA have
demonstrated that positive INHBA expression is significantly
associated with increasing pathological tumor stage, lymph node
metastasis, higher histological grade and vascular invasion in
urothelial carcinoma and gastric cancer (14,15).
Moreover, Seder et al (17)
suggested that in stage I lung ADC, INHBA may encourage cell
proliferation, and that a worse outcome may be associated with
upregulated INHBA expression (17).
However, it remains unclear as to whether INHBA participates in the
regulation of metastasis and invasion of NSCLC.
Previous studies have suggested that the subcellular
localization of YAP implies its functional diversity in lung
cancer. For example, Wang et al (31) revealed that the majority of YAP was
accumulated in the nucleus, and that YAP overexpression contributed
to the progression and poor prognosis of NSCLC (31). Conversely, in lung ADC, high levels
of YAP in the cytoplasm were associated with better survival
(32). The results of the present
study demonstrated that, in NSCLC, INHBA expression was inversely
associated with YAP expression in the cytoplasm and was positively
associated with nuclear YAP levels. Moreover, it was revealed that,
in NSCLC, the expression of INHBA was significantly increased, and
it was positively associated with worse clinicopathological
features, such as poor carcinoma differentiation and advanced TNM
stage. These findings support the two of previously reported
studies, which indicated that INHBA and activin expression levels
were elevated in NSCLC compared with those in non-malignant lungs,
and were associated with advanced stages of primary human NSCLC
(17,18). Furthermore, the present study
demonstrated that patients with INHBA positivity had an unfavorable
prognosis and that INHBA expression was an independent predictive
factor of prognosis. These results prompted the hypothesis that
INHBA affects the invasion and metastasis of NSCLC. It is worth
noting that there was a limitation in comparing INHBA expression
between NSCLC and adjacent normal lung tissues. Due to insufficient
materials, most of the NSCLC samples were not associated with
adjacent normal lung tissue; thus, 30 unpaired adjacent tissues
were used as the control. Previous studies have suggested that YAP
may be vital for TGF-β-mediated induction of certain target genes,
thus indicating that the expression of INHBA, as a member of the
TGF-β superfamily, may be associated with YAP expression (21). The present study determined the
association between the nuclear and cytoplasmic levels of YAP and
INHBA. The expressions levels of INHBA were negatively associated
with the expression levels of YAP in the cytoplasm and were
positively associated with the expression levels of YAP in the
nucleus. These findings suggested that INHBA may regulate
the subcellular localization of YAP and could be involved in lung
cancer development; this hypothesis warranted further exploration.
Therefore, the present study assessed the underlying molecular
mechanism by which INHBA affects NSCLC invasion and metastasis.
As expected, overexpression of INHBA promoted
NSCLC cell invasion via inhibiting the Hippo pathway.
Mechanistically, INHBA inhibited LATS1/2 phosphorylation and
induced YAP nuclear translocation by downregulating Merlin levels.
Notably, in the Hippo pathway, Merlin is an upstream regulator and
a core component of the Merlin/KIBRA/FRMD6 complex, which promotes
the phosphorylation of YAP and activates the Hippo pathway
(33). Additionally, Merlin is
encoded by the NF2 gene. Alcantara and Garcia (34) revealed that downregulation of
NF2 promoted the migration and proliferation of lung cancer
cells. Sánchez et al (35)
also reported that invasive and metastatic lung adenocarcinoma
exhibited lower Merlin protein levels compared with noninvasive
tumors, and suggested that Merlin could be a promising therapeutic
target to inhibit the progression of lung adenocarcinoma.
Therefore, it may be hypothesized that Merlin has a cancer
suppressive role in NSCLC, whereas INHBA may abrogate the
cancer-suppressing effect of Merlin, and promote the invasion and
metastasis of NSCLC.
Notably, in the present study, INHBA only
downregulated Merlin at the protein level, but not at the mRNA
level. Moreover, INHBA could bind to Merlin. These results
suggested that INHBA may downregulate Merlin levels via
post-translational modification. Previous studies have confirmed
that the intracellular regulatory function of Merlin is lost via
ubiquitination-mediated degradation (36,37);
however, as a member of the TGF-β superfamily, INHBA itself has no
ubiquitination function. Mota et al (38) confirmed that Merlin expression was
associated with Smad7. Moreover, previous studies revealed that
Smad7 is the ligation factor of E3 ubiquitin ligase of Smurf2, and
the PPXY domain of Smad7 combines with the WW domain of Smurf2 to
form an E3 ligase complex that regulates the
ubiquitination-mediated degradation of related proteins (39,40).
Therefore, it was hypothesized that INHBA may mediate the
ubiquitination-mediated degradation of Merlin via the Smad7/Smurf2
E3 ligase complex. However, these speculations require further
experimental verification.
In conclusion, the present study demonstrated that
the invasive and metastatic potential of lung cancer cells was
promoted by INHBA via the downregulation of Merlin expression,
which may cause negative regulation of Hippo signaling, and then
inhibit phosphorylation and promote nuclear translocation of YAP.
These results suggested that INHBA may represent a potential
therapeutic target for NSCLC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 82002668 and
81772489).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ and EW conceived the research. YZ, SY and YaL
designed the methodology. YZ, SY, YaY, JZ, YuL, PL, YoL, and YH
performed the experiments. YZ wrote the original draft of the
manuscript. EW reviewed and edited the manuscript, and supervised
the study. YZ and EW confirm the authenticity of all the raw data
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All procedures performed in studies involving human
participants were in accordance with the ethical standards of the
institutional and/or national research committee, and with the 1964
Helsinki Declaration and its later amendments or comparable ethical
standards. The present study was approved by the both the Institute
Research Medical Ethics Committee of Sun Yat-sen University and
China Medical University, and informed consent (written or verbal)
was obtained from the patients in this study for retrospective
analysis of tissue samples. All samples were anonymized.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Spira A and Ettinger DS: Multidisciplinary
management of lung cancer. N Engl J Med. 350:379–392. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Govindan R, Page N, Morgensztern D, Read
W, Tierney R, Vlahiotis A, Spitznagel EL and Piccirillo J: Changing
epidemiology of small-cell lung cancer in the United States over
the last 30 years: Analysis of the surveillance, epidemiologic, and
end results database. J Clin Oncol. 24:4539–4544. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sun S, Schiller JH, Spinola M and Minna
JD: New molecularly targeted therapies for lung cancer. J Clin
Invest. 117:2740–2750. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jemal A, Siegel R, Ward E, Murray T, Xu J,
Smigal C and Thun MJ: Cancer statistics, 2006. CA Cancer J Clin.
56:106–130. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
de Mello RA, Madureira P, Carvalho LS,
Araújo A, O'Brien M and Popat S: EGFR and KRAS mutations, and ALK
fusions: Current developments and personalized therapies for
patients with advanced non-small-cell lung cancer.
Pharmacogenomics. 14:1765–1777. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wagner G, Stollenwerk HK, Klerings I,
Pecherstorfer M, Gartlehner G and Singer J: Efficacy and safety of
immune checkpoint inhibitors in patients with advanced non-small
cell lung cancer (NSCLC): A systematic literature review.
Oncoimmunology. 9:17743142020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gaddy-Kurten D, Tsuchida K and Vale W:
Activins and the receptor serine kinase superfamily. Recent Prog
Horm Res. 50:109–129. 1995.PubMed/NCBI
|
|
8
|
Loomans HA and Andl CD: Intertwining of
activin A and TGFβ signaling: Dual roles in cancer progression and
cancer cell invasion. Cancers (Basel). 7:70–91. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Green JB, New HV and Smith JC: Responses
of embryonic xenopus cells to activin and FGF are separated by
multiple dose thresholds and correspond to distinct axes of the
mesoderm. Cell. 71:731–739. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vale W, Rivier C, Hsueh A, Campen C,
Meunier H, Bicsak T, Vaughan J, Corrigan A, Bardin W, Sawchenko P,
et al: Chemical and biological characterization of the inhibin
family of protein hormones. Recent Prog Horm Res. 44:1–34.
1988.PubMed/NCBI
|
|
11
|
Kelner N, Rodrigues PC, Bufalino A,
Fonseca FP, Santos-Silva AR, Miguel MC, Pinto CA, Leme AF, Graner
E, Salo T, et al: Activin A immunoexpression as predictor of occult
lymph node metastasis and overall survival in oral tongue squamous
cell carcinoma. Head Neck. 37:479–486. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fu S, Zhang N, Yopp AC, Chen D, Mao M,
Chen D, Zhang H, Ding Y and Bromberg JS: TGF-beta induces Foxp3 +
T-regulatory cells from CD4 + CD25-precursors. Am J Transplant.
4:1614–1627. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ogawa K and Funaba M: Activin in humoral
immune responses. Vitam Horm. 85:235–253. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang Q, Wen YG, Li DP, Xia J, Zhou CZ, Yan
DW, Tang HM and Peng ZH: Upregulated INHBA expression is associated
with poor survival in gastric cancer. Med Oncol. 29:77–83. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee HY, Li CC, Huang CN, Li WM, Yeh HC, Ke
HL, Yang KF, Liang PI, Li CF and Wu WJ: INHBA overexpression
indicates poor prognosis in urothelial carcinoma of urinary bladder
and upper tract. J Surg Oncol. 111:414–422. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Peng S, Wang J, Hu P, Zhang W, Li H and Xu
L: INHBA knockdown inhibits proliferation and invasion of
nasopharyngeal carcinoma SUNE1 cells in vitro. Int J Clin Exp
Pathol. 13:854–868. 2020.PubMed/NCBI
|
|
17
|
Seder CW, Hartojo W, Lin L, Silvers AL,
Wang Z, Thomas DG, Giordano TJ, Chen G, Chang AC, Orringer MB and
Beer DG: Upregulated INHBA expression may promote cell
proliferation and is associated with poor survival in lung
adenocarcinoma. Neoplasia. 11:388–396. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wamsley JJ, Kumar M, Allison DF, Clift SH,
Holzknecht CM, Szymura SJ, Hoang SA, Xu X, Moskaluk CA, Jones DR,
et al: Activin upregulation by NF-κB is required to maintain
mesenchymal features of cancer stem-like cells in non-small cell
lung cancer. Cancer Res. 75:426–435. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Varelas X, Samavarchi-Tehrani P, Narimatsu
M, Weiss A, Cockburn K, Larsen BG, Rossant J and Wrana JL: The
Crumbs complex couples cell density sensing to Hippo-dependent
control of the TGF-β-SMAD pathway. Dev Cell. 19:831–844. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Narimatsu M, Samavarchi-Tehrani P, Varelas
X and Wrana JL: Distinct polarity cues direct Taz/Yap and TGFβ
receptor localization to differentially control TGFβ-induced smad
signaling. Dev Cell. 32:652–656. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fujii M, Toyoda T, Nakanishi H, Yatabe Y,
Sato A, Matsudaira Y, Ito H, Murakami H, Kondo Y, Kondo E, et al:
TGF-β synergizes with defects in the Hippo pathway to stimulate
human malignant mesothelioma growth. J Exp Med. 209:479–494. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang J, Wu S, Barrera J, Matthews K and
Pan D: The Hippo signaling pathway coordinately regulates cell
proliferation and apoptosis by inactivating yorkie, the drosophila
homolog of YAP. Cell. 122:421–434. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Edgar BA: From cell structure to
transcription: Hippo forges a new path. Cell. 124:267–273. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pan D: The Hippo signaling pathway in
development and cancer. Dev Cell. 19:491–505. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Han Q, Lin X, Zhang X, Jiang G, Zhang Y,
Miao Y, Rong X, Zheng X, Han Y, Han X, et al: WWC3 regulates the
Wnt and Hippo pathways via dishevelled proteins and large tumour
suppressor 1, to suppress lung cancer invasion and metastasis. J
Pathol. 242:435–447. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Travis WD, Brambilla E, Burke AP, Marx A
and Nicholson AG: Introduction to the 2015 World Health
Organization classification of tumors of the lung, pleura, thymus,
and heart. J Thorac Oncol. 10:1240–1242. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sobin LH, Gospodarowicz MK and Christian
Wittekind C: International Union Against Cancer (UICC): TNM
classification of malignant tumours. 8th edition. Oxford:
Wiley-Blackwell; 2017
|
|
28
|
Zhang Y, Zhao Y, Jiang G, Zhang X, Zhao H,
Wu J, Xu K and Wang E: Impact of p120-catenin isoforms 1A and 3A on
epithelial mesenchymal transition of lung cancer cells expressing
E-cadherin in different subcellular locations. PLoS One.
9:e880642014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang Y, Yan S, Chen J, Gan C, Chen D, Li
Y, Wen J, Kremerskothen J, Chen S, Zhang J and Cao Y: WWC2 is an
independent prognostic factor and prevents invasion via Hippo
signalling in hepatocellular carcinoma. J Cell Mol Med.
21:3718–3729. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang Y, Dong Q, Zhang Q, Li Z, Wang E and
Qiu X: Overexpression of yes-associated protein contributes to
progression and poor prognosis of non-small-cell lung cancer.
Cancer Sci. 101:1279–1285. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sun PL, Kim JE, Yoo SB, Kim H, Jin Y,
Jheon S, Kim K, Lee CT and Chung JH: Cytoplasmic YAP expression is
associated with prolonged survival in patients with lung
adenocarcinomas and epidermal growth factor receptor tyrosine
kinase inhibitor treatment. Ann Surg Oncol. 21 (Suppl 4):S610–S618.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Su T, Ludwig MZ, Xu J and Fehon RG: Kibra
and Merlin activate the Hippo pathway spatially distinct from and
independent of expanded. Dev Cell. 40:478–490.e3. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Alcantara KMM and Garcia RL: MicroRNA-92a
promotes cell proliferation, migration and survival by directly
targeting the tumor suppressor gene NF2 in colorectal and lung
cancer cells. Oncol Rep. 41:2103–2116. 2019.PubMed/NCBI
|
|
35
|
Sánchez NC, Medrano-Jiménez E,
Aguilar-León D, Pérez-Martínez L and Pedraza-Alva G: Tumor necrosis
factor-induced miR-146a upregulation promotes human lung
adenocarcinoma metastasis by targeting Merlin. DNA Cell Biol.
39:484–497. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wei Y, Yee PP, Liu Z, Zhang L, Guo H,
Zheng H, Anderson B, Gulley M and Li W: NEDD4L-mediated Merlin
ubiquitination facilitates Hippo pathway activation. EMBO Rep.
21:e506422020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tang X, Jang SW, Wang X, Liu Z, Bahr SM,
Sun SY, Brat D, Gutmann DH and Ye K: Akt phosphorylation regulates
the tumour-suppressor Merlin through ubiquitination and
degradation. Nat Cell Biol. 9:1199–1207. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mota MSV, Jackson WP, Bailey SK, Vayalil
P, Landar A, Rostas JW III, Mulekar MS, Samant RS and Shevde LA:
Deficiency of tumor suppressor Merlin facilitates metabolic
adaptation by co-operative engagement of SMAD-Hippo signaling in
breast cancer. Carcinogenesis. 39:1165–1175. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kavsak P, Rasmussen RK, Causing CG, Bonni
S, Zhu H, Thomsen GH and Wrana JL: Smad7 binds to Smurf2 to form an
E3 ubiquitin ligase that targets the TGF beta receptor for
degradation. Mol Cell. 6:1365–1375. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang Z, Liu C, Chen B, Tang W, Liu Z, Cao
W and Li X: Smad7 down-regulation via ubiquitin degradation
mediated by Smurf2 in fibroblasts of hypertrophic scars in burned
patients. Burns. 47:1333–1341. 2021. View Article : Google Scholar : PubMed/NCBI
|





















