Introduction
In recent years, stroke has become one of the
commonest causes of mortality worldwide (1,2).
Stroke occurs when the blood supply to the brain is interrupted or
decreased, which prevents the brain tissue from receiving oxygen
and nutrients (3). In total, ~85%
of stroke cases are caused by ischemia (4). Stroke can impair neural circuits and
function (5). It not only disrupts
the infarct area but also the surrounding peri-ischemic areas
(6). The lack of blood flow during
stroke leads to neural damage, including excitotoxicity,
mitochondrial dysfunction, calcium overload, oxidative stress,
protein misfolding, inflammatory changes and neuronal apoptosis
(7,8). At present, clinical treatments of
stroke in the acute phase mainly include thrombolysis, restoration
of blood flow in the penumbral area, neurotrophic factor
administration to protect neurons and symptomatic treatment.
However, a large number of patients with stroke are not suitable
for these treatments, due to the narrow time window of the acute
phase (9,10). In addition, short-term blood flow
recovery usually causes more damage to the neurons (11,12).
Stroke is a medical emergency; therefore, early preventive action
to reduce brain damage is crucial. However, a limited number of
drugs can protect against stroke progression. Thus, identifying
alternative therapeutic agents is necessary.
Neurogenesis is the generation of new neurons in the
brain, which occurs through the division, maturation and
differentiation of neural stem cells (13). Neurogenesis is particularly
important in stroke, due to the need for the replacement of
cortical neurons destroyed by stroke by new neurons, in order to
rebuild neuronal connections (14).
Autophagy, the main degradation pathway, is essential for
maintaining cellular homeostasis (15). Autophagy primarily occurs in
peri-ischemic areas during stroke (16). However, the role of autophagy in
neuroprotection remains controversial. Certain studies have
indicated that the activation of autophagy can promote
neuroprotection in stroke (17,18),
while others have reported opposite findings (19). Several studies have shown that
autophagy serves an important role in neurogenesis while a lack of
autophagy-related genes can reduce neurogenesis (20,21).
Proteins from the sirtuins (SIRT) family can mediate
autophagy (22). As a type of
histone deacetylase, the SIRT family has become an important
regulator of metabolism and life span, with SIRT1 being a critical
regulator of autophagy (23). SIRT1
can regulate the autophagic pathway under different conditions
(24,25). In addition, SIRT1 may serve an
important role during stroke progression.
Buyang Huanwu Decoction (BHD) has been used for the
treatment of stroke in China for several years (26). Clinical trials have indicated that
BHD could ameliorate the outcomes of patients who had suffered a
stroke (5,27,28).
BHD can protect against cerebral I/R injury by promoting
neurogenesis (29–31), inhibiting neural apoptosis and
inflammation (32), promoting
angiogenesis and improving cerebral circulation (33) in middle cerebral artery occlusion
and reperfusion (MCAO-R) rats. Multiple components of BHD could
ameliorate the negative effect of stroke (34). Whether the SIRT1/autophagy pathway
is involved in the protective effect of BHD against stroke remains
to be elucidated.
In the present study, a rat model of MCAO-R was used
to determine the neuroprotective effect of BHD in stroke. In
addition, it was hypothesized that this effect may be associated
with the SIRT1/autophagy pathway.
Materials and methods
Animals
Male Sprague-Dawley rats (n=60; age, 8 weeks;
weight, 270–280 g) were supplied by the Experimental Animal Centre
of Guangzhou University of Chinese Medicine (Guangzhou, China). All
rats were raised in a specific-pathogen free room under controlled
temperature (24 ± 1°C) and humidity (55–70%) with a 12-h light-dark
cycle, and were given free access to food and water. All
experimental procedures were carried out according to the
guidelines of the Administrative Panel on Laboratory Animal Care of
Guangzhou University of Chinese Medicine (Guangzhou, China). After
1 week of adaptive housing and feeding, animals were randomly
divided into six groups (n=10 each group): i) Sham surgery
(control); ii) MCAO-R surgery (model); iii) MCAO-R + rapamycin
(Rapa); iv) MCAO-R + 5 g/kg BHD; v) MCAO-R + 10 g/kg BHD; and vi)
MCAO-R + 20 g/kg BHD.
Rat model of MCAO-R
A transient focal cerebral ischemia model (MCAO-R
model) was established as previously described (18,35).
Briefly, rats were anesthetized with 4% isoflurane and maintained
with 1.5% isoflurane via an isoflurane vaporizer (RWD Life
Science). A midline neck incision was then performed to expose the
right common carotid artery, external carotid artery (ECA) and
internal carotid artery (ICA). A 4-0 silicone rubber-coated nylon
monofilament (cat. no. MSRC43B280PK100; RWD Life Science) was
inserted into the ECA and then gently advanced into the ICA, 18–19
mm from the carotid bifurcation, to occlude the beginning of the
MCA. After 2 h of occlusion, the monofilament was gently removed to
restore blood flow. Throughout the surgery, the body temperature of
all rats was maintained at ~37°C. Rats were anaesthetized by 1%
sodium pentobarbital (40 mg/kg) and euthanized by cervical
dislocation on day 5 following surgery.
Preparation of BHD
BHD was prepared according to previously described
and the quality control was also achieved (Fig. S1) (36). Briefly, the powdered sample of BHD
(143 g) was mixed with Radix astragali, Radix angelicae
sinensis, Radix paeoniae rubra, Rhizoma ligustici chuanxiong, Flos
carthami, Semen persicae and Lumbricus at a
120:6:5:3:3:3:3 ratio. All ingredients were purchased from
Guangzhou Zhixin Chinese Herbal Medicine Co. Ltd. and verified by
the Department of Pharmacy, Guangzhou University of Chinese
Medicine. The decoction was made by boiling the mixture in 10 times
the amount of distilled water at 100°C for 60 min. The drug
solution was removed for use and the residue was boiled once more.
The two solutions were combined and concentrated on a rotary
evaporator at ~60°C. The concentrated medicinal solution was
vacuum-cooled and dried twice to form a powder and dissolved in
distilled water and the final concentration was 2.0 g/ml
(equivalent to the dry weight of the raw material).
Drug administration
All groups of rats were treated with an
intraperitoneal injection of hydroxychloroquine (20 mg/kg; cat. no.
S4430; Selleck Chemicals) 30 min after surgery (37). BHD powder and Rapa (cat. no. S1039;
Selleck Chemicals) were dissolved with 0.9% saline. At the onset of
reperfusion (38), the treatment
groups, including the MCAO-R + BHD and MCAO-R + Rapa groups, were
treated with BHD (5, 10 and 20 g/kg) by gavage and Rapa (10 mg/kg)
via intraperitoneal injection, respectively.
Neurological scores
The modified neurological severity score, which uses
different scores to evaluate motor, sensory, reflex and balance
functions, was used to assess neurological deficits on day 5
following surgery, as previously described (39–41).
The motor test used a six-point scale to assess the movement and
walking ability of the rats (muscle state, abnormal motion and tail
lifting test). The sensory test used a two-point scale to assess
superficial and deep sensations in the rats (vision, touch and
proprioception). The reflex test used a four-point scale to assess
shallow and deep reflection in rats. The balance functions test
used a six-point scale to assess the movement of rats on the
balance beam. The neurological function scores ranged between 0–18
points, and were graded as follows: Mild damage (1–6),
moderate damage (7–12) and severe damage (13–18).
Cerebral infarct size measurement
Following euthanasia, rat brains were rapidly sliced
using a rat brain matrix (RWD Life Science) to measure the cerebral
infarct size. Continuously cut 5 sections of each brain tissue, 2
mm each, were made (n=4). The sections were stained with 2, 3,
5-triphenyltetrazolium hydrochloride (TTC; cat. no. T8877;
MilliporeSigma) at 37°C for 15 min (42). Normal tissues stained red and
ischemic tissues, white. Image-Pro Plus 6.0 (Media Cybernetics,
Inc.) image analysis software was used for image analysis.
Malondialdehyde (MDA), catalase (CAT)
and glutathione peroxidase (GSH-PX) expression measurements
The tissue of the ischemic hemisphere of the rats
was chosen for the oxidative stress kit testing (n=3). The brain
tissues were homogenized with ice-cold saline and centrifuged at
14,000 × g for 10 min at 4°C. The supernatant was then used to
detect the levels of CAT (cat. no. A007-1-1; Nanjing Jiancheng
Bioengineering Institute), MDA (cat. no. A003-1-2; Nanjing
Jiancheng Bioengineering Institute) and GSH-PX (cat. no. A005-1-1;
Nanjing Jiancheng Bioengineering Institute), according to the
manufacturer's instructions. Absorbance was measured using a
microplate reader with the wavelength of 532, 405 and 412 nm.
Hematoxylin and eosin (H&E) and
Nissl staining
The tissue of the ischemic hemisphere of the rats
was chosen for H&E and Nissl staining testing (n=3). Brain
paraffin-embedded sections were deparaffinized and rehydrated in
xylene and gradient alcohol. The sections were then washed in PBS
(Beyotime Institute of Biotechnology) and underwent H&E
(Beyotime Institute of Biotechnology) or Nissl (Nanjing Jiancheng
Bioengineering Institute) staining for 10 min at 37°C. The sections
were then washed with PBS. Images were captured using a light
microscope (Leica Microsystems, Inc.). Image-Pro Plus 6.0 (Media
Cybernetics, Inc.) software was used for image analysis.
Western blot analysis
The tissue of the ischemic hemisphere of the rats
was chosen for western blotting (n=3). Brain tissue was homogenized
in ice-cold RIPA lysis buffer (cat. no. P0013B; Beyotime Institute
of Biotechnology) and centrifuged at 12,000 × g for 10 min at 4°C.
The supernatant was then extracted to determine the total protein
concentration using a bicinchoninic acid protein assay (cat. no.
P0012S; Beyotime Institute of Biotechnology). Next, the appropriate
volume of loading buffer (cat. no. BL511B; Biosharp Life Sciences)
was added, followed by boiling for 10 min at 100°C. Proteins
samples (30 µg per well) were separated using 8, 10 and 12%
SDS-PAGE gels and transferred onto a PVDF (cat. no. ISEQ00010; cat.
no. IPVH00010; MilliporeSigma) membrane. The membrane was blocked
with 5% skimmed milk (cat. no. 1172GR500; BioForxx) at 37°C for 1
h. Then, incubated with primary antibodies against SIRT1 (cat. no.
ab189494; 1:1,000; Abcam), LC3 (cat. no. 2775; 1:1,000; Cell
Signaling Technology, Inc.), beclin 1 (cat. no. 3738, 1:1,000; Cell
Signaling Technology, Inc.), p62 (cat. no. 39749; 1:1,000; Cell
Signaling Technology, Inc.), doublecortin on the X chromosome (DCX;
cat. no. ab18723; 1:1,000; Abcam), β-actin (cat. no. 58169;
1:1,000; Cell Signaling Technology, Inc.) at 4°C overnight and
incubated with goat anti-rabbit IgG (cat. no. S0001; 1:3,000;
Affinity Biosciences) or goat anti-mouse IgG (S0002; 1:3,000;
Affinity Biosciences) at 37°C for 1 h. ECL reagent (cat. no.
WBKLS0500; MilliporeSigma) was added to the membrane for
visualizing the target bands. Digital images of the blots were
visualized using Image Lab 3.0 software (Bio-Rad Laboratories,
Inc.).
Immunofluorescence
The tissue of the ischemic hemisphere of the rats
was chosen for immunofluorescence testing (n=3). Rat sections (10
µm each) were blocked with 5% BSA (Beyotime Institute of
Biotechnology) and incubated with primary antibodies for nestin
(cat. no. 4760; 1:300; Cell Signaling Technology, Inc.), LC3 (cat.
no. 2775; 1:300; Cell Signaling Technology, Inc.), brain-derived
neurotrophic factor (BDNF; cat. no. ab108319; 1:300; Abcam) and DCX
(cat. no. ab18723; 1:300; Abcam) overnight at 4°C. The slices were
incubated with fluorescence-coupled secondary antibody, anti-mouse
IgG (cat. no. 4408; 1:1,000; Cell Signaling Technology, Inc.),
anti-mouse IgG (cat. no. 4409; 1:1,000; Cell Signaling Technology,
Inc.) or anti-rabbit IgG (cat. no. 4412; 1:1,000; Cell Signaling
Technology, Inc.) for 2 h at 37°C. Following rinsing, sections were
incubated with DAPI (cat. no. P0131; Beyotime Institute of
Biotechnology). Fluorescence was detected using a laser scanning
confocal microscope (Carl Zeiss AG). Image-Pro Plus 6.0 (Media
Cybernetics, Inc.) image analysis software was used for image
analysis.
Statistical analysis
Statistical analysis was performed using SPSS
version 17 (SPSS, Inc.). Data are presented as the mean ± standard
deviation. One-way ANOVA was applied to analyze differences in data
for the biochemical parameters among the different groups, followed
by Dunnett's post hoc test, and an unpaired Student's t-test was
also used to determine statistical differences. P<0.05 was
considered to indicate a statistically significant difference.
Results
BHD ameliorates infarction and reduces
neurological scores following MCAO-R
The design of the present study is shown in Fig. 1A. First, the infarct volume was
measured. TTC staining demonstrated that the infarct volume in the
MCAO-R + BHD group was markedly decreased in a dose-dependent
manner compared with that in the MCAO-R group (Fig. 1B and C). The neurological scores
following MCAO-R in two BHD groups were improved (Fig. 1D), particularly in the 20 g/kg BHD
group. A dosage of 20 g/kg BHD was selected for the next
experiments. These data demonstrated that BHD could effectively
ameliorate infarction and reduce neurological scores following
MCAO-R.
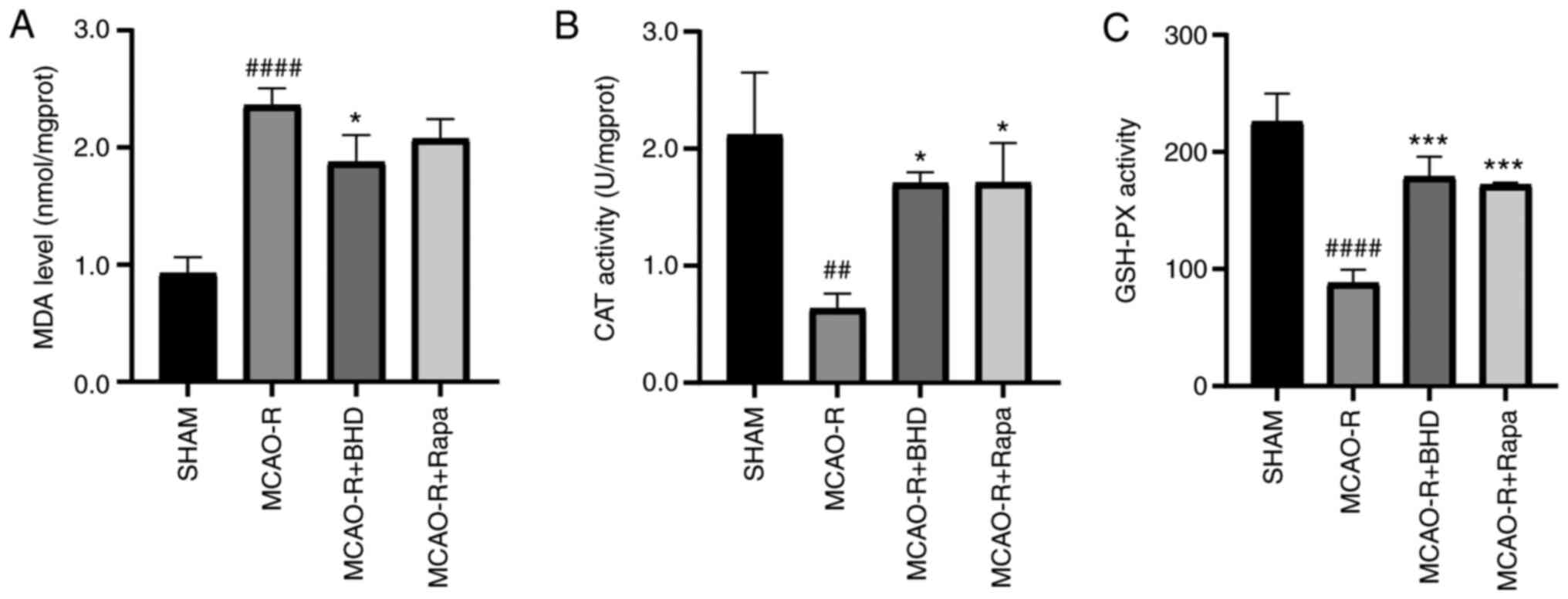
BHD relieves neuronal oxidative stress
damage following MCAO-R
To determine whether BHD exerted protective effects
against oxidative stress, the level of MDA and the activity of CAT
and GSH-PX were detected next (Fig.
2). Compared with the sham group, the MDA level in the MCAO-R
group was significantly increased and the activity of CAT and
GSH-PX was decreased. However, after the oral administration of
BHD, the MDA levels in MCAO-R rats were significantly reduced and
the activity of CAT and GSH-PX were significantly increased. These
data demonstrated that BHD could relieve neuronal oxidative stress
damage following MCAO-R.
BHD protects against neuronal death
following MCAO-R
As shown in Fig. 3A and
B (H&E and Nissl staining, respectively), large areas of
neuronal necrosis were induced by cerebral I/R. The MCAO-R group
exhibited extensive neuronal death accompanied by the disappearance
of cytoplasmic bodies, swelling of cell bodies, nuclear
condensation and sparse Nissl bodies. Conversely, sham group
neurons exhibited clear and large cell nuclei and bodies, abundant
Nissl bodies and strong staining. Notably, BHD reversed these
changes. The expression of BDNF (Fig.
3C and D) was further determined by immunostaining. MCAO-R
downregulated BDNF expression. Post-surgical treatment of MCAO-R
rats with BHD caused a significant increase in BDNF. Collectively,
these data demonstrated that BHD could protect against neuronal
death following MCAO-R.
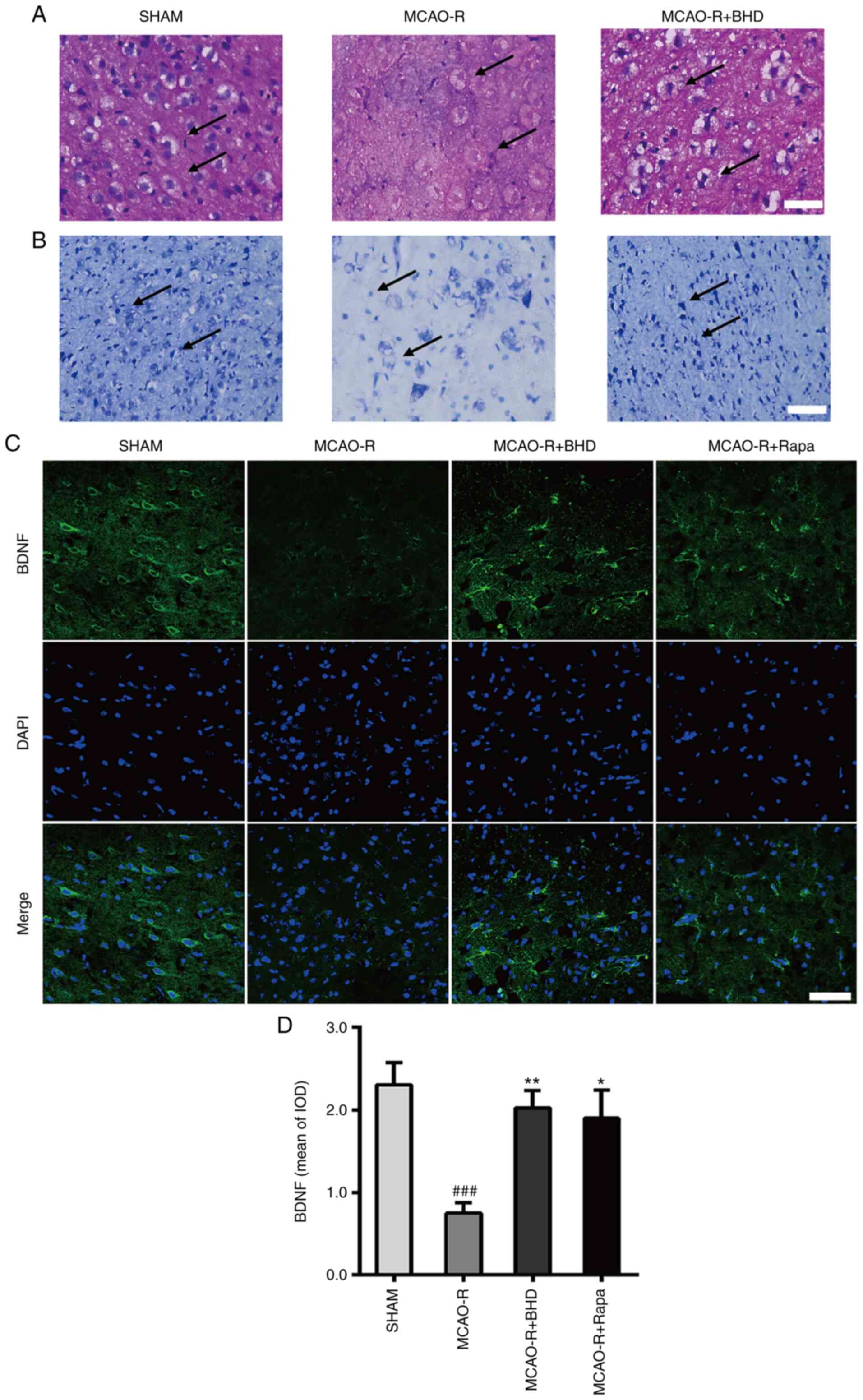 | Figure 3.BHD protects against neural death
after MCAO-R. (A) Hematoxylin and eosin staining of the ischemic
penumbra. Scale bar=50 µm. The arrow points to the cytoplasm and
nucleus of neurons of sham group, MCAO-R group and MCAO-R + BHD
group rats. Sham group neurons demonstrated clear and large cell
nucleus and bodies. MCAO-R group neurons demonstrated disappearance
of the cytoplasmic bodies, swelling of cell bodies and nuclear
condensation. The MCAO-R + BHD group demonstrated neurons clear
cell nucleus and bodies. (B) Nissl staining of the ischemic
penumbra. Scale bar=50 µm. The arrow points to the Nissl body of
sham group, MCAO-R group and MCAO-R + BHD group rats. Sham group
neurons demonstrated a lot of Nissl bodies and deep staining.
MCAO-R group neurons demonstrated sparse Nissl bodies and weak
staining. The MCAO-R + BHD group demonstrated more Nissl bodies and
deeper staining. (C) Immunofluorescence analysis performed with
BDNF antibody (green), nuclei were stained with DAPI (blue). Scale
bar=20 µm. (D) The bar graph represents the IOD quantification of
BDNF. The data are expressed as means ± standard deviation (n=3).
Statistical comparisons were performed with one-way ANOVA followed
by Dunnett's post hoc test. ###P<0.001 vs. sham
group; *P<0.05, **P<0.01 vs. MCAO-R group. BHD, Buyang Huanwu
Decoction; MCAO-R, middle cerebral artery occlusion and
reperfusion; BDNF, brain-derived neurotrophic factor; Rapa,
rapamycin; IOD, integrated optical density. |
BHD promotes neurogenesis in MCAO-R
rats
As shown in Fig. 4,
the expression of DCX, a protein expressed by neural precursor
cells, was detected using immunostaining and western blot analysis.
MCAO-R downregulated DCX. Post-surgical treatment with BHD caused a
significant increase in DCX expression in MCAO-R rats. Thus, these
results supported that BHD promotes neurogenesis in MCAO-R
rats.
BHD activates SIRT1 and autophagy in
the cerebral peri-ischemic area of rats following MCAO-R
In order to determine whether the SIRT1/autophagy
pathway was involved in the protective effect of BHD, SIRT1 and
autophagic markers (LC3, p62 and beclin 1) were detected using
western blot analysis (Fig. 5A-E).
The results demonstrated that the expression of SIRT1 in the MCAO-R
and MCAO-R + BHD groups was elevated. SIRT1 expression was
significantly increased in the MCAO-R + BHD group, compared with
that in the MCAO-R group. The expression of LC3-II and beclin 1 was
increased, while that of p62 was slightly decreased in the cerebral
peri-ischemic area of rats in the MCAO-R group. Post-surgical
treatment with BHD markedly increased autophagy, when compared with
the MCAO-R group. Post-surgical treatment with Rapa had a similar
effect to that of BHD treatment. In addition, the distribution
pattern of nestin and LC3 was elucidated using tissue
immunostaining. The expression pattern of LC3 was similar to that
observed following western blot analysis (Fig. 6A-C). The expression of nestin, a
protein marker for neural stem cells, was also elevated by BHD.
Therefore, these data suggested that the neuroprotective effect of
BHD might be associated with the SIRT1/autophagy pathway.
Discussion
In the present study, it was shown that autophagy
was activated on day 5 following cerebral I/R in vivo and
post-surgical treatment with BHD demonstrated similar trends in
regulating autophagy with those of Rapa treatment. Furthermore, the
present study found that SIRT1 was upregulated on day 5 following
MCAO-R and BHD exacerbated this phenomenon. In addition, BHD
treatment increased nestin and DCX expression in MCAO-R rats,
suggesting that BHD promoted neurogenesis. Therefore, these results
demonstrated that BHD exerts a neuroprotective effect against
stroke and promotes neurogenesis, potentially through the
activation of the SIRT1/autophagy pathway.
Autophagy, a dynamic process, in which a cell
degrades its own cytoplasm through a surrounding lysosome and a
bilayer membrane, fluctuates constantly (43). In the central nervous system,
moderate autophagy activation may be a manifestation of endogenous
neuroprotective mechanisms (44).
The formation of autophagosomes, as a process of cell repair and
damage limitation following cerebral ischemia, serves a key role in
neuronal survival (45,46). In China, BHD has been used for the
clinical treatment of stroke for a number of years. BHD protects
cerebral ischemia-injured neurons, blood vessels, glial cells and
the brain microenvironment through a variety of mechanisms
(47–50). In the present study, LC3-II and
beclin 1 expression was found to be significantly increased, while
p62 expression was found to be decreased, in the peri-ischemic area
of rat brains in the MCAO-R + BHD and MCAO-R + Rapa groups,
compared with the MCAO-R group. Thus, BHD was shown to activate
autophagy in MCAO-R rats, a finding similar to that for Rapa.
Oxidative stress is an important pathological
mechanism of stroke (51). When I/R
occurs, a large amount of reactive oxygen species (ROS) is produced
(52). Mitochondrial dysfunction
cannot clear the excessive ROS, thus triggering further
pathological changes, such as calcium overload and excitotoxicity
(53). There is a negative feedback
regulation response between ROS and autophagy in mitochondria;
mitochondria-produced ROS can activate autophagy and when autophagy
is activated, ROS is eliminated (54). MDA is an important target that
reflects the body's anti-oxidative potential and can indirectly
reflect tissue peroxidation damage. CAT and GSH-PX are two
important peroxidases in the body. BHD can decrease the MDA level
in the ischemic penumbra and increase the CAT and GSH-PX levels.
Therefore, BHD can reduce oxidative stress damage following MCAO-R,
which might be associated with the activation of the
SIRT1/autophagy pathway.
Neurological deficits, such as hemiplegia and
sensory disturbances, are the most common sequelae after stroke
(55). Apoptosis is a process of
programmed cell death, which is activated following cerebral
ischemia injury and the production of ROS and inflammation during
reperfusion (56). Autophagy and
apoptosis both a form of cell self-regulation and the association
between them is complex. Studies have shown that the inhibition of
autophagy may lead to a shortage of bioenergy, thereby triggering
cell apoptosis (57,58). BDNF, a critical growth factor, has
been shown to promote neuronal survival and regulate different
neuronal functions (42). BHD
significantly ameliorated neurological deficit and the level of
brain damage in rats after MCAO-R. In the present study, following
MCAO-R, extensive neuronal death was observed, which was
accompanied by the disappearance of cytoplasmic bodies, swelling of
cell bodies, nuclear condensation and sparse Nissl bodies, whereas
sham group neurons exhibited clear and large cell nuclei and Nissl
bodies. However, treatment with BHD reversed these changes. The
immunofluorescence results demonstrated that MCAO-R significantly
decreased the expression of BDNF, whereas post-surgical treatment
with BHD could markedly increase it compared with the MCAO-R group.
Thus, BHD could protect neurons against MCAO-R, which may have been
associated with the activation of the SIRT1/autophagy pathway.
Neurogenesis is hypothesized to be restricted to
embryonic development, ceasing after birth. However, adult
neurogenesis has been detected to occur throughout the lifetime of
various mammals (59). Adult neural
stem cells in the subventricular zone of the lateral ventricle and
the dentate gyrus of the hippocampus can be activated following
stroke, to then proliferate and produce neuroblasts for the repair
of damaged neurons (60,61). A number of studies have investigated
the role of autophagy in embryonic and adult neural stem cells. In
the adult mammalian brain, the most studied neural stem cells, such
as those located in the subventricular zone of the lateral
ventricle and the subgranular zone of the hippocampal dentate
gyrus, are located in a relatively hypoxic environment, which is a
necessary condition for stem cells (62,63).
Through autophagy, a low level of ROS appears to be maintained, to
ensure the slow circulation of neural stem cells (64). DCX is a microtubule and actin
filament-associated protein (65).
Due to its specific expression in neural precursors and newly
generated immature neurons in several regions of the brain, DCX is
used as a specific marker to assess potential neurogenesis in the
adult brain (66,67). Nestin is expressed in neural stem
and progenitor cells, which may participate in neurogenesis
following stroke (18,68). Several studies have shown that BHD
can induce neurogenesis during the occurrence of stroke (30,69).
In the present study, the expression of DCX in the MCAO-R + BHD
group was increased compared with the MCAO-R group. These data
demonstrated that the neuroprotective effect of BHD may be related
to neurogenesis and autophagy. A double stain of a protein marker
for neural stem cell (nestin) and autophagy-related protein (LC3)
were performed to illustrate whether the neurogenesis of BHD is
related to its regulation of autophagy. Confocal microscopy
demonstrated that nestin and LC3 isoforms were located in living
post-ischemic cells. These data provided evidence to suggest that
BHD increased neurogenesis in the peri-ischemic area of rat brains
on day 5 following MCAO-R, which might be associated with the
activation of the SIRT1/autophagy pathway. SIRT1, a nicotinamide
adenine dinucleotide-positive-dependent deacetylase, is a
well-known modulator of aging (70). SIRT1 has been shown to exert
anti-inflammatory, anti-apoptotic and anti-oxidative effects,
promote DNA repair, maintain energy metabolism and regulate
autophagy following the occurrence of stroke (71). SIRT1 can interact with several
essential components of autophagy (22). Furthermore, research has shown that
SIRT1 protects the brain during stroke, potentially through the
activation of autophagy pathways (72). In the present study, BHD was proven
to elevate SIRT1 expression, which may be an upstream autophagic
protein.
In conclusion, the present study found that BHD may
exert a neuroprotective effect and promote neurogenesis in MCAO-R
rats by regulating the SIRT1/autophagy pathway in the peri-ischemic
area of the brain. The findings of this study suggest that BHD may
be a promising treatment for stroke, and that the SIRT1/autophagy
pathway serves as a potential target for future therapies. However,
the main limitation of this experiment is that the association
between BHD and SIRT1/autophagy was not examined in-depth.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81673717) and
Guangdong Provincial Key Laboratory of Research on Emergency in TCM
(grant no. 2017B030314176).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LG, QW and SJZ designed the experiments. HL, DP and
YZ performed the experiments. HL wrote the manuscript. DP and SJZ
modified the manuscript. HL and DP confirmed the authenticity of
all the raw data. All authors reviewed and approved the final
manuscript.
Ethics approval and consent to
participate
All rat experiments and protocols were approved by
the Experimental Animal Centre of Guangzhou University of Chinese
Medicine of China (IACUC Approval no. 20190310001) on March 10,
2019.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Benjamin EJ, Virani SS, Callaway CW,
Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling
FN, Deo R, et al: Heart disease and stroke statistics-2018 update:
A report from the American heart association. Circulation.
137:e67–e492. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhou M, Wang H, Zhu J, Chen W, Wang L, Liu
S, Li Y, Wang L, Liu Y, Yin P, et al: Cause-specific mortality for
240 causes in China during 1990–2013: A systematic subnational
analysis for the Global Burden of Disease Study 2013. Lancet.
387:251–272. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
GBD 2016 Lifetime Risk of Stroke
Collaborators, ; Feigin VL, Nguyen G, Cercy K, Johnson CO, Alam T,
Parmar PG, Abajobir AA, Abate KH, Abd-Allah F, et al: Global,
regional, and country-specific lifetime risks of stroke, 1990 and
2016. N Engl J Med. 379:2429–2437. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dai W, Liu X, Zhang Z, Chen J, Guo R,
Zheng H, Jin X, Wen S, Gao Y, Li T, et al: A two-level model for
the analysis of syndrome of acute ischemic stroke: From diagnostic
model to molecular mechanism. Evid Based Complement Alternat Med.
2013:2930102013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Han CH, Kim M, Cho SY, Jung WS, Moon SK,
Park JM, Ko CN, Cho KH and Kwon S: Adjunctive herbal medicine
treatment for patients with acute ischemic stroke: A systematic
review and meta-analysis. Complement Ther Clin Pract. 33:124–137.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hodges H: Graft-induced recovery of
cognitive function after diffuse and focal brain damage:
Implications for neural transplantation in man. Zh Vyssh Nerv Deiat
Im I P Pavlova. 45:29–58. 1995.PubMed/NCBI
|
|
7
|
Hossmann KA: Pathophysiology and therapy
of experimental stroke. Cell Mol Neurobiol. 26:1057–1083. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Patel RAG and McMullen PW: Neuroprotection
in the treatment of acute ischemic stroke. Prog Cardiovasc Dis.
59:542–548. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
George PM and Steinberg GK: Novel stroke
therapeutics: Unraveling stroke pathophysiology and its impact on
clinical treatments. Neuron. 87:297–309. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Prabhakaran S, Ruff I and Bernstein RA:
Acute stroke intervention: A systematic review. JAMA.
313:1451–1462. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang S, Boyd J, Delaney K and Murphy TH:
Rapid reversible changes in dendritic spine structure in vivo gated
by the degree of ischemia. J Neurosci. 25:5333–5338. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nakagiri A, Sunamoto M and Murakami M:
NADPH oxidase is involved in ischaemia/reperfusion-induced damage
in rat gastric mucosa via ROS production-role of NADPH oxidase in
rat stomachs. Inflammopharmacology. 15:278–281. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gage FH: Adult neurogenesis in mammals.
Science. 364:827–828. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cavallucci V, Fidaleo M and Pani G:
Nutrients and neurogenesis: The emerging role of autophagy and gut
microbiota. Curr Opin Pharmacol. 50:46–52. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Duan X, Chen B, Cui Y, Zhou L, Wu C, Yang
Z, Wen Y, Miao X, Li Q, Xiong L and He J: Ready player one?
Autophagy shapes resistance to photodynamic therapy in cancers.
Apoptosis. 23:587–606. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liang K, Zhu L, Tan J, Shi W, He Q and Yu
B: Identification of autophagy signaling network that contributes
to stroke in the ischemic rodent brain via gene expression.
Neurosci Bull. 31:480–490. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sheng R, Zhang LS, Han R, Liu XQ, Gao B
and Qin ZH: Autophagy activation is associated with neuroprotection
in a rat model of focal cerebral ischemic preconditioning.
Autophagy. 6:482–494. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Su J, Zhang T, Wang K, Zhu T and Li X:
Autophagy activation contributes to the neuroprotection of remote
ischemic perconditioning against focal cerebral ischemia in rats.
Neurochem Res. 39:2068–2077. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gao L, Jiang T, Guo J, Liu Y, Cui G, Gu L,
Su L and Zhang Y: Inhibition of autophagy contributes to ischemic
postconditioning-induced neuroprotection against focal cerebral
ischemia in rats. PLoS One. 7:e460922012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vázquez P, Arroba AI, Cecconi F, de la
Rosa EJ, Boya P and de Pablo F: Atg5 and Ambra1 differentially
modulate neurogenesis in neural stem cells. Autophagy. 8:187–199.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lv X, Jiang H, Li B, Liang Q, Wang S, Zhao
Q and Jiao J: The crucial role of Atg5 in cortical neurogenesis
during early brain development. Sci Rep. 4:60102014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen X, Pan Z, Fang Z, Lin W, Wu S, Yang
F, Li Y, Fu H, Gao H and Li S: Omega-3 polyunsaturated fatty acid
attenuates traumatic brain injury-induced neuronal apoptosis by
inducing autophagy through the upregulation of SIRT1-mediated
deacetylation of Beclin-1. J Neuroinflammation. 15:3102018.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tang Q, Len Q, Liu Z and Wang W:
Overexpression of miR-22 attenuates oxidative stress injury in
diabetic cardiomyopathy via Sirt 1. Cardiovasc Ther. 36:2018.
View Article : Google Scholar
|
|
24
|
Carloni S, Albertini MC, Galluzzi L,
Buonocore G, Proietti F and Balduini W: Melatonin reduces
endoplasmic reticulum stress and preserves sirtuin 1 expression in
neuronal cells of newborn rats after hypoxia-ischemia. J Pineal
Res. 57:192–199. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Q, Liu M, Liu WW, Hao WB, Tashiro S,
Onodera S and Ikejima T: In vivo recovery effect of silibinin
treatment on streptozotocin-induced diabetic mice is associated
with the modulations of Sirt-1 expression and autophagy in
pancreatic β-cell. J Asian Nat Prod Res. 14:413–423. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Guo Q, Zhong M, Xu H, Mao X, Zhang Y and
Lin N: A systems biology perspective on the molecular mechanisms
underlying the therapeutic effects of buyang huanwu decoction on
ischemic stroke. Rejuvenation Res. 18:313–325. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hao CZ, Wu F, Shen J, Lu L, Fu DL, Liao WJ
and Zheng GQ: Clinical efficacy and safety of buyang huanwu
decoction for acute ischemic stroke: A systematic review and
meta-analysis of 19 randomized controlled trials. Evid Based
Complement Alternat Med. 2012:6301242012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zheng XW, Shan CS, Xu QQ, Wang Y, Shi YH,
Wang Y and Zheng GQ: Buyang huanwu decoction targets SIRT1/VEGF
pathway to promote angiogenesis after cerebral ischemia/reperfusion
injury. Front Neurosci. 12:9112018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu B, Cai G, Yi J and Chen X: Buyang
huanwu decoction regulates neural stem cell behavior in ischemic
brain. Neural Regen Res. 8:2336–2342. 2013.PubMed/NCBI
|
|
30
|
Luo L, Deng S, Yi J, Zhou S, She Y and Liu
B: Buyang huanwu decoction ameliorates poststroke depression via
promoting neurotrophic pathway mediated neuroprotection and
neurogenesis. Evid Based Complement Alternat Med. 2017:40726582017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang Y, Liu X, Hu T, Li X, Chen Y, Xiao G,
Huang J, Chang Y, Zhu Y, Zhang H and Wang Y: Astragalus saponins
improves stroke by promoting the proliferation of neural stem cells
through phosphorylation of Akt. J Ethnopharmacol. 277:1142242021.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li JH, Liu AJ, Li HQ, Wang Y, Shang HC and
Zheng GQ: Buyang huanwu decoction for healthcare: Evidence-based
theoretical interpretations of treating different diseases with the
same method and target of vascularity. Evid Based Complement
Alternat Med. 2014:5067832014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang ZQ, Song JY, Jia YQ and Zhang YK:
Buyanghuanwu decoction promotes angiogenesis after cerebral
ischemia/reperfusion injury: Mechanisms of brain tissue repair.
Neural Regen Res. 11:435–440. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang WW, Xu F, Wang D, Ye J and Cai SQ:
Buyang huanwu decoction ameliorates ischemic stroke by modulating
multiple targets with multiple components: In vitro evidences. Chin
J Nat Med. 16:194–202. 2018.PubMed/NCBI
|
|
35
|
Luo C, Ouyang MW, Fang YY, Li SJ, Zhou Q,
Fan J, Qin ZS and Tao T: Dexmedetomidine protects mouse brain from
ischemia-reperfusion injury via inhibiting neuronal autophagy
through Up-Regulating HIF-1α. Front Cell Neurosci. 11:1972017.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shen J, Zhu Y, Huang K, Jiang H, Shi C,
Xiong X, Zhan R and Pan J: Buyang Huanwu Decoction attenuates
H2O2-induced apoptosis by inhibiting reactive oxygen
species-mediated mitochondrial dysfunction pathway in human
umbilical vein endothelial cells. BMC Complement Altern Med.
16:1542016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Huang YT, Chen YY, Lai YH, Cheng CC, Lin
TC, Su YS, Liu CH and Lai PC: Resveratrol alleviates the
cytotoxicity induced by the radiocontrast agent, ioxitalamate, by
reducing the production of reactive oxygen species in HK-2 human
renal proximal tubule epithelial cells in vitro. Int J Mol Med.
37:83–91. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chong ZZ, Shang YC, Zhang L, Wang S and
Maiese K: Mammalian target of rapamycin: Hitting the bull's-eye for
neurological disorders. Oxid Med Cell Longev. 3:374–391. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xia D, Zhang Z and Zhao Y: Acteoside
attenuates oxidative stress and neuronal apoptosis in rats with
focal cerebral ischemia-reperfusion injury. Biol Pharm Bull.
41:1645–1651. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yang J, Yan H, Li S and Zhang M: Berberine
ameliorates MCAO induced Cerebral Ischemia/Reperfusion injury via
activation of the BDNF-TrkB-PI3K/Akt signaling pathway. Neurochem
Res. 43:702–710. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li YQ, Hui ZR, Tao T, Shao KY, Liu Z, Li M
and Gu LL: Protective effect of hypoxia inducible factor-1α gene
therapy using recombinant adenovirus in cerebral
ischaemia-reperfusion injuries in rats. Pharm Biol. 58:438–446.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Song M, Mohamad O, Gu X, Wei L and Yu SP:
Restoration of intracortical and thalamocortical circuits after
transplantation of bone marrow mesenchymal stem cells into the
ischemic brain of mice. Cell Transplant. 22:2001–2015. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lee JH, Yu WH, Kumar A, Lee S, Mohan PS,
Peterhoff CM, Wolfe DM, Martinez-Vicente M, Massey AC, Sovak G, et
al: Lysosomal proteolysis and autophagy require presenilin 1 and
are disrupted by Alzheimer-related PS1 mutations. Cell.
141:1146–1158. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hou K, Xu D, Li F, Chen S and Li Y: The
progress of neuronal autophagy in cerebral ischemia stroke:
Mechanisms, roles and research methods. J Neurol Sci. 400:72–82.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Carloni S, Girelli S, Scopa C, Buonocore
G, Longini M and Balduini W: Activation of autophagy and Akt/CREB
signaling play an equivalent role in the neuroprotective effect of
rapamycin in neonatal hypoxia-ischemia. Autophagy. 6:366–377. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang Y, Cao Y and Liu C: Autophagy and
ischemic stroke. Adv Exp Med Biol. 1207:111–134. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kim KJ and Namgung U: Facilitating effects
of Buyang Huanwu decoction on axonal regeneration after peripheral
nerve transection. J Ethnopharmacol. 213:56–64. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhang M, Chai Y, Liu T, Xu N and Yang C:
Synergistic effects of Buyang Huanwu decoction and embryonic neural
stem cell transplantation on the recovery of neurological function
in a rat model of spinal cord injury. Exp Ther Med. 9:1141–1148.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Min L, Ling W, Hua R, Qi H, Chen S, Wang
H, Tang L and Shangguan W: Anti-angiogenic therapy for
normalization of tumor vasculature: A potential effect of Buyang
Huanwu decoction on nude mice bearing human hepatocellular
carcinoma xenografts with high metastatic potential. Mol Med Rep.
13:2518–2526. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wu L, Zhang W, Li H, Chen BY, Zhang GM,
Tang YH, He FY and Deng CQ: Inhibition of aortic intimal
hyperplasia and cell cycle protein and extracellular matrix protein
expressions by BuYang HuanWu Decoction. J Ethnopharmacol.
125:423–435. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Rodrigo R, Fernández-Gajardo R, Gutiérrez
R, Matamala JM, Carrasco R, Miranda-Merchak A and Feuerhake W:
Oxidative stress and pathophysiology of ischemic stroke: Novel
therapeutic opportunities. CNS Neurol Disord Drug Targets.
12:698–714. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Orellana-Urzúa S, Rojas I, Líbano L and
Rodrigo R: Pathophysiology of ischemic stroke: Role of oxidative
stress. Curr Pharm Des. 26:4246–4260. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wu MY, Yiang GT, Liao WT, Tsai AP, Cheng
YL, Cheng PW, Li CY and Li CJ: Current mechanistic concepts in
ischemia and reperfusion injury. Cell Physiol Biochem.
46:1650–1667. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Filomeni G, De Zio D and Cecconi F:
Oxidative stress and autophagy: The clash between damage and
metabolic needs. Cell Death Differ. 22:377–388. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yamashita T and Abe K: Recent progress in
therapeutic strategies for ischemic stroke. Cell Transplant.
25:893–898. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kalogeris T, Bao Y and Korthuis RJ:
Mitochondrial reactive oxygen species: A double edged sword in
ischemia/reperfusion vs preconditioning. Redox Biol. 2:702–714.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Lum JJ, DeBerardinis RJ and Thompson CB:
Autophagy in metazoans: Cell survival in the land of plenty. Nat
Rev Mol Cell Biol. 6:439–448. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kroemer G and Jäättelä M: Lysosomes and
autophagy in cell death control. Nat Rev Cancer. 5:886–897. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Eriksson PS, Perfilieva E, Björk-Eriksson
T, Alborn AM, Nordborg C, Peterson DA and Gage FH: Neurogenesis in
the adult human hippocampus. Nat Med. 4:1313–1317. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhang RL, Zhang ZG, Zhang L and Chopp M:
Proliferation and differentiation of progenitor cells in the cortex
and the subventricular zone in the adult rat after focal cerebral
ischemia. Neuroscience. 105:33–41. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Jin K, Minami M, Lan JQ, Mao XO, Batteur
S, Simon RP and Greenberg DA: Neurogenesis in dentate subgranular
zone and rostral subventricular zone after focal cerebral ischemia
in the rat. Proc Natl Acad Sci USA. 98:4710–4715. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Doetsch F, Caillé I, Lim DA,
García-Verdugo JM and Alvarez-Buylla A: Subventricular zone
astrocytes are neural stem cells in the adult mammalian brain.
Cell. 97:703–716. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Palmer TD, Takahashi J and Gage FH: The
adult rat hippocampus contains primordial neural stem cells. Mol
Cell Neurosci. 8:389–404. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Boya P, Codogno P and Rodriguez-Muela N:
Autophagy in stem cells: Repair, remodelling and metabolic
reprogramming. Development. 145:dev1465062018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Dhaliwal J, Xi Y, Bruel-Jungerman E,
Germain J, Francis F and Lagace DC: Doublecortin (DCX) is not
essential for survival and differentiation of Newborn Neurons in
the adult mouse dentate gyrus. Front Neurosci. 9:4942015.PubMed/NCBI
|
|
66
|
Fasemore TM, Patzke N, Kaswera-Kyamakya C,
Gilissen E, Manger PR and Ihunwo AO: The Distribution of Ki-67 and
doublecortin-immunopositive cells in the brains of three
strepsirrhine primates: Galago demidoff, perodicticus potto, and
Lemur catta. Neuroscience. 372:46–57. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Shahsavani M, Pronk RJ, Falk R, Lam M,
Moslem M, Linker SB, Salma J, Day K, Schuster J, Anderlid BM, et
al: An in vitro model of lissencephaly: Expanding the role of DCX
during neurogenesis. Mol Psychiatry. 23:1674–1684. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Bojnordi MN, Azizi H, Skutella T,
Movahedin M, Pourabdolhossein F, Shojaei A and Hamidabadi HG:
Differentiation of spermatogonia stem cells into functional mature
neurons characterized with differential gene expression. Mol
Neurobiol. 54:5676–5682. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Chen X, Chen H, He Y, Fu S, Liu H, Wang Q
and Shen J: Proteomics-guided study on buyang huanwu decoction for
its neuroprotective and neurogenic mechanisms for transient
ischemic stroke: Involvements of EGFR/PI3K/Akt/Bad/14-3-3 and
Jak2/Stat3/Cyclin D1 signaling cascades. Mol Neurobiol.
57:4305–4321. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Lapierre LR, Kumsta C, Sandri M, Ballabio
A and Hansen M: Transcriptional and epigenetic regulation of
autophagy in aging. Autophagy. 11:867–880. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Zhang JF, Zhang YL and Wu YC: The role of
sirt1 in ischemic stroke: Pathogenesis and therapeutic strategies.
Front Neurosci. 12:8332018. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Wang P, Guan YF, Du H, Zhai QW, Su DF and
Miao CY: Induction of autophagy contributes to the neuroprotection
of nicotinamide phosphoribosyltransferase in cerebral ischemia.
Autophagy. 8:77–87. 2012. View Article : Google Scholar : PubMed/NCBI
|