Introduction
Asthma is a common chronic inflammatory disease in
the respiratory system that is characterized by wheeze, shortness
of breath, chest tightness, cough and obvious expiratory airflow
limitation (1). Previous statistics
have indicated that >334 million individuals suffer from asthma,
at the risk of high mortality and global economic burden (2). Airway hyperresponsiveness (AHR),
airway inflammation, inflammatory cytokine infiltration and mucus
hypersecretion are commonly associated with asthma (3). T helper (Th)2 cell-mediated type 2
inflammation and eosinophil abundance are associated with the
progression and exacerbation of asthma (4,5).
Currently, inhaled corticosteroids and bronchodilators are used as
the main treatment for asthma (6).
However, some asthmatic patients who overuse these may suffer from
various side effects, including osteoporosis, infection and drug
dependence (7). Therefore, it is
important to identify other effective and safe therapeutics for
asthmatic patients.
Autophagy, like self-eating, is involved in the
innate and adaptive immune responses of asthma (8). In the presence of allergens, damaged
proteins and organelles decompose themselves, which are captured by
autophagosomes and degraded by lysosomes to achieve the immune
balance in asthma (9). It has been
proposed that in the initiation of autophagy, Beclin 1, a major
activator of autophagy (10),
participates in the recruitment of autophagy proteins to form
autophagosomes (11).
Microtubule-associated proteins light chain (LC) 3 is hydrolyzed to
LC3I by autophagy-related gene (ATG) 4, which is subsequently
hydrolyzed to LC3II during autophagosome formation (12). Furthermore, sequestosome 1
(SQSTM1/p62, p62) can reflect the level of autophagy, and a reduced
level of p62 is generally considered as a marker of activated
autophagy, as the enhancement of autophagy leads to the degradation
of the stress-inducible cellular adapter protein p62 (13). The stable state of autophagy can
regulate both energy homeostasis and the quality of proteins and
organelles in airway inflammation (14). Otherwise, overactivation of
autophagy causes the deterioration of inflammation (15). Previous studies reported that
autophagy is activated in asthma and acts as a double-edged sword,
whereby either enhancement or decrease of autophagy can ease the
airway inflammation of asthma (16,17).
Liu et al (18) proved that
inhibition of autophagy alleviates airway inflammation and AHR in
severe asthmatic mice. Furthermore, McAlinden et al
(19) demonstrated that in the
airway remodeling of asthma, the autophagy inhibitor represses
airway smooth muscle proliferation and profibrotic signaling.
However, the specific mechanism by which autophagy mediates the
airway inflammation of asthma remains unclear.
Traditional Chinese medicine is an effective method
to treat asthma (20). A series of
studies (21,22) have reported that Astragalus
membranaceus (huangqi) has anti-asthma effects, whereby it
reduces inflammatory cytokines and improves efficacy by modulating
immune balance. Aastragaloside IV, as the main active component of
Astragalus membranaceus also exerts strong anti-allergic
effects (23), whereby it protects
mice with allergic rhinitis from inflammation (24), enhances Th1-associated
anti-inflammatory cytokines and diminishes Th2-associated
pro-inflammatory cytokines (25).
Cycloastragenol (CAG), as the main metabolite of Astragaloside IV
in vivo, is a potent small molecule telomerase activator
(26). CAG has been reported to
exert anti-inflammatory effects in cardiovascular, hepatic, skin
and aging diseases (27,28). Notably, CAG is also considered a
modulator of autophagy, associated with the balance between
pro-inflammation and anti-inflammation. However, whether CAG
regulates airway inflammatory conditions remains unclear as the
anti-asthmatic effects of CAG have not yet been investigated. Thus,
the present study used ovalbumin (OVA)-induced asthmatic mice to
investigate the anti-inflammatory effects of CAG in asthma and
determine its potential molecular mechanisms.
Materials and methods
Animals
A total of 20 BALB/c female mice [6-weeks-old
(29); body weight, 18±2 g] were
purchased from Jiesijie Laboratory Animal Co., Ltd. [license no.
SYXK(Hu)2020-0032; http://www.jsj-lab.com/]. All mice were maintained
under specific pathogen-free conditions with a 12-h light/dark
cycle and a free access to food and water at a controlled
temperature of 22±2°C with 55% relative humidity. All animal
experiments were ethically reviewed and approved by the Animal Care
and Use Committee of the Fudan University (authorization no.
2018-10-HSYY-DJC-01; Shanghai, China).
OVA-induced asthmatic mice and
treatment
Age- and sex-matched BALB/c mice were randomly
divided into five groups (4 mice/group), including the normal
control (NC), OVA-induced asthma model (Asthma), low CAG dose
(Asthma/31.25 mg/kg CAG), middle CAG dose (Asthma/62.5 mg/kg CAG)
and high CAG dose (Asthma/125 mg/kg CAG) groups. The OVA and three
doses of CAG groups were immunized on days 0, 7 and 14
intraperitoneally by OVA (100 ug/mouse, grade V, Sigma-Aldrich;
Merck KGaA) mixed with 10 mg aluminum hydroxide (Thermo Fisher
Scientific, Inc.), which was dissolved in 0.2 ml sterile saline.
Furthermore, mice were intranasally challenged with 50 µg OVA
(dissolved in 50 µl PBS) on days 21–25 (30–32).
The NC group was immunized with saline and challenged by PBS
instead. CAG (Winherb Medical Technology Co., Ltd.; http://www.sh-winherb.com/Index.aspx)
was respectively administrated to the three doses of CAG groups
intragastrically (dissolved in 0.2 ml 0.5% sodium carboxymethyl
cellulose/mouse) on days 21–25. The mice were anesthetized with 2%
phenobarbital sodium (50 mg/kg) intraperitoneally and sacrificed
after 24 h.
Measurement of AHR
The mice were tracheostomized, intubated and placed
in a single-chamber, whole-body plethysmograph connected to the
ventilator (DSI Buxco Electronics; http://www.datasci.com/products/buxco-respiratory-products/finepointe-resistance-and-compliance).
To evaluate airway responsiveness, changes in total lung resistance
(RL) and dynamic lung compliance (Cdyn) were measured in
response to aerosolized methacholine (Mch, Sigma-Aldrich; Merck
KGaA) at increasing doses of 0, 6.25, 12.5, 50 and 100 mg/ml. The
mice were subsequently euthanatized with 2% phenobarbital sodium
(150 mg/kg) intraperitoneally.
Collection of bronchoalveolar lavage
fluid (BALF) and leukocyte classification and counts
Following measurement of AHR, lungs were lavaged
using the tracheal cannula (https://www.biomart.cn/infosupply/76901595.htm) with
300 µl aliquots of ice-cold PBS twice, and centrifuged at 500 × g
for 10 min at 4°C. The supernatants were stored at −80°C until
further analyses of cytokines. Total cells were resuspended in 50
µl PBS and counted using the Mindray BC-5000Vet automated
hematology analyzer (Mindray; http://www.mindray.com/cn/product/BC-5000.html).
Lung histology
At room temperature, lung sections (4%
phosphate-buffered and formalin-fixed for 24 h) of the middle lobe
of the left lung (4-µm thick) were embedded in paraffin, stained
with hematoxylin and eosin (H&E; cat. no. G1003; Wuhan
Servicebio Technology Co., Ltd.) for total 10 min or periodic
acid-schiff (PAS; cat. no. G1008; Wuhan Servicebio Technology Co.,
Ltd.) for total 40 min and dehydrated with 100% ethanol (cat. no.
100092683; Sinopharm Chemical Reagent Co., Ltd.) for 5 min three
times and xylene (cat. no. 10023418, SCRC) for 5 min twice,
according to the manufacturer's instructions. Inflammation score of
H&E staining (33) and the
percentage of PAS+ bronchial cells (34) were determined as previously
described.
ELISA
The levels of interleukin (IL)-5, IL-13 and
immunoglobulin E (IgE) in the BALF were determined using ELISA kits
(IL-5, Mouse, cat. no. 70-EK205-48; IL-13, Mouse, cat. no.
70-EK213/2-48; IgE, Mouse, cat. no. 70-EK275-48; MultiSciences),
according to the manufacturer's instructions.
Molecular docking simulation
The 3D structure of CAG was obtained from PubChem
Compound (https://www.ncbi.nlm.nih.gov/pccompound, PubChem CID:
13943286) (35). The X-ray crystal
structure of the ATG4-LC3 complex [Protein Data Bank (PDB) ID:
2Z0D] was acquired from RCSB PDB (https://www.rcsb.org) (36). Subsequently, both of them were
converted into pdbqt formats via AutoDockTools 1.5.6 (37) and were optimized by removing water
molecules and adding polar hydrogen atoms. The potential binding
sites of the ATG4-LC3 complex within CAG were determined using the
molecular docking study employing the docking program AutoDock Vina
(38). The coordinates of the
target active pocket were center_x=−5.906, center_y=−15.694 and
center_z=27.844. Size_x=40, size_y=40 and size_z=40 were applied.
The docking process was also calculated using AutoDock Vina (all
default values). The highly scored docking result was visualized
using PyMoL 2.3.0 (39) and
Discovery Studio 2017 R2 Client (40).
Western blotting
To extract protein, lung tissues were minced and
homogenized in ice-cold RIPA lysis buffer containing phosphatase
inhibitors and a protease inhibitor (Beyotime Institute of
Biotechnology) and centrifuged at 14,000 × g for 10 min at 4°C.
Protein concentrations were quantified using the Pierce BCA Protein
Assay kit (Thermo Fisher Scientific, Inc.). Protein (30 µg) was
loaded into each well and separated via 12% SDS-PAGE. The separated
proteins were transferred onto 0.45 µm PVDF membranes and blocked
with 5% milk for 1 h at room temperature. The membranes were
incubated with the following primary antibodies; Anti-rabbit LC3B
(1:1,000; cat. no. 3868S; Cell Signaling Technology, Inc.),
anti-rabbit SQSTM1/p62 (1:1,000; cat. no. 5114T; Cell Signaling
Technology, Inc.) and anti-rabbit Beclin 1 (1:1,000; cat. no.
11306-1-AP; ProteinTech Group, Inc.) overnight at 4°C. Following
the primary incubation, membranes were incubated with
HRP-conjugated secondary antibodies (1:10,000; cat. no. SA00001-2;
ProteinTech Group, Inc.) for 1.5 h at room temperature. Protein
bands were visualized using ImageQuant LAS-4000 mini (Cytiva) and
analyzed using ImageJ 1.53 software (National Institutes of
Health).
Immunohistochemistry
Paraffin-embedded sections of lungs (4%
phosphate-buffered and formalin-fixed for 24 h at room temperature;
4-µm thick) were dewaxed in xylene, rehydrated in ethanol and
blocked with 3% BSA (cat. no. G5001; Wuhan Servicebio Technology
Co., Ltd.) for 30 min at room temperature. Following antigen
retrieval, sections were incubated with anti-LC3B antibody (1:300;
cat. no. 14600-1-AP; ProteinTech Group, Inc.), anti-SQSTM1/p62
antibody (1:400; cat. no. 88588S; Cell Signaling Technology, Inc.)
and anti-Beclin 1 antibody (1:400; cat. no. 11306-1-AP; ProteinTech
Group, Inc.) for 12 h at 4°C. Then, sections were incubated with
HRP-conjugated secondary antibodies (1:200; cat. no. GB23303; Wuhan
Servicebio Technology Co., Ltd.) for 50 min at 24°C. Cells were
counted in five randomly selected fields using an optical
microscope (magnification, ×400) and analysis was performed using
ImageJ 1.53 software (National Institutes of Health), as previously
described (41).
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 8.0 software (GraphPad Software, Inc.). Each experiment was
repeated ≥3 times. Data are presented as the mean ± SEM. One-way
ANOVA followed by Tukey's post hoc test was used for intragroup and
intergroup comparisons (42,43).
Kruskal-Wallis test followed by Dunn's post hoc test was used for
infla0mmation score. P<0.05 was considered to indicate a
statistically significant difference.
Results
CAG attenuates AHR in the OVA-induced
murine asthma model
Mice were sensitized, intranasally challenged and
administrated treatment according to the protocol presented in
Fig. 1A. At 24 h after the final
OVA challenge, lung function was evaluated through direct
measurements of RL and Cdyn. The results demonstrated
that compared with the NC group, dose-dependent increases of
RL at doses 6.25 (P<0.05), 12.5 (P<0.01), 50
(P<0.01) and 100 mg/ml Mch (P<0.001) were observed in the
Asthma group (Fig. 1B), as well as
significant dose-dependent declines of Cdyn at doses of 6.25
(P<0.05), 12.5 (P<0.01), 50 (P<0.05) and 100 mg/ml Mch
(P<0.001) (Fig. 1C).
Alternatively, compared with the Asthma group, notable reductions
of RL (P<0.05; Fig.
1B) and enhancements of Cdyn (P<0.05; Fig. 1C) were observed in both the
Asthma/62.5 mg/kg CAG and Asthma/125 mg/kg CAG groups at doses of
12.5 and 50 mg/ml Mch. Notably, at 100 mg/ml Mch, significant
decreases of RL (Fig.
1D) and elevations of Cdyn (Fig.
1E) were observed in the Asthma/62.5 mg/kg CAG (P<0.05) and
Asthma/125 mg/kg CAG (P<0.01) groups. Although the Cdyn of mice
in the Asthma/31.25 mg/kg CAG group increased at 100 mg/ml Mch
(P<0.05), the low dose of CAG had no significant effect on the
decrease of RL. Taken together, these results suggest
that 62.5 and 125 mg/kg CAG have the ability to attenuate AHR and
improve dynamic lung compliance, particularly 125 mg/kg CAG.
CAG alleviates immune cell abundance
and eosinophil recruitment
To investigate whether CAG effects immune cells in
asthma, BALF was collected and inflammatory cell classification and
counts were determined. The results demonstrated that compared with
the NC group, the Asthma group displayed significantly higher
numbers of total leucocytes (P<0.01), neutrophils (P<0.05),
lymphocytes (P<0.05), monocytes (P<0.05) and eosinophils
(P<0.01). Notably, 125 mg/kg CAG suppressed the levels of these
cells (P<0.05), particularly eosinophils (P<0.01). Notably,
62.5 mg/kg CAG significantly inhibited the levels of total
leucocytes and eosinophils (P<0.05) compared with the Asthma
group (Fig. 2A).
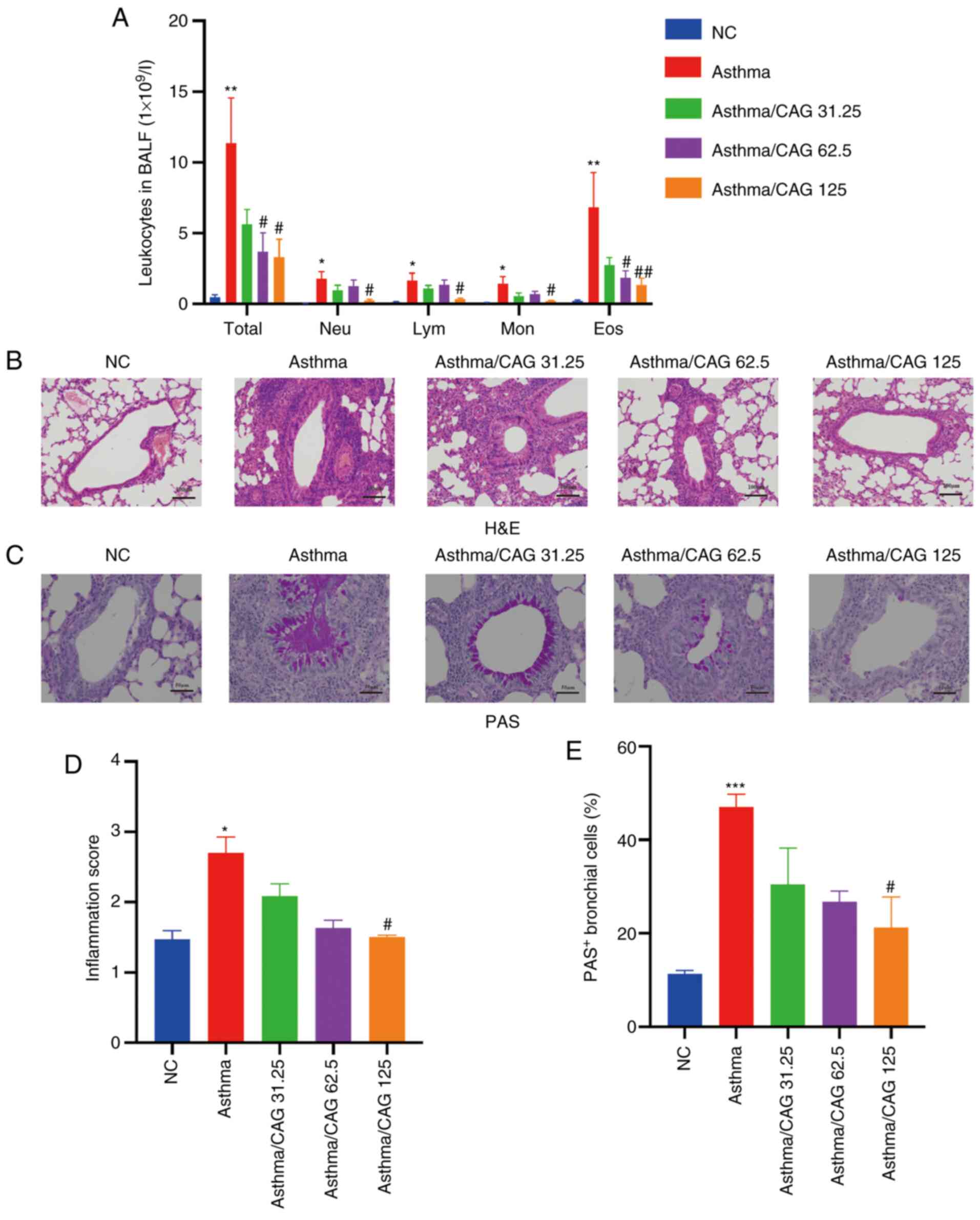 | Figure 2.Effects of CAG on inflammatory cells,
airway inflammation and mucus hypersecretion in ovalbumin-induced
asthmatic mice (n=4 mice/group). (A) Number of Total, Neu, Lym, Mon
and Eos in BALF. (B) Histological examination of H&E staining
(magnification, ×100; scale bar, 100 µm). (C) Histological
examination of PAS staining (magnification, ×200; scale bar, 50
µm). (D) Inflammation score acquired with H&E staining. (E) The
percentage of PAS+ bronchial cells. Data are presented
as the mean ± SEM. *P<0.05, **P<0.01, ***P<0.001 vs. the
NC group; #P<0.05, ##P<0.01 vs. the
Asthma group. CAG, cycloastragenol; Total, total leucocytes; Neu,
neutrophils; Lym, lymphocytes; Mon, monocytes; Eos, eosinophils;
BALF, bronchoalveolar lavage fluid; H&E, hematoxylin and eosin;
PAS, periodic acid-schiff; NC, negative control. |
CAG decreases inflammatory cell
infiltration and mucus hypersecretion
To assess the inflammation of bronchus in lung
tissues, histological changes were detected via H&E (Fig. 2B) and PAS (Fig. 2C) staining. After OVA induction,
there was excessive mucus secretion in the Asthma group, while this
elevated mucus secretion was reversed in the 125 mg/kg CAG group
(Fig. 2C). According to the H&E
staining results, inflammatory cells were significantly infiltrated
in the Asthma group compared with the NC group (P<0.05).
Notably, only 125 mg/kg CAG significantly suppressed inflammation
compared with the Asthma group (P<0.05; Fig. 2D). The PAS staining results
demonstrated that the PAS+ bronchial cell count
significantly increased in the Asthma group following OVA induction
(P<0.001; Fig. 2E), which
suggests that mucus secretion of the Asthma group is extremely
excessive. As expected, 125 mg/kg CAG significantly relieved mucus
secretion (P<0.05). Although the low dose of 31.25 mg/kg CAG had
modest relief in both lung function and airway inflammation, the
tendency was not significant. Thus, according to the results of the
measurement of RL, the counts of total cells and
eosinophils and H&E staining, 62.5 and 125 mg/kg CAG were
selected for subsequent experimentation.
CAG alleviates inflammatory cytokines
and IgE in BALF
The effects of 62.5 and 125 mg/kg CAG on the levels
of Th2 cytokines and IgE, which are common in allergic asthma
(44), were investigated. The
results demonstrated that the levels of IL-5 (P<0.05; Fig. 3A), IL-13 (P<0.01; Fig. 3B) and IgE (P<0.01; Fig. 3C) were significantly higher in the
Asthma group compared with the NC group. Notably, 125 mg/kg CAG
significantly decreased the levels of IL-5 (P<0.05), IL-13
(P<0.05) and IgE (P<0.01). However, 62.5 mg/kg CAG
significantly decreased the level of IL-5 (P<0.05).
Collectively, these results suggest that 125 mg/kg CAG regulates
Th2-associated inflammation.
Molecular docking study
After confirming the anti-inflammatory function of
CAG (Fig. 4A) in asthma, the
present study investigated the specific mechanism and performed
molecular docking between the ATG4-LC3B complex and CAG to
determine whether CAG can modulate autophagy-related proteins, and
the potential interaction between them. The highest binding energy
of CAG towards the ATG4-LC3B complex was −8.0 kcal/mol, and the
docking analysis predicted that CAG made hydrogen-bonding
interactions with LEU232 and GLN43 at the active site (Fig. 4B). Furthermore, CAG probably formed
a pi-alkyl with LYS42.
CAG inhibits autophagy-related
proteins in lung tissues
To further verify the regulation of CAG on the
expression of autophagy-related proteins, the present study
examined the major autophagy-related factors, LC3B, p62 and Beclin
1. According to the results of western blotting, it was found that
the expression levels of LC3B and Beclin 1 were enhanced, while the
expression of p62 was diminished in the Asthma group. Moreover, 125
mg/kg CAG restored these expressions (Fig. 5A). The results demonstrated that
LC3B protein expression was significantly higher in the Asthma
group compared with the NC group (P<0.01; Fig. 5B). Furthermore, p62 protein
expression was significantly lower in the Asthma group compared
with the NC group (P<0.01; Fig.
5C). Notably, LC3B protein expression was relieved following
treatment with 62.5 mg/kg CAG (P<0.05) and 125 mg/kg CAG,
particularly in the higher dose (P<0.01). In addition, 62.5
mg/kg CAG (P<0.05) and 125 mg/kg CAG (P<0.01) restored the
p62 expression, which suggests that high doses of CAG can
significantly inhibit autophagy in the asthma model.
Immunohistochemistry analysis (Fig.
5D-F) revealed a notable increase in the expression levels of
LC3B (P<0.0001; Fig. 5G) and
Beclin 1 (P<0.05) (Fig. 5I) with
OVA challenge, while p62 expression significantly decreased in the
Asthma group (P<0.01; Fig. 5H).
Taken together, these results confirm that 62.5 and 125 mg/kg CAG
decrease LC3B expression (P<0.001) and Beclin 1 expression
(P<0.05). In addition, 125 mg/kg CAG significantly increased p62
expression (P<0.001), which were consistent with the western
blot results. Overall, 125 mg/kg CAG had the potential to alleviate
the levels of inflammation to attenuate AHR and mucus secretion in
asthma pathogenesis, probably via the inhibition of the levels of
autophagy (Fig. 6).
 | Figure 5.Effects of CAG on the expression
levels of LC3B, p62 and Beclin 1 in ovalbumin-induced asthmatic
mice (n=4 mice/group). (A) Protein expression levels of LC3B, p62
and Beclin 1 in the assessed groups. Relative density
quantifications of (B) LC3B and (C) p62. Data are presented as a
ratio of LC3B and p62 relative to β-actin. Immunohistochemistry
analysis for positive expression levels of (D) LC3B, (E) p62 and
(F) Beclin 1 (magnification, ×400; scale bar, 100 µm). Positive
areas of protein expression levels of (G) LC3B, (H) p62 and (I)
Beclin 1. Data are presented as the mean ± SEM. *P<0.05,
**P<0.01, ****P<0.0001 vs. the NC group;
#P<0.05, ##P<0.01,
###P<0.001 vs. the Asthma group. CAG,
cycloastragenol; LC3B, light chain 3B; NC, negative control. |
Discussion
Asthma is characterized by airway inflammation, AHR
and airway remodeling (45).
Although corticosteroids are used to treat airway inflammation of
asthma, they still have multiple adverse reactions, such as
infections, diabetes and osteoporosis (46). Thus, other safe and effective
therapies are required to relieve inflammation of asthma that
contribute to improved quality of life and reduce social
burden.
CAG, an active sapogenin of Astragaloside IV, has
been proposed to function on multiple pharmacological effects and
has been gradually developed as a modern dietary ingredient
(47). Recent studies (48–52)
have demonstrated that CAG exerts protective effects in
inflammation and oxidation. However, whether CAG can prevent the
progress of asthma in murine remains unknown. Thus, the present
study investigated the course of airway inflammation in asthma and
established an OVA-induced acute asthmatic murine model to assess
the anti-asthmatic effect of CAG in vivo. Notably, all mice
survived and had no loss of body weight. Furthermore, AHR, immune
cell infiltration and the metaplasia and hypersecretion of goblet
cells were restored via CAG, potentially through inhibition of
autophagy.
RL and Cdyn reflect the state of lung
ventilation, whereby high RL is associated with airflow
obstruction of main bronchus and low Cdyn is associated with
narrowing of peripheral bronchus (53). The results of the present study
confirmed that both 62.5 and 125 mg/kg CAG triggered the notable
decrease of RL and elevation of Cdyn, which ameliorated
the aggravation of lung function in asthma.
Immune cell counts, as well as H&E and PAS
staining, are the main indicators of airway inflammation and mucus
production (54). Overactivation of
immune cells, particularly eosinophil recruitment and infiltration
promote the progress of asthma (55). In addition, hyperplastic goblet
cells produce excessive mucus plugs, exudation and cell debris to
cause further airway occlusion (56). It has been demonstrated that high
doses of CAG can suppress immune cells to prevent the development
of asthma (50); however, 31.25
mg/kg CAG had little efficacy in both lung function and immune cell
counts. Similar results were observed following H&E and PAS
staining.
Then we observed the effects of 62.5 mg/kg CAG and
125 mg/kg CAG on Th2-associated cytokines (IL-5 and IL-13). IL-5 is
dominant in Th2-mediated eosinophilic asthma and can reflect the
vitality of eosinophils as well as AHR while IL-13 promotes B cells
to produce IgE, mucus secretion and exacerbates AHR (57). It was found that they were both
repressed by CAG. So, we further measured IgE, a central player in
the allergy response, and proved that the enhancement of IgE in
asthma was also controlled by CAG (58). These results were consistent with
the results of the lung function mentioned above and 125 mg/kg CAG
was suggested to be an effective therapy for asthma.
It has been reported that CAG can regulate the
levels of autophagy in myocardial cells (59), thus the present study investigated
the probable binding between CAG and autophagy-associated targets,
based on molecular docking. The results suggest that CAG may bind
to the ATG4-LC3 complex to exert anti-inflammatory effects.
Autophagy is the degradation of organelles and protein aggregates
that are not degradable by proteasomes or invading microorganisms,
such as viruses and bacteria (60).
Autophagy-associated pathways and proteins play crucial roles in
immunity and inflammation, acting as a central pivot to balance the
beneficial and harmful effects of the host on infection and stimuli
(61). Currently, the evaluation of
autophagy is based on the autophagy markers, LC3, p62 and Beclin 1,
which participate in the formation of autophagosome and phagophore
(62). Previous studies have
proposed that autophagy is promoted in the pathogenesis of asthma
(63,64), which is consistent with the results
of the present study. The present study further investigated the
modulation of autophagy by CAG in lung tissues of asthmatic mice.
The results demonstrated that both 62.5 and 125 mg/kg CAG reverted
the increased protein expression levels of LC3B and restored the
decreased protein expression levels of p62 in asthma. Notably, 125
mg/kg CAG triggered the regulation of autophagic flux to suppress
autophagy, which might be associated with the attenuation of the
development of asthma.
Due to the limitations of the experimental design,
the present study only simulated the probable bond with CAG and
autophagy-related targets, but failed to confirm their direct
association, which can be verified via knockdown experiments. In
addition, the mechanism by which cells express autophagy-related
proteins, and are modulated by CAG in the lungs, remain
unclear.
In conclusion, the results of the present study
verified CAG as a potential therapeutic target for AHR, airway
inflammation and mucus hypersecretion in asthma, and suggested that
these functions may be associated with the regulation of autophagic
flux, mainly including decreased LC3B protein expression and
increased p62 protein expression. However, further studies are
required to confirm whether CAG alleviates airway inflammation by
modulating autophagy. The results of the present study demonstrated
that CAG exerted anti-inflammatory effects and inhibited autophagy
in OVA-induced asthmatic murine, which provides the basis for
further research on the target of CAG in the treatment of
asthma.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81774074), Shanghai
Science and Technology Commission (grant nos. 17401930300 and
18401971300) and the Expert Workstation for Jingcheng Dong in
Yunnan Province (grant no. 20210101).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XZ, YC and JD designed the present study. XZ, MS,
MC, CL, LY, JQ, WT, FT, YZ, WT and SW performed the experiments and
confirmed the authenticity of all the raw data. XZ and YC analyzed
the data and drafted the initial manuscript. JD revised the
manuscript for important intellectual content. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
All experimental procedures were approved by the
Animal Care and Use Committee of the Fudan University (Shanghai,
China; authorization no. 2018-10-HSYY-DJC-01).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cevhertas L, Ogulur I, Maurer DJ, Burla D,
Ding M, Jansen K, Koch J, Liu C, Ma S, Mitamura Y, et al: Advances
and recent developments in asthma in 2020. Allergy. 75:3124–3146.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Papi A, Brightling C, Pedersen SE and
Reddel HK: Asthma. Lancet. 391:783–800. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bao W, Zhang Y, Zhang M, Bao A, Fei X,
Zhang X and Zhou X: Effects of ozone repeated short exposures on
the airway/lung inflammation, airway hyperresponsiveness and mucus
production in a mouse model of ovalbumin-induced asthma. Biomed
Pharmacother. 101:293–303. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Busse W, Kraft M, Rabe KF, Deniz Y, Rowe
PJ, Ruddy M and Castro M: Understanding the key issues in the
treatment of uncontrolled persistent asthma with type 2
inflammation. Eur Respir J. 58:20033932021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Piñeros YS, Bal SM, Dijkhuis A, Majoor CJ,
Dierdorp BS, Dekker T, Hoefsmit EP, Bonta PI, Picavet D, van der
Wel NN, et al: Eosinophils capture viruses, a capacity that is
defective in asthma. Allergy. 74:1898–1909. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Reddel HK, Bateman ED, Becker A, Boulet
LP, Cruz AA, Drazen JM, Haahtela T, Hurd SS, Inoue H, de Jongste
JC, et al: A summary of the new GINA strategy: A roadmap to asthma
control. Eur Respir J. 46:622–639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Heffler E, Madeira LNG, Ferrando M,
Puggioni F, Racca F, Malvezzi L, Passalacqua G and Canonica GW:
Inhaled corticosteroids safety and adverse effects in patients with
asthma. J Allergy Clin Immunol Pract. 6:776–781. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu Y and Eissa NT: Autophagy in innate and
adaptive immunity. Proc Am Thorac Soc. 7:22–28. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Levine B, Mizushima N and Virgin HW:
Autophagy in immunity and inflammation. Nature. 469:323–335. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang S, Wuniqiemu T, Tang W, Teng F, Bian
Q, Yi L, Qin J, Zhu X, Wei Y and Dong J: Luteolin inhibits
autophagy in allergic asthma by activating PI3K/Akt/mTOR signaling
and inhibiting Beclin-1-PI3KC3 complex. Int Immunopharmacol.
94:1074602021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hill S, Wrobel L and Rubinsztein D:
Post-translational modifications of Beclin 1 provide multiple
strategies for autophagy regulation. Cell Death Differ. 26:617–629.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Matsuzawa-Ishimoto Y, Hwang S and Cadwell
K: Autophagy and Inflammation. Ann Rev Immunol. 36:73–101. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kirkin V, McEwan D, Novak I and Dikic I: A
role for ubiquitin in selective autophagy. Mol Cell. 34:259–269.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Deretic V: Autophagy in inflammation,
infection, and immunometabolism. Immunity. 54:437–453. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Racanelli AC, Kikkers SA, Choi AMK and
Cloonan SM: Autophagy and inflammation in chronic respiratory
disease. Autophagy. 14:221–232. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Renz H: Autophagy: Nobel prize 2016 and
allergy and asthma research. J Allergy Clin Immunol. 140:1548–1549.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Silveira JS, Antunes GL, Kaiber DB, da
Costa MS, Ferreira FS, Marques EP, Schmitz F, Gassen RB, Breda RV,
Wyse ATS, et al: Autophagy induces eosinophil extracellular traps
formation and allergic airway inflammation in a murine asthma
model. J Cell Physiol. 235:267–280. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu JN, Suh DH, Trinh HK, Chwae YJ, Park
HS and Shin YS: The role of autophagy in allergic inflammation: A
new target for severe asthma. Exp Mol Med. 48:e2432016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
McAlinden KD, Deshpande DA, Ghavami S,
Xenaki D, Sohal SS, Oliver BG, Haghi M and Sharma P: Autophagy
activation in asthma airways remodeling. Am J Respir Cell Mol Biol.
60:541–553. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chan HHL and Ng T: Traditional Chinese
medicine (TCM) and allergic diseases. Curr Allergy Asthma Rep.
20:672020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang W, Liu QB and Jing W: Astragalus
membranaceus improves therapeutic efficacy of asthmatic children by
regulating the balance of Treg/Th17 cells. Chin J Nat Med.
17:252–263. 2019.PubMed/NCBI
|
|
22
|
Wang W, Jing W and Liu Q: Astragalus oral
solution ameliorates allergic asthma in children by regulating
relative contents of CD4+ CD25high
CD127low treg cells. Front Pediatr. 6:2552018.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li L, Hou X, Xu R, Liu C and Tu M:
Research review on the pharmacological effects of astragaloside IV.
Fundam Clin Pharmacol. 31:17–36. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li K, Chen Y, Jiang R, Chen D, Wang H,
Xiong W, Li D, Liu Z, Li X, Li J and Yuan K: Protective effects of
astragaloside IV against ovalbumin-induced allergic rhinitis are
mediated by T-box protein expressed in T cells/GATA-3 and forkhead
box protein 3/retinoic acid-related orphan nuclear receptor γt. Mol
Med Rep. 16:1207–1215. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang X, Tang L, Wang F and Song G:
Astragaloside IV attenuates allergic inflammation by regulation
Th1/Th2 cytokine and enhancement CD4(+)CD25(+)Foxp3 T cells in
ovalbumin-induced asthma. Immunobiology. 219:565–571. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Calis I, Gazar H, Piacente S and Pizza C:
Secondary metabolites from the roots of Astragalus zahlbruckneri. J
Nat Prod. 64:1179–1182. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu Y, Zhou L, Yang Y and Liu Y:
Cycloastragenol: An exciting novel candidate for age-associated
diseases. Exp Ther Med. 16:2175–2182. 2018.PubMed/NCBI
|
|
28
|
Wan Y, Xu L, Wang Y, Tuerdi N, Ye M and Qi
R: Preventive effects of astragaloside IV and its active sapogenin
cycloastragenol on cardiac fibrosis of mice by inhibiting the NLRP3
inflammasome. Eur J Pharmacol. 833:545–554. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Melgert BN, Postma DS, Kuipers I,
Geerlings M, Luinge MA, van der Strate BW, Kerstjens HAM, Timens W
and Hylkema MN: Female mice are more susceptible to the development
of allergic airway inflammation than male mice. Clin Exp Allergy.
35:1496–1503. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li Y, Chen S, Chi Y, Yang Y, Chen X, Wang
H, Lv Z, Wang J, Yuan L, Huang P, et al: Kinetics of the
accumulation of group 2 innate lymphoid cells in IL-33-induced and
IL-25-induced murine models of asthma: A potential role for the
chemokine CXCL16. Cell Mol Immunol. 16:75–86. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Peng H, Ning H, Wang Q, Lu W, Chang Y,
Wang TT, Lai J, Kolattukudy PE, Hou R, Hoft DF, et al: Monocyte
chemotactic protein-induced protein 1 controls allergic airway
inflammation by suppressing IL-5-producing TH 2 cells
through the Notch/Gata3 pathway. J Allergy Clin Immunol.
142:582–594. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen S, Yun F, Yao Y, Cao M, Zhang Y, Wang
J, Song X and Qian Y: USP38 critically promotes asthmatic
pathogenesis by stabilizing JunB protein. J Exp Med. 215:2850–2867.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tel BC, Telli G, Onder S, Nemutlu E and
Bozkurt TE: Investigation of the relationship between chronic
montelukast treatment, asthma and depression-like behavior in mice.
Exp Ther Med. 21:272021.PubMed/NCBI
|
|
34
|
Tamaru S, Mishina H, Watanabe Y, Watanabe
K, Fujioka D, Takahashi S, Suzuki K, Nakamura T, Obata JE, Kawabata
K, et al: Deficiency of phospholipase A2 receptor exacerbates
ovalbumin-induced lung inflammation. J Immunol. 191:1021–1028.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim S, Thiessen PA, Bolton EE, Chen J, Fu
G, Gindulyte A, Han L, He J, He S, Shoemaker BA, et al: PubChem
substance and compound databases. Nucleic Acids Res.
44:D1202–D1213. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Berman H, Westbrook J, Feng Z, Gilliland
G, Bhat TN, Weissig H, Shindyalov IN and Bourne PE: The protein
data bank. Nucleic Acids Res. 28:235–242. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Morris G, Huey R, Lindstrom W, Sanner MF,
Belew RK, Goodsell DS and Olson AJ: AutoDock4 and AutoDockTools4:
Automated docking with selective receptor flexibility. J Comput
Chem. 30:2785–2791. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Trott O and Olson A: AutoDock vina:
Improving the speed and accuracy of docking with a new scoring
function, efficient optimization, and multithreading. J Comput
Chem. 31:455–461. 2010.PubMed/NCBI
|
|
39
|
Seeliger D and de Groot B: Ligand docking
and binding site analysis with PyMOL and autodock/vina. J Comput
Aided Mol Des. 24:417–422. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang C, Tu M, Wu D, Chen H, Chen C, Wang Z
and Jiang L: Identification of an ACE-inhibitory peptide from
walnut protein and its evaluation of the inhibitory mechanism. Int
J Mol Sci. 19:11562018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Feng FB and Qiu HY: Effects of Artesunate
on chondrocyte proliferation, apoptosis and autophagy through the
PI3K/AKT/mTOR signaling pathway in rat models with rheumatoid
arthritis. Biomed Pharmacother. 102:1209–1220. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wei Y, Liu B, Sun J, Lv Y, Luo Q, Liu F
and Dong J: Regulation of Th17/Treg function contributes to the
attenuation of chronic airway inflammation by icariin in
ovalbumin-induced murine asthma model. Immunobiology. 220:789–797.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tang W, Dong M, Teng F, Cui J, Zhu X, Wang
W, Wuniqiemu T, Qin J, Yi L, Wang S, et al: TMT-based quantitative
proteomics reveals suppression of SLC3A2 and ATP1A3 expression
contributes to the inhibitory role of acupuncture on airway
inflammation in an OVA-induced mouse asthma model. Biomed
Pharmacother. 134:1110012021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kubo M: Innate and adaptive type 2
immunity in lung allergic inflammation. Immunol Rev. 278:162–172.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhu X, Cui J, Yi L, Qin J, Tulake W, Teng
F, Tang W, Wei Y and Dong J: The role of T cells and macrophages in
asthma pathogenesis: A new perspective on mutual crosstalk.
Mediators Inflamm. 2020:78352842020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bleecker ER, Menzies-Gow AN, Price DB,
Bourdin A, Sweet S, Martin AL, Alacqua M and Tran TN: Systematic
literature review of systemic corticosteroid use for asthma
management. Am J Respir Crit Care Med. 201:276–293. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhu J, Lee S, Ho MK, Hu Y, Pang H, Ip FC,
Chin AC, Harley CB, Ip NY and Wong YH: In vitro intestinal
absorption and first-pass intestinal and hepatic metabolism of
cycloastragenol, a potent small molecule telomerase activator. Drug
Metab Pharmacokinet. 25:477–486. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Li M, Li SC, Dou BK, Zou YX, Han HZ, Liu
DX, Ke ZJ and Wang ZF: Cycloastragenol upregulates SIRT1
expression, attenuates apoptosis and suppresses neuroinflammation
after brain ischemia. Acta Pharmacol Sin. 41:1025–1032. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Gu M, Zhang S, Zhao Y, Huang J, Wang Y, Li
Y, Fan S, Yang L, Ji G, Tong Q and Huang C: Cycloastragenol
improves hepatic steatosis by activating farnesoid X receptor
signalling. Pharmacol Res. 121:22–32. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wang Y, Chen C, Wang Q, Cao Y, Xu L and Qi
R: Inhibitory effects of cycloastragenol on abdominal aortic
aneurysm and its related mechanisms. Br J Pharmacol. 176:282–296.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Liu J, Gao D, Dan J, Liu D, Peng L, Zhou R
and Luo Y: The protective effect of cycloastragenol on aging mouse
circadian rhythmic disorder induced by d-galactose. J Cell Biochem.
120:16408–16415. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Deng G, Chen W, Wang P, Zhan T, Zheng W,
Gu Z, Wang X, Ji X and Sun Y: Inhibition of NLRP3
inflammasome-mediated pyroptosis in macrophage by cycloastragenol
contributes to amelioration of imiquimod-induced psoriasis-like
skin inflammation in mice. Int Immunopharmacol. 74:1056822019.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kanehiro A, Takeda K, Joetham A, Tomkinson
A, Ikemura T, Irvin CG and Gelfand EW: Timing of administration of
anti-VLA-4 differentiates airway hyperresponsiveness in the central
and peripheral airways in mice. Am J Respir Crit Care Med.
162:1132–1139. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Dong L, Wang Y, Zheng T, Pu Y, Ma Y, Qi X,
Zhang W, Xue F, Shan Z, Liu J, et al: Hypoxic hUCMSC-derived
extracellular vesicles attenuate allergic airway inflammation and
airway remodeling in chronic asthma mice. Stem Cell Res Ther.
12:42021. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Hammad H and Lambrecht BN: The basic
immunology of asthma. Cell. 184:1469–1485. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Chen W, Sivaprasad U, Gibson AM, Ericksen
MB, Cunningham CM, Bass SA, Kinker KG, Finkelman FD, Wills-Karp M
and Hershey GK: IL-13 receptor α2 contributes to development of
experimental allergic asthma. J Allergy Clin Immunol. 132:951–958.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Lambrecht BN, Hammad H and Fahy JV: The
cytokines of asthma. Immunity. 50:975–991. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Gould HJ and Sutton BJ: IgE in allergy and
asthma today. Nat Rev Immunol. 8:205–217. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wang J, Wu ML, Cao SP, Cai H, Zhao ZM and
Song YH: Cycloastragenol ameliorates experimental heart damage in
rats by promoting myocardial autophagy via inhibition of
AKT1-RPS6KB1 signaling. Biomed Pharmacother. 107:1074–1081. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Cadwell K: Crosstalk between autophagy and
inflammatory signalling pathways: Balancing defence and
homeostasis. Nat Rev Immunol. 16:661–675. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Mizushima N and Levine B: Autophagy in
human diseases. N Engl J Med. 383:1564–1576. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Maneechotesuwan K, Kasetsinsombat K,
Wongkajornsilp A and Barnes PJ: Role of autophagy in regulating
interleukin-10 and the responses to corticosteroids and statins in
asthma. Clin Exp Allergy. 19:138252021. View Article : Google Scholar
|
|
63
|
Xia F, Deng C, Jiang Y, Qu Y, Deng J, Cai
Z, Ding Y, Guo Z and Wang J: IL4 (interleukin 4) induces autophagy
in B cells leading to exacerbated asthma. Autophagy. 14:450–464.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zhang Y, Do DC, Hu X, Wang J, Zhao Y,
Mishra S, Zhang X, Wan M and Gao P: CaMKII oxidation regulates
cockroach allergen-induced mitophagy in asthma. J Allergy Clin
Immunol. 147:1464–1477. 2021. View Article : Google Scholar : PubMed/NCBI
|




















