Introduction
Currently, besides surgery and chemotherapy,
radiotherapy remains one of the most commonly used treatment
methods for thoracic malignant tumors such as lung cancer, breast
cancer, neck cancer and lymphoma (1,2).
Radiation-induced normal lung tissue injury is a common
complication of chest radiotherapy and is an important reason for
the limited application of radiotherapy in patients with thoracic
malignancies (3,4). Main radiation-induced injury is
reported to result in acute pneumonitis and, later, fibrosis in
lung tissue (4). In addition,
studies have indicated that radiation could result in injury of
alveolar epithelial cells (AECs) and subsequently induce
epithelial-mesenchymal transition (EMT) of AECs. This is one of the
possible pathological mechanisms of pulmonary fibrosis after
radiotherapy (5,6). Therefore, suppression of the EMT
process in AECs would be beneficial for controlling or preventing
the development of pulmonary fibrosis under radiotherapy.
Jiawei-Maxing-Shigan decoction (JMSD), is
composed of Roasted ephedra (Herba Ephedrae), Gypsum
(Gypsum Fibrosum), Paeoniae radix (Radix Paeoniae
Rubra), Apricot seed (Semen Armeniacae Amarum), Mulberry
(Cortex mori Radicis), Honeysuckle (Flos Lonicerae
Japonicae) and Licorice (Radix Et Rhizoma Glycyrrhizae)
(7) and is a clinically proven
recipe for treating pediatric asthma (8), bronchitis (9), infantile mycoplasma pneumonia
(10) and radioactive lung injury
(7,11). Our previous study found that the
main constituents of JMSD are farnesene, dihydrotanshinone I,
paeonol, emodin, schisanhenol, tanshinone IIA, cryptotanshinone,
columbianadin, uridine and liquiritigenin (7). Furthermore, JMSD administration can
attenuate radiation-induced EMT in AECs by regulating the
expression of TGF-β/Smad signaling (7). Protein phosphatase
Mg2+/Mn2+-dependent 1A (PPM1A) serves an
important role in the signal transduction of TGF-β/Smad signaling
and can inactivate TGF-β/Smad signaling by dephosphorylating
Smad2/3. PPM1A functions as a tumor suppressor in bladder and
breast cancer via regulating cell invasion, EMT and cell cycle
progression (12–14). As part of a continuing study on the
molecular mechanisms of JMSD, the present study further examined
the role of PPM1A in the anti-EMT activity of JMSD on AECs.
Materials and methods
Herbal medicines
The seven herbal medicines were supplied and the
JMSD water extract was prepared by the School of Pharmacy, Zhejiang
Chinese Medicine University. The composition of JMSD is presented
in Table I. The seven herbs were
decocted by boiling in distilled water for 1 h twice as described
in our previous paper (7). Then,
the water extract was filtered and concentrated to 32 ml. As JMSD
was a mixture, its purity could not be determined.
 | Table I.Composition of
Jiawei-Maxing-Shigan decoction. |
Table I.
Composition of
Jiawei-Maxing-Shigan decoction.
| Herbal
medicines | Weight (g) |
|---|
| Roasted ephedra
(Herba Ephedrae) | 9 |
| Gypsum (Gypsum
Fibrosum) | 18 |
| Paeoniae radix
(Radix Paeoniae Rubra) | 12 |
| Apricot seed
(Semen Armeniacae Amarum) | 12 |
| Mulberry (Cortex
mori Radicis) | 12 |
| Honeysuckle
(Flos Lonicerae Japonicae) | 9 |
| Licorice (Radix
Et Rhizoma Glycyrrhizae) | 6 |
Liquid chromatography coupled with
electrospray mass spectrometry (HPLC/ESI-MS) analysis of JMSD
The aqueous extract of JMSD was analyzed with an
Agilent 1100 HPLC system (Agilent Technologies, Inc.) coupled with
electrospray mass spectrometry as previously described (7). The separation was performed on a
GS-120-5-C18-BIO chromatographic column (5 µm; 250×4.6 mm i.d.;
Global Chromatography Co., Ltd.) with the column temperature set at
35°C. A linear gradient elution of A (0.1% formic acid water) and B
(acetonitrile) was used with the gradient procedure as follows: B
5% at 0 min, B 40% at 60 min (v/v). The flow rate was 1.0 ml/min
and the injection volume was 10 µl. DAD was on and the target
wavelength was simultaneously set at 210 nm. The split ratio to the
mass spectrometer was 1:3. The acquisition parameters for negative
ion mode were: collision gas, ultra high-purity helium (He),
nebulizer gas (N2), 35 psi, drying gas (N2), 10 l/min, drying
temperature, 350°C, HV, 3500 V, mass scan range, m/z 100–2200,
target mass, 500 m/z, compound stability, 100%, trap drive level,
100%. All the data were analyzed using the Chemstation software
(version B.04.03; Agilent Technologies, Inc.).
Animals
Male Sprague-Dawley (SD) rats (6–8 weeks; 200±20 g;
n=3) and Wistar rats (6–8 weeks; 200 ± 20 g; n=6) were acquired
from the Shanghai Experimental Animal Center (Shanghai, China).
Rats were maintained at 25°C with 60% humidity and a 12-h
light/dark cycle, and free access to food and water. The
experimental protocols were approved by the animal ethics committee
of Zhejiang Chinese Medicine University (Hangzhou, China; approval
no. 2019485).
Chemicals
The TRIzol® kit, BCA protein quantitative
kit, SYBR Green PCR kit and reverse transcription kit used in the
study were bought from the Thermo Fisher Scientific, Inc. The ECL
kit (cat. no. WBKLS0100) was purchased from EDM Millipore. The
primary antibodies for PPM1A and phosphorylated (p-)Smad2/3 were
purchased from Abcam. The primary antibodies for Smad2/3,
p-Smad2/3, E-cadherin, vimentin, α-SMA and
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were purchased
from Cell Signaling Technology, Inc. PLKO.1, psPAX2 and pMD2G were
acquired from Addgene, Inc. DH5α competent cells were purchased
from Beijing Transgen Biotech Co., Ltd.; 293T cells, from ATCC and
pLVX-Puro from Clontech Laboratories, Inc.
Preparation of the JMSD-medicated
serum and control serum
Wistar rats were randomly divided into the JMSD and
control groups (n=3 per group). In the rats in the control and JMSD
groups, 4 ml of 0.9% sodium chloride and JMSD were intragastrically
administered twice per day for 3 days, respectively. At 2 h after
the last administration, the rats were euthanized with
intraperitoneal injections of pentobarbital sodium (100 mg/kg) and
a blood sample (~6 ml) was drawn from the abdominal aorta, pooled
and clotted for 2 h at room temperature. Serum was isolated
carefully by centrifuging the clotted blood at 2,000 x g for 20 min
at 4°C and stored at −70°C until use. In accordance with previous
literature, the dose for preparation of medicated serum can be
calculated as: Dose=clinical dose x animal equivalent dose x
dilution (15). In the present
study, the animals were given 15 x adult dose to prepare the
JMSD-medicated serum. Primary type II AECs were treated the
JMSD-medicated serum at doses of ~2-10%, which was diluted to
~2/100-10/100. Therefore, the dose used in this present study was
not a very high dose. During the experiments, animal health and
behavior were monitored daily to minimize suffering and distress.
Greater than 20% weight loss, dehydration, or loss of ability to
ambulate, were the signs we used to determine the time at which the
animals should be euthanized. Confirmation of death was evaluated
with vital signs including heart beats, pupillary response and
respiratory pattern. No animals showed signs of humane endpoints
and no rats were dead prior to the end of the experiments.
Preparation of type II AECs and cell
culture
Primary type II AECs were prepared from SD rats as
previously described (10); after
the rats were euthanized with intraperitoneal injections of
pentobarbital sodium (100 mg/kg). Dispase was instilled into the
lung via a tracheal catheter for 15 min at 37°C. The lungs were
removed, carefully teased apart and treated with DNase I for 5 min
at 37°C. The cell suspension was passed through 150, 15 and 7.5 µm
metal strainers and then centrifuged at 100 x g at 4°C for 8 min.
The cell pellet was resuspended in Dulbecco's modified Eagle's
medium (DMEM) and plated to a culture dish precoated with rat IgG.
After 1 h of culture, the non-adherent cells were collected and
plated to another culture dish. After culturing for another 20 min,
the non-adherent cells were centrifuged at 100 x g at 4°C for 8
min. The cell pellet was resuspended in DMEM containing 20% fetal
bovine serum (HyClone; Cytiva) and cultured at 37°C in a 5%
CO2 incubator. The purity of the isolated cells was
>90% as determined with nitroblue
tetrazolium/5-bromo-4-chloro-3-indolyl phosphate staining.
Constructions of the overexpression
and low expression level of lentivirus
A lentivirus was constructed commercially by
Genewiz, Inc. Briefly, the plasmid vectors were extracted and
subsequently co-transfected into 293T cells with the packaging
plasmids using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.). After 72 h of culture at 37°C,
supernatant of 293T cells was collected and filtered using a
0.45-µm filter. The short interfering (si)RNA sequences used are as
follows: siPPM1A-1: 5′-CCAAGUGGACUUGAGACAUUU-3′; siPPM1A-2:
5′-GCCUGAAGUCCAUGAUAUUUU-3′; siPPM1A-3′:
5′-CCUUGAGAAAGUUUGCAAUUU-3′; and siNC:
5′-CAGUACUUUUGUGUAGUACAA-3′.
Cell treatment
Primary type II AECs were divided into four groups.
Groups 1 and 2 were cultured with 10% serum collected from control
rats. The cells in group 3 were incubated with 2% JMSD-medicated
serum (J) and 8% normal control rat serum (N). Those in group 4
were incubated with 6% J serum and 4% N serum, whereas those in
group 5 were incubated with 10% J serum. Groups 2–5 were stimulated
with 8 Gy of 60Co γ-rays (Hangzhou Cancer Hospital) at 3.64 Gy/min.
After 24 h of culture, reverse transcription-quantitative (RT-q)
PCR assay and western blotting analyses were performed.
RT-qPCR
Total RNA was isolated from cells with
TRIzol® and subsequently reversed transcribed into cDNA
with RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. Then, a
real-time PCR analysis was performed using an ABI 7300 instrument
(Applied Biosystems; Thermo Fisher Scientific, Inc.) at the
following parameters: 95°C for 10 min, followed by 40 cycles of
annealing at 95°C for 15 sec and amplification at 60°C for 45 sec.
GAPDH was used as an internal control. The primers used were:
PPM1A: 5′-TGCCAAATACTGCTGTGAG-3′ (forward),
5′-CTGTTGACCCACTTCTATCTG-3′ (reverse); and GAPDH:
5′-GGAGTCTACTGGCGTCTTCAC-3′ (forward), 5′-ATGAGCCCTTCCACGATGC-3′
(reverse). The mRNA level in each sample was evaluated using the
ΔΔCT method (16). The
experiments were repeated three times.
Western blotting assay
The proteins in the cells were prepared using a
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology) and the protein concentrations were measured using
the bicinchoninic acid method. Equal amounts of protein (30 µg)
were loaded on 10% sodium dodecyl sulfate-polyacrylamide gel and
then transferred onto a nitrocellulose membrane and blocked with 5%
skimmed milk at room temperature for 1 h. The membranes were then
incubated with primary antibodies and subsequently incubated with
HRP-conjugated secondary antibodies at room temperature for 1 h.
Lastly, the targeting bands were detected using ECL reagents. The
primary antibodies were anti-PPM1A (cat. no. ab154489, 1:1,000) and
anti-p-Smad2/3 (cat. no. ab63399, 1:1,000), purchased from Abcam.
Anti-Smad2/3 (cat. no. 5678, 1:1,000), anti-E-cadherin (cat. no.
14472, 1:1,000), anti-vimentin (cat. no. 5741, 1:1,000), anti-α-SMA
(cat. no. 19245, 1:5,000) and anti-GAPDH (cat. no. 5174, 1:1,000)
were obtained from Cell Signaling Technology, Inc. Densitometry was
performed using ImageJ software (version 1.48; National Institutes
of Health) with GAPDH as the loading control.
Statistical analysis
All experimental data are shown in mean ± standard
deviation. One-way ANOVA followed by Sidak's post hoc test was
performed to analyze statistical comparisons. P<0.05 was
considered to indicate a statistically significant difference.
Results
Identification of 10 compounds
The aqueous extract from the JMSD was measured with
high-performance liquid chromatography coupled with electrospray
mass spectrometry (HPLC/ESI-MS) in positive- and negative-ion modes
(Fig. 1, Table II). The following 10 compounds were
identified: ephedrine hydrochloride (1), levistilide A (2), liquiritigenin (3), pseudoephedrine hydrochloride (4), paeoniflorin (5), astilbin (6), cycloastragenol (7), diosgenin (8), rosmarinic acid (9) and paeonol (10), by comparing the retention times and
MS data with the reference standards.
 | Figure 1.Screening the components in the
aqueous extract of Jiawei-Maxing-Shigan decoction. (A)
High-performance liquid chromatography coupled with electrospray
mass spectrometry chromatogram of the aqueous extract in positive
and negative modes. (B) The product ion mass spectra of (1) ephedrine hydrochloride, (2) levistilide A, (3) liquiritigenin, (4) pseudoephedrine hydrochloride, (5) paeoniflorin, (6) astilbin, (7) cycloastragenol, (8) diosgenin, (9) rosmarinic acid and (10) paeonol. Conditions: collision gas,
ultrahigh-purity helium (He); nebulizer gas (N2), 35 psi; drying
gas (N2), 10 L/min; drying temperature, 350°C; HV, 3500 V; mass
scan range, 100–2200 m/z; target mass, 500 m/z; compound stability,
100%; and trap drive level, 100%. |
 | Table II.Identification of constituents from
Jiawei-Maxing-Shigan decoction. |
Table II.
Identification of constituents from
Jiawei-Maxing-Shigan decoction.
| Compound | RT/min | Herbal
medicines |
|---|
| 1. Ephedrine
hydrochloride | 0.56 | Honey-fried Herba
Ephedrae |
| 2. Levistilide
A | 0.68 | Gypsum
Fibrosum |
| 3.
Liquiritigenin | 1.01 | Radix Et Rhizoma
Glycyrrhizae |
| 4. Pseudoephedrine
hydrochloride | 3.18 | Honey-fried Herba
Ephedrae |
| 5.
Paeoniflorin | 5.09 | Radix Paeoniae
Rubra |
| 6. Astilbin | 5.70 | Cortex mori from
Morus alba L. |
| 7.
Cycloastragenol | 5.10 | Radix Et Rhizoma
Glycyrrhizae |
| 8. Diosgenin | 5.74 | Stir-baked Semen
Armeniacae Amarum |
| 9. Rosmarinic
acid | 6.32 | Flos Lonicerae
Japonicae |
| 10. Paeonol | 7.54 | Radix Paeoniae
Rubra |
Decreased PPM1A expression level in
type II AECs following radiation treatment
Following radiation treatment (60Co γ-ray at 8 Gy),
the PPM1A expression level in type II AECs was determined using
RT-PCR and western blotting assays. As shown in Fig. 2, the results indicated that the
radiation treatment could decrease the PPMIA expression levels in
AECs both in terms of mRNA and protein expression levels
(P<0.05) in an obvious time-dependent manner.
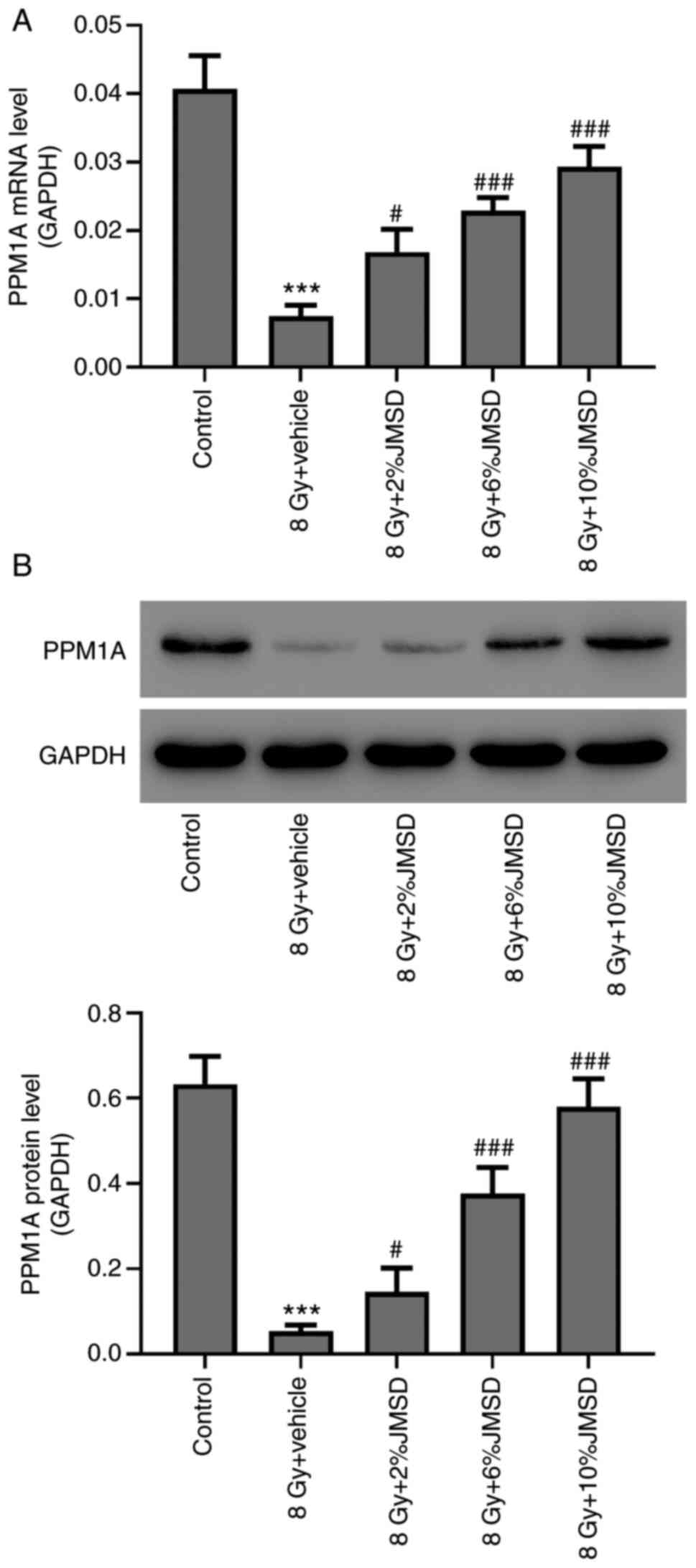
Attenuation of radiation-mediated
decreased PPM1A expression level in type II AECs by JMSD-medicated
serum
Furthermore, the effects of the JMSD-medicated serum
on the PPM1A expressions in radiation-induced type II AECs were
determined using RT-PCR and western blotting assays. The results
shown in Fig. 3 suggested that
JMSD-medicated sera (2, 6 and 10%) could upregulate the PPM1A
expressions as compared with the vehicle (P<0.05, P<0.001 and
P<0.001, respectively) in a concentration-dependent manner.
Attenuation of radiation-induced Smad
activation and epithelial-mesenchymal transition by PPM1A
overexpression
Consequently, PPM1A overexpressed AECs (Fig. 4A and B) were constructed to
investigate the role of PPM1A. The results showed that compared
with vector expression, the PPM1A and E-cadherin expressions were
upregulated, whereas the p-Smad2/3, vimentin and α-SMA expressions
were downregulated in the PPM1A-overexpressed AECs (Fig. 4C). Morphology analysis showed that
elongated spindle-like type cells were increased in
radiation-treated cells, and such morphological alteration was
decreased when PPM1A was overexpressed (Fig. 4D).
 | Figure 4.PPM1A overexpression attenuated the
radiation-induced Smad activation and epithelial-mesenchymal
transition. Type II AEC was transduced with oePPM1A. After 24 h,
the (A) mRNA and (B) protein levels of PPM1A were detected using
reverse transcription-quantitative PCR and western blotting,
respectively. ***P<0.001, vs. vector. Type II AECs were
transduced with oePPM1A and then treated with 8 Gy of
60Co γ-rays. After 24 h, the protein levels were
measured using (C) western blotting and (D) phase contrast images
were obtained (magnification, ×200). *P<0.05, **P<0.01,
***P<0.001, vs. vector. PPM1A, protein phosphatase
Mg2+/Mn2+-dependent 1A; AEC, alveolar
epithelial cells; oe, overexpression; p-, phosphorylated; α-SMA,
α-smooth muscle actin. |
Attenuation of radiation-induced Smad
activation and epithelial-mesenchymal transition by JMSD-medicated
serum via the regulation of PPM1A expression
Subsequently, the role of PPM1A was further studied
using the siRNA interference of PPM1A. The present study designed
three siRNA sequences for the PPM1A and siNC, a scrambled sequence,
was used as a negative control. siPPM1A-2 was selected as the siRNA
sequence for the following studies (Fig. 5A and B).
 | Figure 5.PPM1A knockdown promoted the
epithelial-mesenchymal transition by activating the TGF-β1/Smad
pathway. (A and B) Type II AECs were transduced with siPPM1A. After
24 h, the mRNA (A) and protein levels (B) of PPM1A were detected
with RT-qPCR and western blotting, respectively. ***P<0.001 vs.
siNC. (C) Type II AECs were transduced with siPPM1A and then
treated with 10 µmol/l TGF-β1/Smad inhibitor (SB431542). After 24
h, the protein levels were measured using western blotting.
*P<0.05, **P<0.01, ***P<0.001. PPM1A, protein phosphatase
Mg2+/Mn2+-dependent 1A; AEC, alveolar
epithelial cells; si, short interfering; p-, phosphorylated; α-SMA,
α-smooth muscle actin; NC, negative control. |
Furthermore, the results suggested that compared
with the cells in the vehicle group, the PPM1A siRNA-interfered
AECs showed downregulated PPM1A and E-cadherin expressions but
upregulated p-Smad2/3, vimentin and α-SMA expressions (Fig. 5C). In addition, after treatment with
SB431542, a TGF-β1/Smad signaling inhibitor, the p-Smad2/3,
vimentin and α-SMA expression levels were decreased, whereas the
E-cadherin expression level was increased in the PPM1A knockdown
AECs as compared with the vehicle group. Combined with the
above-mentioned results, it was hypothesized that PPM1A might be a
crucial target for EMT in AECs in TGF-β1/Smad signaling.
Finally, the role of PPM1A in the attenuating
effects of JMSD against radiation-induced EMT was studied in
primary type II VECs. The image depicted in Fig. 6 suggested that JMSD-medicated serum
could increase the E-cadherin expression level and decrease the
p-Smad2/3, vimentin and α-SMA expression levels. However, these
effects could be blocked by siPPM1A-2. Consequently, it is
hypothesized that PPM1A-2 is a crucial target for the anti-EMT
effect of JMSD in VECs.
 | Figure 6.JMSD-medicated serum attenuated
radiation-induced Smad activation and epithelial-mesenchymal
transition by regulating the PPM1A expression. Type II AECs, which
were incubated with 6% JSMD-medicated serum, were transduced with
siPPM1A (or siNC) and then treated with 8 Gy of 60Co
γ-rays. After 24 h, the protein levels were measured using western
blotting. *P<0.05, **P<0.01, ***P<0.001. JMSD,
Jiawei-Maxing-Shigan decoction; PPM1A, protein phosphatase
Mg2+/Mn2+-dependent 1A; AEC, alveolar
epithelial cells; si, short interfering; NC, negative control; p-,
phosphorylated; α-SMA, α-smooth muscle actin. |
Discussion
Herbal medicines are important alternative and
complementary remedies for the treatment of various diseases,
particularly those that cannot be treated with western drugs.
Radiation-induced pulmonary fibrosis is an intractable disease in
clinical practice and an increasing number of researchers have
attempted to search for treatments for pulmonary fibrosis from
herbal medicines (17). Some
extracts/compounds from natural herbal medicines are feasible for
treating lung pulmonary diseases, such as polydatin from
Polygonum cuspidatum (18,19)
and astragaloside IV from Astragalus membranaceus (20,21).
JMSD administration can attenuate the radiation-induced EMT in AECs
via the TGF-β/Smad signaling (7).
The present study on JMSD against radiation-induced EMT found that
PPM1A exhibits a crucial role in its anti-EMT activity.
TGF-β/Smad signaling serves a key effect in the EMT
process of AECs and further induction of pulmonary fibrosis
(22). Under radiation, the TGF-β1
expression level in bronchoalveolar lavage fluids can be increased
and then, TGF-β/Smad signaling is activated (23). First, TGF-β expression can promote
the phosphorylation of Smad2/3 by binding to the TGF-β receptor in
the cell membrane and then the transcription of p-Smad2/3 to the
cell nucleus, resulting in some transcriptional regulation of a
number of genes (24).
Consequently, some of the responding productions such as Snail,
connective tissue growth factor and matrix metalloproteinases would
be released and further result in EMT (25). PPM1A is a phosphatase of the
serine/threonine PPM family and the main substrates of PPM1A
include MAPK, Smad2 and Smad3 and MKKs (12–14).
PPM1A serves an important role in the signal transduction of
TGF-β/Smad signaling and can inactivate TGF-β/Smad signaling by
dephosphorylating Smad2/3 (26,27)
(Fig. 7). The present study found
that PPM1A expression level was reduced in type II AECs following
radiation treatment. PPM1A overexpression attenuated EMT, while
PPM1A knockdown showed reverse effects on EMT. Furthermore,
treatment with a TGF-β1/Smad signaling inhibitor blocked PPM1A
knockdown-induced EMT. Therefore, PPM1A upregulation is a potential
strategy for treating EMT and pulmonary fibrosis through inhibiting
TGF-β1/Smad signaling.
Previously, we have reported that JMSD attenuated
radiation-induced EMT in AECs by regulating TGF-β/Smad signaling
(7). The present study found that
JMSD-medicated serum attenuated the radiation-mediated inhibition
of PPM1A expression in type II AECs. The role of PPM1A in the
anti-EMT effects of JMSD in AECs was then examined and it was found
that JMSD treatment could increase the PPM1A and E-cadherin
expression levels. JMSD also decreased the p-Smad2/3, vimentin and
α-SMA expressions in VECs. After knockdown of the PPM1A, the
regulating effects of JMSD on these proteins were blocked. The data
indicated that JMSD acted against radiation-induced EMT through
upregulating PPM1A.
However, the present study has some limitations. It
did not investigate the biological effects of the detailed
components identified using HPLC/ESI-MS owing to the limited time.
In addition, upstream targets of PPM1A should also be investigated
in succeeding works. The research regarding JMSD is continuing and
it is hoped to investigate the detail mechanisms by which JMSD
increase the PPMIA expression and the biological effects of detail
components of JMSD and present the related results in the
future.
Together, the present study suggested that PPM1A is
a key target of JMSD administration for the attenuation of the
radiation-induced EMT in primary type II alveolar epithelial cells
via TGF-β1/Smad signaling.
Acknowledgements
Not applicable.
Funding
The present study was supported by Science
Technology Department of Zhejiang Province (grant no. 2018C03025),
Zhejiang Provincial Natural Science Foundation of China (grant no.
LY15H290002) and Zhejiang Province Traditional Chinese Medicine
Science and Technology Planning Project (grant no. 2021ZA099).
Availability of data and materials
The data used to support the findings of this study
are available from the corresponding author upon request.
Authors' contributions
SL and JL conceived and designed the study, JL, ZL,
XL, YS and JS collected and analyzed the data and SL and JL wrote
the manuscript. All authors read and approved the final manuscript.
SL and JL confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The experimental protocols were approved by the
Animal ethics committee of Zhejiang Chinese Medicine University
(Hangzhou, China; approval no. 2019485).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tian S, Zhang X, Jiang R, Pillai RN,
Owonikoko TK, Steuer CE, Saba NF, Pakkala S, Patel PR, Belani CP,
et al: Survival outcomes with thoracic radiotherapy in
extensive-stage small-cell lung cancer: A propensity score-matched
analysis of the national cancer database. Clin Lung Cancer.
20:484–493.e6. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rodrigues G and Movsas B: Future
directions in palliative thoracic radiotherapy. Curr Opin Support
Palliat Care. 6:91–96. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Simone CB II: Thoracic radiation normal
tissue injury. Semin Radiat Oncol. 27:370–377. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Giuranno L, Ient J, De Ruysscher D and
Vooijs MA: Radiation-Induced Lung Injury (RILI). Front Oncol.
9:8772019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ota C, Ng-Blichfeldt JP, Korfei M,
Alsafadi HN, Lehmann M, Skronska-Wasek W, M De Santis M, Guenther
A, Wagner DE and Königshoff M: Dynamic expression of HOPX in
alveolar epithelial cells reflects injury and repair during the
progression of pulmonary fibrosis. Sci Rep. 8:129832018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nagarajan D, Wang L, Zhao W and Han X:
Trichostatin A inhibits radiation-induced epithelial-to-mesenchymal
transition in the alveolar epithelial cells. Oncotarget.
8:101745–101759. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lu J, Zhong Y, Lin X, Lin Z, Chen Z, Wu X,
Wang N and Lin S: Jiawei Maxing Shigan Decoction (JMSD) attenuates
radiation-induced epithelial-mesenchymal transition of primary rat
type II alveolar epithelial cells. Int J Clin Exp Med.
10:16292–16300. 2017.
|
|
8
|
Yan-He LI: Clinical effect of Jiawei
Maxingshigan decoction treatment for pediatric asthma. Laboratory
Medicine & Clinic. 2013.
|
|
9
|
Lin T and Geng R: To Observe the Curative
Effect of Western Medicine in the Treatment Combined With Jiawei
Maxingshigan Decoction in Treating Acute Attack of Chronic
Bronchitis. China Continuing Medical Education. 2015.
|
|
10
|
Li J: Clinical observation of Jiawei
Maxing Shigan Decoction in complementary treatment of 48 cases of
infantile mycoplasma pneumonia. J Pediatr Traditional Chin Med.
3:40–41. 2007.(In Chinese).
|
|
11
|
Wang J, Li YQ, Zhang WP, Zhang T and Zhou
X: The effects of Jiawei Maxing Shigan Decoction on radioactive
lung injury Tianjin. J Traditional Chin Med. 28:377–378. 2011.
|
|
12
|
Geng J, Fan J, Ouyang Q, Zhang X, Zhang X,
Yu J, Xu Z, Li Q, Yao X, Liu X and Zheng J: Loss of PPM1A
expression enhances invasion and the epithelial-to-mesenchymal
transition in bladder cancer by activating the TGF-β/Smad signaling
pathway. Oncotarget. 5:5700–5711. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lammers T, Peschke P, Ehemann V, Debus J,
Slobodin B, Lavi S and Huber P: Role of PP2Calpha in cell growth,
in radio- and chemosensitivity, and in tumorigenicity. Mol Cancer.
6:642007. View Article : Google Scholar
|
|
14
|
Mazumdar A, Tahaney WM, Reddy Bollu L,
Poage G, Hill J, Zhang Y, Mills GB and Brown PH: The phosphatase
PPM1A inhibits triple negative breast cancer growth by blocking
cell cycle progression. NPJ Breast Cancer. 5:222019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang JT, Wang P, Liu AF, Yang G,
Yuan-Dong LI and Zhang C: Overview about preparation methods of
serum containing Chinese medicine. China J Traditional Chin Med
Pharm. 2015.
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ghasemian M, Owlia S and Owlia MB: Review
of anti-inflammatory herbal medicines. Adv Pharmacol Sci.
2016:91309792016.PubMed/NCBI
|
|
18
|
Peng W, Qin R, Li X and Zhou H: Botany,
phytochemistry, pharmacology, and potential application of
Polygonum cuspidatum Sieb.et Zucc.: A review. J Ethnopharmacol.
148:729–745. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zeng H, Wang Y, Gu Y, Wang J, Zhang H, Gao
H, Jin Q and Zhao L: Polydatin attenuates reactive oxygen
species-induced airway remodeling by promoting Nrf2-mediated
antioxidant signaling in asthma mouse model. Life Sci. 218:25–30.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fu J, Wang Z, Huang L, Zheng S, Wang D,
Chen S, Zhang H and Yang S: Review of the botanical
characteristics, phytochemistry, and pharmacology of Astragalus
membranaceus (Huangqi). Phytother Res. 28:1275–1283. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qian W, Cai X, Qian Q, Zhang W and Wang D:
Astragaloside IV modulates TGF-β1-dependent epithelial-mesenchymal
transition in bleomycin-induced pulmonary fibrosis. J Cell Mol Med.
22:4354–4365. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lu J, Zhong Y, Chen J, Lin X, Lin Z, Wang
N and Lin S: Radiation enhances the Epithelial-Mesenchymal
transition of A549 cells via miR3591-5p/USP33/PPM1A. Cell Physiol
Biochem. 50:721–733. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Barthelemy-Brichant N, Bosquée L, Cataldo
D, Corhay JL, Gustin M, Seidel L, Thiry A, Ghaye B, Nizet M, Albert
A, et al: Increased IL-6 and TGF-beta1 concentrations in
bronchoalveolar lavage fluid associated with thoracic radiotherapy.
Int J Radiat Oncol Biol Phys. 58:758–767. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hu HH, Chen DQ, Wang YN, Feng YL, Cao G,
Vaziri ND and Zhao YY: New insights into TGF-beta/Smad signaling in
tissue fibrosis. Chem Biol Interact. 292:76–83. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kasai H, Allen JT, Mason RM, Kamimura T
and Zhang Z: TGF-beta1 induces human alveolar epithelial to
mesenchymal cell transition (EMT). Respir Res. 6:562005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu F, Liu C, Zhou D and Zhang L:
TGF-β/Smad Pathway and its regulation in hepatic fibrosis. J
Histochem Cytochem. 64:157–167. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhu F, Xie N, Jiang Z, Li G, Ma L and Tong
T: The cellular Senescence-Inhibited gene is essential for PPM1A
Myristoylation to modulate transforming growth factor β signaling.
Mol Cell Biol. 38:e00414–18. 2018. View Article : Google Scholar
|