Introduction
Laryngeal squamous cell carcinoma (LSCC) represents
>90% of histological subtypes of laryngeal carcinoma (1). The incidence and mortality of
laryngeal cancer were 177,422 and 94,771 cases worldwide in 2018
(2). Despite major improvements in
diagnosis and treatment, nearly 60% of patients present at an
advanced (III or IV) stage when diagnosed (3). Unfortunately, the 5-year survival rate
of laryngeal cancer has decreased from 66 to 63% over the past 40
years (4). Hence, it is of
importance to identify the exact molecular mechanisms and effective
therapeutic strategies of LSCC.
Epithelial-mesenchymal transition (EMT) is a
fundamental developmental process during tumor progression
characterized by the loss of epithelial characteristics and the
gain of mesenchymal features, ultimately leading to the acquisition
of enhanced migratory and invasive capacities (5). Among the multiple inducers of EMT,
transforming growth factor-β (TGF-β) plays a key role in initiating
and driving EMT (6). Previous
reports have identified that FOXP4 is involved in EMT process and
facilitates invasion and metastasis in breast cancer and
hepatocellular carcinoma (7,8).
However, whether FOXP4 can promote EMT in LSCC remains unclear, and
the potential regulatory mechanism of FOXP4 in this process needs
to be clarified.
Forkhead box (FOX) proteins are a superfamily of
transcriptional regulators that have been demonstrated to be
implicated in cancer initiation, maintenance, progression and drug
resistance (9). FOXP4, a member of
the FOXP subfamily, is located on human chromosome 6p21.1 and
encodes 680 amino acids protein (7). It has been demonstrated that FOXP4 is
significantly upregulated in breast cancer, hepatocellular
carcinoma and oral squamous cell carcinoma, and was observed to
participate in tumorigenesis and progression through various
molecular mechanisms (7,8,10).
However, the expression level and functional role of FOXP4 in LSCC
has not yet been characterized.
In the current study, the expression of FOXP4, its
potential functional role in TGF-β-induced EMT and the downstream
regulatory mechanisms of FOXP4 were explored in the pathogenesis of
LSCC.
Materials and methods
Bioinformatics analysis
FOXP4 was predicted using the Search Tool for Gene
Expression Profiling Interactive Analysis (GEPIA) online dataset
(http://gepia.cancer-pku.cn/index.html). FOXP4 was
identified as a transcription factor of LEF-1 using the Animal TFDB
3.0 database (https://ngdc.cncb.ac.cn/databasecommons/database/id/8)
and JASPAR database (http://jaspar.genereg.net).
Patients and specimens
Tumor tissues and paired adjacent normal tissues
(distance from tumor margin, >2 cm) were acquired from 81
patients undergoing surgical resection at the Fourth Hospital of
Hebei Medical University (Shijiazhuang, China) between September
2008 and December 2013. The patients had not received radiotherapy
or chemotherapy prior to surgery. Patients provided signed informed
consent prior to the study. Each surgical resection specimen was
divided into two parts, one part was fixed in 10% neutral formalin
solution for the conventional wax block; the other part was placed
in a fresh state at −80°C to extract genomic RNA and DNA. Ethical
approval was acquired by the ethics committee of the Fourth
Hospital of Hebei Medical University. Information on
clinicopathological features and survival data were available from
hospital recordings.
Cell culture
Three laryngeal cancer cell lines (TU177, TU686 and
TU212) were purchased from American Type Culture Collection. All
cell lines were cultured in RPMI-1640 medium (Invitrogen; Thermo
Fisher Scientific, Inc.), while AMC-HN-8 cells were cultured in
Dulbecco's modified Eagle's medium (Invitrogen; Thermo Fisher
Scientific, Inc.) containing 10% FBS (Invitrogen; Thermo Fisher
Scientific, Inc.) and antibiotics (100 U/ml penicillin and 100
mg/ml streptomycin) under conventional culture conditions. All cell
lines were authenticated via DNA fingerprinting using the short
tandem repeat (STR) method from Procell Life Science &
Technology Co., Ltd. The cDNA of 10 LSCC-adjacent tissues were
randomly mixed in equal proportion as the control group (pools).
For RNA sequencing, TU177 cells were starved in serum-free medium
overnight before the addition of recombinant TGF-β1 (10 ng/ml,
R&D Systems, Inc.), and cells were routinely cultured for 7
days at 37°C in a humidified atmosphere with 5% CO2.
Total RNA from TU177 cells was used for sequencing on a HiSeq 2000
system (Illumina, Inc.). Cuffdiff (version 2.2.1.2) was used to
compare the log ratio of Fragments Per Kilobase of transcription
per Million mapped reads in the two conditions (11). The differential expression results
were constructed by GraphPad Prism7 software (GraphPad Software,
Inc.).
Reverse transcription-quantitative PCR
(RT-qPCR) assay
Total RNA was extracted from LSCC tissues and cells
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). RNA concentration was quantified using a
NanoDrop™ spectrophotometer (NanoDrop Technologies; Thermo Fisher
Scientific, Inc.) RNA (1 µg) was reverse transcribed at 65°C for 10
min and 85°C for 5 min using the Transcriptor Fist Strand cDNA
Synthesis kit (Roche Life Science Co., Ltd.). RT-qPCR was performed
using GoTaq® qPCR Master Mix (Promega Corporation) in
the StepOnePlus™ Real-Time PCR System (Applied Biosystems; Thermo
Fisher Scientific, Inc.). The following thermocycling conditions
were used for qPCR: Initial denaturation at 95°C for 10 min;
followed by 40 cycles at 95°C for 15 sec, 54°C for 30 sec and 72°C
for 30 sec. Relative gene expression was quantified using the
2−ΔΔCq method (12)
normalized to GAPDH. The gene-specific primers are listed in
Table SI.
Cell transfection
The small interfering RNAs (siRNAs) specifically
targeting FOXP4 were synthesized by Shanghai GenePharma Co., Ltd.
TU177 cells were divided into four groups: i) si1-FOXP4 (cells
transfected with 5 µg si1-FOXP4; 5′-TGTAGAACTCATGATTCTGGGTT-3′);
ii) si2-FOXP4 (cells transfected with 5 µg si2-FOXP4;
5′-CAGAATCATGAGTTCTACAAGTT-3′); iii) si3-FOXP4 (cells transfected
with 5 µg si3-FOXP4; 5′-CCTGGGCCAGTTTATCAAATT-3′); and iv) si-NC
(cells transfected with 5 µg si-NC; 5′-TTCTCCGAACGTGTCACGTTT-3′).
AMC-HN-8 cells were divided into two groups: pcDNA3.1-NC (cells
transfected with 4 µg pcDNA3.1-NC vector) and pcDNA3.1-FOXP4 (cells
transfected with 4 µg pcDNA3.1-FOXP4 vector). The pcDNA3.1-FOXP4
was constructed by Sangon Biotech Co., Ltd. TU177 and AMC-HN-8
cells were cultured until 70–80% confluence in 6-well plate and
were transfected using Lipofectamine® 2000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. After transfection at 37°C for 6 h, the
medium was replaced with complete medium. Following incubation at
37°C for 48 h, transfection efficiencies were assessed and cells
were used for subsequent experiments.
Cell viability assay and clone
formation assay
The MTS assay was used to determine the cell
viability by using CellTiter96® AQueous One Solution
Cell Proliferation assay kit (Promega Corporation). TU177 and
AMC-HN-8 cells were seeded in 96-well plates with 1×103
per well following transfection for 24 h. After 0, 24, 48, 72 and
96 h, 20 µl MTS reagent was added to cultured mediums and then
cells were incubated for 2 h. The optical density at 490 nm was
measured for each well. For the clone formation assay, following
transfection for 24 h, 3×103 TU177 or AMC-HN-8 cells per
well were routinely cultured for 1 week. The clones were fixed in
4% paraformaldehyde at room temperature for 20 min and then stained
with crystal violet solution at room temperature for 20 min. The
total clones (≥50 cells) were calculated manually under a
microscope.
Transwell migration and invasion
assays
For the cell migration assay, 1×105 TU177
or AMC-HN-8 cells were seeded into the upper chamber of a Transwell
insert (Costar; Corning, Inc.) with 200 µl serum-free medium, while
medium with 600 µl 10% FBS was added into the lower chamber. The
migratory cells were fixed with 4% paraformaldehyde at room
temperature for 20 min and stained with crystal violet solution at
room temperature for 20 min after incubation at 37°C for 24 h. For
the cell invasion assay, the steps were the same as described
above, except that the membrane of the upper chamber was pre-coated
with Matrigel® Basement Membrane Matrix (Costar;
Corning, Inc.). Following a 24-h incubation at 37°C, the invading
cells on the bottom surface of the filter were fixed with 4%
paraformaldehyde at 4°C for 30 min and stained with crystal violet
solution at room temperature for 20 min. Cell migration and
invasion were analyzed in three randomly selected fields using a
light microscope (magnification, ×20).
Western blot analysis
RIPA buffer containing PMSF (Beijing Solarbio
Science & Technology Co., Ltd.) was used to extract total
protein from transfected cells. The quantification of protein was
determined using the BCA Protein Assay Kit [Hangzhou Multi Sciences
(Lianke) Biotech Co., Ltd.]. The lysates (40 µg) were resolved by
10% SDS-PAGE and transferred onto polyvinylidene difluoride
membranes (MilliporeSigma). Then, membranes were blocked with 5%
skimmed milk at room temperature for 60 min. Following which, the
membranes were incubated overnight at 4°C with specific primary
antibodies against: β-actin (1:10,000; cat. no. AC026; ABclonal
Biotech Co., Ltd.), E-cadherin (1:1,000; cat. no. E-AB-31261;
Elabscience Biotechnology, Inc.), N-cadherin (1:1,000; cat. no.
E-AB-64011; Elabscience Biotechnology, Inc.), Vimentin (1:1,000;
cat. no. bs-0756R; BIOSS), Twist (1:1,000; cat. no. bs-2441R;
BIOSS). After washing, the membranes were incubated at room
temperature for 45 min with the appropriate IgG HRP-conjugated
secondary antibody (1:10,000; cat. no. SA00001-9; ProteinTech
Group, Inc.) and detected with enhanced chemiluminescence (ECL)
reagent [Hangzhou Multi Sciences (Lianke) Biotech Co., Ltd.] and
analyzed using ImageJ software (version 1.48; National Institutes
of Health).
Chromatin immunoprecipitation (ChIP)
assay
LSCC cells (1×106) were crosslinked by 1%
formaldehyde (Sigma-Aldrich; Merck KGaA) by centrifugation at 300 ×
g for 3 min at 25°C and washed in pre-cooled PBS for 10 min at
25°C. The formaldehyde was quenched by the addition of glycine
(Beijing Solarbio Science & Technology Co., Ltd.). Then three
sets of 20-sec pulses were used to obtain chromatin fragments.
EZ-Magna ChIP A/G kit (MilliporeSigma; 60 µl) was used to perform
the ChIP assay according to the manufacturer's protocols.
Subsequently, 5 µg anti-IgG (1:40; cat. no. sc-2025; Santa Cruz
Biotechnology, Inc.) or anti-FOXP4 (cat. no. ab242127; Abcam)
antibodies were used to immunoprecipitate chromatin fragments at
4°C overnight. The IgG antibody was used as control. Then the
immunoprecipitated DNA was purified using a ChIP DNA purification
kit (cat. no. D0033; Beyotime Institute of Biotechnology). The fold
enrichment of the DNAs amplified by ChIP was assessed the promoter
region of the LEF-1 gene. The recovered DNA fragments were
evaluated using RT-qPCR with the following primers: Forward,
5′-CAGCCACGCAAATAGAGCAAGG-3′ and reverse,
5′-TCCTGGTTCCTCGGCCCGAGA−3′.
Luciferase reporter assay
The promoter regions (−2,308~+91; −1,782~+91;
−1,186~+91) of lymphoid enhancer-binding factor 1 (LEF-1) were
respectively subcloned into pGL3-Basic vector (E1761; Promega
Corporation), and the recombinant plasmids were sequenced and
confirmed. LSCC cells were cotransfected with 2 µg pGL3-LEF-1-luc,
2 ng Renilla and 2 µg si-NC or si-FOXP4 using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. At 48 h
post-transfection, the Dual-Luciferase Reporter Assay System
(Promega Corporation) was used to determine luciferase activity.
Firefly luciferase activity was normalised to Renilla
luciferase activity.
Statistical analysis
SPSS 22.0 software package (IBM Corp.) was used to
analyze the data in this study and the data are presented as the
mean ± standard deviation. Differences between the paired tissue
samples from patients were assessed using a paired Student's
t-test, whereas comparisons between two unpaired groups were
determined using an unpaired Student's t-test. Differences between
multiple groups were analyzed using one-way ANOVA followed by
Tukey's post hoc test. Pearson's correlation analysis was used to
detect the correlation between FOXP4 and LEF-1 expression.
Chi-square test was used to analyze the association between FOXP4
expression level and clinicopathological features. The correlation
between patient survival and FOXP4 expression was determined by the
Kaplan-Meier method and the difference was analyzed using log-rank
tests. Cox regression analysis was performed to determine the
independent factors affecting survival. A two-sided P<0.05 was
considered to indicate a statistically significant difference.
Results
FOXP4 expression is significantly
upregulated and closely related with the survival of patients with
LSCC
By scanning the GEPIA online dataset (13), it was found that FOXP4 was expressed
at relatively high levels in head and neck squamous cell carcinoma
(Fig. 1A). A significantly
increased expression level of FOXP4 was observed in all laryngeal
cancer cell lines (Fig. 1B).
Similarly, FOXP4 expression in 81 pairs of LSCC tissues was
significantly higher than that in adjacent normal tissues (Fig. 1C). It was identified that the high
FOXP4 expression, defined as 200% higher FOXP4 expression in LSCC
tissues, was significantly related to lymph node metastasis, TNM
stage and pathological differentiation (Table I). In addition, patients with LSCC
with high FOXP4 expression level demonstrated poorer survival rate
(Fig. 1D). As analyzed by
univariate analysis, lymph node metastasis, TNM stage, pathological
differentiation and FOXP4 expression were significantly associated
with overall survival (Table II).
Furthermore, FOXP4 was an independent prognostic factor for
patients with LSCC, as determined via multivariate Cox regression
analysis (Table II).
 | Figure 1.FOXP4 expression is significantly
upregulated and closely related with the survival of patients with
LSCC. (A) Relative expression of FOXP4 in various tumor types cited
from the Gene Expression Profiling Interactive Analysis dataset.
(B) FOXP4 expression in laryngeal cancer cell lines detected via
RT-qPCR assay. **P<0.01 vs. pools group. (C) FOXP4 expression in
81 pairs of LSCC tissues and adjacent normal tissues detected via
RT-qPCR. (D) Overall survival of patients with LSCC according to
FOXP4 expression by Kaplan-Meier analysis. *P<0.05 vs. normal
group. ACC, adrenocortical carcinoma; BLCA, bladder urothelial
carcinoma; BRCA, breast invasive carcinoma; CESC, cervical squamous
cell carcinoma and endocervical adenocarcinoma; CHOL,
cholangiocarcinoma; COAD, colon adenocarcinoma; DLBC, lymphoid
neoplasm diffuse large B-cell lymphoma; ESCA, esophageal carcinoma;
GBM, glioblastoma multiforme; HNSC, head and neck squamous cell
carcinoma; KICH, kidney chromophobe; KIRC, kidney renal clear cell
carcinoma; KIRP, kidney renal papillary cell carcinoma; LAML, acute
myeloid leukemia; LGG, brain lower-grade glioma; LIHC, liver
hepatocellular carcinoma; LUAD, lung adenocarcinoma; LUSC, lung
squamous cell carcinoma; OV, ovarian serous cystadenocarcinoma;
PAAD, pancreatic adenocarcinoma; PCPG, pheochromocytoma and
paraganglioma; PRAD, prostate adenocarcinoma; READ, rectum
adenocarcinoma; SARC, sarcoma; SKCM, skin cutaneous melanoma; STAD,
stomach adenocarcinoma; TGCT, testicular germ cell tumors; THCA,
thyroid carcinoma; THYM, thymoma; UCEC, uterine corpus endometrial
carcinoma; UCS, uterine carcinosarcoma; FOXP4, forkhead box P4;
LSCC, laryngeal squamous cell carcinoma; RT-qPCR, reverse
transcription-quantitative PCR. |
 | Table I.Association between the expression of
FOXP4 and the clinicopathological features of patients with
laryngeal squamous cell carcinoma. |
Table I.
Association between the expression of
FOXP4 and the clinicopathological features of patients with
laryngeal squamous cell carcinoma.
|
|
| FOXP4
expression |
|
|---|
|
|
|
|
|
|---|
|
Characteristics | Number of
patients | Low, n (%) | High, n (%) | P-value |
|---|
| Age, years |
|
|
| 0.224 |
|
<60 | 37 | 21 (56.76) | 16 (43.24) |
|
|
≥60 | 44 | 19 (43.18) | 25 (56.82) |
|
| Smoking |
|
|
| 0.094 |
| No | 33 | 20 (60.61) | 13 (39.39) |
|
|
Yes | 48 | 20 (41.67) | 28 (58.33) |
|
| Lymph node
metastasis |
|
|
| 0.027a |
|
Negative | 51 | 30 (58.82) | 21 (41.18) |
|
|
Positive | 30 | 10 (33.33) | 20 (66.67) |
|
| TNM stage |
|
|
| 0.001b |
|
I+II | 37 | 26 (70.27) | 11 (29.73) |
|
|
III+IV | 44 | 14 (31.82) | 30 (68.18) |
|
| Pathological
differentiation |
|
|
| 0.037a |
|
Well/moderate | 56 | 32 (57.14) | 24 (42.86) |
|
|
Poor | 25 | 8 (32.00) | 17 (68.00) |
|
 | Table II.Univariate and multivariate Cox
regression analysis for clinicopathological features associated
with prognosis of 81 patients with laryngeal squamous cell
carcinoma. |
Table II.
Univariate and multivariate Cox
regression analysis for clinicopathological features associated
with prognosis of 81 patients with laryngeal squamous cell
carcinoma.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age, years, <60
vs. ≥60 | 1.642
(0.752–3.588) | 0.214 | 0.416
(0.144–1.203) | 0.106 |
| Smoking, no vs.
yes | 1.508
(0.677–3.359) | 0.314 | 1.766
(0.764–4.079) | 0.183 |
| Lymph node
metastasis, negative vs. positive | 3.529
(1.629–7.647) | 0.001c | 1.014
(0.368–2.795) | 0.978 |
| TNM stage, I+II vs.
III+IV | 10.768
(3.226–35.945) | 0.000c | 6.295
(1.615–24.538) | 0.008b |
| Pathological
differentiation, well/moderate vs. poor | 5.800
(2.642–12.734) | 0.000c | 4.555
(1.516–13.685) | 0.007b |
| FOXP4 expression,
low vs. high | 3.859
(1.626–9.159) | 0.002c | 2.691
(1.083–6.687) | 0.033a |
FOXP4 promotes laryngeal cancer cell
viability, migration and invasion in vitro
To better understand whether FOXP4 plays a
biological role in LSCC development, FOXP4 expression was silenced
in TU177 cells with two independent si-FOXP4 sequences, si-FOXP4-1
and si-FOXP4-2 successfully knocked down FOXP4 expression compared
with the si-NC group (Fig. 2A).
Whereas, AMC-HN-8 cells were transfected with pcDNA3.1-FOXP4 to
increase FOXP4 expression, which was successful, as shown in
Fig. 2B.
The MTS assay demonstrated that transfection of
si-FOXP4 or pcDNA3.1-FOXP4 led to a significant inhibition or
promotion of laryngeal cancer cell viability, respectively
(Fig. 2C). The effects of
FOXP4-knockdown were only apparent up to 72 h, as siRNA produces
short-term inhibition of gene expression. The clone formation assay
showed that clone formation was significantly inhibited in the
si-FOXP4-1 and si-FOXP4-2 groups compared with the si-NC group,
whereas it was significantly increased in the pcDNA3.1-FOXP4 group
compared with the pcDNA3.1 group (Fig.
2D). Furthermore, transfection of si-FOXP4 or pcDNA3.1-FOXP4
affected the migratory and invasive abilities of TU177 and AMC-HN-8
cells in vitro (Fig. 2E and
F).
FOXP4 contributes to EMT process
induced by TGF-β1
An increasing number of studies have shown the
important role of EMT in regulating tumor invasiveness and
metastasis (14–16), thus the effects of FOXP4 on EMT
characteristics were further examined in the present study. The
phenotype of TU177 cells in the presence of TGF-β1 was observed and
it was found that cells exhibited a spindle-shaped morphology
characteristic of EMT (Fig. 3A).
Subsequently, the expression levels of EMT-related markers and
FOXP4 were found to be upregulated in TGF-β1-treated TU177 cells
compared with untreated cells, whereas epithelial marker
(E-cadherin) was downregulated (Fig. 3B
and C). These findings indicated that the TU177 cells displayed
EMT-associated characteristics and FOXP4 could be induced by
TGF-β1.
Moreover, the role of FOXP4 on the mRNA expression
levels of EMT-related markers was explored. It was shown that
knockdown of FOXP4 significantly increased E-cadherin and reduced
N-cadherin, Vimentin, Twist, Snail and zinc finger E-box-binding
homeobox 1 (ZEB1) expression in TU177 cells compared with the si-NC
group (Fig. 3D). Meanwhile, the
expression of E-cadherin was decreased, and N-cadherin, Vimentin,
Twist, Snail and ZEB1 expression levels were upregulated following
FOXP4 overexpression in AMC-HN-8 cells compared with the pcDNA3.1
group (Fig. 3E). The expression of
E-cadherin in TU177 cell lines was significantly upregulated in the
si-FOXP4 groups compared with that in the si-NC group, whereas
N-cadherin, Vimentin, Twist and Snail expression levels were
significantly downregulated. Besides, the expression of E-cadherin
was significantly downregulated in AMC-HN-8 cell lines with FOXP4
overexpression compared with those in the pcDNA3.1-control group,
whereas N-cadherin, Vimentin, Twist and Snail expression levels
were markedly upregulated (Fig.
3F). Taken together, the aforementioned results indicated that
FOXP4 promoted EMT in laryngeal cancer cells.
FOXP4 directly targets LEF-1 and
activates Wnt signaling pathway
To further investigate the downstream targets of
FOXP4, the Animal TFDB 3.0 database was screened and potential
FOXP4-binding elements in the LEF-1 proximal promoter were
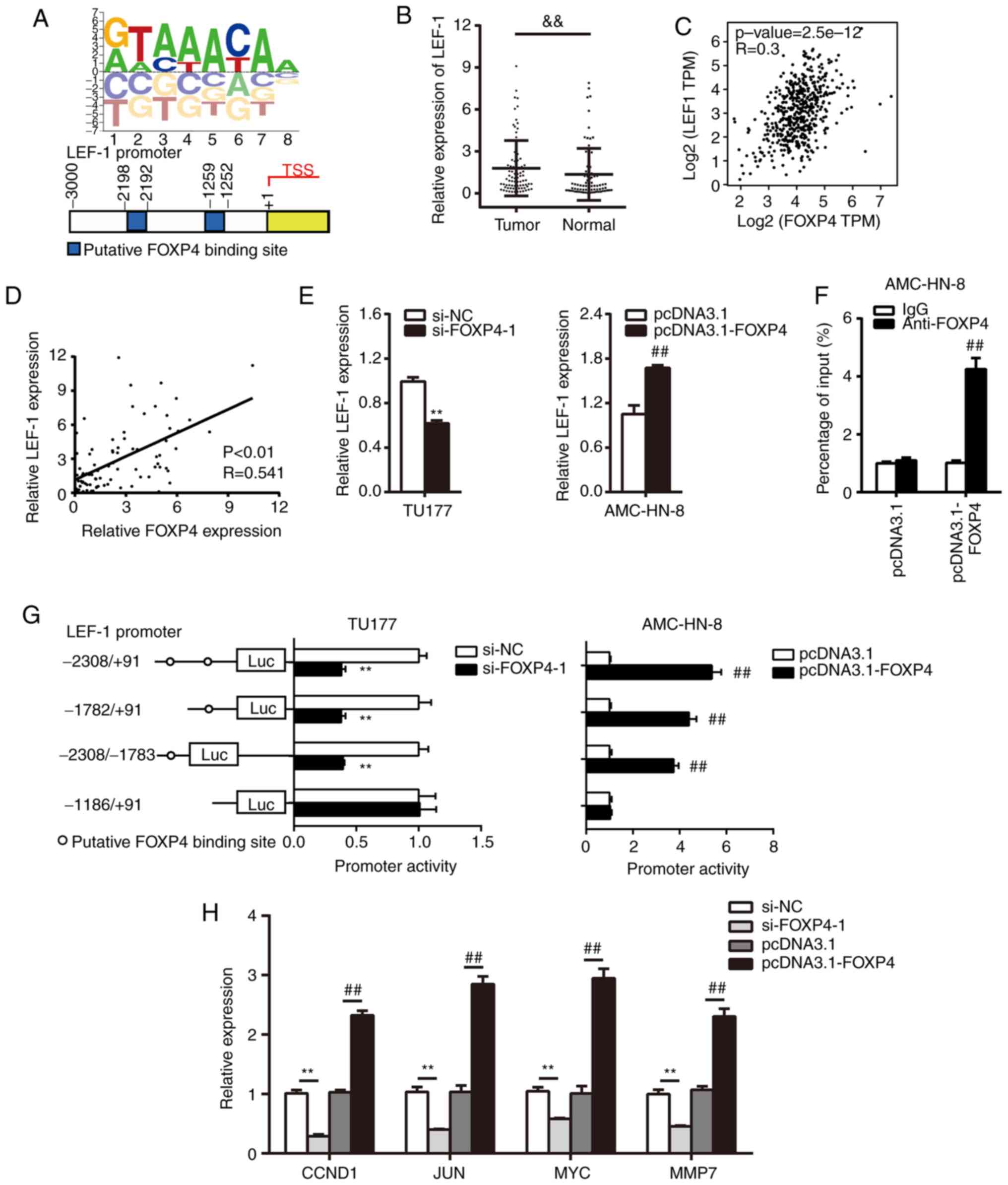
predicted (17) (Fig. 4A).
First, the expression of LEF-1 in LSCC tissues was
detected and it was found that LEF-1 expression was significantly
upregulated in tumor tissues compared with the normal tissues
(Fig. 4B). In addition, a moderate
positive correlation between FOXP4 and LEF-1 expression in head and
neck squamous cell carcinoma was predicted using data from GEPIA
(Fig. 4C). Similarly, a moderate
positive correlation was also confirmed between FOXP4 and LEF-1 in
LSCC tissues (Fig. 4D).
Furthermore, transfection with si-FOXP4-1 led to the significant
downregulation of LEF-1 expression compared with the si-NC group,
while transfection with pcDNA3.1-FOXP4 caused significant
upregulation of LEF-1 expression compared with the pcDNA3.1 group
(Fig. 4E). The ChIP assay confirmed
that FOXP4 was directly recruited to the LEF-1 promoter in AMC-HN-8
cells transfected with pcDNA3.1-FOXP4 (Fig. 4F). Consistently, silencing FOXP4
significantly decreased the relative luciferase activity of LEF-1
promoter in the group that contained the FOXP4-binding site,
whereas FOXP4 overexpression had the opposite effects (Fig. 4G). These results indicated that
FOXP4 directly targeted and positively regulated LEF-1.
Due to the fact that LEF-1 plays crucial roles in
Wnt signaling, it was hypothesized whether FOXP4 could modulate Wnt
signaling downstream genes. The results demonstrated that FOXP4
positively regulated the expression levels of G1/S-specific
cyclin-D1 (CCND1), JUN, MYC and MMP7 (Fig. 4H), which are well-established
downstream target genes of the Wnt pathway (18). Collectively, these data suggested
that FOXP4 activates the Wnt signaling pathway.
Discussion
Abnormal gene expression is involved in the
malignant biological behavior of LSCC. In the present study, it was
found that FOXP4 was significantly upregulated in LSCC tissues and
laryngeal cancer cell lines and closely associated with the poor
survival rate of patients with LSCC. Besides, FOXP4 possessed roles
as an oncogene in promoting cell viability, migration, invasion and
EMT process, and directly bound to the promoters of LEF-1 to
activate Wnt signaling. Thus, this study delineated a molecular
mechanism of FOXP4 in facilitating LSCC progression and improves
our understanding of this transcription factor.
The human FOX gene family consists of at least 43
members, which are mainly involved in tumorigenesis through gene
amplification, retroviral integration, chromosomal translocation
and transcriptional regulation (19). At present, an increasing number of
studies have revealed that FOX family genes fulfil their roles as
transcriptional activators or repressors in cancer initiation,
progression and chemoresistance. For example, FOXA1 has been found
to occupy the upstream enhancer of the transforming growth factor
β-3 proprotein promoter to repress its transcription in
castration-resistant prostate cancer (20). FOXA2 can target and simulate the
E-cadherin promoter and recruit transducin-like enhancer protein 3
to the ZEB2 promoter to inhibit ZEB2 expression in breast cancer
(21). Furthermore, it has been
observed that FOXC2 can target the angiopoietin-2 promoter to
regulate its expression in hepatocellular carcinoma cells (22). FOXM1 can directly activate
chromosome-associated kinesin KIF4A (KIF4A) gene transcription by
binding to the KIF4A promoter in hepatocellular carcinoma (23).
Members of the FOXP family have also been
demonstrated to function as oncogenes or tumor suppressors in
tumorigenesis and progression. Sheng et al (24) reported that FOXP1 was downregulated
and contributed to lung adenocarcinoma cell progression via
chemokine signaling pathways. Chen et al (25) indicated that FOXP2 acted as a novel
suppressor in regulating EMT process via the TGFβ/SMAD signaling
pathway in breast cancer. Zhang et al (26) corroborated that FOXP3 suppressed
breast cancer metastasis and directly bound to the promoter of CD44
to inhibit its expression. Similarly, FOXP4 has been demonstrated
to promote cell growth and metastasis in breast cancer,
hepatocellular carcinoma and oral squamous cell carcinoma (7,8,10).
However, to the best of our knowledge, there are no findings
concerning the role of FOXP4 in regulating gene transcription in
LSCC, thus the present study examined the expression level of FOXP4
and potential regulatory mechanisms in LSCC. It was demonstrated
that FOXP4 was upregulated in LSCC tissue and laryngeal cancer cell
lines and displayed an oncogenic role in LSCC tumorigenesis.
EMT is a complex molecular process that contributes
to tumor progression and metastasis that is characterized by the
decreased expression of E-cadherin and increased expression of
vimentin and N-cadherin (5,27). Besides, EMT-initiating transcription
factors are implicated in inducing the EMT phenotype (28). Most previous studies have
demonstrated that TGF-β-induced genes play pivotal roles in EMT to
regulate the malignant biological behavior of different cancer
types. Cui et al (29)
reported that MIR155HG induced by TGF-β participated in LSCC
progression and EMT by regulating the miR-155-5p/SOX10 axis. Feng
et al (30) observed that
TGF-β-dependent upregulation of FAM83H-AS1 promoted EMT process in
esophageal squamous cell carcinoma progression. In the present
study, TGF-β1-induced FOXP4 expression increased N-cadherin,
Vimentin, Twist, Snail and ZEB1 expression at the mRNA and protein
levels in laryngeal cancer cells, which indicated that FOXP4 was a
TGF-β-induced transcription factor and promoted EMT process in
LSCC.
As a transcriptional factor, FOXP4 is involved in
the activation of several genes that have a crucial role in various
biological processes for cancer progression (7,8). For
example, Ma and Zhang (7) found
that FOXP4 promoted EMT through transcriptionally activating Snail
expression in breast cancer. Another study revealed that FOXP4 was
significantly upregulated and facilitated EMT by transcriptionally
regulating Slug in hepatocellular carcinoma (8). The current study verified that FOXP4
directly targeted the LEF-1 promoter and regulated its
transcriptional activity, as confirmed via ChIP and luciferase
reporter assays. LEF-1 is a member of the LEF/TCF transcription
factor family and is a well-known binding partner of β-catenin,
which promotes the activation of Wnt signaling (18). Whether FOXP4 exerted its
carcinogenic effect via regulating Wnt signaling was further
examined in the present study by measuring the expression levels of
the Wnt/β-catenin signaling-related target genes. It was found that
FOXP4 positively regulated CCND1, JUN, MYC and MMP7 expression,
illustrating that FOXP4 may mediate tumorigenesis and progression
of LSCC via Wnt signaling.
However, there were several limitations of the
present study. First, the detailed mechanism of FOXP4 in LSCC
progression requires further investigation. Furthermore, in
vivo assays are necessary to verify the present conclusions,
which were not performed in this study.
In conclusion, this study demonstrated that FOXP4
was induced by TGF-β and functioned as an oncogene in LSCC.
Besides, FOXP4 was associated with tumor progression and poor
prognosis. FOXP4 was demonstrated to regulate the EMT process, and
could directly bind to the LEF-1 promoter to activate Wnt
signaling. Thus, FOXP4 may be a potential strategy for inhibiting
LSCC progression and metastasis.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the
Livelihood Technology Special Project (grant no. 20377709D) and the
Financial Support Program of Hebei Province (grant nos. 20201511
and 20180588).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ designed the study and revised the manuscript. JS
and JW wrote the manuscript. JS, JW, HC, SL, XH, LL, GW and ML
recruited the patients, performed the experiments, analyzed the
data and drafted the paper. YZ and JS confirm the authenticity of
all the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was approved by the ethics committee of
the Fourth Hospital of Hebei Medical University (approval no.
2020k-1147; Shijiazhuang, China). Patients provided signed informed
consent prior to the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liu C, Lu ZJ, Liu H, Zhuang S and Guo P:
LncRNA XIST promotes the progression of laryngeal squamous cell
carcinoma via sponging miR-125b-5p to modulate TRIB2. Biosci Rep.
40:BSR201931722020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Groome PA, O'Sullivan B, Irish JC,
Rothwell DM, Schulze K, Warde PR, Schneider KM, Mackenzie RG,
Hodson DI, Hammond JA, et al: Management and outcome differences in
supraglottic cancer between Ontario, Canada, and the surveillance,
epidemiology, and end results areas of the United States. J Clin
Oncol. 21:496–505. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2009. View Article : Google Scholar
|
|
5
|
Thiery JP, Acloque H, Huang RYJ and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Morikawa M, Derynck R and Miyazono K:
TGF-β and the TGF-β Family: Context-dependent roles in cell and
tissue physiology. Cold Spring Harb Perspect Biol. 8:a0218732016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ma T and Zhang J: Upregulation of FOXP4 in
breast cancer promotes migration and invasion through facilitating
EMT. Cancer Manag Res. 11:2783–2793. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang G and Zhang G: Upregulation of FoxP4
in HCC promotes migration and invasion through regulation of EMT.
Oncol Lett. 17:3944–3951. 2019.PubMed/NCBI
|
|
9
|
Lam EW, Brosens JJ, Gomes AR and Koo CY:
Forkhead box proteins: Tuning forks for transcriptional harmony.
Nat Rev Cancer. 13:482–495. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu Y, Liu Y, Xiao W, Yue J, Xue L, Guan Q,
Deng J and Sun J: MicroRNA-299-3p/FOXP4 axis regulates the
proliferation and migration of oral squamous cell carcinoma.
Technol Cancer Res Treat. 18:15330338198748032019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Trapnell C, Williams BA, Pertea G,
Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Barbara JW and
Pachter L: Transcript assembly and quantification by RNA-Seq
reveals unannotated transcripts and isoform switching during cell
differentiation. Nat Biotechnol. 28:511–515. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tang Z, Li C, Kang B, Gao G, Li C and
Zhang Z: GEPIA: A web server for cancer and normal gene expression
profiling and interactive analyses. Nucleic Acids Res. 45:W98–W102.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pastushenko L and Blanpain C: EMT
Transition states during tumor progression and metastasis. Trends
Cell Biol. 29:212–226. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mittal V: Epithelial mesenchymal
transition in tumor metastasis. Annu Rev Pathol. 13:395–412. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jin W: Role of JAK/STAT3 signaling in the
regulation of metastasis, the transition of cancer stem cells, and
chemoresistance of cancer by epithelial-mesenchymal transition.
Cells. 9:2172020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hu H, Miao YR, Jia LH, Yu QY, Zhang Q and
Guo AY: AnimalTFDB 3.0: A comprehensive resource for annotation and
prediction of animal transcription factors. Nucleic Acids Res.
47:D33–D38. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Eliason S, Sharp T, Sweat M, Sweat YY and
Amendt BA: Ectodermal organ development is regulated by a
microRNA-26b-Lef-1-wnt signaling axis. Front Physiol. 11:7802020.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Katoh M and Katoh M: Human FOX gene family
(Review). Int J Oncol. 25:1495–1500. 2004.PubMed/NCBI
|
|
20
|
Song B, Park SH, Zhao JC, Fong KW, Li S,
Lee Y, Yang YA, Sridhar S, Lu X, Abdulkadir SA, et al: Targeting
FOXA1-mediated repression of TGF-β signaling suppresses
castration-resistant prostate cancer progression. J Clin Invest.
129:569–582. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang Z, Yang C, Gao W, Chen T, Qian T, Hu
J and Tan Y: FOXA2 attenuates the epithelial to mesenchymal
transition by regulating the transcription of E-cadherin and ZEB2
in human breast cancer. Cancer Lett. 361:240–250. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen J, Rong X, Liu X, Zheng D, Rong X,
Chen F, Zhao P, Liu F and Ruan J: FOXC2 is a prognostic biomarker
and contributes to the growth and invasion of human hepatocellular
carcinoma. Cancer Cell Int. 20:1962020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hu G, Yan Z, Zhang C, Cheng M, Yan Y, Wang
Y, Deng L, Lu Q and Luo S: FOXM1 promotes hepatocellular carcinoma
progression by regulating KIF4A expression. J Exp Clin Cancer Res.
38:1882019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sheng H, Li X and Xu Y: Knockdown of FOXP1
promotes the development of lung adenocarcinoma. Cancer Biol Ther.
20:537–545. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen MT, Sun HF, Li LD, Zhao Y, Yang LP,
Gao SP and Jin W: Downregulation of FOXP2 promotes breast cancer
migration and invasion through TGFβ/SMAD signaling pathway. Oncol
Lett. 15:8582–8588. 2018.PubMed/NCBI
|
|
26
|
Zhang C, Xu Y, Hao Q, Wang S, Li H, Li J,
Gao Y, Li M, Li W, Xue X, et al: FOXP3 suppresses breast cancer
metastasis through downregulation of CD44. Int J Cancer.
137:1279–1290. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zeisberg M and Neilson EG: Biomarkers for
epithelial-mesenchymal transitions. J Clin Invest. 119:1429–1437.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cui W, Meng W, Zhao L, Cao H, Chi W and
Wang B: TGF-β-induced long non-coding RNA MIR155HG promotes the
progression and EMT of laryngeal squamous cell carcinoma by
regulating the miR-155-5p/SOX10 axis. Int J Oncol. 54:2005–2018.
2019.PubMed/NCBI
|
|
30
|
Feng B, Wang G, Liang X, Wu Z, Wang X,
Dong Z, Guo Y, Shen S, Liang J and Guo W: LncRNA FAM83H-AS1
promotes oesophageal squamous cell carcinoma progression via
miR-10a-5p/Girdin axis. J Cell Mol Med. 24:8962–8976. 2020.
View Article : Google Scholar : PubMed/NCBI
|