Introduction
Breast cancer (BC) is a malignant tumor occurring in
the epithelial tissues of the mammary gland. The incidence of BC
worldwide has been on the rise since the late 1970s, with ~1.7
million new cases detected annually (1). Furthermore, the incidence and
mortality of BC are predicted to increase significantly over the
next 5–10 years, along with a decrease in the age of patients with
BC (2). At present, the treatment
of BC mainly comprises surgical resection and postoperative
chemotherapy or radiotherapy, but the overall survival rate after
treatment is low and the prognosis is poor (3). Therefore, identifying effective
targets for the treatment of BC at the molecular level may
significantly improve the survival rates of patients.
Low expression of microRNA (miR/miRNA)-302d-3p has
been reported in endometrial cancer cells, while overexpression of
miR-302d-3p was revealed to inhibit the epithelial-to-mesenchymal
transformation, viability and migration and promote the apoptosis
of endometrial cancer cells (4,5).
However, it was found that the expression of miR-302d-3p was
abnormally increased in the tumor tissues of patients with
hepatocellular carcinoma (HCC), and the survival time of patients
with HCC and lower expression of miR-302d was longer compared with
that of patients with HCC and higher expression of miR-302d
(6). Therefore, the role of
miR-302d in cancer varies according to the type of the cancer. Chen
and Yang (7) observed that miR-302d
was closely associated with the occurrence and development of BC
through analyzing microarray data and identifying differentially
expressed genes in BC. However, the specific role of miR-302d-3p in
BC and its possible underlying mechanism have yet to be
reported.
Therefore, the aim of the present study was to
investigate the effect of miR-302d-3p on the viability, migration
and apoptosis of BC cells and discuss the underlying mechanism, in
order to provide a valuable reference for identifying novel
therapeutic targets for BC.
Materials and methods
BC tissue samples
Between August 2019 and August 2020, a total of 30
pairs of BC tissue samples and matched adjacent normal tissue
(distance, 2 cm) were collected from female patients (age range,
20–55 years) who underwent surgery at the Baoan Central Hospital of
Shenzhen (Shenzhen, China). All fresh specimens were immediately
placed in liquid nitrogen following surgery. All cases were
diagnosed using postoperative pathological examination, and other
major diseases were excluded. The present study was approved by the
Ethics Committee of Baoan Central Hospital of Shenzhen and all
patients provided written informed consent. All the procedures
complied with the principles outlined in the Declaration of
Helsinki and relevant policies in China. Inclusion criteria were as
follows: i) Diagnosis by postoperative pathology; ii) imaging
examination showed no active lesions; iii) aged 18–65 years; iv) no
history of smoking, drinking or recreational drug use; v) provision
of signed informed consent. Exclusion criteria were as follows: i)
Patients with diabetes or other diseases undergoing treatment; ii)
surgery, chemotherapy, nuclear medicine or immunotherapy <3
months before enrollment; iii) other malignant tumors; iv) drug
treatment <7 days before enrollment; v) pregnancy and lactation;
vi) blood transfusions <4 months before enrollment; vii)
hepatitis C, syphilis or HIV antibody-positive.
Cell culture
All cell lines (MCF-10A, MCF7 and MDA-MB-231) were
purchased from The Cell Bank of Type Culture Collection of the
Chinese Academy of Sciences. The cells were cultured and stored as
follows: DMEM (Gibco; Thermo Fisher Scientific, Inc.) was
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.)
for these cell lines, according to the manufacturer's instructions.
All cell lines were maintained in a humidified cell incubator at
37°C with an atmosphere of 5% CO2. ERK pathway inhibitor
U0126 (10 ng/ml) and agonist EGF (30 ng/ml; both pretreatment for 2
h at 37°C; both 98% pure; both MedChem Express) were used to induce
cells.
Reverse transcription-quantitative PCR
(RT-qPCR) analysis
The cells were seeded into 6-well plates at a
density of 1×106 cells/well. Total RNA was extracted
using RNA Purified Total RNA Extraction Kit (Invitrogen; Thermo
Fisher Scientific, Inc.), and then subjected to reverse
transcription to cDNA using SMART MMLC Reverse Transcriptase
(Takara Biotechnology Co., Ltd.) at ~65°C for 10 min. The qPCR
reaction mixture contained 10 µl 2X Power Taq PCR MasterMix (cat.
no. PR1702; BioTeke Corporation), 0.5 µl of each primer
[miR-302d-3p forward, 5′-GCGTAAGTGCTTCCATGTTTGTGTGT-3′;
transmembrane Bax inhibitor motif containing 6 (TMBIM6) forward,
5′-TCCCTCGACACAGCAGCACCT-3′ and reverse,
5′-CCCCAGAGAGGACAGGAGCAT-3′; GAPDH forward,
5′-AACTTTGGCATTGTGGAAGG-3′ and reverse, 5′-GGATGCAGGGATGATGTTCT-3′;
and U6 forward, 5′-AACTTTGGCATTGTGGAAGG-3′ and reverse,
5′-GGATGCAGGGATGATGTTCT-3′], 1 µl cDNA template and 8 µl RNase free
H2O. GAPDH acted as an endogenous control of TMBIM6 and
U6 was used as an endogenous control of miR-302d-3p. The
thermocycling conditions were as follows: 95°C for 10 min, 40
cycles of 95°C for 10 sec, 55°C for 10 sec, and 72°C for 30 sec.
The amplification was performed with the Exicycler™ 96 (Bioneer
Corporation) and relative expression levels were calculated
according to the 2−∆∆Cq method (8).
Cell Counting Kit-8 (CCK-8) assay
The CCK-8 assay (Dojindo Molecular Technologies,
Inc.) was used for the detection of cell viability, according to
the manufacturer's protocol. Briefly, cells were seeded in 96-well
plates (1×104 cells/well) and examined at 24 h. The
detection buffer (100 µl; ratio of medium to CCK-8, 9:1) was added
to each well. After incubation for 3 h at 37°C, the absorbance at
450 nm was detected by a microplate reader.
Wound healing assay
Cells were seeded at 6-well plates (1×106
cells/well)and grown to 80% confluence in 12-well plates. Two
linear scratches were created in the cell monolayer with a 200-µl
micropipette tip in each well, after which time the cells were
treated in serum-free media and allowed to migrate for 24 h. Images
of the wound areas were captured under an AxioVert 200M
fluorescence microscope (magnification, ×100; Carl Zeiss AG) at 0
and 24 h. Migration was calculated according to the following
formula: Cell mobility=(0 h scratch width-scratch width after
culture)/0 h scratch width.
TUNEL assay
The cells were collected and washed with PBS three
times. Cells were fixed with 4% paraformaldehyde for 30 min at room
temperature and washed with PBS. Then, 0.3% Triton X-100 in PBS was
added and incubated for 5 min at room temperature. Subsequently, 50
µl TUNEL assay solution (Roche Diagnostics GmbH) was added to the
cells and incubated at 37°C in the dark for 60 min. Next, cells
were incubated with DAB and stained with hematoxylin and eosin for
5 min at room temperature. The detection solution was then
discarded and the cells were washed with PBS three times and sealed
with anti-fluorescence quenched sealing solution. A total of 3
visual fields were randomly selected for observation under a
fluorescence microscope (magnification, ×200; Carl Zeiss AG). The
available excitation wavelength range was 450–500 nm and the
emission wavelength range was 515–565 nm (green fluorescence).
Western blot analysis
The samples were treated with RIPA lysis buffer
(Beyotime Institute of Biotechnology), incubated on ice for 30 min
and later centrifuged at 300 × g for 20 min (4°C). The protein
concentration was determined using a bicinchoninic acid assay
protein assay kit (Beyotime Institute of Biotechnology). Total
protein (30 µg) was collected and separated via 10% SDS-PAGE, and
subsequently transferred to PVDF membranes and blocked in 5%
non-fat milk at room temperature for 1 h. The membranes were
incubated overnight at 4°C with polyclonal rabbit anti-Bcl-2
antibody (1:1,000; cat. no. ab32124; Abcam), rabbit anti-Bax
antibody (1:1,000; cat. no. ab32503; Abcam), anti-caspase-3
antibody (1:1,000; cat. no. ab197202; Abcam), anti-cleaved
caspase-3 (1:1,000; cat. no. PA5-17913; Thermo Fisher Scientific,
Inc.), anti-caspase-9 antibody (1:1,000; cat. no. ab219590; Abcam),
anti-cleaved caspase-9 (1:1,000; cat. no. PA5-17605; Thermo Fisher
Scientific, Inc.), anti-extracellular signal-regulated kinase (ERK)
antibody (1:1,000; cat. no. ab32537; Abcam), anti-phosphorylated
(p)-ERK antibody (1:1,000; cat. no. ab192591; Abcam) and
anti-β-actin antibody (1:1,000; cat. no. ab179467; Abcam), followed
by incubation with HRP-labeled goat anti-rabbit secondary antibody
(1:5,000; cat. no. A-11012; Thermo Fisher Scientific, Inc.) for 1 h
at room temperature. Proteins were visualized using ImageQuant™ LAS
4000 (Cytiva) and semi-quantified using ImageJ software (version
1.46; National Institutes of Health).
Dual-luciferase reporter assay
Bioinformatics software TargetScan (http://www.targetscan.org) was used to predict the
target genes of miR-302d-3p, and TMBIM6 was determined as a
potential target. The wild-type (WT) or mutant (MUT)
miR-302d-3p-binding site was subcloned into a pCDNA3.1 plasmid
purchased from Thermo Fisher Scientific, Inc. The cells were plated
in the 24-well plates (1×104 cells/well) 24 h before
transfection. miR-302d-3p-mimic, miR-302d-3p-inhibitor and
corresponding controls were co-transfected with 10 µg
pCDNA3.1-WT-miR-302d-3p or pCDNA3.1-MUT-miR-302d-3p for 48 h at
37°C using Lipofectamine® 2000 (Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. The
luciferase activity was measured using a plate reader (BD
Biosciences) and was normalized to Renilla luciferase
activity (pRL-TK) using the Luc-Screen™ Extended-Glow Luciferase
Reporter Gene Assay system (cat. no. E1910; Promega
Corporation).
Immunohistochemical analyses
Tissue samples (0.2 cm) were fixed in 10% formalin
for 24 h at room temperature, embedded in paraffin, and analyzed by
immunohistochemistry. The samples were blocked in 5% normal goat
serum (Gibco; Thermo Fisher Scientific, Inc.) for 1 h at room
temperature, probed with anti-TMBIM6 (1:150; cat. no. ab18852;
Abcam) at 4°C overnight and labeled with biotinylated secondary
antibodies (1:2,000; cat. no. ab205718; Abcam) for 1 h at 37°C. The
immunoreaction signal was developed with DAB staining, and the
slides were counterstained in hematoxylin for 5 min at room
temperature. Stained tissue sections were viewed under a light
microscope (magnification, ×200; ECLIPSE Ni-U; Nikon Corporation).
Histological score (H-score) was used to calculate staining score
as follows: H-score=intensity × total number of positive cells ×
100%.
Cell transfection
The mimic and the inhibitor of hsa-miR-302d-3p,
miRNA negative controls (miR-302d-3p-NC), TMBIM6-smalll interfering
(si)RNA and negative control vector (TMBIM6-NC) at a concentration
of 20 nM were synthesized by Shanghai GenePharma Co., Ltd. Cells at
a final concentration of 25 nM (1×106 cells/well) were
transfected using Lipofectamine® 3000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. Following incubation for 48 h at 37°C,
cells were used for subsequent experiments. Transfection efficiency
was detected via RT-qPCR. The sequences of mimics/inhibitors/siRNAs
were as follows: NC-mimic, 5′-UUCUCCGAACGUGUCACGUTT-3′;
NC-inhibitor, 5′-CAGUACUUUUGUGUAGUACAA-3′; miR-302d-3p mimic,
5′-UAAGUGCUUCCAUGUUUGAGUGU-3′; miR-302d-3p inhibitor,
5′-ACACUCAAACAUGGAAGCACUUA-3′; si-TMBIM6 forward,
5′-GUGGAAGGCCUUCUUUCUA-3′ and reverse, 5′-UAGAAAGAAGGCCUUCCAC-3′;
and TMBIM6-NC forward, 5′-CUGAACAACCAAUGCAAAU-3′ and reverse,
5′-AUUUGCAUUGGUUGUUCAG-3′. The cells were grouped as follows:
Control, miR-302d-3p-NC, TMBIM6-NC, miR-302d-3p mimics, miR-302d-3p
mimics + si-TMBIM6, miR-302d-3p inhibitor, miR-302d-3p inhibitor +
si-TMBIM6 and si-TMBIM6. Subsequently, the cells were divided into
control, miR-302d-3p-NC, miR-302d-3p mimics, miR-302d-3p mimics +
U0126, miR-302d-3p mimics + EGF, miR-302d-3p inhibitor, miR-302d-3p
inhibitor + U0126 and miR-302d-3p inhibitor + EGF groups.
Statistical analysis
All data are presented as the mean ± standard
deviation. Multiple comparisons among three groups were performed
using one-way ANOVA followed by Tukey's post hoc test. Error bars
represent standard deviation. P<0.05 was considered to indicate
a statistically significant difference.
Results
miR-302d-3p inhibits viability and
migration and promotes apoptosis of BC cells
The expression of miR-302d-3p in the BC cell lines
MCF7 and MDA-MB-231 was significantly downregulated compared with
normal breast epithelial cells, as determined via RT-qPCR (Fig. 1A). The decrease in miR-302d-3p
expression levels was greatest in MCF-7 cells, therefore MCF7 cells
were selected for subsequent experiments. miR-302d-3p mimics,
miR-302d-3p inhibitor and miR-302d-3p-NC were transfected into MCF7
BC cells. The transfection efficiency was confirmed by RT-qPCR
analysis (Fig. 1B). Subsequently,
the CCK-8 assay demonstrated that, compared with the
miR-302d-3p-NC, the cell survival rate of the miR-302d-3p mimics
group was decreased, whereas that of the miR-302d-3p inhibitor
group was increased (Fig. 1C). The
wound healing assay also demonstrated that the cell migration rate
of the miR-302d-3p mimics group was decreased, whereas that of the
miR-302d-3p inhibitor group was increased (Fig. 1D and E). The TUNEL assay revealed
increased apoptosis in the miR-302d-3p mimics group, whereas no
apoptosis was observed in the miR-302d-3p inhibitor group (Fig. 2A). Western blot analysis of the
expression levels of the apoptosis-related proteins Bcl-2, Bax,
caspase-3 and caspase-9 revealed a consistent trend. Compared with
miR-302d-3p-NC group, Bcl-2 was decreased and Bax, caspase-3 and
caspase-9 were increased in miR-302d-3p mimic group. Compared with
miR-302d-3p-NC group, Bcl-2 was increased and Bax, caspase-3 and
caspase-9 were decreased in miR-302d-3p inhibitor group (Fig. 2B). The aforementioned results
indicated that miR-302d-3p may inhibit the viability and migration,
and promote the apoptosis, of BC cells.
miR-302d-3p inhibits the viability and
migration of BC cells and promotes apoptosis by targeting the
expression of TMBIM6
The binding site of miR-302d-3p and the
3′-untranslated region (UTR) of TMBIM6 were predicted using
TargetScan (Fig. 3A) and the
binding site of miR-302d-3p and TMBIM6 was verified with a
dual-luciferase reporter assay (Fig.
3B). Subsequently, immunohistochemistry was used to detect the
expression of TMBIM6 in BC samples and normal adjacent tissues, and
it was observed that the expression of TMBIM6 in BC tissues was
increased (Fig. 3C and D). In
addition, RT-qPCR assays were performed to detect the expression of
TMBIM6 in the serum of 30 samples from patients with BC (Fig. 3E) and in BC cell lines (Fig. 3F), which were both consistent with
the results presented in Fig.
3D.
In order to further test the role of TMBIM6 in the
viability, migration and apoptosis of BC cells, miR-302d-3p mimics,
miR-302d-3p inhibitor, si-TMBIM6 and the corresponding NC groups
miR-302d-3p-NC and TMBIM6-NC were synthesized and transfected into
MCF7 cells. It was observed that the expression of TMBIM6 decreased
when miR-302d-3p was overexpressed and increased when miR-302d-3p
was inhibited compared with miR-302d-3p-NC (Fig. 4A). Compared with miR-302d-3p-NC,
expression of miR-302d-3p increased in miR-302d-3p mimic, whereas
expression of miR-302d-3p decreased in miR-302d-3p inhibitor group
(Fig. 4B). This finding further
indicated that miR-302d-3p may target TMBIM6. As shown in Fig. 4C, cell viability significantly
decreased when cells were transfected with miR-302d-3p mimics
compared with miR-302d-3p-NC, and a further decrease in the cell
survival rate was observed following transfection with si-TMBIM6
compared with miR-302d-3p mimics. Cell viability was increased in
the miR-302d-3p inhibitor group compared with that of the
miR-302d-3p-NC group, and further interference with TMBIM6 resulted
in a significant decrease in cell viability. Compared with the
TMBIM6-NC group, the cell viability of the si-TMBIM6 group
decreased. The wound healing assay revealed that, compared with the
si-TMBIM6 group, cell migration exhibited a decreasing trend in the
miR-302d-3p mimics + si-TMBIM6 group, whereas cell migration
increased in the miR-302d-3p inhibitor + si-TMBIM6 group. Moreover,
the rate of cell migration was always decreased following
transfection with si-TMBIM6 compared with miR-302d-3p mimics,
despite miR-302d-3p knockdown or overexpression (Fig. 4D and E). The results revealed that,
compared with TMBIM6-NC, the si-TMBIM6 group had fewer apoptotic
cells. After knockdown or overexpression of miR-302d-3p, the rate
of apoptosis was notably increased after further interference with
si-TMBIM6 (Fig. 5A). Next, western
blotting was used to detect the expression of apoptosis-related
proteins (Fig. 5B and C), and the
findings were consistent with those shown in Fig. 5A. These results suggested that
miR-302d-3p inhibited viability and migration, and promoted
apoptosis of BC cells by targeting the expression of TMBIM6.
 | Figure 5.miR-302d-3p promotes apoptosis by
targeting TMBIM6. (A) TUNEL assay was performed to determine the
apoptosis rate of cells following transfection (scale bar, 100 µm).
(B and C) Western blotting was performed to detect the expression
of apoptosis-related proteins. *P<0.05, **P<0.01,
***P<0.001 vs. miR-302d-3p-NC; ∆P<0.05,
∆∆P<0.01, ∆∆∆P<0.001 vs. miR-302d-3p
mimics or inhibitor; #P<0.05, ##P<0.01,
###P<0.001 vs. TMBIM6-NC. miR, microRNA; NC, negative
control; TMBIM6, transmembrane Bax inhibitor motif containing 6;
si-, small interfering RNA. |
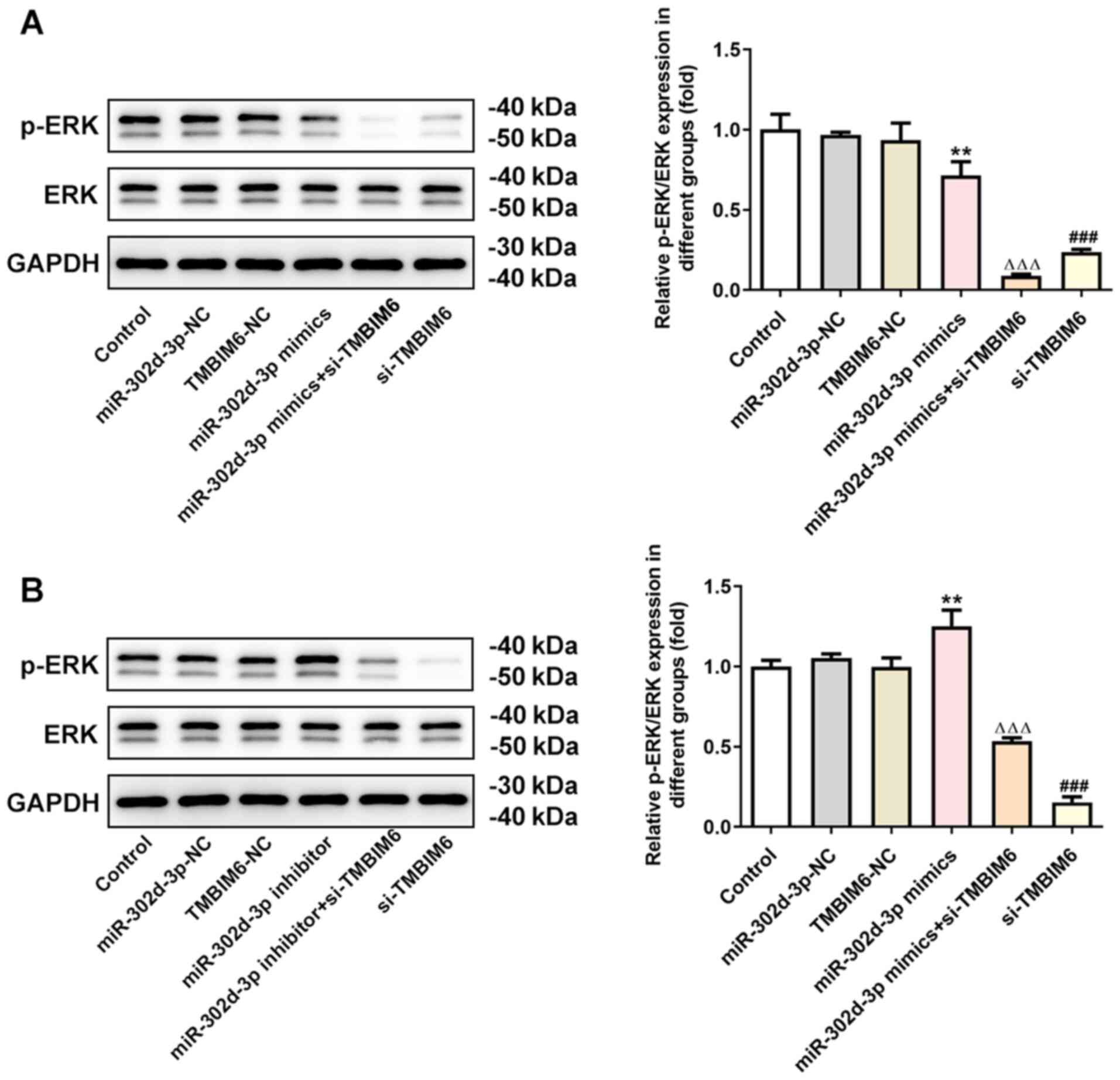
miR-302d-3p regulates the ERK
signaling pathway via targeting TMBIM6 to inhibit viability and
migration and promote apoptosis of BC cells
In this experiment, changes were also found in the
expression of ERK pathway-related proteins. It was observed that,
compared with the miR-302d-3p-NC group, the expression of p-ERK was
decreased following overexpression of miR-302d-3p (Fig. 6A), whereas p-ERK expression was
significantly increased after knockdown of miR-302d-3p expression
(Fig. 6B). Compared with TMBIM6-NC,
the expression of p-ERK significantly decreased after transfection
with si-TMBIM6. Compared with miR-302d-3p mimics, the expression of
p-ERK was further decreased after knockdown of TMBIM6. Compared
with miR-302d-3p inhibitor, the expression of p-ERK decreased after
transfection with si-TMBIM6. These results suggested that
miR-302d-3p inhibited viability and migration and promoted
apoptosis of BC cells via targeting TMBIM6, which may be related to
the ERK signaling pathway. Therefore, in the following experiments,
an inhibitor of the ERK pathway, U0126, and an EGF agonist were
added after cell transfection to further test the underlying
mechanism. After overexpression or knockdown of miR-302d-3p through
cell transfection, there was no significant change in the
expression of the ERK protein, whereas the expression of p-ERK was
altered following the addition of U0126 or EGF. Compared with the
miR-302d-3p-NC group, the expression of p-ERK decreased after
overexpression of miR-302d-3p. Compared with the miR-302d-3p mimics
group, the expression of p-ERK was further inhibited after the
addition of U0126, and the decreasing trend in p-ERK expression was
reversed following the addition of EGF (Fig. 7A). Compared with the miR-302d-3p-NC
group, the expression of p-ERK increased after the inhibition of
miR-302d-3p. This increase in p-ERK was reversed following the
addition of U0126, whereas the expression of p-ERK was enhanced
after the addition of EGF, compared with the miR-302d-3p inhibition
group (Fig. 7B).
Subsequently, it was observed that, compared with
miR-302d-3p mimics and miR-302d-3p inhibitor, the addition of U0126
reduced cell viability (Fig. 8A)
and migration (Fig. 8B and C), and
notably increased apoptosis (Fig.
9A). The addition of EGF promoted an increase in cell viability
and cell migration, and cell apoptosis was markedly decreased.
Subsequently, the expression levels of apoptosis-related proteins
were detected by western blotting. The results demonstrated that,
compared with miR-302d-3p-NC, the expression of Bcl-2 was decreased
and the expression levels of Bax and caspase-3/9 were increased
following overexpression of miR-302d-3p. Furthermore, after the
addition of U0126, the expression of Bcl-2 was further decreased,
whereas the expression levels of Bax and caspase3/9 were further
increased, compared with the miR-302d-3p mimics group. Following
the addition of EGF, the changes in the expression of all proteins
were the opposite of those induced by U0126 (Fig. 9B). Compared with miR-302d-3p-NC,
expression of Bcl-2 increased, and the expression levels of Bax and
caspase-3/9 decreased following inhibition of miR-302d-3p.
Following the addition of U0126, compared with the miR-302d-3p
inhibition group, the expression of Bcl-2 was decreased, and the
expression levels of Bax and caspase-3/9 were increased, which were
both reversed after the addition of EGF (Fig. 9C). Therefore, these results
indicated that miR-302d-3p regulates the ERK signaling pathway via
targeting TMBIM6 to inhibit viability and migration and promote
apoptosis of BC cells.
Discussion
miR-302d-3p is a recently identified
cancer-regulating gene that plays a key role in cancer development
(9). miR-302d may bind to the
3′-UTR of cyclin D1 mRNA, thus inhibiting the viability of bladder
cancer cells (10). The expression
of miR-302d was found to be downregulated in human glioblastoma
cells, and miR-302d inhibited the occurrence of tumors in xenograft
model mice (11). However, the
effect of miR-302d-3p on the viability, migration and apoptosis of
BC cells and its underlying mechanism have not been reported to
date. The present study demonstrated that the expression of
miR-302d-3p in BC cell lines was decreased. Subsequently, the
expression of miR-302d-3p was promoted or inhibited through cell
transfection, and it was observed that miR-302d-3p inhibited
viability and migration, and promoted apoptosis of BC cells.
Using TargetScan bioinformatics software, it was
predicted that the 3′-UTR region of TMBIM6 had binding sites for
miR-302d-3p. TMBIM6, also referred to as Bax inhibitor-1, is an
evolutionarily conserved transmembrane protein that is primarily
located in the endoplasmic reticulum (12). TMBIM6 has been identified as an
anti-apoptotic protein due to its protective effect against the
pro-apoptotic protein Bax (13).
TMBIM6 is upregulated in a number of tumors and is involved in
metastasis, whereas the downregulation of TMBIM6 may lead to cancer
cell death (14). It has been
reported that TMBIM6 is highly expressed in the cells and serum of
patients with BC (15). In the
present study, the targeting association between miR-302d-3p and
TMBIM6 was first verified through a dual-luciferase reporter assay.
Moreover, the expression of TMBIM6 was found to be significantly
increased in BC tissues and cell lines. Next, miR-302d-3p
overexpression and interference with the expression of miR-302d-3p
and TMBIM6 was achieved through cell transfection, and cell
viability, migration and apoptosis were detected. The findings
demonstrated that knockdown of TMBIM6 could further inhibit cell
viability and migration and further promote apoptosis induced by
miR-302d-3p.
ERK is an important member of the mitogen-activated
protein kinase (MAPK) family and plays an important role in
regulating different biological processes in different cells,
including proliferation, differentiation, survival and apoptosis
(16). The results of the present
study indicated that the activation of the ERK signaling pathway
exerted anti-apoptotic effects on cancer cells, which could
therefore promote the occurrence and development of BC, as commonly
observed in other types of cancer, including pancreatic cancer,
esophageal squamous cell carcinoma and thyroid cancer (17–20).
Zhao et al (21)
investigated the sensitivity of BC cells to Adriamycin and found
that miR-302d regulated cell sensitivity to Adriamycin by
regulating MAPK/ERK kinase kinase 1 to inhibit the expression of
p-glycoprotein. Therefore, the ERK protein may be a regulatory
target for miR-302d-3p. Moreover, overexpression of TMBIM6 can
activate the ERK signaling pathway in order to inhibit apoptosis
(22). It was observed that, after
promoting the expression of miR-302d-3p, the expression of p-ERK
decreased, while further inhibiting the expression of TMBIM6
further decreased the expression of p-ERK. When inhibiting the
expression of miR-302d-3p, p-ERK increased, while upon further
inhibition of TMBIM6, the expression of p-ERK decreased. Therefore,
these results suggested that miR-302d-3p may regulate the ERK
signaling pathway by targeting TMBIM6 to affect the viability,
migration and apoptosis of BC cells. Addition of the inhibitor
U0126 further aggravated the miR-302d-3p-induced decrease in cell
viability and migration. Furthermore, U0126 could further increase
miR-302d-3p-induced apoptosis, whereas this phenomenon was reversed
following the addition of an EGF agonist. These results indicated
that miR-302d-3p may regulate the ERK signaling pathway via
targeting TMBIM6 to inhibit viability and migration and promote
apoptosis of BC cells.
In conclusion, the present study revealed that
miR-302d-3p regulated the viability, migration and apoptosis of BC
cells via the regulation of TMBIM6-mediated ERK signaling, which
may provide a novel experimental basis for targeted gene therapy
for BC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL made substantial contributions to the conception
and design of the study, and the acquisition of data. ZQ and LB
made substantial contributions to analysis and interpretation of
data. YL and ZQ confirm the authenticity of all the raw data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Baoan Central
Hospital of Shenzhen (Shenzhen, China), and all patients provided
written informed consent. All of the procedures were in compliance
with The Declaration of Helsinki and relevant policies in
China.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Anastasiadi Z, Lianos GD, Ignatiadou E,
Harissis HV and Mitsis M: Breast cancer in young women: An
overview. Updates Surg. 69:313–317. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Merino Bonilla JA, Torres Tabanera M and
Ros Mendoza LH: Breast cancer in the 21st century: From early
detection to new therapies. Radiologia. 59:368–379. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tian Z, Tang J, Liao X, Yang Q, Wu Y and
Wu G: An immune-related prognostic signature for predicting breast
cancer recurrence. Cancer Med. 9:7672–7685. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li Y, Huo J, Pan X, Wang C and Ma X:
MicroRNA 302b-3p/302c-3p/302d-3p inhibits epithelial-mesenchymal
transition and promotes apoptosis in human endometrial carcinoma
cells. Onco Targets Ther. 11:1275–1284. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Erratum: MicroRNA 302b-3p/302c-3p/302d-3p
inhibits epithelial-mesenchymal transition and promotes apoptosis
in human endometrial carcinoma cells [Erratum]. Onco Targets Ther.
11:22032018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ecevit CO, Aktas S, Tosun Yildirim H,
Demirağ B, Erbay A, Karaca İ, Çelik A, Demir AB, Erçetin AP and
Olgun N: MicroRNA-17, MicroRNA-19b, MicroRNA-146a, MicroRNA-302d
expressions in hepatoblastoma and clinical importance. J Pediatr
Hematol Oncol. 41:7–12. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen D and Yang H: Integrated analysis of
differentially expressed genes in breast cancer pathogenesis. Oncol
Lett. 9:2560–2566. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang S, Zheng Y, Hu Z, Wang Z, Zhang Y and
Wei L: Downregulated miR302d3p promotes chondrocyte proliferation
and migration by regulation of Unc-51-like kinase 1. Int J Mol Med.
44:1039–1047. 2019.PubMed/NCBI
|
|
10
|
Xu J, Wang Y, Hua X, Xu J, Tian Z, Jin H,
Li J, Wu XR and Huang C: Inhibition of PHLPP2/cyclin D1 protein
translation contributes to the tumor suppressive effect of NFκB2
(p100). Oncotarget. 7:34112–34130. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang F, Yang L, Sun J, Zheng J, Shi L,
Zhang G and Cui N: Tumor suppressors microRNA-302d and microRNA-16
inhibit human glioblastoma multiforme by targeting NF-κB and FGF2.
Mol Biosyst. 13:1345–1354. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ishikawa T, Watanabe N, Nagano M,
Kawai-Yamada M and Lam E: Bax inhibitor-1: A highly conserved
endoplasmic reticulum-resident cell death suppressor. Cell Death
Differ. 18:1271–1278. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu Q and Reed JC: Bax inhibitor-1, a
mammalian apoptosis suppressor identified by functional screening
in yeast. Mol Cell. 1:337–346. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Junjappa RP, Kim HK, Park SY, Bhattarai
KR, Kim KW, Soh JW, Kim HR and Chae HJ: Expression of TMBIM6 in
cancers: The involvement of Sp1 and PKC. Cancers (Basel).
11:9742019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee GH, Chae HJ and Kim HR: Monoamine
carboxylate transporters are involved in BI-1-associated cancer
metastasis in HT1080 colon fibrosarcoma cells. Int J Oncol.
39:209–216. 2011.PubMed/NCBI
|
|
16
|
Li D, Wei Y, Xu S, Niu Q, Zhang M, Li S
and Jing M: A systematic review and meta-analysis of bidirectional
effect of arsenic on ERK signaling pathway. Mol Med Rep.
17:4422–4432. 2018.PubMed/NCBI
|
|
17
|
Yang C, Yu H, Chen R, Tao K, Jian L, Peng
M, Li X, Liu M and Liu S: CXCL1 stimulates migration and invasion
in ERnegative breast cancer cells via activation of the ERK/MMP2/9
signaling axis. Int J Oncol. 55:684–696. 2019.PubMed/NCBI
|
|
18
|
Sheng W, Chen C, Dong M, Wang G, Zhou J,
Song H, Li Y, Zhang J and Ding S: Calreticulin promotes EGF-induced
EMT in pancreatic cancer cells via Integrin/EGFR-ERK/MAPK signaling
pathway. Cell Death Dis. 8:e31472017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Maehara O, Suda G, Natsuizaka M, Ohnishi
S, Komatsu Y, Sato F, Nakai M, Sho T, Morikawa K, Ogawa K, et al:
Fibroblast growth factor-2-mediated FGFR/Erk signaling supports
maintenance of cancer stem-like cells in esophageal squamous cell
carcinoma. Carcinogenesis. 38:1073–1083. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Buffet C, Hecale-Perlemoine K, Bricaire L,
Dumont F, Baudry C, Tissier F, Bertherat J, Cochand-Priollet B,
Raffin-Sanson ML, Cormier F and Groussin L: DUSP5 and DUSP6, two
ERK specific phosphatases, are markers of a higher MAPK signaling
activation in BRAF mutated thyroid cancers. PLoS One.
12:e01848612017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao L, Wang Y, Jiang L, He M, Bai X, Yu L
and Wei M: MiR-302a/b/c/d cooperatively sensitizes breast cancer
cells to adriamycin via suppressing P-glycoprotein(P-gp) by
targeting MAP/ERK kinase kinase 1 (MEKK1). J Exp Clin Cancer Res.
35:252016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim JH, Lee ER, Jeon K, Choi HY, Lim H,
Kim SJ, Chae HJ, Park SH, Kim S, Seo YR, et al: Role of BI-1
(TEGT)-mediated ERK1/2 activation in mitochondria-mediated
apoptosis and splenomegaly in BI-1 transgenic mice. Biochim Biophys
Acta. 1823:876–888. 2012. View Article : Google Scholar : PubMed/NCBI
|