Introduction
Acute lung injury (ALI), a severe heterogeneous
clinical syndrome of lung injury, is mainly responsible for most
acute respiratory symptoms (1).
Pathologically, it is often characterized by diffuse alveolar
injury and regional alveolar hypoxia (1,2). Acute
inflammation is closely implicated in the pathogenesis of ALI
(3). The diagnosis of ALI is
usually based on clinical and imaging criteria with great
diagnostic uncertainty (1).
Currently, standard therapies for ALI, such as prone position and
neuromuscular blockade, are mainly focused on lung protection, but
with limited efficacy (4,5). Therefore, novel diagnostic markers and
effective treatments for ALI at the molecular level are required to
be explored from a pathophysiological perspective.
Vitamin A and its metabolites such as retinoic acid
(RA) and all-trans RA (ATRA) serve a vital role in maintaining the
balance of immune responses and regulating developmental
abnormalities, such as respiratory system defects and lung agenesis
(6,7). As an endocrine hormone, ATRA can
trigger genomic effects (8).
Compelling evidence reveals that ATRA can effectively alleviate
organ injury, including kidney and liver injury (9,10). In
addition, ATRA can relieve chronic inflammation-induced emphysema
characterized by alveolar wall destruction (11). However, the effect of ATRA on ALI
remains to be elucidated.
Alveolar macrophages, a type of tissue-resident
macrophage, can regulate pathogen-induced immune responses and
contribute to maintaining the homeostasis of the lung (12). Alveolar macrophages possess powerful
phagocytosis that can keep the airway free of bacteria and foreign
particles, thereby protecting against lung injury (13). In addition, alveolar macrophages are
intimately associated with lung inflammation (14). Mechanically, the role of macrophages
is mediated by a series of receptors on their surface, of which
toll-like receptor 4 (TLR4) and cluster of differentiation (CD) 14
are the two most important (15).
Therefore, it is reasonable to hypothesize that ATRA
serves a vital role in lipopolysaccharide (LPS)-induced ALI with
the involvement of CD14 and TLR4 in macrophages. Consequently, the
present study sought to identify the role of ATRA in ALI and to
explore the related regulatory mechanism, which may provide novel
insights into the therapies for ALI.
Materials and methods
Ethics statement
All animal procedures were performed in accordance
with the guidelines of the Animal Ethics Committee of Shanxi
Provincial People's Hospital and ethics approval was received
(approval no. 2019-56). Significant efforts were made to minimize
animal numbers and their suffering.
Bioinformatics analysis
Signaling pathway enrichment analysis was performed
using the Kyoto Encyclopedia of Genes and Genomes (KEGG) database
(https://www.kegg.jp).
Establishment of the ALI rat model and
rat grouping
A total of 30 adult male Sprague-Dawley (SD) rats
(8–10 weeks; 220–270 g) were provided by Shanghai SLAC Laboratory
Animal Co., Ltd. [SYXK (Shanghai) 2018-0038]. Rats were placed in a
clean environment (20°C; 50–60% humidity) and raised under a 12-h
light/dark cycle. All animals were euthanized by an intraperitoneal
injection of pentobarbital sodium (≥100 mg/kg).
SD rats were randomly allocated to the ALI, ATRA and
control groups, with 10 rats in each group. LPS solution (10 mg/ml;
Sigma-Aldrich; Merck KGaA) was prepared with normal saline. Rats in
the ALI and ATRA groups were injected with 5 mg/kg LPS solution via
the caudal vein. In addition, 5 days before LPS injection, rats in
the ALI group were administered olive oil (0.5 ml/kg/time) by
gavage once a day and then intraperitoneally injected with 1 ml/kg
olive oil after LPS injection. Meanwhile, 5 days before LPS
injection, rats in the ATRA group were administered olive oil
containing 30 mg/kg ATRA (0.5 ml/kg/time) by gavage once a day and
then intraperitoneally injected with 1 ml/kg olive oil containing 5
mg/kg ATRA. Rats in the control group were administered equal
amount of normal saline for 7 days. On day 7, 24 h after an
intraperitoneal injection of ATRA, rats in each group were
euthanized and sampled (16).
Detection of arterial partial pressure
of oxygen (PaO2)
The rats were anesthetized by intraperitoneal
injection of 50 mg/kg pentobarbital sodium (17). Then, 2 ml blood was collected from
the external carotid artery using a blood gas needle. Blood gas was
analyzed using an ABL 700 Radiometer (Radiometer Trading) blood gas
analyzer.
Detection of lung wet/dry weight (W/D)
ratio
After the rats were euthanized, the lung tissues
were removed, and water and blood were removed from the surface of
the lung tissues. The lung tissues were weighed using an electronic
balance (wet weight), wrapped in tin foil paper, dried in an oven
at 70°C for 72 h and weighed (dry weight). The W/D ratio of the
lung tissues was calculated.
Detection of the protein content in
the bronchial alveolar lavage fluid (BALF)
After the rats were euthanized, the lung tissues of
rats in each group were removed and subjected to lavage three times
repeatedly with phosphate-buffered saline at 4°C and 5 ml each time
with a total perfusion amount of 15 ml/rat and then BALF was
harvested. Following centrifugation (4°C; 2,500 × g; 10 min), the
supernatant was collected for the determination of the total
protein level using the bicinchoninic acid (BCA) kit (Thermo Fisher
Scientific, Inc.) (18).
Hematoxylin and eosin (H&E)
staining
The right lungs of rats were immersed in formalin at
25°C for 24 h, embedded in paraffin and sliced into 4-µm-thick
sections. The sections were then dewaxed in xylene at 25°C and
rehydrated with gradient ethanol. The sections were subsequently
stained with 100 µl hematoxylin solution (cat. no. PT001; Shanghai
Bogoo Biological Technology Co., Ltd.) for 10 min and eosin
solution (cat. no. G1424; Beijing Solarbio Science & Technology
Co., Ltd.) for 3 min, both at 25°C. The sections were observed and
photographed under a light microscope (magnification, ×200). The
lung injury score was assessed according to the American Thoracic
Society ALI pathological score system (19). Lung injury included the following
categories: Alveolar congestion, bleeding, infiltration or
aggregation of interstitial neutrophils or neutrophils in the
vessel wall, alveolar septum thickening, or hyaline membrane
formation. It was classified into four grades: 0, no or very slight
injury; 1, mild injury; 2, moderate injury; 3, severe injury; and
4, very severe injury. The total score of cumulative lesions was
used as the pathological score for ALI. A high score indicated
serious injury (19).
Preparation and grouping of the
macrophages
A total of three rats were randomly selected from
each group. The BALF was collected and centrifuged (20°C; 2,000 ×
g; 30 min) and the precipitate harvested. The cells were
resuspended in RMPI-1640 medium (Thermo Fisher Scientific, Inc.)
containing 10% fetal bovine serum (Sangon Biotech Co., Ltd.). The
cell suspension was placed in a glass culture dish and incubated
for 2 h (37°C; 5% CO2; 95% humidity). The cell
suspension was removed and the culture dish was repeatedly washed
with normal saline. The washing solution containing adherent cells
was collected and centrifuged at 4°C and 250 × g for 10 min to
obtain alveolar macrophages. The cell concentration was adjusted to
1×106 cells/ml using PBS for subsequent experiments
(20).
The macrophages extracted from normal, ALI and
ATRA-treated ALI rats were grouped into control, ALI and ATRA
groups, respectively. The TLR4 inhibitor IAXO-102 (10 µM)
(MedChemExpress) was added to the ALI and ATRA groups for
interference, with the addition of PBS as control, which were
recorded as ALI + PBS, ALI + IAXO-102, ATRA + PBS and ATRA +
IAXO-102 groups, respectively.
Detection of myeloperoxidase (MPO)
activity
Macrophages were re-suspended in a 4-fold volume of
MPO buffer and then centrifuged (4°C; 13,000 g; 10 min). The
supernatant was collected, transferred to a clean tube and placed
on ice. MPO activity was determined using the MPO activity assay
kit (cat. no. ab105136, Abcam). The optical density (OD) at 460 nm
was determined using a microplate reader (Bio-Rad Laboratories,
Inc.).
Detection of the phagocytic function
of the macrophages
The chicken erythrocyte phagocytosis method was
used. Briefly, the prepared macrophages were suspended in culture
medium and mixed well. Following the addition of Hanks solution,
the macrophages were evenly mixed with an appropriate amount of
chicken erythrocytes (Thermo Fisher Scientific, Inc.). The ratio of
macrophages to chicken erythrocytes was adjusted to approximately
1:600. The mixture was incubated at 37°C for 0.5 h after being
agitated and mixed every 10 min and then centrifuged (25°C; 1,000 ×
g; 10 min). Afterwards, the cell suspension was dropped onto a
slide for a cell suspension smear preparation. The slides were
fixed using acetone and methanol (1:1) at room temperature for 10
min and then Giemsa staining solution was added for an 8-min
incubation at room temperature. The number of macrophages
phagocytizing chicken erythrocytes in 100 macrophages was observed
under a light microscope (magnification, ×400). The phagocytic
rate=the number of macrophages phagocytizing chicken erythrocytes
in 100 macrophages/100 and the phagocytic index=the total number of
chicken erythrocytes phagocytized by 100 macrophages/100.
Flow cytometry was performed. Macrophages in each
group were seeded onto 12-well plates (5×104 cells/well)
and 1 µl fluorescein isothiocyanate (FITC)-labeled fluorescent
microspheres (4.55×107 microspheres with a diameter of 1
µm) was added to each well for 2-h at 37°C. Following removal of
the culture supernatant, macrophages were detached with 0.25%
trypsin, washed three times with PBS and fixed with 4%
paraformaldehyde at room temperature for 20 min. The mean
fluorescence intensity (MFI) of the macrophages and the percentage
of phagocytizing fluorescent microspheres (phagocytic fluorescent
microspheres) were measured using a flow cytometer (MoFlo Astrios
EQ; Beckman Coulter, Inc.). Higher MFI values and percentages of
phagocytic positive cells indicated a stronger phagocytic
ability.
Reverse transcription quantitative
(RT-q) PCR
Total RNA was extracted using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and reverse
transcribed into cDNA using the PrimeScript RT kit (cat. no.
RR037A, Takara Bio, Inc.). The reaction volume was 10 µl. The
reaction solution was then subjected to fluorescence qPCR using the
SYBR® Premix ExTaq II kit (cat. no. RR820A, Takara Bio,
Inc.) on a fluorescent qPCR instrument (ABI 7500; Applied
Biosystems; Thermo Fisher Scientific, Inc.). The following
thermocycling conditions were used for the qPCR: Initial
denaturation for 1 min at 95°C; followed by 40 cycles of 15 sec at
95°C, 30 sec at 55°C, and 35 sec at 72°C; followed by a final
extension for 30 sec at 95°C and 35 sec at 55°C (21). Relative expression of genes was
examined using the 2−ΔΔCq method (22), with GAPDH as the internal reference.
The experiments were repeated three times. The experiment was
repeated three times. The primers (Table I) were synthesized by Sangon Biotech
Co., Ltd.
 | Table I.Primer sequences used for reverse
transcription-quantitative PCR. |
Table I.
Primer sequences used for reverse
transcription-quantitative PCR.
| Gene | Primer sequence
(5′→3′) |
|---|
| Toll-like | F:
ACAAGGCATGGCATGGCTTACAC |
| receptor 4 | R:
TGTCTCCACAGCCACCAGATTCTC |
| CD14 | F:
ACTTCTCAGATCCGAAGCCAG |
|
| R:
CCGCCGTACAATTCCACAT |
| IκB | F:
GCTGAAGAAGGAGCGGCTACT |
|
| R:
TCGTACTCCTCGTCTTTCATGGA |
| P65 | F:
ACAACCCCTTCCAAGTTCCT |
|
| R:
ATCTTGAGCTCGGCAGTGTT |
| GAPDH | F:
GGGTGATGCTGGTGCTGAGTATGT |
|
| R:
AAGAATGGGAGTTGCTGTTGAAGT |
Cell Counting Kit-8 (CCK-8) assay
Macrophage cells (5,000 cells/well) in each group
were seeded onto 96-well plates. After 48 h of culture, cell
viability was measured according to the manufacturer's instructions
of the CCK-8 detection kit (R&D Systems, Inc.). The OD value at
450 nm of each well was detected.
TUNEL staining
The macrophages were rinsed with PBS for 5 min,
fixed in 4% paraformaldehyde at room temperature for 30 min and
washed with PBS three times (5 min/time). Next, the macrophages
were permeated with 0.1% Triton X-100 for 10 min, rinsed twice with
PBS (5 min/time) and then added to the TUNEL detection solution
(Sigma-Aldrich; Merck KGaA) for incubation (37°C; 60 min) in the
dark. After being washed with PBS for 5 min, the macrophages were
stained with 5 mg/l 4′,6-diamidino-2-phenylindole (DAPI) for 10 min
at room temperature and washed with PBS three times (5 min/time).
An anti-fade mounting medium was used for sealing. The images were
collected under a fluorescence inverted microscope (Olympus
Corporation). A total of five visual fields (magnification, ×200)
were randomly selected. The images were analyzed using Image-Pro
Plus 6.0 (Media Cybernetics, Inc.) and the apoptosis rate was
calculated.
Detection of apoptosis using flow
cytometry
Cell apoptosis was analyzed using an Annexin-V-FITC
cell apoptosis detection kit (BioVision Inc.), according to the
manufacturer's protocol. Annexin-V-FITC, propidium iodide (PI) and
HEPES buffer solution were prepared as the Annexin-V-FITC/PI
staining solution at the ratio of 1:2:50. Macrophages were detached
using trypsin, washed with PBS and stained with Annexin-V-FITC/PI
staining solution at room temperature for 15 min in the dark. The
apoptosis rate was detected using a flow cytometer (MoFlo Astrios
EQ, Beckman Coulter, Inc.). The lower left quadrant (Q4) on the
scatter plot showed healthy living cells; the upper left quadrant
(Q1) showed mechanically damaged cells; the lower right quadrant
(Q3) showed early apoptotic cells and the upper right quadrant (Q2)
showed necrotic and late apoptotic cells. The apoptotic rate=early
apoptosis percentage (Q3) + late apoptosis percentage (Q2).
Enzyme-linked immunosorbent assay
(ELISA)
Carotid artery blood was collected for the
assessment of IL-6 (cat. no. DY406), IL-1β (cat. no. MLB00C) and
macrophage inflammatory protein-2 (MIP-2; cat. no. 1084-M2) in
plasma using ELISA kits (R&D Systems, Inc.). The OD value at
450 nm was determined.
Immunofluorescence
The frozen tissues (−15°C) were sectioned (10-µm
thick) using a freezing microtome. The frozen sections were washed
with PBS and the membranes were permeated with 0.3% Triton X-100
(50 µl) by gavage at room temperature for 15 min. The antigen was
extracted using sodium citrate solution and the sections were
incubated with normal goat serum (Sangon Biotech Co., Ltd.). The
sections were cultured with the antibodies against TLR4 (cat. no.
ab13556, 1:500, Abcam) and CD14 (cat. no. ab183322, 1:100, Abcam)
at 4°C overnight. After 1 h of incubation at room temperature, the
sections were cultured with goat anti-rabbit antibody
immunoglobulin G (IgG) (cat. no. ab205718, 1:2,000, Abcam)
containing FITC for 1 h at room temperature. The nuclei were
stained with DAPI at room temperature for 5 min and the cells were
observed under a Biorevo BZ9000 fluorescence microscope (Keyence
Corporation; magnification, ×200) in five randomly selected fields
of view.
Western blot (WB analysis)
Total protein was extracted using RIPA lysis buffer
containing phenylmethanesulfonyl fluoride (Beyotime Institute of
Biotechnology). Protein concentration was determined using the BCA
protein quantitative kit (Wuhan Boster Biological Technology, Ltd.)
and 50 µg protein/lane was separated by 10% SDS-PAGE, transferred
to polyvinylidene fluoride membranes and blocked with 5% bovine
serum albumin (Sangon Biotech Co., Ltd.) at room temperature for 2
h to block nonspecific binding. The diluted primary antibodies
rabbit anti-mouse TLR4 (cat. no. ab217274, 1:300, Abcam), CD14
(cat. no. ab221678, 1:1,000, Abcam), P65 (cat. no. ab16502,
1:1,000, Abcam), p-P65 (cat. no. ab194726, 1:500, Abcam), IκBα
(cat. no. ab32518, 1:1,000, Abcam), p-IκBα (cat. no. ab133462,
1:1,000, Abcam) and β-actin (cat. no. ab8227, 1:2,000, Abcam) were
added for overnight incubation at 4°C. The membranes were washed
and then cultured with horseradish peroxidase-labeled goat
anti-rabbit IgG (cat. no. ab205718, 1:2,000, Abcam) for 1 h at room
temperature. An enhanced chemiluminescence working solution (EMD
Millipore) was used for membrane development. Image-Pro Plus 6.0
(Media Cybernetics, Inc.) was used to quantify the gray value of
bands in WB images. β-actin served as the internal control.
Statistical analysis
All data were analyzed using SPSS v21.0 (IBM Corp.).
First, the normality and homogeneity of variance tests verified
that the data were normally distributed and showed homogeneity of
variance. The measurement data were represented as mean ± standard
deviation. Comparison between two groups was performed using an
independent sample t-test. Comparisons among groups were analyzed
using one-way or two-way analysis of variance followed by Tukey's
multiple comparisons test. P<0.05 was considered to indicate a
statistically significant difference.
Results
ATRA exhibits protective effects on
LPS-induced ALI rats
The rat model of ALI was induced by LPS injection.
Compared with the ALI + saline group, there were no significant
differences in PaO2, BALF protein content and W/D ratio
in the ALI + olive group (Supplementary materials and methods;
Supplementary results; Fig. S1).
In addition, 5 mg/kg ATRA demonstrated an improved protective
effect on ALI rats following LPS injection (Supplementary materials
and methods; Fig. S2). Therefore,
5 mg/kg ATRA was used in the following experiment. The arterial
blood and BALF of ALI rats and control rats at 24 h after modeling
were collected to determine PaO2 concentration and
protein content. ALI rats showed significantly increased protein
content in BALF and decreased PaO2 concentration (both
P<0.01; Fig. 1A and B). The lung
W/D ratio was measured, and it was observed that the lung W/D ratio
was significantly increased in ALI rats (P<0.01; Fig. 1C). The above results indicated that
the ALI rat model was successfully induced.
 | Figure 1.ATRA possesses protective effects on
LPS-induced ALI rats. (A) Protein content in BALF detected using a
kit. (B) PaO2 in arterial blood detected using a blood
gas analyzer. (C) Lung W/D ratio. (D) Lung tissue injury observed
with hematoxylin and eosin staining. Magnification, ×200. (E) Lung
injury score assessed with high score indicative of severe injury.
N=10 in each group. A total of three independent experiments were
repeatedly conducted and the data are expressed as mean ± standard
deviation. Data analyzed using one-way analysis of variance,
followed by Tukey's multiple comparisons test. *P<0.05,
**P<0.01 vs. control group; #P<0.05,
##P<0.01 vs. ALI group. ATRA, all-trans retinoic
acid; LPS, lipopolysaccharide; ALI, acute lung injury; BALF,
bronchial alveolar lavage fluid; PaO2, arterial partial
pressure of oxygen; W/D, wet/dry weight. |
ATRA has a variety of biological functions,
including affecting the cell proliferation cycle, immune cell
functional molecule expression and various cytokine secretions
(23). However, the effect and
mechanism of ATRA in LPS-induced ALI rats require further study.
Therefore, LPS-induced ALI rats were treated with ATRA. ATRA
treatment markedly increased PaO2 concentration and
reduced protein content and lung W/D ratio (all P<0.05; Fig. 1A-C). Additionally, the pathological
changes in lung tissues were measured using H&E staining to
assess the tissue injury. The results revealed that ALI rats
exhibited abundant inflammatory cells in the alveolar cavity of
lung tissues, massive exudate and clearly dilated and congested
pulmonary interstitial capillaries (P<0.01), while ATRA
treatment effectively reversed all these trends (P<0.01;
Fig. 1D). The lung injury score of
rats in the ATRA group was significantly reduced (Fig. 1E). Thus, ATRA possessed protective
effects in LPS-induced ALI rats.
ATRA enhances macrophage phagocytosis
in ALI rats
A previous study hypothesized that the phagocytic
function of macrophages is crucial for maintaining tissue
homeostasis and is closely associated with inflammation and immune
response (24). Therefore, it was
important to explore the function of alveolar macrophages in ALI.
BALF was collected and alveolar macrophages were isolated and
purified. MPO activity of alveolar macrophages was measured using
an MPO kit. As demonstrated by the results, macrophages in the ALI
group showed noticeably increased MPO activity compared with the
control cells, which then decreased after ATRA treatment (both
P<0.01; Fig. 2A). Next, the
phagocytic rate and phagocytic index of macrophages were detected
using the chicken erythrocyte phagocytosis method. It was observed
that the phagocytic rate and phagocytic index were notably
decreased in ALI-induced macrophages and ATRA treatment effectively
enhanced the phagocytic rate and index of alveolar macrophages (all
P<0.01; Fig. 2B and C). Flow
cytometry was used to detect phagocytosis of fluorescent
microspheres by macrophages. It was found that macrophages in the
ALI group exhibited clearly reduced MFI values and percentage of
phagocytic positive cells and ATRA treatment partially reversed
these trends (all P<0.01; Fig. 2D
and E). Briefly, it was demonstrated that ATRA enhanced the
phagocytic function of macrophages in ALI rats.
ATRA increases viability and inhibits
the apoptosis of macrophages and inflammation in ALI rats
The effects of ATRA on the viability and apoptosis
of macrophages in ALI rats were assessed. CCK-8 assay revealed that
ATRA treatment markedly increased the viability of macrophages (all
P<0.01; Fig. 3A). According to
TUNEL and flow cytometry, macrophages in the ALI group showed
markedly elevated apoptosis, which was then notably reduced after
ATRA treatment (all P<0.01; Fig. 3B
and C).
Macrophage activation is closely associated with
inflammatory responses (25).
Hence, levels of inflammatory cytokines (IL-6, IL-1β and MIP-2) in
the plasma of rats in each group were detected using ELISA kits.
ATRA-treated ALI rats exhibited noticeably reduced levels of
inflammatory cytokines (all P<0.01; Fig. 3D-F). These results revealed that
ATRA increased the viability and inhibited apoptosis of macrophages
and decreased inflammatory cytokine levels in ALI rats.
ATRA inhibits CD14 and TLR4
upregulation and downstream NF-κB pathway activation in LPS-induced
ALI rat macrophages
CD14 and TLR4 are important receptors in the LPS
signal transduction pathway (26).
Therefore, CD14 and TLR4 expression levels on the macrophage
membrane were detected using immunofluorescence and CD14 and TLR4
protein levels were detected using WB. CD14 and TLR4 expression
levels were clearly upregulated in macrophages in the ALI group and
notably decreased following ATRA treatment (Fig. 4A). RT-qPCR and WB results further
confirmed the above results (all P<0.01; Fig. 4C and D). Briefly, it was
demonstrated that ATRA treatment inhibited CD14 and TLR4
expressions in macrophages. Furthermore, according to the KEGG
database, there are many pathways downstream of CD14/TLR4 receptors
(Fig. 4B), among which the NF-κB
pathway serves a key role in ALI (27,28).
Therefore, the mRNA and proteins of the NF-κB pathway were detected
using RT-qPCR and WB. The mRNA expression of P65 and IκBα was
increased in macrophages in the ALI group and the protein ratios of
p-P65/P65 and p-IκBα/IκBα were also clearly elevated, while ATRA
treatment effectively reduced these mRNA expressions and protein
ratios (all P<0.01; Fig. 4C).
Taken together, ATRA inhibited CD14/TLR4 expression and NF-κB
pathway activation in macrophages in LPS-induced ALI rats.
 | Figure 4.ATRA inhibits CD14/TLR4 upregulation
and the downstream NF-κB pathway activation in macrophages in
LPS-induced ALI rats. (A) CD14 and TLR4 expressions on macrophage
membrane are detected using immunofluorescence. (B) Key pathways in
LPS signal transduction through the Kyoto Encyclopedia of Genes and
Genomes database (https://www.kegg.jp/). (C) mRNA expressions of TLR4,
CD14, P65 and IκBα are detected using reverse transcription
quantitative PCR. (D) Protein levels of TLR4, CD14, p-P65, P65,
p-IκBα and IκBα are detected using western blotting. The cell
experiments were repeated three times independently. Data are
expressed as mean ± standard deviation and analyzed using one-way
or two-way analysis of variance, followed by Tukey's multiple
comparisons test. *P<0.05, **P<0.01 vs. control group;
#P<0.05, ##P<0.01 vs. ALI group. ATRA,
all-trans retinoic acid; CD, cluster of differentiation; TLR,
Toll-like receptor; LPS, lipopolysaccharide; ALI, acute lung
injury; p-, phosphorylated. |
ATRA enhances macrophage phagocytosis
and reduces inflammation via inhibiting CD14/TLR4 in ALI rats
To clarify the specific regulatory mechanism of ATRA
in macrophage phagocytosis in ALI rats, the ALI group (ALI +
IAXO-102) and ATRA group (ATRA + IAXO-102) were treated with the
TLR4 inhibitor IAXO-102, with the addition of PBS as the control.
TLR4 expression, and mRNA expression and protein levels of the
NF-κB pathway were detected using RT-qPCR and WB. The results
revealed that TLR4 expression, mRNA expression of P65 and IκBα, and
protein levels of p-P65/P65 and p-IκBα/IκBα were notably decreased
after the addition of TLR4 inhibitor IAXO-102 (all P<0.01;
Fig. 5A and B). Compared with those
in the ALI + PBS group, macrophages in the ALI + IAXO-102 group and
the ATRA + PBS group showed clearly decreased MPO activity, and
macrophages in the ATRA + IAXO-102 group exhibited the most reduced
MPO activity (all P<0.01; Fig.
5C). According to the flow cytometry results, the MFI value and
percentage of phagocytic positive cells were markedly higher in the
ALI + IAXO-102 group and the ATRA + PBS group compared with the ALI
+ PBS group and the ATRA + IAXO-102 group showed the highest MFI
value and percentage of phagocytic positive cells (all P<0.01;
Fig. 5D and E). ELISA results
revealed that the ALI + IAXO-102 group and ATRA + PBS group
presented markedly reduced levels of inflammatory cytokines in
plasma and the ATRA + IAXO-102 group exhibited the lowest levels of
inflammatory cytokines (all P<0.01; Fig. 5F). Meanwhile, no notable difference
in the indicators was found between the ALI + IAXO-102 group and
the ATRA + PBS group (P>0.05). In summary, ATRA enhanced
macrophage phagocytosis and reduced inflammatory cytokine levels by
inhibiting CD14/TLR4 in LPS-induced ALI rats.
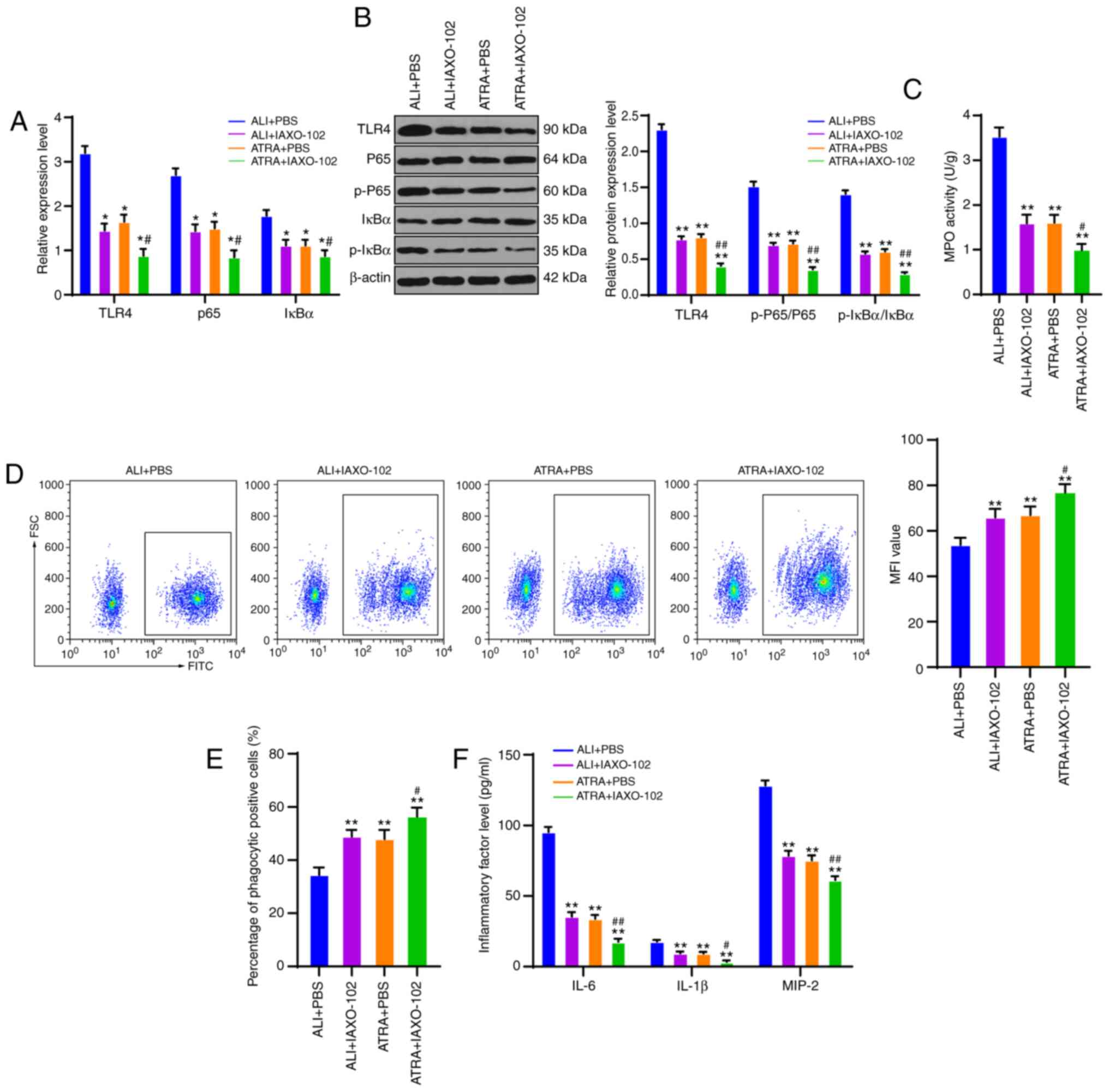 | Figure 5.ATRA enhances macrophage phagocytosis
and reduces inflammatory cytokine levels via inhibiting CD14/TLR4
expressions in LPS-induced ALI rats. (A) mRNA expressions of TLR4,
CD14, P65 and IκBα are detected using reverse transcription
quantitative PCR. (B) Western blotting was used to detect protein
levels of TLR4, p-P65, P65, p-IκBα and IκBα. (C) MPO activity in
macrophages was measured using a kit. (D) MFI value of macrophages
was measured using flow cytometry. (E) Percentage of macrophage
phagocytic positive cells was determined using flow cytometry. (F)
Levels of inflammatory cytokines (IL-6, IL-1β and MIP-2) in rat
plasma were detected using ELISA kits. The cell experiments were
repeated three times independently. Data were expressed as mean ±
standard deviation and analyzed using one-way or two-way analysis
of variance, followed by Tukey's multiple comparisons test.
*P<0.05, **P<0.01 vs. the ALI + PBS group;
#P<0.05, ##P<0.01 vs. the ATRA +
IAXO-102 group. ATRA, all-trans retinoic acid; CD, cluster of
differentiation; TLR, Toll-like receptor; LPS, lipopolysaccharide;
ALI, acute lung injury; p-, phosphorylated; MPO, myeloperoxidase;
MFI, mean fluorescence intensity; MIP-2, macrophage inflammatory
protein-2. |
Discussion
ALI is a serious heterogeneous lung disease often
accompanied by lung inflammation and alveolar macrophages have been
demonstrated to participate in lung inflammation owing to their
phagocytosis (4,29). ATRA has been demonstrated to serve a
potential regulatory role in multiple cellular processes such as
cell activity and inflammation (30,31).
The present study demonstrated that ATRA promoted macrophage
phagocytosis and reduced inflammation in LPS-induced ALI rats by
inhibiting the CD14/TLR4-NF-κB pathway.
ATRA is a carboxyl form of vitamin A (32) and exerts protective effects against
liver and kidney injury (33,34).
In addition, ATRA is closely associated with lung diseases
(35,36). However, little is known about the
specific role of ATRA in ALI. It has been demonstrated that
biomarkers in the plasma and BALF can be used to evaluate ALI
severity (37). Decreased lung W/D
ratio and protein content in BALF, together with increased
PaO2, are indicators of attenuated ALI (38,39).
According to the findings of the present study, ATRA markedly
increased PaO2 in arterial blood and reduced the protein
content in BALF and lung W/D ratio in ALI rats, along with
effectively alleviating pathological lung injury. In accordance
with this, a previous study demonstrated that RA possesses
beneficial effects on injured lung tissues following ALI (40). ATRA may help to alleviate
transfusion-related ALI (41).
According to previous studies (32,42–44),
olive oil is usually selected as the control of ATRA treatment.
Accordingly, the present study used olive oil as the control of
ATRA treatment. Meanwhile, preliminary experiments were conducted
to exclude the possible influence of olive oil treatment on this
experiment (Fig. S2). The results
of the present study demonstrated that ATRA had protective effects
on LPS-induced ALI rats.
It has been demonstrated that macrophages are
closely implicated in ALI pathogenesis as a crucial organizer
(45). Alveolar macrophages
alleviate ALI, which may be attributed to their phagocytic
functions (29). In the present
study, BALF was collected and alveolar macrophages were isolated
and purified. It was found that ALI rats showed notably decreased
macrophage phagocytosis, which was then clearly enhanced following
ATRA treatment. ATRA efficiently improves the phagocytic function
of macrophages (46). The effects
of ATRA on macrophage viability and apoptosis in ALI rats were
further assessed. It was shown that ATRA treatment markedly
increased macrophage viability and reduced apoptosis. In agreement
with these findings, alveolar macrophages exhibit reduced cell
viability and augmented apoptosis rate in LPS-induced ALI (47). Furthermore, macrophage activity is
closely associated with the inflammatory response in ALI (48). Hence, levels of inflammatory
cytokines (IL-6, IL-1β and MIP-2) were detected in the plasma of
ALI rats and were observed to be noticeably reduced in ATRA-treated
ALI rats. A previous study identified that RA helps to reduce
levels of proinflammatory cytokines, thereby attenuating lung
dysfunction (49). Taken together,
the present study confirmed that ATRA increased phagocytosis and
promoted the viability and inhibited apoptosis of macrophages as
well as decreased inflammation in ALI rats.
Subsequently, the underlying mechanism of ATRA in
ALI was explored. ALI may develop as a consequence of LPS binding
to its receptors, such as CD14 and TLR4 (50). TLR4 and CD14 serve crucial roles in
responding to inflammatory stimuli in macrophages (51,52).
Based on the results of the present study, CD14 and TLR4 expression
were clearly upregulated in ALI rat macrophages and notably
decreased following ATRA treatment. A previous study showed that
CD14 and TLR4 expression is increased in LPS-induced ALI (53). RA receptor agonists help to inhibit
CD14 and TLR4 signaling and then suppress the NF-κB pathway
(44). It has been demonstrated
that TLR4 mediates the NF-κB pathway in macrophages and they serve
a crucial role in ALI (27,28,52).
The present study confirmed that the NF-κB pathway was notably
activated in ALI and ATRA treatment effectively inhibited NF-κB
pathway activation. A previous study demonstrated that ATRA
administration suppresses the TLR4/NF-κB inflammatory pathway in
diabetic nephropathy (54). In
addition, according to the functional rescue experiment results of
the present study, inhibition of TLR4 markedly enhanced the
efficacy of ATRA on ALI, as manifested by notably increased
macrophage phagocytosis and decreased inflammation in ALI rats.
Similarly, TLR4 downregulation in the lung is closely associated
with suppressed inflammation and lung injury (55). Suppression of TLR4 helps RA to
attenuate the LPS-induced inflammatory response (56). Taken together, ATRA inhibited
CD14/TLR4 expression and the downstream NF-κB pathway activation in
macrophages, thereby enhancing macrophage phagocytosis and
decreasing inflammation in ALI.
Overall, the present study found that ATRA promoted
macrophage phagocytosis and decreased inflammation via inhibition
of CD14/TLR4 expression and NF-κB pathway activation in LPS-induced
ALI. ATRA could affect macrophage function, and it exerted an
effect on the expression of CD14/TLR4 and alleviated the process of
ALI. These results revealed a novel ATRA-based therapy for ALI
patients. Although the present study provides therapeutic value for
ALI treatment, the experimental results and clinical application
need further validation.
Supplementary Material
Supporting Data
Acknowledgments
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SL made substantial contributions to the conception
of the present study. YL and JL performed the experiments and wrote
the manuscript. HL contributed to the design of the present study
and interpreted the data. SL and HL confirm the authenticity of all
the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All animal procedures were performed in accordance
with the guidelines of the Animal Ethics Committee of Shanxi
Provincial People's Hospital and ethics approval was received
(approval no. 2019-56). Significant efforts were made to minimize
animal numbers and their suffering.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Elicker BM, Jones KT, Naeger DM and Frank
JA: Imaging of acute lung injury. Radiol Clin North Am.
54:1119–1132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fröhlich S, Boylan J and McLoughlin P:
Hypoxia-induced inflammation in the lung: A potential therapeutic
target in acute lung injury? Am J Respir Cell Mol Biol. 48:271–279.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gouda MM and Bhandary YP: Acute lung
injury: IL-17A-mediated inflammatory pathway and its regulation by
curcumin. Inflammation. 42:1160–1169. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mokra D, Mikolka P, Kosutova P and Mokry
J: Corticosteroids in acute lung injury: The dilemma continues. Int
J Mol Sci. 20:47652019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Perl M, Lomas-Neira J, Venet F, Chung CS
and Ayala A: Pathogenesis of indirect (secondary) acute lung
injury. Expert Rev Respir Med. 5:115–126. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Barbalho SM, de Goulart RA and Batista G:
Vitamin A and inflammatory bowel diseases: From cellular studies
and animal models to human disease. Expert Rev Gastroenterol
Hepatol. 13:25–35. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen F, Cao Y, Qian J, Shao F,
Niederreither K and Cardoso WV: A retinoic acid-dependent network
in the foregut controls formation of the mouse lung primordium. J
Clin Invest. 120:2040–2048. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nagpal I and Wei LN: All-trans retinoic
acid as a versatile cytosolic signal modulator mediated by CRABP1.
Int J Mol Sci. 20:36102019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sierra-Mondragon E, Rodriguez-Munoz R,
Namorado-Tonix C, Molina-Jijon E, Romero-Trejo D, Pedraza-Chaverri
J and Reyes JL: All-trans retinoic acid attenuates fibrotic
processes by downregulating TGF-β1/Smad3 in early diabetic
nephropathy. Biomolecules. 92:5252019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu D, Cai SY, Mennone A, Vig P and Boyer
JL: Cenicriviroc, a cytokine receptor antagonist, potentiates
all-trans retinoic acid in reducing liver injury in cholestatic
rodents. Liver Int. 38:1128–1138. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Uniyal S, Dhasmana A, Tyagi A and Muyal
JP: ATRA reduces inflammation and improves alveolar epithelium
regeneration in emphysematous rat lung. Biomed Pharmacother.
108:1435–1450. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kawasaki T, Ito K, Miyata H, Akira S and
Kawai T: Deletion of PIKfyve alters alveolar macrophage populations
and exacerbates allergic inflammation in mice. EMBO J.
36:1707–1718. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang P, Wei S, Huang W, Wu P, Chen S, Tao
A, Wang H, Liang Z, Chen R, Yan J and Zhang Q: Hydrogen gas
inhalation enhances alveolar macrophage phagocytosis in an
ovalbumin-induced asthma model. Int Immunopharmacol. 74:1056462019.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Moser EK, Field NS and Oliver PM: Aberrant
Th2 inflammation drives dysfunction of alveolar macrophages and
susceptibility to bacterial pneumonia. Cell Mol Immunol.
15:480–492. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bonhomme D, Santecchia I, Vernel-Pauillac
F, Caroff M, Germon P, Murray G, Adler B, Boneca IV and Werts C:
Leptospiral LPS escapes mouse TLR4 internalization and
TRIFassociated antimicrobial responses through O antigen and
associated lipoproteins. PLoS Pathog. 16:e10086392020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu X, Kong Q, Zhan L, Qiu Z, Huang Q and
Song X: TIPE2 ameliorates lipopolysaccharide-induced apoptosis and
inflammation in acute lung injury. Inflamm Res. 68:981–992. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Intengan HD and Smyth DD: Renal alpha
2a/d-adrenoceptor subtype function: Wistar as compared to
spontaneously hypertensive rats. Br J Pharmacol. 121:861–866. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun A, Wang W, Ye X, Wang Y, Yang X, Ye Z,
Sun X and Zhang C: Protective effects of methane-rich saline on
rats with lipopolysaccharide-induced acute lung injury. Oxid Med
Cell Longev. 2017:74301932017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xia H, Ge Y, Wang F, Ming Y, Wu Z, Wang J,
Sun S, Huang S, Chen M, Xiao W and Yao S: Protectin DX ameliorates
inflammation in sepsis-induced acute lung injury through mediating
PPARγ/NF-κB pathway. Immunol Res. 68:280–288. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li Q, Ran Q, Sun L, Yin J, Luo T, Liu L,
Zhao Z, Yang Q, Li Y, Chen Y, et al: Lian Hua Qing Wen Capsules, a
potent epithelial protector in acute lung injury model, block
proapoptotic communication between macrophages, and alveolar
epithelial cells. Front Pharmacol. 11:5227292020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yi R, Wei Y, Tan F, Mu J, Long X, Pan Y,
Liu W and Zhao X: Antioxidant capacity-related preventive effects
of shoumei (Slightly Fermented Camellia sinensis) polyphenols
against hepatic injury. Oxid Med Cell Longev. 2020:93293562020.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li B, Gao MH, Lv CY, Yang P and Yin QF:
Study of the synergistic effects of all-transretinoic acid and
C-phycocyanin on the growth and apoptosis of A549 cells. Eur J
Cancer Prev. 25:97–101. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Miao J, Ye S, Lan J, Ye P, Wen Q, Mei L,
Liu X, Lin J, Zhou X, Du S, et al: Nuclear HMGB1 promotes the
phagocytic ability of macrophages. Exp Cell Res. 393:1120372020.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang C, Petriello MC, Zhu B and Hennig B:
PCB 126 induces monocyte/macrophage polarization and inflammation
through AhR and NF-κB pathways. Toxicol Appl Pharmacol. 367:71–81.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Leláková V, Beraud-Dufour S, Hošek J,
Šmejkal K, Prachyawarakorn V, Pailee P, Widmann C, Václavík J,
Coppola T, Mazella J, et al: Therapeutic potential of prenylated
stilbenoid macasiamenene F through its anti-inflammatory and
cytoprotective effects on LPS-challenged monocytes and microglia. J
Ethnopharmacol. 263:1131472020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu TY, Zhao LL, Chen SB, Hou BC, Huang J,
Hong X, Qing L, Fang Y and Tao Z: Polygonatum sibiricum
polysaccharides prevent LPS-induced acute lung injury by inhibiting
inflammation via the TLR4/Myd88/NF-κB pathway. Exp Ther Med.
20:3733–3739. 2020.PubMed/NCBI
|
|
28
|
Zhang Y, Zhu Y, Gao G and Zhou Z:
Knockdown XIST alleviates LPS-induced WI-38 cell apoptosis and
inflammation injury via targeting miR-370-3p/TLR4 in acute
pneumonia. Cell Biochem Funct. 37:348–358. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mohning MP, Thomas SM, Barthel L, Mould
KJ, McCubbrey AL, Frasch SC, Bratton DL, Henson PM and Janssen WJ:
Phagocytosis of microparticles by alveolar macrophages during acute
lung injury requires MerTK. Am J Physiol Lung Cell Mol Physiol.
314:L69–L82. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu ZM, Wang KP, Ma J and Zheng SG: The
role of all-trans retinoic acid in the biology of Foxp3+ regulatory
T cells. Cell Mol Immunol. 12:553–557. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhou TB, Ou C, Jiang ZP, Xiong MR and
Zhang F: Potential signal pathway between all-trans retinoic acid
and LMX1B in hypoxia-induced renal tubular epithelial cell injury.
J Recept Signal Transduct Res. 36:53–56. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Amengual J, Ribot J, Bonet ML and Palou A:
Retinoic acid treatment increases lipid oxidation capacity in
skeletal muscle of mice. Obesity (Silver Spring). 16:585–591. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Elshal M, Abu-Elsaad N, El-Karef A and
Ibrahim T: Retinoic acid modulates IL-4, IL-10 and MCP-1 pathways
in immune mediated hepatitis and interrupts CD4+ T cells
infiltration. Int Immunopharmacol. 75:1058082019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu J, Wan X, Zhang H, Li W, Ma M, Pan B,
Liang X and Cao C: Retinoic acid attenuates contrast-induced acute
kidney injury in a miniature pig model. Biochem Biophys Res Commun.
512:163–169. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Leem AY, Shin MH, Douglas IS, Song JH,
Chung KS, Kim EY, Jung JY, Kang YA, Chang J, Kim YS and Park MS:
All-trans retinoic acid attenuates bleomycin-induced pulmonary
fibrosis via downregulating EphA2-EphrinA1 signaling. Biochem
Biophys Res Commun. 491:721–726. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang Y, Zhao J, Sun J, Huang L and Li Q:
Targeting lung cancer initiating cells by all-trans retinoic
acid-loaded lipid-PLGA nanoparticles with CD133 aptamers. Exp Ther
Med. 16:4639–4649. 2018.PubMed/NCBI
|
|
37
|
Mokra D and Kosutova P: Biomarkers in
acute lung injury. Respir Physiol Neurobiol. 209:52–58. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu W, Liu K, Zhang S, Shan L and Tang J:
Tetramethylpyrazine showed therapeutic effects on sepsis-induced
acute lung injury in rats by inhibiting endoplasmic reticulum
stress protein kinase RNA-Like endoplasmic reticulum kinase (PERK)
signaling-induced apoptosis of pulmonary microvascular endothelial
cells. Med Sci Monit. 24:1225–1231. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhou H, Li F, Niu JY, Zhong WY, Tang MY,
Lin D, Cui HH, Huang XH, Chen YY, Wang HY and Tu YS: Ferroptosis
was involved in the oleic acid-induced acute lung injury in mice.
Sheng Li Xue Bao. 71:689–697. 2019.PubMed/NCBI
|
|
40
|
Yang C, Yang X, Du J, Wang H, Li H, Zeng
L, Gu W and Jiang J: Retinoic acid promotes the endogenous repair
of lung stem/progenitor cells in combined with simvastatin after
acute lung injury: A stereological analysis. Respir Res.
16:1402015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jeddi R, Mansouri R, Kacem K, Gouider E,
Abid HB, Belhadjali Z and Meddeb B: Transfusion-related acute lung
injury (TRALI) during remission induction course of acute myeloid
leukemia: A possible role for all-transretinoic-acid (ATRA)? Pathol
Biol (Paris). 57:500–502. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Uniyal S, Tyagi AK and Muyal JP: All trans
retinoic acid (ATRA) progresses alveolar epithelium regeneration by
involving diverse signalling pathways in emphysematous rat. Biomed
Pharmacother. 131:1107252020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Seifart C, Muyal JP, Plagens A, Yildirim
AÖ, Kohse K, Grau V, Sandu S, Reinke C, Tschernig T, Vogelmeier C
and Fehrenbach H: All-trans retinoic acid results in irregular
repair of septa and fails to inhibit proinflammatory macrophages.
Eur Respir J. 38:425–439. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ramirez-Moreno A, Escorza MA, Garza RG,
Hady K, Valenzuela AM, Marszalek JE, Sharara-Núñez I and
Delgadillo-Guzmán D: All-trans retinoic acid improves pancreatic
cell proliferation on induced type 1 diabetic rats. Fundam Clin
Pharmacol. 34:345–351. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chen X, Tang J, Shuai W, Meng J, Feng J
and Han Z: Macrophage polarization and its role in the pathogenesis
of acute lung injury/acute respiratory distress syndrome. Inflamm
Res. 69:883–895. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lo HM, Wang SW, Chen CL, Wu PH and Wu WB:
Effects of all-trans retinoic acid, retinol, and beta-carotene on
murine macrophage activity. Food Funct. 5:140–148. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang Z, Zhang Y and Zhou R: Loss of
annexin A5 expression attenuates the lipopolysaccharide-induced
inflammatory response of rat alveolar macrophages. Cell Biol Int.
44:391–401. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Huang X, Xiu H, Zhang S and Zhang G: The
role of macrophages in the pathogenesis of ALI/ARDS. Mediators
Inflamm. 2018:12649132018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
James ML, Ross AC, Nicola T, Steele C and
Ambalavanan N: VARA attenuates hyperoxia-induced impaired alveolar
development and lung function in newborn mice. Am J Physiol Lung
Cell Mol Physiol. 304:L803–L812. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Jeyaseelan S, Chu HW, Young SK, Freeman MW
and Worthen GS: Distinct roles of pattern recognition receptors
CD14 and toll-like receptor 4 in acute lung injury. Infect Immun.
73:1754–1763. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chen Z, Ding X, Jin S, Pitt B, Zhang L,
Billiar T and Li Q: WISP1-αvβ3 integrin signaling positively
regulates TLR-triggered inflammation response in sepsis induced
lung injury. Sci Rep. 6:288412016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ren W, Wang Z, Hua F and Zhu L:
Plasminogen activator inhibitor-1 regulates LPS-induced TLR4/MD-2
pathway activation and inflammation in alveolar macrophages.
Inflammation. 38:384–393. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ma L, Wu XY, Zhang LH, Chen WM, Uchiyama
A, Mashimo T and Fujino Y: Propofol exerts anti-inflammatory
effects in rats with lipopolysaccharide-induced acute lung injury
by inhibition of CD14 and TLR4 expression. Braz J Med Biol Res.
46:299–305. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Sierra-Mondragon E, Molina-Jijon E,
Namorado-Tonix C, Rodriguez-Munoz R, Pedraza-Chaverri J and Reyes
JL: All-trans retinoic acid ameliorates inflammatory response
mediated by TLR4/NF-κB during initiation of diabetic nephropathy. J
Nutr Biochem. 60:47–60. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Tang SE, Wu SY, Chu SJ, Tzeng YS, Peng CK,
Lan CC, Perng WC, Wu CP and Huang KL: Pre-treatment with ten-minute
carbon dioxide inhalation prevents lipopolysaccharide-induced lung
injury in mice via down-regulation of toll-like receptor 4
expression. Int J Mol Sci. 20:62932019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Gu B, Miao J, Fa Y, Lu J and Zou S:
Retinoic acid attenuates lipopolysaccharide-induced inflammatory
responses by suppressing TLR4/NF-kappaB expression in rat mammary
tissue. Int Immunopharmacol. 10:799–805. 2010. View Article : Google Scholar : PubMed/NCBI
|



















