Introduction
As an effective herbicide, paraquat (PQ,
1,1-dimethyl-4,4-bipyridinium) is a highly toxic pro-oxidant that
is widely used worldwide (1). To
date, human PQ intoxication due to accidental exposure or suicide
intention has been observed (2).
PQ poisoning induces multi-organ failure involving lung,
gastrointestinal tract, pancreas, kidney, liver, heart and brain
injury (3). Pulmonary fibrosis is
the most typical feature of PQ poisoning and continues for several
days to weeks after PQ ingestion (4). Although the underlying mechanism of
PQ-induced pulmonary fibrosis remains unclear, inflammation,
oxidative stress, epithelial-to-mesenchymal transition (EMT) and
fibrogenic pathways, such as transforming growth factor
(TGF)-β/SMAD and PI3K/Akt/mTOR signaling pathways can be involved
in the pathogenesis of pulmonary fibrosis induced by PQ (1,5).
TGF-β1 is considered a ‘master switch’ in the fibrosis process. Kan
et al (6) observed that TGF-β1
expression was elevated in the serum and lung tissues of rats
exposed to PQ. Han et al (7)
demonstrated that the TGF-β/SMAD pathway was an important process
in the development of PQ-induced pulmonary fibrosis. Recent studies
have focused on the TGF-β/SMAD pathway as an important target for
drugs such as doxycycline and tacrolimus to attenuate PQ-induced
pulmonary fibrosis (8,9).
TGF-β1 has been reported to be the key growth factor
that initiates tissue repair, and its sustained production is
involved in the development of tissue fibrosis (7). TGF-β1 activates the downstream
transcription factor SMAD and triggers the intracellular signaling
pathway (10). An early study by
Sato et al (11) showed that
targeted disruption of TGF-β1/SMAD3 signaling protected against
renal tubulointerstitial fibrosis induced by unilateral ureteral
obstruction in mice lacking SMAD3 (SMAD3 ex8/ex8). Several other
studies using different models of kidney disease further confirmed
the central role of the SMAD3 pathway in the pathogenesis of
interstitial fibrosis (12,13). The peroxisome proliferator
activated receptor γ (PPARγ) is well-known for its ability to
regulate glucose and lipid metabolism. The interaction between
TGF-β1 and PPARγ is involved in the development of fibrosis. TGF-β1
controls PPARγ expression, transcriptional potential and activity
partly through SMAD3 signaling in murine lung fibroblasts (14). PPARγ is an inhibitory regulator of
the TGF-β1/SMAD pathway, while TGF-β1 induces fibrosis-related
genes that suppress PPARγ. PPARγ is an important regulator of
TGF-β1-associated diseases, such as pulmonary arterial
hypertension, parenchymal lung diseases and Marfan's syndrome
(15).
5-Aminosalicylic acid (5-ASA) is an
anti-inflammatory agent. Over the past decades, 5-ASA preparations
have been specific and first-line therapeutic drugs for mild to
moderate active inflammatory bowel disease (IBD) (16). As a PPAR-γ agonist, 5-ASA is a
widely used first-line medication for the treatment of ulcerative
colitis (17). Activation of
PPAR-γ signaling plays an important role in alleviating the effects
of 5-ASA on colitis (18). Tissue
damage and inflammation are important triggers for regeneration and
fibrosis. PPAR-γ has been demonstrated not only to be able to
downregulate pro-inflammatory cytokine production, such as
interleukin (IL)-4, −5 and −6, but also to interfere with
profibrotic molecules, including platelet-derived growth factor,
IL-1 and TGF-β, the main promoters of fibrosis (19). Moreover, 5-ASA was found to reduce
TGF-β signaling, as indicated by the reduction in TGF-β-specific
reporter gene activity (20). The
intestinal anti-inflammatory effect of 5-ASA is dependent on PPARγ
(18,21), and its anti-neoplastic effect in
the intestine is also mediated by PPARγ (20). Thus, it is clear that PPARγ is the
key mediator of the anti-inflammatory and antineoplastic effects of
5-ASA. As PPARγ is also involved in the development of fibrotic
diseases, there is interest in determining whether 5-ASA could play
a role in the interaction of PPARγ and the TGF-β1/SMAD3 pathway in
the pathogenesis of pulmonary fibrosis, and whether it could be
used as a potential drug for PQ poisoning. As pulmonary fibrosis is
the most typical feature of PQ poisoning, whether the agonist of
PPAR-γ, 5-ASA, may be used as a potential drug for alleviating
pulmonary fibrosis induced by PQ is worthy of investigation.
To date, to the best of our knowledge, there have
been no reports on the clinical use of 5-ASA in the treatment of
human PQ poisoning; however, experimental studies in animals have
been performed. Wang et al (22)
found that 5-ASA could attenuate PQ-induced acute renal injury
damage by activating the Nrf2-antioxidant response element
signaling pathway. A recent study by Ramadan et al (23) revealed that mesalazine, with 5-ASA
as its main ingredient, had potential as a novel anti-fibrotic
agent by reducing oxidative damage and altering the TNF-α pathway
as an anti-inflammatory drug, which was demonstrated by its ability
to downregulate TGF-β1, osteopontin, α-SMA and caspase-3 signaling
pathways in liver fibrosis in rats (23). A previous study by Hoffmann et al
(24) also provided experimental
pre-clinical evidence for the antifibrotic effects of mesalazine in
an in vitro model of cardiac fibrosis.
Therefore, the aim of the present study was to
explore the effects of 5-ASA on pulmonary fibrosis progression in a
PQ intoxication rat model. It was first investigated whether 5-ASA
exerted protective effects against PQ-induced pulmonary fibrosis.
Following which, the putative mechanism of action of 5-ASA in
preventing PQ-induced pulmonary fibrosis was explored. This study
provided insights for the treatment of PQ poisoning.
Materials and methods
Reagents
PQ (33.5%) was purchased from Syngenta Nantong Crop
Protection Co., Ltd. 5-ASA (98.5%, chemical purity) was purchased
from J&K Scientific Ltd. The primary antibodies used were as
follows: Rabbit anti-human PPARγ (cat. no. P37231; Bioworld
Technology, Inc.), rabbit anti-human TGF-β1 antibodies (cat. no.
P01137; Bioworld Technology, Inc.), rabbit anti-human SMAD3 (cat.
no. AF6362; Affinity Biosciences, Ltd.), rabbit anti-human
phosphorylated (p)-SMAD3 antibody (cat. no. AF3362; Affinity
Biosciences, Ltd.) and mouse anti-human β-actin monoclonal antibody
(cat. no. sc-47778; Santa Cruz Biotechnology, Inc.).
Animals
In this study, 100 healthy male Wistar rats aged 6–8
weeks and weighing 180–220 g were purchased from the Animal Center
of Hebei Medical University (Shijiazhuang, China). The animals were
placed in a ventilated room at 22±2°C with a 12 h light/dark cycle,
with ad libitum access to food and water. All animal experiments
conformed to the guidelines of the Ethics Committee for Laboratory
Animals of Hebei Medical University. All experiments were performed
in compliance with the Guide for the Care and Use of Laboratory
Animals of the National Institutes of Health (25) and were reviewed and approved by
the Ethics Committee for the Use of Experimental Animals at Hebei
Medical University (approval no. 1608303).
Animal models and tissue sampling
The Wistar rats were randomly divided into four
groups, with 25 rats in each group: i) Control; ii) PQ; iii) 5-ASA
and iv) PQ + 5-ASA. The concentration of PQ in this study was
selected based on the results of our preliminary experiments and
related literature (26,27). On the first day, the rats in the
PQ and PQ + 5-ASA groups were administered doses of 80 mg/kg PQ by
gavage, whereas, in the control and 5-ASA groups, the rats were
treated with distilled water, and 2 h later, equal amounts of
distilled water were administered to the control and PQ groups,
while 30 mg/kg 5-ASA in distilled water was intragastrically
administered to the 5-ASA and PQ + 5-ASA groups. On the second day,
only equal amounts of distilled water were administered to the
control and PQ groups. At the same time, 30 mg/kg 5-ASA was
administered to the 5-ASA and PQ + 5-ASA groups and the steps of
the second day were repeated once a day for up to 14 days. Rats
were sacrificed by cervical dislocation after anesthesia by
intraperitoneal injection of a saturated pentobarbital sodium
solution (40 mg/kg) at 3, 14 and 28 days after PQ administration.
General pathological changes in the rats in each group were
observed, including size, congestion and dot bleeding. To
distinguish the rats' left and right lungs, each left lung was
placed in a frozen pipette and stored in a −70°C liquid nitrogen
freezer to detect hydroxyproline (HYP). The right lung was immersed
in 10% formalin and embedded in paraffin.
Comparison of relative weight
Before the rats were sacrificed, the rats were
weighed and the ratio of the weight at day 3, 14 and 28 to the
weight of the rats on the first day, that is, the relative weight,
was calculated.
Lung coefficient
The whole lung and trachea of the rats were
collected, the trachea was cut between the 5 and 6 cartilage rings
above the tracheal bifurcation, clean filter paper was used to
absorb the blood and tissue fluid on the lung surface, and it was
weighed with a high-precision balance. The pulmonary coefficient
was calculated as follows: Lung coefficient (LI)= total lung wet
weight (mg)/body weight (g) ×100%.
Histopathological examination
The right lung tissue samples were fixed in 10%
formalin at room temperature for 4–6 h, embedded in paraffin and
sectioned (thickness of 5 µm). The slides were subsequently stained
with hematoxylin solution at room temperature for 5 min followed by
five immersions in 1% acid ethanol (1% HCl in 70% ethanol) and then
rinsed in distilled water. Then, the sections were stained at room
temperature with eosin solution for 3 min, followed by dehydration
with graded alcohol and washing in xylene. The slides were then
examined under a light microscope (RM2245; Leica Microsystems GmbH)
by an experienced pathologist who was blinded to the treatment
received by each animal.
Masson's trichrome staining
The slides were deparaffinized and subjected to
Masson staining to detect fibrosis. Briefly, the tissue sections
(thickness, 5 µm) were cut and placed on standard microscopy
slides. After deparaffinization and rehydration, the slides were
immersed in Bouin's solution (cat. no. HT10132; Sigma-Aldrich;
Merck KGaA) at 56°C for 15 min. Subsequently, the slides were
washed with tap water for 5 min. Next, the sections were stained in
Weigert's hematoxylin for 5 min at room temperature and then washed
again with tap water for 5 min and rinsed in distilled water. Next,
the slides were stained in Biebrich scarlet-acid fuchsin for 5 min
at room temperature, rinsed in distilled water, incubated in
phosphotungstic-phosphomolybdic acid for 5 min at room temperature,
dyed with aniline blue for 5 min at room temperature and fixed in
1% acetic acid for 2 min at room temperature. Finally, the slides
were rinsed in distilled water, dehydrated and mounted in synthetic
resin. Slides stained with Masson's trichrome stain were observed
using the Leica microscope. The smooth muscle cell cytoplasm was
stained red, while the collagenous fibrous tissue was stained
blue.
Pathology scores were assessed using the method
described by Szapiel et al (28).
The pathological score consisted of alveolar inflammation and
pulmonary fibrosis scores. The criteria for alveolitis were as
follows: i) Grade 0, normal alveolar morphology, no alveolar
inflammation; ii) grade I, mild alveolitis, alveolar septum widened
by inflammatory cell infiltration; iii) grade II, moderate
alveolitis; and iv) grade III, severe alveolitis, a large number of
infiltrating inflammatory cells and diffusely distributed lesions.
The criteria for pulmonary fibrosis were as follows: i) Grade 0,
normal lung tissue, with few or no filamentous collagen fibers; ii)
grade I, slight increase in collagen fiber amount, with thin bundle
morphology; iii) grade II, moderate increase in collagen fiber
amount fused into fine bands, with alveolar structure disorder; and
iv) grade III, substantial increase in collagen fiber amount into a
broadband or flaky morphology, with alveolar collapse and fusion,
as well as structural disorder. Alveolar inflammation and pulmonary
fibrosis scores were 0 points for grade 0, 2 points for grade I, 3
points for grade II and 4 points for grade III; these scores were
used for statistical analysis.
Determination of HYP
According to the manufacturer's instructions for the
Hydroxyproline Assay kit (cat. no. BC0250; Beijing Solarbio Science
& Technology Co., Ltd.), lung tissues were isolated to
determine the optical density (OD) of the samples at a wavelength
of 550 nm using a microplate reader (Thermo Fisher Scientific,
Inc.) and the level of HYP was calculated accordingly.
Cell culture and treatment
Human lung fibroblasts WI-38 VA13 purchased from
American Type Culture Collection were grown in DMEM/F12 (Gibco;
Thermo Fisher Scientific, Inc.) at 37°C supplemented with 10% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) and 100
U/ml penicillin and streptomycin in 5% CO2/95% air. When
the cells reached 80% confluence, they were randomly divided into
four groups: i) Control; ii) PQ; iii) 5-ASA; and iv) PQ + 5-ASA
groups. The cells were incubated with 200 µM of PQ for 12, 24 and
48 h, with or without pretreatment with 5-ASA (10 mM) at 37°C for 2
h. Cells were collected at 12, 24 and 48 h after PQ treatment.
Western blotting
After treatment, cells were washed with ice-cold
PBS. Total cell proteins were extracted using lysis buffer (1%
Triton X-100, 150 mM NaCl, 2 mM EDTA, 50 mM Tris-HCl, 10%
phosphatase inhibitors and 1% cocktail). Total protein
concentration was quantified using the Bradford Protein Assay Kit
(Bio-Rad Laboratories, Inc.). A total of 40 µg protein/lane protein
was separated via 10% SDS-PAGE and then transferred to a PVDF
membrane. Subsequently, the membranes were blocked with 5% skimmed
milk with PBS with 0.05% Tween-20 for 1 h at room temperature and
then incubated overnight at 4°C with specific primary antibodies at
a 1:1,000 dilution in blocking solution. After washing, the
membranes were incubated with horseradish peroxidase-conjugated
goat anti-rabbit IgG secondary antibody (cat. no. sc-2030; Santa
Cruz Biotechnology, Inc.) at a 1:5,000 dilution for 1 h at room
temperature and visualized using an ECL chemiluminescent detection
system (Santa Cruz Biotechnology, Inc.). Band density was
semi-quantified using GeneTools Image Analysis Software (version
4.02; Syngene Europe) and normalized to β-actin.
Immunohistochemical (IHC)
staining
Tissues were fixed in 10% neutralized formalin for
48 h at room temperature and embedded in paraffin blocks. Sections
(4-µm thick) were prepared from paraffin blocks. After
deparaffinization, antigen retrieval was performed in 10 mmol/l of
citrate buffer at room temperature for 15 min. Endogenous
peroxidase activity was blocked with 3% hydrogen peroxide in
methanol for 10 min at room temperature. Blocking was carried out
at room temperature for 1 h using 10% normal goat serum (Vector
Laboratories, Ltd.). Incubation with primary antibodies against
TGF-β (1:100) and PPARγ (1:100) was conducted overnight at 4°C in a
humidified chamber and then washed with PBS. Subsequently, sections
were incubated in PBS with Tween-20 (0.5%) containing a
biotin-conjugated secondary antibody (cat. no. bs-0346R-Bio; BIOSS)
at 1:200 dilution for 1 h at room temperature and
3,3′diaminoenzidine was used to locate the specific antigens in
each section. Slides were counterstained with hematoxylin for 30
sec at room temperature (29).
The primary antibody was replaced with PBS as a negative control.
All slides were scored by an experienced pathologist. Images were
captured using an Olympus AH2 Vanox Microscope System (light
microscope; Olympus Corporation).
The levels of TGF-β1 and PPARγ are described based
on the ratio of positive cells to the intensity of the reaction.
The parameter was classified and a combined score was used to
determine positive or negative results according to previously
defined criteria (30). For the
score of positive cell ratio, 0–1, 1–10, 10–50, 50–80 and 80–100%
were scored as 0, 1, 2, 3 and 4, respectively. For the intensity
score, negative, weakly positive, positive and strongly positive
were scored as 0, 1, 2 and 3, respectively. IHC score=positive cell
ratio score × intensity score.
Statistical analysis
Statistical analysis was performed using one-way
analysis of variance (ANOVA) with SPSS 16.0 (SPSS, Inc.). One-way
ANOVA followed by the Tukey's post-hoc test was used to compare
differences between groups. The time-effect relationship was
analyzed using Pearson's correlation analysis. All results were
confirmed using at least three independent experiments. The results
are presented as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference.
Results
Rat general conditions after PQ
exposure
In the PQ group, the rats displayed a series of
symptoms, including sluggishness, lethargy, irritability and bloody
discharge in the mouth, nose and eyes 2 h after PQ exposure. The
poisoning symptoms progressively worsened from 3 to 28 days after
PQ exposure, including dyspnea, abdominal breathing and perioral
cyanosis in the respiratory system, diarrhea in the alimentary
system and oliguria, anuresis and hematuria in the urinary system.
The poisoning manifestations, both general and systemic, were
alleviated in the PQ + 5-ASA group (data not shown).
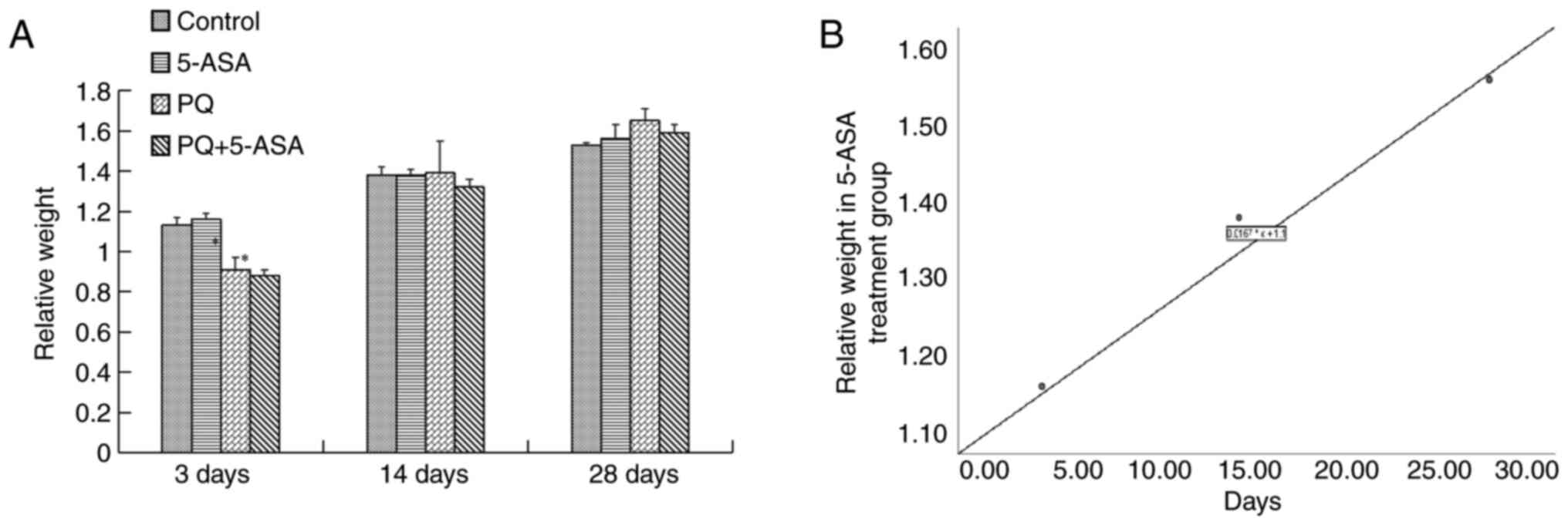
Relative body weight gradually increased with time
in the 5-ASA group (r=0.992). It showed no changes compared with
the control group. After PQ exposure, the relative weight decreased
to a minimum at 3 days and gradually increased to the control level
at 14 days. In the PQ + 5-ASA group, the relative body weight
decreased to a minimum at 3 days. The body weight decrease was
statistically significant when compared with the control group and
5-ASA group at 3 days (Fig. 1A and
B). Therefore, the PQ-induced decrease in relative body weight
could be improved by 5-ASA.
5-ASA attenuates PQ-induced pulmonary
damage
The lungs in the control and 5-ASA groups were
normal in size and pink in color, while lungs in the PQ group
notably increased in size and congestion and dot bleeding could be
seen within 14 days after PQ exposure, turning grayish with uneven
surface 28 days after PQ exposure (data not shown). The changes in
the lungs in the PQ + 5-ASA group were markedly alleviated; no
obvious bleeding was observed and the lung surface was smooth.
There was no significant difference in lung
coefficient between the 5-ASA group and control group. After PQ
exposure, the lung coefficient increased significantly, reached a
peak on day 3, decreased on day 14 compared with the 3 days, but
increased again on day 28, showing a biphasic increase. The lung
coefficient in each time period in the PQ group was significantly
higher compared with the control group. Compared with the control
group, the lung coefficient of the PQ group was significantly
increased, especially at day 3. However, the lung coefficient at
each time point in the PQ + 5-ASA group was significantly lower
compared with the PQ group. In conclusion, lung coefficient
measurements further confirmed that 5-ASA treatment could alleviate
the changes caused by PQ in the lungs (Fig. 2).
Histologically, the morphological changes in the
lungs of rats in different groups were evaluated by H&E
staining (Fig. 3A). Except for a
few phagocytes in the lumens of the alveoli, no changes, including
edema, congestion, bleeding and inflammatory modifications, were
observed in the lungs of the control and 5-ASA groups. Alveolitis
was observed in the lungs of rats after PQ exposure. Congestion,
edema and inflammatory cell infiltration were observed in the
bronchial and alveolar walls at 3 days after PQ exposure. Alveolar
septum thickening with alveolar lumen narrowing, diffuse pulmonary
hemorrhage and hyaline membrane formation were observed at 14 days
after PQ exposure. At the later stage, fibroblast proliferation,
increased collagen fiber amount and fibrous thickening of the
alveolar walls was also observed at 28 days after PQ exposure. All
pulmonary injury changes observed in the lungs of rats in the PQ
group were notably attenuated in the PQ + 5-ASA groups, as
evidenced by a decrease in the degree of congestion, inflammatory
cell infiltration, bleeding at an early stage (<14 days) and
fibrous proliferation at the late stage (28 days) after PQ
exposure.
5-ASA attenuates PQ-induced fibrosis
in lung tissues of rats HYP content analysis of the lung
tissues
Pulmonary fibrosis is characterized by the
accumulation of collagen. HYP is a non-essential amino acid found
in collagen that serves a crucial role in collagen synthesis and is
frequently used as a biomarker of tissue fibrosis (31). HPY content changes were measured
in lung tissues in different groups. The results showed that HYP
content significantly increased in the lung tissues of the rats at
14 days after PQ treatment and reached a peak at 28 days compared
with the control group. Compared with the HYP changes in the PQ
group, HYP content of lung tissue was significantly lower in the
lung tissues of rats at 14 and 28 days after PQ + 5-ASA treatment
(Fig. 4), suggesting that 5-ASA
treatment could significantly alleviate the degree of collagen
accumulation induced by PQ.
Masson's trichrome staining
Masson's trichrome staining revealed that the
collagen fibers were stained blue. The results (Fig. 5A) showed that, compared with those
in the control group and 5-ASA group, the amount of blue-stained
collagen fibers in the lung tissues of rats in the PQ group
increased at 14 and 28 days. Collagen fibers were mainly
concentrated in the alveolar thickening area and bronchioles. With
5-ASA treatment, collagen deposition (blue staining) of lung tissue
in the PQ + 5-ASA groups was lower than that in the PQ group alone,
further confirming the alleviating effect of 5-ASA on the increase
in collagen fiber amount induced by PQ at 14 and 28 days.
No pathological changes were found in the lungs of
the rats in the control and 5-ASA groups, while injury and fibrosis
changes with different severity were observed in rats of the other
two groups. Further quantitative analysis showed that the
inflammation score in the PQ group increased from 3 to 28 days
after PQ exposure compared with the control group. Inflammation
scores at all time points (3, 14 and 28 days) in the PQ + 5-ASA
groups all decreased compared with those in the PQ group,
suggesting that 5-ASA treatment could partly alleviate the degree
of inflammation induced by PQ (Fig.
3B). The fibrosis score increased at 14 and 28 days after PQ
exposure compared with the control group, while that in the PQ +
5-ASA group significantly decreased compared with the PQ group,
suggesting that 5-ASA treatment could partly alleviate the degree
of fibrosis induced by PQ (Fig.
5B).
5-ASA attenuates the upregulation of
TGF-β1 and p-SMAD3 and the reduction of PPARγ induced by PQ in the
lung tissue of rats in vivo and human lung fibroblasts WI-38 VA13
cells in vitro
The expression of fibrosis-related factors in the
lung tissue of rats were further evaluated by IHC staining to
explore the putative anti-fibrotic mechanism of 5-ASA. The IHC
staining results showed that TGF-β1 expression increased from days
3 to 28 after PQ exposure compared with the control group. Compared
with that in the PQ group, the expression level of TGF-β1 decreased
in the PQ + 5-ASA group at each time point (Fig. 6A and B). The expression of PPARγ
in the lung tissue of rats in the PQ group obviously decreased
compared with that in the control group. This downregulation of
PPARγ induced by PQ could be partly inhibited by 5-ASA treatment in
the lung tissue of rats (Fig. 7A and
B). In addition, the activation of SMAD3, which is another key
member in the TGF-β1/SMAD3 pathway, was further analyzed. The
active form of SMAD3, p-SMAD3 was determined by western blotting.
The results showed that PQ exposure significantly increased p-SMAD3
expression in lung tissues compared with that in the control group
at each time point. The p-SMAD3 level was significantly
downregulated in the lung tissue of PQ + 5-ASA-treated rats
compared with that in the PQ treatment group (Fig. 8A and C). PQ treatment had no
significant effects on regulating the protein levels of SMAD3
(Fig. 8B and D). Furthermore, the
p-SMAD3/total SMAD3 ratio was significantly downregulated in the
lung tissue of PQ + 5-ASA-treated rats compared with that in the PQ
treatment group (Fig. 8E).
Studies have shown that myofibroblast proliferation
in the lung tissue of patients is the basis for pulmonary fibrosis
(32,33). Based on the aforementioned
results, the effect of 5-ASA on the TGF-β1 pathway after PQ
exposure was further explored in human lung fibroblasts WI-38 VA13
cells in vitro. The results, as determined by western blotting,
were consistent with those in vivo and revealed that pretreatment
with 5-ASA significantly reversed the reduction of PPARγ (Fig. 9A) and the increase in TGF-β1
(Fig. 9B) and p-SMAD3 (Fig. 9C) levels induced by PQ. PQ
treatment had no significant effects on regulating the protein
levels of SMAD3 (Fig. 9D).
Furthermore, the p-SMAD3/total SMAD3 ratio was significantly
downregulated in the PQ + 5-ASA group compared with that in the PQ
treatment group (Fig. 9D).
Discussion
Pulmonary fibrosis is an irreversible stage of the
pathological development of PQ poisoning, leading to high mortality
(34). No safe and effective
treatment has been found to reverse the fibrosis process. In the
present study, it was found that intragastric administration of PQ
induced significant injury and fibrotic changes in the lungs of
experimental rats, including congestion, edema, hemorrhage,
increased lung coefficient, increased HYP content, accumulation of
collagen, increase in the amount of collagen fibers and fibrous
thickening of the alveolar walls. 5-ASA treatment notably
alleviated the pulmonary injury and fibrotic changes induced by PQ
in rats, as evidenced by decrease in the degree of congestion,
inflammatory cell infiltration, hemorrhage, lung coefficient,
collagen accumulation and collagen fiber amount. Thus, this study
confirmed that 5-ASA could markedly attenuate the injury and
fibrotic changes induced by PQ, suggesting that 5-ASA could be used
in the alleviation of pulmonary fibrosis in PQ poisoning.
TGF-β1 plays a crucial role in the induction of
fibrosis. TGF-β1 signaling exerts its biological effects via the
TGF-β1/SMAD/Snail signaling pathway, serving an important
pathogenic role in several fibrotic diseases, such as pulmonary
fibrosis and cardiac fibrosis (13,35). In the current study, the
upregulation of TGF-β1 and SMAD3 activation was confirmed by
observing the increased p-SMAD3 levels after PQ exposure,
suggesting that the TGF-β1/SMAD signaling pathway was involved in
the fibrotic changes induced by PQ. These results were in
accordance with those reported in the literature on tissue fibrosis
(36).
PPARs are recognized as versatile members of the
ligand-activated nuclear hormone receptor superfamily of
transcription factors, including steroids, thyroid hormone,
retinoic acid and vitamin D (37). There are three subtypes of PPARs:
PPARα, PPARβ/δ and PPARγ. Of the three PPARs identified to date,
PPARγ represents the most promising PPAR target in lung diseases in
view of emerging reports implicating this molecule in various
pulmonary processes, for example, pulmonary thromboembolectomy,
lung transplantation and severe viral pneumonia (38). Additionally, it has been reported
that PPARγ activators can inhibit TGF-β1-induced myofibroblast
transdifferentiation (39). In
diseased tissues, PPARγ expression is inversely related to that of
TGF-β1 (27). Thus, it appears
that the balance between TGF-β1 and PPARγ may determine whether
fibrogenesis predominates after tissue injury. In the present
study, it was found that the expression of PPARγ significantly
decreased in the lung tissues of rats after PQ intragastric
treatment. At the same time, it was found that PQ exposure
decreased the expression of PPARγ in human lung fibroblasts WI-38
VA13 cells in vitro. Therefore, this study suggested that PPARγ was
involved in the regulation of the TGF-β1/SMAD3 signaling pathway in
PQ-induced pulmonary fibrosis.
5-ASA is an anti-inflammatory agent commonly used in
the treatment of IBD (40).
Although the exact mechanisms of action of 5-ASA have not yet been
completely elucidated, recent studies have revealed that the basic
mechanism of action of 5-ASA relies on increased expression of
PPARγ (21,41). As a ligand of PPAR, 5-ASA can
increase PPARγ expression, promote its translocation from the
cytoplasm to the nucleus and induce activation of the downstream
signaling pathway (18,20).
The results of the current study showed that 5-ASA
treatment significantly relieved PQ-induced pulmonary fibrotic
changes in rats. To explore the putative mechanism of the
attenuating effects of 5-ASA on PQ-induced pulmonary fibrotic
changes, the expression and interaction of TGF-β1/SMAD3 signaling
and PPARγ were studied both in vivo in rats and in vitro in human
lung fibroblasts. The results showed that 5-ASA treatment
significantly prevented the upregulation of TGF-β1, the
phosphorylation level of SMAD3 and the downregulation of PPARγ
induced by PQ in human lung fibroblasts. Collectively, these data
suggested that 5-ASA treatment could attenuate PQ-induced pulmonary
fibrosis progression by upregulating the expression of PPARγ and
inhibiting the TGF-β1/SMAD3 signaling pathway.
In conclusion, to the best of our knowledge, this
study showed for the first time that 5-ASA had significant
inhibitory effects on pulmonary fibrosis progression in a PQ
intoxication rat model, and these effects may be partly ascribed to
the inhibition of the TGF-β1/SMAD3 signaling pathway. Thus, 5-ASA
may have potential value in the treatment of PQ-induced pulmonary
fibrosis.
Acknowledgements
Not applicable.
Funding
This work was supported by the Natural Science Foundation of
China (grant no. 81672706).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HC and JC conducted the experiments and wrote the
manuscript. JW, YW, FT, YT, YG, YM and LL collected the data and
performed the experiments and XZ designed the study. HC and XZ
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
All experiments were performed in compliance with
the Guide for the Care and Use of Laboratory Animals of the
National Institutes of Health (NIH Publication No. 8023, Revised
1978) and were reviewed and approved by the Ethics Committee for
the Use of Experimental Animals at Hebei Medical University
(approval no. 1608303; Shijiazhuang, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sun B and Chen YG: Advances in the
mechanism of paraquat-induced pulmonary injury. Eur Rev Med
Pharmacol Sci. 20:1597–1602. 2016.PubMed/NCBI
|
|
2
|
Zhou Q, Kan B, Jian X, Zhang W, Liu H and
Zhang Z: Paraquat poisoning by skin absorption: Two case reports
and a literature review. Exp Ther Med. 6:1504–1506. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kimbrough RD and Linder RE: The
ultrastructure of the paraquat lung lesion in the rat. Environ Res.
6:265–273. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shadnia S, Ebadollahi-Natanzi A,
Ahmadzadeh S, Karami-Mohajeri S, Pourshojaei Y and Rahimi HR:
Delayed death following paraquat poisoning: Three case reports and
a literature review. Toxicol Res (Camb). 7:745–753. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tsoyi K, Chu SG, Patino-Jaramillo NG,
Wilder J, Villalba J, Doyle-Eisele M, McDonald J, Liu X, El-Chemaly
S, Perrella MA and Rosas IO: Syndecan-2 Attenuates
Radiation-induced pulmonary fibrosis and inhibits fibroblast
activation by regulating PI3K/Akt/ROCK Pathway via CD148. Am J
Respir Cell Mol Biol. 58:208–215. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kan B, Jian X, Zhou Q, Wang J, Yu G, Sun J
and Gao Y: Effect of transforming growth factor-beta1 on acute lung
injury caused by paraquat. Mol Med Rep. 9:1232–1236. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Han YY, Shen P and Chang WX: Involvement
of epithelial-to-mesenchymal transition and associated transforming
growth factor-β/Smad signaling in paraquat-induced pulmonary
fibrosis. Mol Med Rep. 12:7979–7984. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hua XF, Li XH, Li MM, Zhang CY, Liu HJ,
Sun T, Zhou HG and Yang C: Doxycycline attenuates paraquat-induced
pulmonary fibrosis by downregulating the TGF-β signaling pathway. J
Thorac Dis. 9:4376–4386. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ren Y, Jian X, Zhang Z, Ning Q, Kan B and
Kong L: Effects of tacrolimus on the TGF-β1/SMAD signaling pathway
in paraquat-exposed rat alveolar type II epithelial cells. Mol Med
Rep. 22:3687–3694. 2020.PubMed/NCBI
|
|
10
|
Hu Z, Qin F, Gao S, Zhen Y, Huang D and
Dong L: Paeoniflorin exerts protective effect on radiation-induced
hepatic fibrosis in rats via TGF-β1/Smads signaling pathway. Am J
Transl Res. 10:1012–1021. 2018.eCollection 2018. PubMed/NCBI
|
|
11
|
Sato M, Muragaki Y, Saika S, Roberts AB
and Ooshima A: Targeted disruption of TGF-beta1/Smad3 signaling
protects against renal tubulointerstitial fibrosis induced by
unilateral ureteral obstruction. J Clin Invest. 112:1486–1494.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Loboda A, Sobczak M, Jozkowicz A and Dulak
J: TGF-β1/Smads and miR-21 in renal fibrosis and inflammation.
Mediators Inflamm. 2016:83192832016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sisto M, Lorusso L, Ingravallo G, Tamma R,
Ribatti D and Lisi S: The TGF-β1 signaling pathway as an attractive
target in the fibrosis pathogenesis of Sjogren's Syndrome.
Mediators Inflamm. 2018:19659352018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ramirez A, Ballard EN and Roman J: TGFβ1
Controls PPARү expression, transcriptional potential, and activity,
in part, through Smad3 Signaling in Murine lung fibroblasts. PPAR
Res. 2012:3758762012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Calvier L, Chouvarine P, Legchenko E,
Hoffmann N, Geldner J, Borchert P, Jonigk D, Mozes MM and Hansmann
G: PPARү Links BMP2 and TGFβ1 pathways in vascular smooth muscle
cells, regulating cell proliferation and glucose metabolism. Cell
Metab. 25:1118–1134.e7. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Solitano V, D'Amico F, Fiorino G,
Paridaens K, Peyrin-Biroulet L and Danese S: Key strategies to
optimize outcomes in Mild-to-Moderate Ulcerative Colitis. J Clin
Med. 9:29052020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Le Berre C, Roda G, Nedeljkovic Protic M,
Danese S and Peyrin-Biroulet L: Modern use of 5-aminosalicylic acid
compounds for ulcerative colitis. Expert Opin Biol Ther.
20:363–378. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cevallos SA, Lee JY, Velazquez EM,
Foegeding NJ, Shelton CD, Tiffany CR, Parry BH, Stull-Lane AR,
Olsan EE, Savage HP, et al: 5-Aminosalicylic Acid Ameliorates
Colitis and checks Dysbiotic Escherichia coli expansion by
activating PPAR-γ Signaling in the intestinal epithelium. mBio.
12:e03227–20. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vetuschi A, Pompili S, Gaudio E, Latella G
and Sferra R: PPAR-γ with its anti-inflammatory and anti-fibrotic
action could be an effective therapeutic target in IBD. Eur Rev Med
Pharmacol Sci. 22:8839–8848. 2018.PubMed/NCBI
|
|
20
|
Rousseaux C, El-Jamal N, Fumery M,
Dubuquoy C, Romano O, Chatelain D, Langlois A, Bertin B, Buob D,
Colombel JF, et al: The 5-aminosalicylic acid antineoplastic effect
in the intestine is mediated by PPARγ. Carcinogenesis.
34:2580–2586. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Z, Koonen D, Hofker M and Bao Z:
5-aminosalicylic acid improves lipid profile in mice fed a high-fat
cholesterol diet through its dual effects on intestinal PPARgamma
and PPARalpha. PLoS One. 13:e01914852018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang Y, Zhou M, Lu Y, Yu A and Li J:
Protective effect of 5-aminosalicylic acid on the kidney of
paraquat poisoning rats by Nrf2-ARE signal pathway. Zhonghua Wei
Zhong Bing Ji Jiu Yi Xue. 29:961–966. 2017.(In Chinese). PubMed/NCBI
|
|
23
|
Ramadan A, Afifi N, Yassin NZ,
Abdel-Rahman RF, Abd El-Rahman SS and Fayed HM: Mesalazine, an
osteopontin inhibitor: The potential prophylactic and remedial
roles in induced liver fibrosis in rats. Chem Biol Interact.
289:109–118. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hoffmann M, Kant TA, Emig R, Rausch JSE,
Newe M, Schubert M, Künzel K, Winter L, Klapproth E, Peyronnet R,
et al: Repurposing mesalazine against cardiac fibrosis in vitro.
Naunyn Schmiedebergs Arch Pharmacol. 394:533–543. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schaeffer WI: Proposed usage of animal
tissue culture terms (revised 1978). Usage of vertebrate cell,
tissue and organ culture terminology. In Vitro. 15:649–653. 1979.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zheng L, Zhang YL, Dai YC, Chen X, Chen
DL, Dai YT and Tang ZP: Jianpi Qingchang decoction alleviates
ulcerative colitis by inhibiting nuclear factor-ΚB activation.
World J Gastroenterol. 23:1180–1188. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tang H, Xiang D, Wang F, Mao J, Tan X and
Wang Y: 5-ASA-loaded SiO2 nanoparticles-a novel drug delivery
system targeting therapy on ulcerative colitis in mice. Mol Med
Rep. 15:1117–1122. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Szapiel SV, Elson NA, Fulmer JD,
Hunninghake GW and Crystal RG: Bleomycin-induced interstitial
pulmonary disease in the nude, athymic mouse. Am Rev Respir Dis.
120:893–899. 1979.PubMed/NCBI
|
|
29
|
Wei J, Ghosh AK, Sargent JL, Komura K, Wu
M, Huang QQ, Jain M, Whitfield ML, Feghali-Bostwick C and Varga J:
PPARү downregulation by TGFβ in fibroblast and impaired expression
and function in systemic sclerosis: A novel mechanism for
progressive fibrogenesis. PLoS One. 5:e137782010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang Z, Ding L, Wu L, Xu L, Zheng L and
Huang X: Salidroside alleviates paraquat-induced rat acute lung
injury by repressing TGF-β1 expression. Int J Clin Exp Pathol.
7:8841–8847. 2014.eCollection 2014. PubMed/NCBI
|
|
31
|
Srivastava AK, Khare P, Nagar HK,
Raghuwanshi N and Srivastava R: Hydroxyproline: A potential
biochemical marker and its role in the pathogenesis of different
diseases. Curr Protein Pept Sci. 17:596–602. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kuhn C and McDonald JA: The roles of the
myofibroblast in idiopathic pulmonary fibrosis. Ultrastructural and
immunohistochemical features of sites of active extracellular
matrix synthesis. Am J Pathol. 138:1257–1265. 1991.PubMed/NCBI
|
|
33
|
Pache JC, Christakos PG, Gannon DE,
Mitchell JJ, Low RB and Leslie KO: Myofibroblasts in diffuse
alveolar damage of the lung. Mod Pathol. 11:1064–1070.
1998.PubMed/NCBI
|
|
34
|
Dong J, Yu X, Porter DW, Battelli LA,
Kashon ML and Ma Q: Common and distinct mechanisms of induced
pulmonary fibrosis by particulate and soluble chemical fibrogenic
agents. Arch Toxicol. 90:385–402. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhou H, Yu X and Zhou G: NLRC5 silencing
ameliorates cardiac fibrosis by inhibiting the TGF-β1/Smad3
signaling pathway. Mol Med Rep. 16:3551–3556. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xue L, Zhang X, Li Y, Yang H, Li X, Mi J,
Wang H, Wang J and Yan X: Differences of immunophenotypic markers
and signaling molecules between adenocarcinomas of gastric cardia
and distal stomach. Hum Pathol. 42:594–601. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu Y, Wang J, Luo S, Zhan Y and Lu Q: The
roles of PPARγ and its agonists in autoimmune diseases: A
comprehensive review. J Autoimmun. 113:1025102020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Carvalho MV, Goncalves-de-Albuquerque CF
and Silva AR: PPAR Gamma: From definition to molecular targets and
therapy of lung diseases. Int J Mol Sci. 22:8052021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kulkarni AA, Thatcher TH, Olsen KC,
Maggirwar SB, Phipps RP and Sime PJ: PPAR-γ ligands repress
TGFβ-induced myofibroblast differentiation by targeting the
PI3K/Akt pathway: Implications for therapy of fibrosis. PLoS One.
6:e159092011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Moran CJ, Huang H, Rivas M, Kaplan JL,
Daly MJ and Winter HS: Genetic variants in cellular transport do
not affect mesalamine response in ulcerative colitis. PLoS One.
13:e01928062018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Serra D, Almeida LM and Dinis TC:
Anti-inflammatory protection afforded by cyanidin-3-glucoside and
resveratrol in human intestinal cells via Nrf2 and PPAR-γ:
Comparison with 5-aminosalicylic acid. Chem Biol Interact.
260:102–109. 2016. View Article : Google Scholar : PubMed/NCBI
|