Introduction
Under normal conditions, skeletal bone mass is
maintained by the net balance between the number of bone-forming
osteoblasts and bone-resorbing osteoclasts. If either of these bone
cells undergo a change in activity, an imbalance in bone
maintenance can occur. The dysregulation of bone remodelling can
result in different types of skeletal diseases, such as
osteoporosis and osteopetrosis (1). Osteoporosis, a global public health
concern, is characterised by bone mass reduction and
microarchitectural deterioration of bone, resulting in increased
risk of fragility fractures (2).
Osteoporosis-related fractures can cause disability, substantial
pain and even death in patients with osteoporosis (3).
Osteoclasts are unique bone-resorbing and
multinucleated giant cells derived from the fusion of hematopoietic
precursor cells of the monocyte/macrophage lineage (4). This fusion is regulated by the
fusion-related molecule dendritic cell-specific transmembrane
protein (DC-STAMP). The failure of cell fusion can result in an
increase in bone mass, as observed in osteopetrosis (5). In addition, osteoclast
differentiation is mainly governed by two crucial cytokines:
Macrophage colony-stimulating factor (M-CSF) and receptor activator
of nuclear factor (NF)-κB ligand (RANKL). M-CSF is constitutively
produced by mesenchymal cells in the bone marrow in response to
osteotropic factors; it induces the differentiation of precursor
cells into mature osteoclasts (6). RANKL is most abundantly expressed on
the cell surfaces of bone marrow stromal cells, osteoblasts and
osteocytes (7); it stimulates the
commitment of osteoclast precursors to the osteoclastic phenotype
by interacting with RANK, which is expressed in osteoclast
precursors. The RANKL/RANK interaction leads to the recruitment of
signalling adaptor molecule tumour necrosis factor
receptor-associated factor 6 (TRAF6). This in turn triggers the
activation of a series of downstream signalling pathways,
activating all three mitogen-activated protein kinases (MAPKs)
[c-Jun N-terminal kinase (JNK), extracellular signal-regulated
kinase (ERK) and p38], as well as transcription factors such as
NF-κB (8,9). NF-κB is a key pleiotropic
transcription factor involved in the early stages of RANKL-induced
osteoclast differentiation (10).
Activation of the NF-κB signalling pathway promotes the
phosphorylation and subsequent degradation of inhibitor of κBα
(IκBα), followed by the phosphorylation and translocation of
downstream p65 to the nucleus. In addition, MAPK activation results
in the phosphorylation of c-Jun and its binding to c-Fos to form
the essential activator protein-1 (AP-1) transcription factor,
which ultimately evokes the induction and activation of nuclear
factor of activated T cell c1 (NFATc1), a well-known calcineurin-
and calcium-regulating transcription factor that promotes
osteoclast differentiation and function (11,12). These transcription factors
consequently regulate several target genes involved in bone matrix
degradation, including tartrate resistant acid phosphatase (TRAP),
calcitonin receptor (CTR) and RANK (13).
Bisphosphonates (BPs) are a class of antiresorptive
drugs that have a high affinity with bone. BPs are widely used for
the clinical treatment of osteoporosis, bone metastasis, multiple
myeloma, breast cancer and Paget's disease, as they help prevent
hypercalcemia, pain and pathological fractures (14–16). Furthermore, they have been
revealed to prevent diabetes-induced bone loss, and can enhance
bone density in diabetic animals (17). Zoledronic acid (ZOL) is the most
widely used nitrogen-containing BP. ZOL inhibits the
differentiation and apoptosis of osteoclasts (18–20). In previous years, ZOL has been
applied in stomatology, and numerous studies have focused on the
outcomes of dental extractions in patients using ZOL, either alone
or in combination with steroids (21–23). Data are also available regarding
the outcomes of placing dental implants in these patients (24). Numerous researchers have concluded
that ZOL can reverse the negative effects of osteoporosis and
improve the fixation and osseointegration of dental implants, for
both local and systemic treatments, and in autologous bone grafts
under osteoporotic conditions (21,25). However, the utility of ZOL is
currently limited, as it can give rise to several side effects, one
of which is termed bisphosphonate-related osteonecrosis of the jaw
(BRONJ) (26–28). BRONJ has been associated with
studies in various fields, including general medicine, oncology,
and dental, oral and maxillofacial surgical procedures. Despite
this, researchers have reached a consensus that the benefits of BP
treatment generally outweigh the risks (21,29,30). Importantly, several oral
health-related risk factors, including periodontal disease and oral
infections, implants, poor oral health, tooth extractions and
dentoalveolar surgery (before and during treatment) are considered
to be triggers for BRONJ development. However, the exact molecular
pathways involved in BRONJ pathogenesis require further
investigation (15,28,31). Additionally, the precise molecular
mechanisms of ZOL in the treatment of osteoporosis remain unclear.
Our previous study (32) reported
that ZOL may inhibit NF-κB and JNK signalling by reducing the
levels of p-IκBα, p-p65 and p-JNK in a time-dependent manner, but
the evidence showing that ZOL blocks the RANKL-induced activation
of the NF-κB and JNK signalling pathways is insufficient. The
present study hypothesized that suppressing these signalling
pathways may be an effective therapeutic approach for treating bone
loss diseases, including osteoporosis. Therefore, the present study
further investigated the mechanisms through which ZOL inhibits
osteoclast differentiation and function in RAW264.7 cells. In
particular, focus was given to its effects on the NF-κB and
JNK/c-Jun/c-Fos/NFATc1/DC-STAMP signalling axis, to provide new
strategies for the treatment of osteoporosis.
Materials and methods
Cells, reagents and antibodies
The α-modification of Eagle's medium (α-MEM; cat.
no. SH30265.01B) and fetal bovine serum (FBS; cat. no. 10099141C)
were obtained from Gibco; Thermo Fisher Scientific, Inc. The
RAW264.7 mouse macrophage cell line (osteoclast precursor; cat. no.
TIB71) was purchased from the American Type Culture Collection. The
recombinant murine soluble RANK ligand (sRANKL; cat. no. 315-11C)
and macrophage colony-stimulating factor (M-CSF; cat. no. 315-02)
were obtained from PeproTech, Inc. The TRAP staining kit (cat. no.
386 A) and ZOL (cat. no. SML0223-10MG) were purchased from
Sigma-Aldrich; Merck KgaA. Specific antibodies against IκBα (cat.
no. 4812), phospho-IκBα (p-IkBa; cat. no. 2859) (Ser32), p38 (cat.
no. 8690), phospho-p38 (p-p38; cat. no. 4511) (Thr180/Tyr182),
c-Jun N-terminal kinase (JNK; cat. no. 9252), phospho-JNK (p-JNK;
cat. no. 4668) (Thr183/Tyr185), extracellular signal-regulated
kinase 1/2 (ERK1/2; cat. no. 9102), phospho-ERK (p-ERK; cat. no.
4370) (Thr202/Tyr204), p65 (cat. no. 8242), phospho-p65 (p-p65,
cat. no. 3033), c-Fos (cat. no. 2250), NFATc1 (cat. no. 8032) and
c-Jun (cat. no. 9165) were obtained from Cell Signaling Technology,
Inc. Specific antibodies against DC-STAMP (cat. no. MABF39-1) were
purchased from Sigma-Aldrich; Merck KgaA. Specific antibodies
against GAPDH (cat. no. AB-P-R 001) were obtained from Hangzhou
Xianzhi Biotechnology Co., Ltd., and those against Lamin B1 (cat.
no. bs-1840R) were obtained from Beijing Biosynthesis Biotechnology
Co., Ltd. Horseradish peroxidase-conjugated goat anti-rabbit IgG
secondary anti-body (cat. no. 014-090S) was obtained from PMK
Bioprimacy Co., Ltd. ECL solution (cat. no. WBKLS0100) was obtained
from EMD Millipore.
Osteoclastogenesis
Osteoclast differentiation was performed according
to the method previously described by Chen et al (33). RAW264.7 cells were seeded in
96-well tissue culture plates with α-MEM (10% FBS and 1%
penicillin-streptomycin) at a density of 1.5×103
cells/well and incubated at 37°C under 5% CO2 and 95%
humidity overnight. Cells were divided into four groups as follows:
i) Vehicle; ii) RANKL-only; iii) RANKL + M-CSF and iv) RANKL +
M-CSF + ZOL. Cells were cultured for 5 days. The conditioned medium
was replaced with fresh α-MEM every 2 days, and cells were then
stained for TRAP at 37°C for 1 h using a TRAP staining kit
according to the manufacturer's protocol. TRAP+ cells
with three or more nuclei were manually counted as mature
multinucleated osteoclasts by bright field microscopy.
Detection of osteoclast bone
resorption
To detect osteoclast bone resorption, sterile bovine
bone slices (IDS Nordic) were placed in 96-well plates in
triplicate. RAW264.7 cells were then placed onto the bovine bone
slices in α-MEM complete medium at a density of 1.5×103
cells/well, and the medium was replaced every 48 h. After 10 days
of induction, cells were brushed off the bone slices, and the
resorption pits were observed using a scanning electron microscope
(E-1010; Hitachi, Ltd.). Finally, the number of pits was quantified
using ImageJ software 6.0 (National Institutes of Health) (34).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RAW264.7 cells were seeded onto 6-well plates at a
density of 1×105 cells/well and cultured in complete
α-MEM with 100 ng/ml RANKL. Cells were or were not treated with 1
µM ZOL at 37°C for 0, 1, 3 and 5 days. Total RNA was extracted from
the cells using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.). cDNA was synthesised from 1 mg of total
RNA using the PrimeScript RT reagent kit (Takara Bio, Inc.)
according to the manufacturer's protocol and stored at −70°C until
further use. qPCR was performed using the SYBR® Premix
Ex Taq™ kit (TaKaRa Bio, Inc.). Thermocycling conditions
were as follows: Initial denaturation for 2 min at 95°C, followed
by 40 cycles of denaturation at 95°C for 15 sec and amplification
at 60°C for 1 min and final extension for 15 sec at 95°C, 15 sec at
60°C and 15 sec at 95°C. The 2−∆∆Cq method was used to
calculate the relative mRNA expression, and all reactions were
performed in triplicate (35).
GAPDH was used as a quantitative control for the expression levels
of each gene in all experimental groups (33). Data is expressed as fold-change
relative to the control. The refseq of NFATc1, c-fos, DC-STAMP,
TRAP, RANK, CTR, and GAPDH were as follows: TRAP, 001102405.1;
DS-STAMP, 029422.4; RANK, AF019046.1; NFATc1, 016791.4; CTR,
001355192.1; c-fos, 010234.3; and GAPDH, 001289726.1. The primer
sequences of these osteoclast-specific markers and GAPDH are listed
in Table I.
 | Table I.Sequences of primers used in
quantitative PCR. |
Table I.
Sequences of primers used in
quantitative PCR.
| Primer | Gene sequence
(5′-3′) |
|---|
| Mouse
NFATc1 |
|
|
Forward |
GACCGAAGATACCTGGCTCG |
|
Reverse |
GTCAGAAGTGGGTGGAGTGG |
| Mouse
c-Fos |
|
|
Forward |
CCGGTTCCTTCTATGCAGCA |
|
Reverse |
GCTTGGGAAGGAGTCAGCTT |
| Mouse
CTR |
|
|
Forward |
GTCCAGAGTGAAAAGGCGGA |
|
Reverse |
AGGGCAACTGATGAATCCGG |
| Mouse
TRAP |
|
|
Forward |
AAGAGATCGCCAGAACCGTG |
|
Reverse |
TTCCAGCCAGCACATACCAG |
| Mouse
DC-STAMP |
|
|
Forward |
CCCTTGGGCTGTTCTTCCTT |
|
Reverse |
AGGAATGCAGCTCGGTTCAA |
| Mouse
RANK |
|
|
Forward |
TTCGACTGGTTCACTGCTCC |
|
Reverse |
TCAGGTGCTTTTCAGGGGAC |
| Mouse
GAPDH |
|
|
Forward |
GGTTGTCTCCTGCGACTTCA |
|
Reverse |
TGGTCCAGGGTTTCTTACTCC |
Western blot analysis
Total protein was extracted from cells using RIPA
buffer containing 150 mM NaCl, 5 mM EDTA, 50 mM Tris-HCl, 1 mM
sodium fluoride, 1 mM sodium vanadate, 1% Triton X-100, 1%
phosphatase and 1% protease inhibitors. The protein concentrations
were quantified using the bicinchoninic acid method. Next, total
protein (30 µg per lane) was subjected to 10% SDS-PAGE and
transferred to PVDF membranes. Following transfer, membranes were
blocked with 5% non-fat milk in Tris-buffered saline containing
0.1% Tween-20 at room temperature for 2 h. After incubation with
indicated primary antibodies [p-ERK (1:1,000), ERK (1:1,000), p-p38
(1:1,000), p38 (1:1,000), p-IκBα (1:1,000), IκBα, (1:1,000), p-JNK
(1:1,000), JNK (1:1,000), p-p65 (1:1,000), p65 (1:1,000), NFATc1
(1:1,000), c-Fos (1:600), DC-STAMP (1:800), c-Jun (1:1,000), Lamin
B1 (1:1,000) and GAPDH (1:1,000)] overnight at 4°C, the membranes
were washed and then incubated with horseradish
peroxidase-conjugated secondary antibodies diluted at 1:10,000 for
1 h at room temperature. ECL was used to develop a fluorescent
signal. Antibody reactivity was detected using the Gene Gnome
Imaging System (Syngene Europe) and band densities were quantified
using ImageJ software 6.0 (National Institutes of Health). Only
representative blots are shown.
NFATc1 reporter assay
To examine whether ZOL inhibited NFATc1 activation,
RAW264.7 cells stably transfected with a luciferase reporter
construct NFATc1-Luc were seeded onto 96-well plates at a density
of 1×104 cells/well. These cells were pre-treated with 1
µM ZOL for 1 h, and then incubated in the absence or presence of
100 ng/ml RANKL at 37°C for 8 h. At the end of the treatments,
cells were lysed and luciferase activity was measured using
Luciferase Assay System according to the manufacturer's protocol
(Promega Corporation).
Statistical analysis
All data were collected from at least three
independent experiments. Values are expressed as the mean ±
standard deviation. An unpaired Student's t-test was used for
comparisons between two groups, and one-way ANOVA followed by
Tukey's post-hoc test was used for multiple comparisons, assessed
via GraphPad Prism 6.0 (GraphPad Software, Inc.). P<0.05 was
considered to indicate a statistically significant difference.
Results
ZOL inhibits RANKL-mediated
osteoclastogenesis and bone resorption
Increasing evidence has suggested that RANKL and
M-CSF are sufficient and necessary for the formation and
differentiation of osteoclasts (36–38). Our previous study revealed that
ZOL had no cytotoxic effects on RAW264.7 cells at the
concentrations used in the present study (32). The present study confirmed that
RAW264.7 cells differentiated into TRAP+ multinucleated
osteoclasts (Fig. 1A) and
subsequently displayed enhanced bone resorptive function (Fig. 1B) in the presence of RANKL.
However, no significant difference was observed between the RANKL
and RANKL + M-CSF groups. Therefore, RAW264.7 cells were cultured
in the presence of 100 ng/ml RANKL (with no M-CSF) for subsequent
experiments. The effects of ZOL on osteoclast differentiation and
resorption pit formation were then investigated. Pre-treatment with
ZOL strongly inhibited the RANKL-induced formation of
TRAP+ multinucleated osteoclasts (Fig. 1A) and bone-resorbing pits
(Fig. 1B). The numbers of
osteoclasts (Fig. 1C) and
resorption pits (Fig. 1D) were
both significantly reduced following incubation with 1 µM ZOL.
These findings convincingly demonstrated that ZOL inhibits the
fusion of osteoclast precursors and the bone-resorbing activity of
mature osteoclasts.
ZOL inhibits the expression of
osteoclast-specific markers
The effects of ZOL on osteoclast-specific markers
were further explored by analysing the mRNA expression levels of
RANK, TRAP and CTR in the absence or presence of ZOL.
The stimulation of RAW264.7 cells with RANKL markedly induced the
expression of these osteoclast marker genes. By contrast, treatment
with ZOL after 3 days of RANKL stimulation markedly suppressed the
mRNA expression levels of RANK, TRAP and CTR
(Fig. 2). These results are
consistent with the previous finding that ZOL inhibits
osteoclastogenesis and bone resorption.
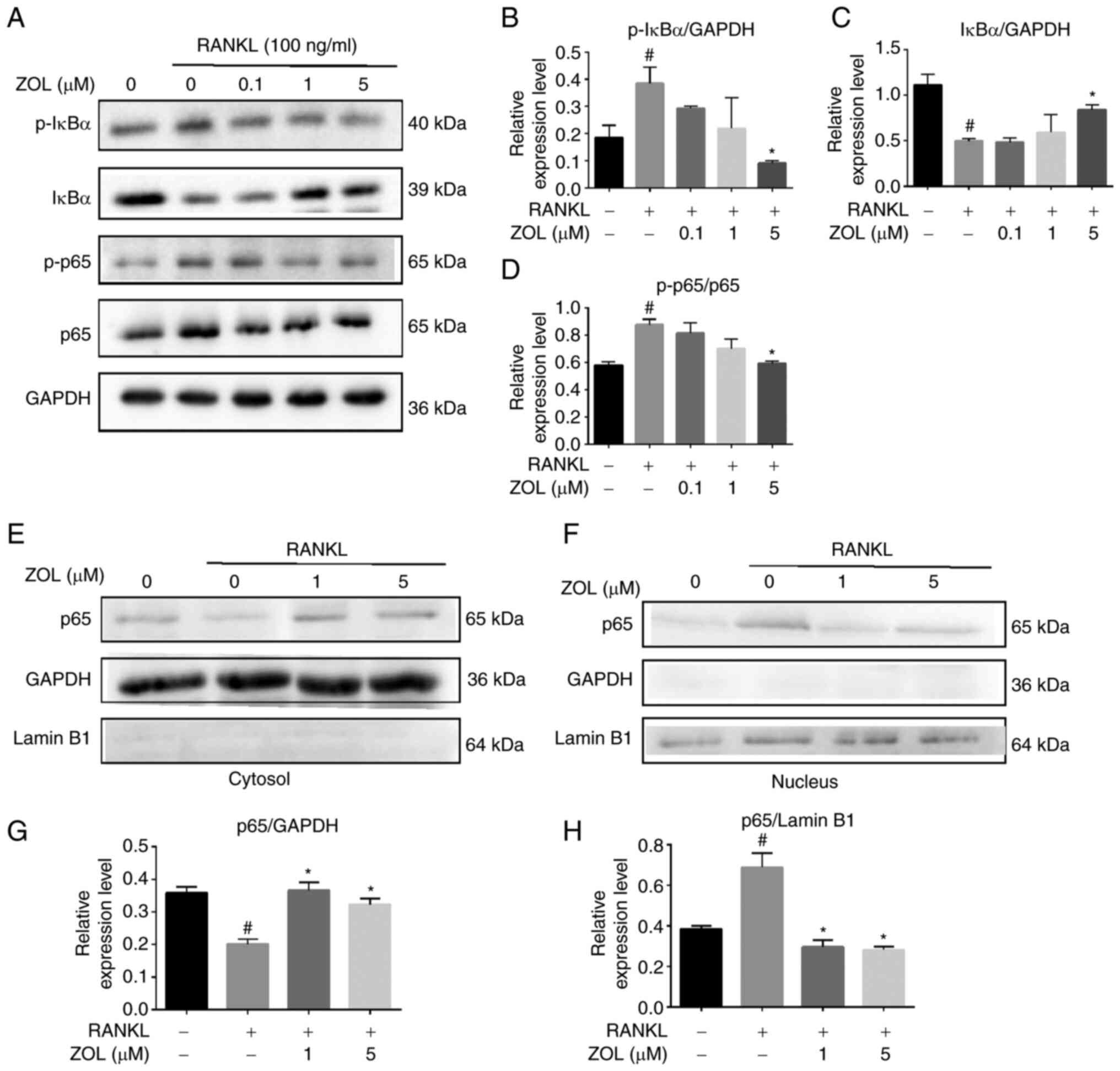
Effect of ZOL on RANKL-induced NF-κB
activation
The NF-κB pathway plays a vital role in
RANKL-induced osteoclastogenesis. To investigate the molecular
mechanism by which ZOL suppresses the proteins associated with
osteoclast differentiation, the present study first focused on the
effect of ZOL on NF-κB activation by analysing the phosphorylation
of IκBα (the inhibitor of NF-κB) and the phosphorylation and
translocation of p65, all of which are critical steps in NF-κB
activation. The rapid phosphorylation of IκBα and the p65 subunit
was detected in RAW264.7 cells treated with RANKL, indicating that
the NF-κB pathway was activated. However, ZOL downregulated the
RANKL-induced phosphorylation of IκBα, while upregulating the level
of non-phosphorylated IκBα. Similarly, RANKL-induced
phosphorylation of p65 was attenuated by ZOL in a dose-dependent
manner (Fig. 3A-D). To further
confirm this finding, the nuclear translocation of p65 was assessed
by western blotting of cytosolic and nuclear extracts, which
revealed that RANKL treatment increased p65 levels in nuclear
extracts. By contrast, treatment with ZOL, followed by stimulation
with RANKL, inhibited the nuclear translocation of p65 (Fig. 3E-H). These results suggested that
the inhibitory effects of ZOL on RANKL-induced osteoclast
differentiation and bone resorption may occur in part due to
inhibition of the NF-κB pathway.
ZOL inhibits RANKL-induced JNK
phosphorylation
MAPKs are located downstream of TRAF6 signalling
complexes and play a significant role in RANKL-mediated osteoclast
differentiation (39). Therefore,
the effects of ZOL on MAPK family proteins were examined. Treating
RAW264.7 cells with RANKL increased the phosphorylation of p38, JNK
and ERK. By contrast, ZOL treatment downregulated JNK
phosphorylation in a dose-dependent manner. However, the
phosphorylation of the p38 and ERK proteins was not significantly
affected by ZOL (Fig. 4). These
results suggested that ZOL suppresses RANKL-induced JNK
phosphorylation during osteoclast differentiation.
ZOL suppresses RANKL-induced
downstream expression of c-Jun, c-Fos and NFATc1
As ZOL can suppress RANKL-induced activation of JNK
and NF-κB during osteoclastogenesis, the ability of ZOL to inhibit
the downstream expression of c-Jun, NFATc1 and c-Fos was further
explored. The expression of c-Jun, which is downstream of JNK, was
increased in RANKL-stimulated RAW264.7 cells and was reduced
significantly by exposure to ZOL. Consistent with aforementioned
results, the mRNA and protein expression levels of c-Fos and NFATc1
both increased from the first day of RANKL treatment. However, ZOL
treatment significantly reduced the RANKL-induced mRNA and protein
expression of these two transcription factors (Fig. 5A-J). Furthermore, a luciferase
reporter assay revealed that the transcriptional activity of NFATc1
signalling was also significantly inhibited by ZOL treatment
(Fig. 5K). Collectively, these
results suggested that ZOL targets the upstream kinase to inhibit
the expression of downstream functional transcription factors.
 | Figure 5.Suppression of RANKL-induced
expression of downstream c-Jun, c-Fos and NFATc1 by ZOL. (A)
RAW264.7 cells incubated in 100 ng/ml RANKL with or without 1 µM
ZOL for 0, 1 or 3 days. The protein expression level was detected
by western blotting. Only representative blots are shown (c-Fos is
from a different membrane to the other bands). Band intensity
ratios of (B) NFATc1/GAPDH, (C) c-Jun/GAPDH and (D) c-Fos/GAPDH.
(E) RAW264.7 cells cultured in 100 ng/ml RANKL with 0, 1 or 5 µM
ZOL for 1 day. Band intensity ratios of (F) NFATc1/GAPDH, (G)
c-Fos/GAPDH and (H) c-Jun/GAPDH. Expression of osteoclast-specific
genes (I) c-Fos and (J) NFATc1, as detected via
quantitative PCR. (K) ZOL inhibits NFATc1 transcriptional activity,
as determined by the Promega Luciferase Assay System. #P<0.05
and ##P<0.01 vs. vehicle group; *P<0.05 and **P<0.01 vs.
RANKL-only group. ZOL, zoledronic acid; RANKL, receptor activator
of nuclear factor-κB ligand; NFATc1, nuclear factor of activated T
cells 1. |
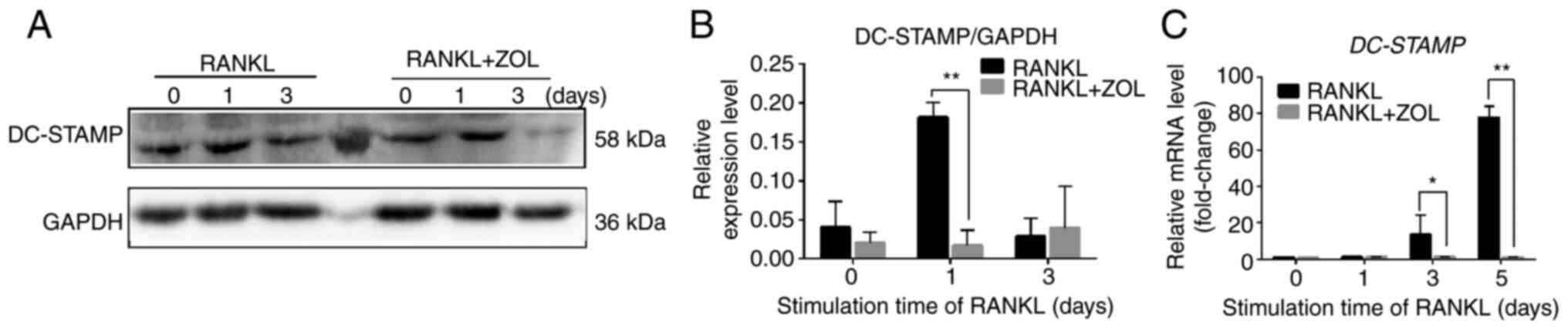
ZOL negatively regulates RANKL-induced
expression of fusion-related molecules DC-STAMP
Cell-cell fusion is crucial for the formation of
osteoclasts, and fusion-related molecule DC-STAMP is involved in
this process (38). To determine
whether the suppressive effect of ZOL on osteoclast differentiation
and bone resorption arose from the inhibition of cell-cell fusion,
DC-STAMP expression was examined using qPCR and western blot
analyses. The results indicated that RANKL-induced protein
expression of DC-STAMP was significantly inhibited by ZOL compared
with the RANKL control (Fig. 6A and
B). ZOL treatment also suppressed the RANKL-induced mRNA
expression of DC-STAMP (Fig. 6C).
These results indicated that the inhibitory effect of ZOL on the
RANKL-induced expression of DC-STAMP may also suppress
RANKL-induced osteoclast differentiation and bone resorption.
Discussion
Osteoclasts are known to mediate physiological bone
remodelling during tooth eruption, bone growth and fracture healing
(40,41). Notably, to date, no endogenous
factors other than RANKL have been found to induce osteoclast
formation without RANKL participation. Additionally, M-CSF is vital
for providing osteoclast precursor cells with proliferation and
survival signals, and increasing RANK expression, which is a
prerequisite for the differentiation and function of osteoclasts
(3). However, no significant
difference was here observed between the RANKL and RANKL + M-CSF
groups during osteoclast differentiation in RAW264.7 cells.
Therefore, in the follow-up study, the RANKL-induced osteoclast
differentiation platform was adopted to examine the effect of ZOL
on osteoclast formation in RAW264.7 cells. The results of the
present study revealed that ZOL significantly inhibited
RANKL-induced osteoclastogenesis without any cytotoxic effects.
Bone resorption is known to occur in conjunction with
osteoclastogenesis. The present study confirmed that ZOL
significantly suppressed both mature osteoclast formation and bone
resorptive function. These findings partly explain the
effectiveness of ZOL in treating bone-destroying skeletal
diseases.
The role of NF-κB in osteoclastogenesis and bone
homeostasis has been widely investigated (42). NF-κB-knockout mice have shown
defects in osteoclast differentiation and severe osteopetrosis
(39). In addition, in an
unstimulated state, NF-κB exists in the cytoplasm as a complex with
IκB, an inhibitory protein. When the NF-κB signalling pathway is
activated by RANKL, IκB is phosphorylated and degraded. The p65/p50
heterodimer then translocates to the nucleus, thereby activating
the transcription of osteoclastogenesis-related genes. In the
present study, western blot analysis revealed that the suppressive
effect of ZOL on osteoclastogenesis was dependent on the NF-κB
signalling pathway. NF-κB signalling in RAW264.7 cells was
activated by RANKL treatment but was suppressed by ZOL treatment.
In particular, pre-treatment with ZOL markedly attenuated
RANKL-induced NF-κB activation by inhibiting IκBα degradation and
the phosphorylation and nuclear translocation of p65 in RAW264.7
cells. These results suggested that ZOL inhibits RANKL-induced
osteoclast differentiation by blocking NF-κB activation.
Downstream of RANKL/RANK signalling, JNK, p38 and
ERK have been implicated as prominent regulators of various
cellular responses, such as apoptosis, differentiation and cell
proliferation (43,44). Specifically, RANKL-activated MAPKs
play vital roles in osteoclastogenesis. Thus, they are essential
molecular targets for therapeutic applications in inflammatory bone
diseases (33,45,46). The inhibition of p38, ERK and JNK
can disturb RANKL-induced osteoclastogenesis (11,47,48). In the present study, the effects
of ZOL on the MAPK signalling pathway were investigated,
demonstrating that ZOL significantly inhibited the phosphorylation
of JNK, but did not significantly inhibit ERK or p38 signalling in
RAW264.7 cells. The significance of JNK signalling in
osteoclastogenesis has been previously reported. The activated JNK
phosphorylates c-Jun and c-Fos bind to form heterodimers of AP-1, a
crucial transcription factor during osteoclast differentiation
(49). NFATc1 is another
transcription factor that plays a crucial role in osteoclast
formation by upregulating several genes responsible for osteoclast
acidification, migration and adhesion, and for the degradation of
inorganic and organic bone matrix (50). NFATc1-deficient embryonic stem
cells cannot form mature osteoclasts via RANKL exposure, and the
overexpression of NFATc1 in osteoclast precursors induces
osteoclast differentiation (39,51). The results presented in the
current study revealed that ZOL treatment not only suppressed JNK
phosphorylation, but also downregulated c-Jun levels. It was also
identified that ZOL treatment reduced RANKL-induced NFATc1
activation in a luciferase activity assay, which is consistent with
the evidence demonstrating that the stimulation of RAW264.7 cells
with RANKL significantly upregulated the mRNA and protein
expression levels of c-Fos and NFATc1, and this effect could be
significantly suppressed by ZOL treatment. Additionally, the
present study demonstrated that ZOL suppressed the expression of
specific phenotypic markers, including TRAP, RANK and CTR. This
indicated that the JNK/c-Jun/c-Fos/NFATc1 signalling axis may be
involved in the inhibitory effects of ZOL on osteoclast
differentiation.
In osteoclast precursors, RAW264.7 cells first
proliferate and are then induced to become TRAP+
mononuclear cells, which are termed preosteoclasts. These
preosteoclasts then fuse together under the regulation of the
fusion-related molecule DC-STAMP to form mature osteoclasts, and
cell-cell fusion determines osteoclast size and initiates
osteoclast bone resorptive activity (52). Without fusion-related molecules,
RAW264.7 cells will only proliferate. After the effects of these
molecules, RAW264.7 cells were found to preferentially
differentiate into osteoclasts instead of proliferating. An
anti-DC-STAMP monoclonal antibody has been reported to strongly
inhibit osteoclast formation in vitro (53). Furthermore, DC-STAMP-deficient
cells cannot differentiate into multinucleated osteoclasts and
suffer from impaired bone resorptive function (54). As the expression of DC-STAMP is
induced by RANKL/RANK signalling (38), it was hypothesized in the present
study that DC-STAMP may also be involved in the inhibitory effects
of ZOL on osteoclast differentiation. The data of the present study
revealed that ZOL treatment reduced the expression of DC-STAMP at
both the protein and mRNA levels. Therefore, ZOL may suppress both
RANKL-mediated osteoclastogenesis and bone resorptive capacity by
downregulating the expression of DC-STAMP.
As the RANKL/RANK pathway plays a key role in the
pathological processes that induce bone loss, RANKL-targeted
therapy is a valid approach for treating osteoporosis. In addition
to the results of the present study, it has been previously
demonstrated that the inhibiting effect of ZOL is involved in the
RANKL/RANK pathway (19,55). Denosumab, another effective
medication for osteoporosis, is a fully human monoclonal anti-RANKL
antibody that has high affinity and specificity for RANKL. However,
whether denosumab is a valid alternative for patients unable to
receive standard adjuvant i.v. ZOL remains controversial. Lee et
al (56) conducted a large
population-based cohort study using claims data (2010–15) from two
large US commercial insurance databases to explore ocular outcomes
in patients with osteoporosis in whom treatment was initiated with
either ZOL or denosumab. It was identified that initiation of
denosumab decreased osteoclast activity and exerted a prolonged
effect on calcium metabolism, leading to decreased calcium
deposition in the lens and lower likelihood of necessary cataract
surgery compared with that of ZOL, but that the risk of age-related
macular degeneration was similar between the two drugs. Kondo et
al (57) suggested that
sequential therapy using ZOL could suppress decreases in bone
mineral density and increase of bone turnover marker if the period
of denosumab administration was <3 years. Mori et al
(58) used a simulation model to
evaluate the effectiveness and cost of two treatment strategies. It
was found that annual i.v. ZOL was more economical than biannual
subcutaneous denosumab followed by weekly oral alendronate for 3
years. Notably, both ZOL and denosumab have been associated with
BRONJ. Ikesue et al (59)
evaluated the association between clinical characteristics and
development of BRONJ in patients who underwent dental examinations
before the initiation of treatment with denosumab or ZOL. The data
suggested that BRONJ caused by denosumab resolves faster than that
caused by ZOL. It was also reported that switching from ZOL to
denosumab markedly increased the risk of developing BRONJ in
patients with bone metastases (60). That is likely due to ZOL having
high affinity for bone hydroxyapatite, thus leading to prolonged
drug action and excessive toxic effects. In general, it remains
unknown which treatment strategy exhibits improved value from
economic and health perspectives. In particular, comprehensive
guidelines claim that there is insufficient evidence for
recommending one of these bone-targeting drugs over another for the
treatment of metastatic bone disease (61). Therefore, the clinical utilities
of these two drugs require further exploration.
Accumulating evidence has indicated that ZOL
inhibits osteoclast differentiation in vitro by affecting
various signalling pathways. For example, evidence has indicated
that ZOL acts by inhibiting farnesyl pyrophosphate synthase in the
HMGA-CoA reductase pathway, also known as the mevalonate pathway
(62–64). In addition, certain previous
studies have indicated that ZOL suppresses the non-canonical
Wnt/Ca2+/calmodulin-dependent protein kinase II pathway
(65,66). Certain studies have also
demonstrated that ZOL is involved in the RANKL/RANK pathway.
Specifically, Pan et al (67) revealed that ZOL inhibits
recruitment and osteoclastogenesis by significantly reducing the
protein expression of transmembrane RANKL. It was also found that
it does not markedly affect RANKL gene expression in
osteoblast-like cells. Thus, there are conflicting results
regarding the effects of ZOL on RANKL. In addition, Kimachi et
al (55) reported that ZOL
hinders osteoclast differentiation by inhibiting RANK expression
and the migration of RAW264.7 and bone marrow cells; it was
hypothesized that the inhibitory effects of ZOL on RANK expression
may be associated with the inhibition of the NF-κB pathway. Cheng
et al (68) further
confirmed that ZOL modulates osteoclast apoptosis through
activation of the NF-κB signalling pathway using an ovariectomised
rat model. Furthermore, ZOL has been reported to inhibit the NF-κB
pathway by promoting the deubiquitination of TRAF6 (19). Our previous study also reported
that ZOL reduces the levels of p-IκBα, p-p65 and p-JNK at different
time points following RANKL exposure in RAW264.7 cells (32). In the present study, the
expression levels of these signaling pathways were also found to be
suppressed in a concentration-dependent manner. To the best of our
knowledge, the present study also revealed for the first time that
ZOL inhibits the nuclear translocation of p65 and the levels of the
downstream factors c-Jun, c-Fos and NFATc1, thus decreasing the
expression of the fusion-related molecule DC-STAMP and other
osteoclast-specific markers in RAW264.7 cells. To a certain extent,
the present data confirmed the previous conclusion that NF-κB and
JNK signalling pathways may be involved in the inhibitory effects
of ZOL on osteoclastogenesis.
However, the present study has several limitations.
First, the effects of ZOL on other cell types, such as osteocytes
and osteoblasts, need to be revealed through future investigations.
Second, the findings of the present study need to be validated by
additional assays. For example, luciferase activity assays could be
used to further verify the activation of downstream factors; the
findings could then be compared to establish their consistency with
the present evidence. In addition, molecular docking assays could
be performed to determine whether the expression of the gene
encoding the osteoclast differentiation marker can be silenced by
ZOL. In conclusion, the results of the present study demonstrated
that ZOL suppressed both osteoclast formation and bone resorption
in vitro. Mechanistically, the study confirmed that the
inhibitory effects of ZOL occur via the inhibition of JNK and NF-κB
activation, thus decreasing the downstream expression of c-Jun,
c-Fos and NFATc1. This subsequently reduces the expression of the
fusion-related molecule DC-STAMP, as well as other osteoclastic
marker genes (Fig. 7). These
results highlight the potential usefulness of ZOL in preventing
osteoclast formation and provide further insights into the
mechanism of action of ZOL in this context. Therefore, ZOL may be a
potential target for the treatment of osteoclast-related diseases
such as osteoporosis, and thus warrants further study.
 | Figure 7.Schematic model for the action of ZOL
treatment on the RANKL/RANK signalling pathway during osteoclast
differentiation and bone resorption. The figure summarizes the
results of the present study. Vertical arrows indicate either
downregulation or inhibition of osteoclasts. TRAF6, tumour necrosis
factor-associated factor 6; ZOL, zoledronic acid; JNK, c-Jun
N-terminal kinase; ERK1/2, extracellular regulated protein kinases;
TRAP, tartrate-resistant acid phosphatase; NF-κB, nuclear
factor-κB; DC-STAMP, dendritic cell-specific transmembrane protein;
CTR, calcitonin receptor; NFATc1, nuclear factor of activated T
cells 1; RANKL, receptor activator of nuclear factor-κB ligand;
IκBα, inhibitor of κBα; MAPK, mitogen-activated protein kinase. |
Acknowledgements
All experiments were performed in the Key Laboratory
of Endemic and Ethnic diseases of Guizhou Medical University
(Guizhou, China).
Funding
The present study was supported by the Natural Science
Foundation of China (grant nos. 81660179 and 82060207).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JL and JPL conceived and designed the study; XLH and
YTC performed the experiments; CL wrote the manuscript and designed
the figures; and QZ and XMS analysed the data. XLH and JL confirm
the authenticity of all the raw data. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li DZ, Zhang QX, Dong XX, Li HD and Ma X:
Treatment with hydrogen molecules prevents RANKL-induced osteoclast
differentiation associated with inhibition of ROS formation and
inactivation of MAPK, AKT and NF-kappa B pathways in murine
RAW264.7 cells. J Bone Miner Metab. 32:494–504. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cosman F, de Beur SJ, LeBoff MS, Lewiecki
EM, Tanner B, Randall S and Lindsay R; National Osteoporosis
Foundation, : Clinician's guide to prevention and treatment of
osteoporosis. Osteoporos Int. 25:2359–2381. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang JH, Li B, Wu Q, Lv JG and Nie HY:
Echinocystic acid inhibits RANKL-induced osteoclastogenesis by
regulating NF-κB and ERK signaling pathways. Biochem Biophys Res
Commun. 477:673–677. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Madel MB, Ibáñez L, Wakkach A, de Vries
TJ, Teti A, Apparailly F and Blin-Wakkach C: Immune function and
diversity of osteoclasts in normal and pathological conditions.
Front Immunol. 10:14082019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Islam R, Bae HS, Yoon WJ, Woo KM, Baek JH,
Kim HH, Uchida T and Ryoo HM: Pin1 regulates osteoclast fusion
through suppression of the master regulator of cell fusion
DC-STAMP. J Cell Physiol. 229:2166–2174. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee JW, Kobayashi Y, Nakamichi Y, Udagawa
N, Takahashi N, Im NK, Seo HJ, Jeon WB, Yonezawa T, Cha BY and Woo
JT: Alisol-B, a novel phyto-steroid, suppresses the RANKL-induced
osteoclast formation and prevents bone loss in mice. Biochem
Pharmacol. 80:352–361. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nakashima T and Takayanagi H: New
regulation mechanisms of osteoclast differentiation. Ann NY Acad
Sci. 1240:E13–E18. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
He Y, Zhang Q, Shen Y, Chen X, Zhou F and
Peng D: Schisantherin A suppresses osteoclast formation and wear
particle-induced osteolysis via modulating RANKL signaling
pathways. Biochem Biophys Res Commun. 449:344–350. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yen ML, Hsu PN, Liao HJ, Lee BH and Tsai
HF: TRAF-6 dependent signaling pathway is essential for TNF-related
apoptosis-inducing ligand (TRAIL) induces osteoclast
differentiation. PLoS One. 7:e380482012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Léotoing L, Wauquier F, Guicheux J,
Miot-Noirault E, Wittrant Y and Coxam V: The polyphenol fisetin
protects bone by repressing NF-κB and MKP-1-dependent signaling
pathways in osteoclasts. PLoS One. 8:e683882013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cheng B, Li J, Du J, Lv X, Weng L and Ling
C: Ginsenoside Rb1 inhibits osteoclastogenesis by modulating NF-κB
and MAPKs pathways. Food Chem Toxicol. 50:1610–1615. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Soysa NS, Alles N, Aoki K and Ohya K:
Osteoclast formation and differentiation: An overview. J Med Dent
Sci. 59:65–74. 2012.PubMed/NCBI
|
|
13
|
Lieben L: Bone: The concept of
RANKL-independent osteoclastogenesis refuted. Nat Rev Rheumatol.
12:6232016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cai XJ, Wang Z, Cao JW, Ni JJ, Xu YY, Yao
J, Xu H, Liu F and Yang GY: Anti-angiogenic and anti-tumor effects
of metronomic use of novel liposomal zoledronic acid depletes
tumor-associated macrophages in triple negative breast cancer.
Oncotarget. 8:84248–84257. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Endo Y, Kumamoto H, Nakamura M, Sugawara
S, Takano-Yamamoto T, Sasaki K and Takahashi T: Underlying
mechanisms and therapeutic strategies for bisphosphonate-related
osteonecrosis of the jaw (BRONJ). Biol Pharm Bull. 40:739–750.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Elsayed R, Abraham P, Awad ME, Kurago Z,
Baladhandayutham B, Whitford GM, Pashley DH, McKenna CE and
Elsalanty ME: Removal of matrix-bound zoledronate prevents
post-extraction osteonecrosis of the jaw by rescuing osteoclast
function. Bone. 110:141–149. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Russell RG: Bisphosphonates: The first 40
years. Bone. 49:2–19. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qiao H, Wang TY, Yu ZF, Han XG, Liu XQ,
Wang YG, Fan QM, Qin A and Tang TT: Structural simulation of
adenosine phosphate via plumbagin and zoledronic acid competitively
targets JNK/Erk to synergistically attenuate osteoclastogenesis in
a breast cancer model. Cell Death Dis. 7:e20942016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li X, Sun W, Li J, Wang M, Zhang H, Pei L,
Boyce BF, Wang Z and Xing L: Clomipramine causes osteoporosis by
promoting osteoclastogenesis via E3 ligase Itch, which is prevented
by zoledronic acid. Sci Rep. 7:413582017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li P, Yang H, Jia N, Jin X, Xu D and Shen
Y: Experimental study on inhibitory effect of zoledronic acid on
the action style of the osteoclast. Sheng Wu Yi Xue Gong Cheng Xue
Za Zhi. 34:78–82. 2017.(In Chinese). PubMed/NCBI
|
|
21
|
de Oliveira MA, Asahi DA, Silveira CAE,
Lima LAPA, Glick M and Gallottini M: The effects of zoledronic acid
and dexamethasone on osseointegration of endosseous implants:
Histological and histomorphometrical evaluation in rats. Clin Oral
Implants Res. 26:e17–e21. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Weber JBB, Camilotti RS, Jasper J,
Casagrande LCO and Maito FLDM: Effect of low-level laser therapy on
tissue repair after dental extraction in rats administered
zoledronic acid and dexamethasone. J Biomed Opt. 22:580012017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Allen MR, Chu TM and Ruggiero SL: Absence
of exposed bone following dental extraction in beagle dogs treated
with 9 months of high-dose zoledronic acid combined with
dexamethasone. J Oral Maxillofac Surg. 71:1017–1026. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Subramanian G, Fritton JC, Iyer S and Quek
SY: Atypical dental implant failure with long-term bisphosphonate
treatment-akin to atypical fractures? Oral Surg Oral Med Oral
Pathol Oral Radiol. 114:e30–e35. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qi M, Hu J, Li J, Li J, Dong W, Feng X and
Yu J: Effect of zoledronate acid treatment on osseointegration and
fixation of implants in autologous iliac bone grafts in
ovariectomized rabbits. Bone. 50:119–127. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Khosla S and Shane E: A crisis in the
treatment of osteoporosis. J Bone Miner Res. 31:1485–1487. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim SC, Kim DH, Mogun H, Eddings W,
Polinski JM, Franklin JM and Solomon DH: Impact of the U.S. food
and drug administration's safety-related announcements on the use
of bisphosphonates after hip fracture. J Bone Miner Res.
31:1536–1540. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nakagawa T, Ohta K, Uetsuki R, Kato H,
Naruse T, Murodumi H, Yokoyama S, Sakuma M, Ono S and Takechi M:
Zoledronate inhibits osteoclast differentiation via suppressing
vascular endothelial growth factor receptor 2 expression. Biochem
Genet. 58:473–489. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dundar S, Yaman F, Gecor O, Cakmak O,
Kirtay M, Yildirim TT, Karaman T and Benlidayi ME: Effects of local
and systemic zoledronic acid application on titanium implant
osseointegration: An experimental study conducted on two surface
types. J Craniofac Surg. 28:935–938. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chadha GK, Ahmadieh A, Kumar S and
Sedghizadeh PP: Osseointegration of dental implants and
osteonecrosis of the jaw in patients treated with bisphosphonate
therapy: A systematic review. J Oral Implantol. 39:510–520. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim HJ, Kim HJ, Choi Y, Bae MK, Hwang DS,
Shin SH and Lee JY: Zoledronate enhances osteocyte-mediated
osteoclast differentiation by IL-6/RANKL axis. Int J Mol Sci.
2:14672019. View Article : Google Scholar
|
|
32
|
Huang XL, Huang LY, Cheng YT, Li F, Zhou
Q, Wu C, Shi QH, Guan ZZ, Liao J and Hong W: Zoledronic acid
inhibits osteoclast differentiation and function through the
regulation of NF-κB and JNK signalling pathways. Int J Mol Med.
44:582–592. 2019.PubMed/NCBI
|
|
33
|
Chen G, Huang L, Wu X, Liu X, Xu Q, Li F,
Dai M and Zhang B: Adiponectin inhibits osteoclastogenesis by
suppressing NF-κB and p38 signaling pathways. Biochem Biophys Res
Commun. 503:2075–2082. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jiao Z, Xu W, Zheng J, Shen P, Qin A,
Zhang S and Yang C: Kaempferide prevents titanium particle induced
osteolysis by suppressing JNK activation during osteoclast
formation. Sci Rep. 7:166652017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shibata K, Yoshimura Y, Kikuiri T,
Hasegawa T, Taniguchi Y, Deyama Y, Suzuki K and Iida J: Effect of
the release from mechanical stress on osteoclastogenesis in
RAW264.7 cells. Int J Mol Med. 28:73–79. 2011.PubMed/NCBI
|
|
37
|
Tsubaki M, Komai M, Itoh T, Imano M,
Sakamoto K, Shimaoka H, Takeda T, Ogawa N, Mashimo K, Fujiwara D,
et al: Nitrogen-containing bisphosphonates inhibit RANKL- and
M-CSF-induced osteoclast formation through the inhibition of ERK1/2
and Akt activation. J Biomed Sci. 21:102014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kang MR, Jo SA, Yoon YD, Park KH, Oh SJ,
Yun J, Lee CW, Nam KH, Kim Y, Han SB, et al: Agelasine D suppresses
RANKL-induced osteoclastogenesis via down-regulation of c-Fos,
NFATc1 and NF-κB. Mar Drugs. 12:5643–5656. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kong X, Wu W, Yang Y, Wan H, Li X, Zhong
M, Zhao H, Su X, Jia S, Ju D and Lin N: Total saponin from anemone
flaccida Fr. Schmidt abrogates osteoclast differentiation and bone
resorption via the inhibition of RANKL-induced NF-κB, JNK and p38
MAPKs activation. J Transl Med. 13:912015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Prideaux M, Findlay DM and Atkins GJ:
Osteocytes: The master cells in bone remodelling. Curr Opin
Pharmacol. 28:24–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tseng HC, Kanayama K, Kaur K, Park SH,
Park S, Kozlowska A, Sun S, McKenna CE, Nishimura I and Jewett A:
Bisphosphonate-induced differential modulation of immune cell
function in gingiva and bone marrow in vivo: Role in
osteoclast-mediated NK cell activation. Oncotarget. 6:20002–20025.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Otero JE, Chen T, Zhang K and Abu-Amer Y:
Constitutively active canonical NF-κB pathway induces severe bone
loss in mice. PLoS One. 7:e386942012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kim HK, Kim MG and Leem KH: Osteogenic
activity of collagen peptide via ERK/MAPK pathway mediated boosting
of collagen synthesis and its therapeutic efficacy in osteoporotic
bone by back-scattered electron imaging and microarchitecture
analysis. Molecules. 18:15474–15489. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sui X, Kong N, Ye L, Han W, Zhou J, Zhang
Q, He C and Pan H: p38 and JNK MAPK pathways control the balance of
apoptosis and autophagy in response to chemotherapeutic agents.
Cancer Lett. 344:174–179. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhai ZJ, Li HW, Liu GW, Qu XH, Tian B, Yan
W, Lin Z, Tang TT, Qin A and Dai KR: Andrographolide suppresses
RANKL-induced osteoclastogenesis in vitro and prevents inflammatory
bone loss in vivo. Br J Pharmacol. 171:663–675. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Huh JE, Jung IT, Choi J, Baek YH, Lee JD,
Park DS and Choi DY: The natural flavonoid galangin inhibits
osteoclastic bone destruction and osteoclastogenesis by suppressing
NF-κB in collagen-induced arthritis and bone marrow-derived
macrophages. Eur J Pharmacol. 698:57–66. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yamanaka Y, Clohisy JC, Ito H, Matsuno T
and Abu-Amer Y: Blockade of JNK and NFAT pathways attenuates
orthopedic particle-stimulated osteoclastogenesis of human
osteoclast precursors and murine calvarial osteolysis. J Orthop
Res. 31:67–72. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Park JH, Lee NK and Lee SY: Current
understanding of RANK signaling in osteoclast differentiation and
maturation. Mol Cells. 40:706–713. 2017.PubMed/NCBI
|
|
49
|
Liu X, Qu X, Wu C, Zhai Z, Tian B, Li H,
Ouyang Z, Xu X, Wang W, Fan Q, et al: The effect of enoxacin on
osteoclastogenesis and reduction of titanium particle-induced
osteolysis via suppression of JNK signaling pathway. Biomaterials.
35:5721–5730. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhao Q, Wang X, Liu Y, He A and Jia R:
NFATc1: Functions in osteoclasts. Int J Biochem Cell Biol.
42:576–579. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lee JH, Jin H, Shim HE, Kim HN, Ha H and
Lee ZH: Epigallocatechin-3-gallate inhibits osteoclastogenesis by
down-regulating c-Fos expression and suppressing the nuclear
factor-kappaB signal. Mol Pharmacol. 77:17–25. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhang C, Dou CE, Xu J and Dong S:
DC-STAMP, the key fusion-mediating molecule in osteoclastogenesis.
J Cell Physiol. 229:1330–1335. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chiu YH, Mensah KA, Schwarz EM, Ju Y,
Takahata M, Feng C, McMahon LA, Hicks DG, Panepento B, Keng PC and
Ritchlin CT: Regulation of human osteoclast development by
dendritic cell-specific transmembrane protein (DC-STAMP). J Bone
Miner Res. 27:79–92. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zeng XZ, He LG, Wang S, Wang K, Zhang YY,
Tao L, Li XJ and Liu SW: Aconine inhibits RANKL-induced osteoclast
differentiation in RAW264.7 cells by suppressing NF-κB and NFATc1
activation and DC-STAMP expression. Acta Pharmacol Sin. 37:255–263.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kimachi K, Kajiya H, Nakayama S, Ikebe T
and Okabe K: Zoledronic acid inhibits RANK expression and migration
of osteoclast precursors during osteoclastogenesis. Naunyn
Schmiedebergs Arch Pharmacol. 383:297–308. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lee H, Jin Y, Roh M, Tsacogianis TN, Park
S, Choi NK and Kim SC: Risk of cataract surgery and age-related
macular degeneration after initiation of denosumab vs zoledronic
acid for osteoporosis: A multi-database cohort study. Drugs Aging.
37:311–320. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kondo H, Okimoto N, Yoshioka T, Akahoshi
S, Fuse Y, Ogawa T, Okazaki Y, Katae Y, Tsukamoto M, Yamanaka Y, et
al: Zoledronic acid sequential therapy could avoid disadvantages
due to the discontinuation of less than 3-year denosumab treatment.
J Bone Miner Metab. 38:894–902. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Mori T, Crandall CJ, Fujii T and Ganz DA:
Cost-effectiveness of zoledronic acid compared with sequential
denosumab/alendronate for older osteoporotic women in Japan. Arch
Osteoporos. 16:1132021. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Ikesue H, Mouri M, Tomita H, Hirabatake M,
Ikemura M, Muroi N, Yamamoto S, Takenobu T, Tomii K, Kawakita M, et
al: Associated characteristics and treatment outcomes of
medication-related osteonecrosis of the jaw in patients receiving
denosumab or zoledronic acid for bone metastases. Support Care
Cancer. 29:4763–4772. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ikesue H, Doi K, Morimoto M, Hirabatake M,
Muroi N, Yamamoto S, Takenobu T and Hashida T: Switching from
zoledronic acid to denosumab increases the risk for developing
medication-related osteonecrosis of the jaw in patients with bone
metastases. Cancer Chemother Pharmacol. 87:871–877. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Chen C, Li R, Yang T, Ma L, Zhou S, Li M,
Zhou Y and Cui Y: Denosumab versus zoledronic acid in the
prevention of skeletal-related events in vulnerable cancer
patients: A meta-analysis of randomized, controlled trials. Clin
Ther. 42:1494–1507.e1. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Yang G, Singh S, Chen Y, Hamadeh IS,
Langaee T, McDonough CW, Holliday LS, Lamba JK, Moreb JS, Katz J
and Gong Y: Pharmacogenomics of osteonecrosis of the jaw. Bone.
124:75–82. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Fliefel RM, Entekhabi SA, Ehrenfeld M and
Otto S: Geranylgeraniol (GGOH) as a mevalonate pathway activator in
the rescue of bone cells treated with zoledronic acid: An in vitro
study. Stem Cells Int. 2019:43513272019. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Nakagawa T, Ohta K, Kubozono K, Ishida Y,
Naruse T, Takechi M and Kamata N: Zoledronate inhibits receptor
activator of nuclear factor kappa-B ligand-induced osteoclast
differentiation via suppression of expression of nuclear factor of
activated T-cell c1 and carbonic anhydrase 2. Arch Oral Biol.
60:557–565. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Cui P, Liu H, Sun J, Amizuka N, Sun Q and
Li M: Zoledronate promotes bone formation by blocking
osteocyte-osteoblast communication during bone defect healing.
Histol Histopathol. 33:89–99. 2018.PubMed/NCBI
|
|
66
|
Zhang J, Park J, Lee JW, Kwon YD and Kim
EC: Bisphosphonates hinder osteoblastic/osteoclastic
differentiation in the maxillary sinus mucosa-derived stem cells.
Clin Oral Investig. 22:1933–1943. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Pan B, Farrugia AN, To LB, Findlay DM,
Green J, Lynch K and Zannettino AC: The nitrogen-containing
bisphosphonate, zoledronic acid, influences RANKL expression in
human osteoblast-like cells by activating TNF-alpha converting
enzyme (TACE). J Bone Miner Res. 19:147–154. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Cheng YT, Liao J, Zhou Q, Huo H, Zellmer
L, Tang ZL, Ma H, Hong W and Liao DJ: Zoledronic acid modulates
osteoclast apoptosis through activation of the NF-κB signaling
pathway in ovariectomized rats. Exp Biol Med (Maywood).
246:1727–1739. 2021. View Article : Google Scholar : PubMed/NCBI
|