Introduction
Asthma is one of the most common chronic airway
inflammatory conditions in adults and children (1), affecting 5–10% of the global
population (2). It is
predominantly mediated by T helper (Th) 2 cells and characterized
by eosinophilic airway inflammation, mucus hypersecretion and
airway hyperresponsiveness (AHR) (1). Asthma is an important public health
issue owing to its increasing incidence, with the incidence
increasing from 9.3 to 11.5% in young adults between 2008 and 2016
(3) and substantial economic
burden (4). Patients with asthma
undergoing surgery are at great risk of perioperative morbidity and
mortality due to bronchospasms and hypoxemia (5). Although medical treatment that can
relax bronchial smooth muscle (6)
or inhibit leukotriene production (7) is available, the development of
additional approaches to relieve these life-threatening symptoms
during the perioperative period may be beneficial for patients with
asthma.
Allergic airway inflammatory responses are
characterized by eosinophil and Th2 cell infiltration, secretion of
type-2 cytokines and elevated immunoglobulin E levels (8). Cytokines derived from Th2 cells,
including IL-4, IL-5 and IL-13, enhance airway eosinophilia, mucus
production and AHR, and are involved in the pathogenesis of asthma
(9). Therefore, targeting these
cytokines could represent an effective therapeutic approach, as
previous studies have demonstrated that reduced levels of these
cytokines can relieve the typical symptoms of asthma (10,11).
Various signaling pathways have been studied with
respect to the pathophysiology of asthma. Among them, the toll-like
receptor (TLR) family of conserved pattern-recognition receptors,
has been demonstrated to serve a pivotal role in asthma (12,13). TLR4, in particular, can be
activated by bacterial lipopolysaccharide, which is the main
component of the cell wall of Gram-negative bacteria (14). Activation of TLR4 can initiate
inflammatory responses by targeting myeloid differentiation primary
response gene 88 and NF-κB. The TLR4/NF-κB pathway has been
demonstrated to trigger airway inflammation and AHR by promoting
cytokine production (15–17).
Dexmedetomidine (DEX) is a selective α2 adrenoceptor
that is widely used in the clinic, as it can induce sedation and
analgesia, as well as reduce anxiety, without respiratory
depression (18). In addition,
the anti-inflammatory properties of DEX have also been examined,
since they may attenuate acute multiple organ injury (19). Several studies have reported that
DEX may have a protective effect on pulmonary dysfunction (20,21). However, whether DEX could
alleviate AHR and allergic airway inflammation in asthma is
unclear, and the potential underlying mechanism remains unknown.
Therefore, the aim of the present study was to evaluate the effect
of DEX on airway inflammation and AHR in allergic asthma and to
examine its potential effect on the TLR4/NF-κB pathway.
Materials and methods
Animals
In the present study, 7-week-old specific
pathogen-free female BALB/c mice (weight, 18–22 g) purchased from
Beijing Vital River Laboratory Animal Technology Co., Ltd. Mice
were housed under standard laboratory conditions at 25°C with 50±5%
humidity and 12-h light/dark cycles in the experimental animal
center of the Plastic Surgery Hospital (Chinese Academy of Medical
Sciences and Peking Union Medical College; Beijing, China) for 1
week prior to the start of the experiments The animals were
provided with sterilized food and water ad libitum. All
experimental procedures were performed according to the People's
Republic of China Animal Protection Law. Experimental animals were
handled under a protocol approved by the Institutional Animal Care
and Use Committee of Plastic Surgery Hospital, Chinese Academy of
Medical Sciences and Peking Union Medical College.
Experimental protocols
A total of 30 female BALB/c mice were divided into
six groups (n=5 mice/group): Control, OVA, OVA + DEX (20, 30 or 50
µg/kg) and OVA + TAK-242 (an inhibitor of TLR4) groups. All mice
except those in the control group were sensitized on days 1, 7 and
14 with an intraperitoneal (i.p.) injection of 100 µg ovalbumin
(OVA; Sigma-Aldrich; Merck KGaA), 10 mg aluminum hydroxide
(Sigma-Aldrich; Merck KGaA) in saline (200 µl). The control group
was injected with 200 µl saline. From day 21 to 29, the mice in the
OVA + DEX (Jiangsu Hengrui Medicine Co., Ltd.) groups received
daily i.p. injections of 20, 30 or 50 µg/kg DEX, and those in the
OVA + TAK-242 (MedChemExpress) group received 3 mg/kg TAK-242 daily
i.p. injection; these mice were also challenged by intranasal
administration of 200 µg OVA in 30 µl saline 60 min after the i.p.
injection. From day 21 to 29, the control group was intranasally
administered 30 µl saline once a day, and each OVA group received a
daily intranasal administration of 200 µg OVA in 30 µl saline. The
mice were sacrificed 24 h after the last challenge. After airway
resistance measurement, the mice were euthanized using an i.p.
administered overdose of 2% pentobarbital sodium (150 mg/kg);
bronchoalveolar lavage fluid (BALF) and lung tissue were then
collected.
Histological analysis of lung
tissue
Following BALF collection, lung tissue samples were
fixed at room temperature with 4% paraformaldehyde for 48 h, then
embedded in paraffin. A series of 5-µm thick lung sections were
prepared for hematoxylin and eosin (H&E) and periodic
acid-Schiff (PAS) staining (Beijing Solarbio Science &
Technology Co., Ltd.). The slides were deparaffinized in xylene and
rehydrated in descending alcohol solutions. H&E staining was
performed at room temperature to assess inflammatory cell
infiltration. Briefly, cells were stained with hematoxylin for 1
min, washed with tap water five times, incubated with blue nuclei
in 1X PBS for 1 min, washed three times with distilled water,
counterstained in alcoholic-eosin for 1 min and then dehydrated
using an ascending alcohol series. Subsequently, xylene clearing
was performed. PAS staining was performed at room temperature to
assess goblet cell hyperplasia. Following oxidization in 0.5%
periodic acid solution for 6 min and rinsing with distilled water,
the slides were covered by Schiff regent for 15 min, washed with
tap water for 5 min, stained with hematoxylin for 50 sec, rinsed
with running water for 2 min and then differentiated with
hydrochloric acid for 3 sec. The percentage of PAS-stained cells in
the airway epithelium is indicative of the production of mucus
(22). A total of five fields of
view were examined by two experienced pathologists blindly at ×200
magnification using a BX53 upright fluorescence microscope (Olympus
Corporation) under the mode of bright-field imaging. The evaluation
of peribronchial inflammation was based on a modified six-point
scoring system (23) as follows:
0, normal; 1, a few cells; 2, a ring of inflammatory cells
consisting of one cell layer; 3, a ring of inflammatory cells
consisting of two-four cell layers; 4, a ring of inflammatory cells
consisting of five-seven cell layers; 5, a ring of inflammatory
cells consisting of eight-ten cell layers; 6, a ring of
inflammatory cells consisting of >10 cell layers. A modified
scoring system (24) was used for
the abundance of PAS-positive mucus-containing cells in each airway
as follows: 0, <2%; 1, ≥2% and <20%; 2, ≥20% and <40%; 3,
≥40% and <60%; 4, ≥60% and <80%; and 5, ≥80% PAS-positive
cells.
Analysis of BALF
The mice were anaesthetized with 2% pentobarbital
sodium (150 mg/kg) 24 h after the last challenge. After inserting a
catheter into the trachea, the lungs were flushed with 0.8 ml cold
PBS three times, and 85–95% of the lavage volume was collected
through the catheter. BALF was centrifuged at 187 × g at 4°C for 10
min. The supernatant was collected and stored at −80°C until
analyzed by ELISA. The total count of inflammatory cells in the
BALF was determined using a chamber slide and the eosinophil counts
were determined using ImageJ software (version 1.8.0; National
Institutes of Health) by Wright-Giemsa staining (Beijing Solarbio
Science & Technology Co., Ltd.) at room temperature for 1.5
min.
ELISA
Commercial ELISA kits (IL-4, cat. no. EK0405; IL-5,
cat. no. EK0408; and IL-13, cat. no. EK0425; all from Boster
Biological Technology) were used to detect the levels of IL-4, IL-5
and IL-13 in BALF according to the manufacturer's protocol. The
optical density was spectrophotometrically measured at 450 nm using
a multimode plate reader (PerkinElmer, Inc.).
Assessment of AHR
AHR was detected 24 h after the last challenge. The
mice were anesthetized with 2% pentobarbital sodium (50 mg/kg) and
the airway resistance was measured using FlexiVent (SCIREQ).
Aerosolized methacholine (Mch) at different concentrations (0, 6,
12, 24 and 48 mg/ml) was continuously nebulized through a catheter
in each animal for 6 min. The airway resistance was collected every
minute and the final result of each concentration for each animal
was presented as the average of 6 min. The airway resistance is
presented as respiratory resistance (Rrs) in
cmH2O/ml/sec. The results are presented as the
percentage increase in Rrs at each concentration of Mch over the
baseline (Rrs in the control mice at different concentrations of
Mch).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from homogenized lung tissue
(50 mg) using TRIzol® (Ambion; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. The RNA pellet was
resuspended in DNase/RNase-free water (Beijing Solarbio Science
& Technology Co., Ltd.). RNA concentration was measured at a
wavelength of 260 nm using a NanoDrop® 2000 ultraviolet
spectrophotometer (Thermo Fisher Scientific, Inc.), and the quality
of RNA was evaluated by verifying that the ratio at 260/280 nm was
1.8-2.0. RT was carried out with 5 µg RNA using the
TransScript® First-Strand cDNA Synthesis SuperMix
(Beijing Transgen Biotech Co., Ltd.) in 20-µl reaction volumes,
according to the manufacturer's protocol. The qPCR primers
(Invitrogen; Thermo Fisher Scientific, Inc.) were as follows: IL-4
forward, 5′-CTCACAGCAACGAAGAACACC-3′ and reverse,
5′-CTGCAGCTCCATGAGAACACT-3′; IL-5 forward,
5′-AGAATCAAACTGTCCGTGGGG-3′ and reverse,
5′-TCCTCGCCACACTTCTCTTTT-3′; IL-13 forward,
5′-CTCTTGCTTGCCTTGGTGGTC-3′ and reverse,
5′-TGTGATGTTGCTCAGCTCCTC-3′; TLR4 forward,
5′-TCATCAGTGTATCGGTGGTCAG-3′ and reverse,
5′-TTTCCATCCAACAGGGCTTT-3′; NF-κB forward,
5′-GGGGCCTGCAAAGGTTATC-3′ and reverse, 5′-TGCTGTTACGGTGCATACCC-3′;
and β-actin forward, 5′-CTCTTTTCCAGCCTTCCTTCTT-3′ and reverse,
5′-AGGTCTTTACGGATGTCAACGT-3′. The relative expression levels of the
IL-4, IL-5, IL-13, TLR4 and NF-κB end-products were normalized to
those of β-actin. qPCR was carried out using
LightCycler® 480 SYBR Green I Master mix (Roche
Diagnostics GmbH) on a LightCycler® 96 Instrument (Roche
Diagnostics GmbH). The following thermocycling conditions were used
for qPCR: Initial denaturation at 95°C for 180 sec; followed by a
two-step amplification of 40 cycles of denaturation at 95°C for 10
sec and extension at 60°C for 30 sec. The data were analyzed using
the 2−ΔΔCq method (25) and LightCycler® 96
software version 1.1 (Roche Diagnostics GmbH).
Immunohistochemistry
Paraffin-embedded lung tissue blocks were cut into
5-µm sections, which were used for TLR4, NF-κB and phosphorylated
(p)NF-κB protein detection. For immunohistochemical staining, the
anti-TLR4 (cat. no. K003881P; 1:100) and anti-NF-κB (cat. no.
K003592P; 1:50) primary antibodies and the SABC (Rabbit IgG)-POD
kit (cat. no. SA0021) were obtained from Beijing Solarbio Science
& Technology Co. Ltd. Anti-p-NF-κB (cat. no. 3033T; 1:100)
primary antibody was obtained from Cell Signaling Technology, Inc.
The slides were deparaffinized in xylene and rehydrated in a
descending alcohol series, and the endogenous peroxidase activity
was quenched at room temperature for 8 min using 0.3% hydrogen
peroxide. The sections were boiled in 0.01 mol/l sodium citrate
buffer (pH 6.0) in a microwave oven for 12 min for antigen
retrieval, then rinsed three times with PBS (pH 7.2-7.6) for 5 min.
After blocking nonspecific protein binding with 5% BSA (cat. no.
P1621-25; Applygen Technologies, Inc.) at room temperature for 30
min, the sections were incubated for 12 h with primary antibodies
at 4°C. The sections were then incubated with biotinylated goat
anti-rabbit IgG (cat. no. SE134; 1:100; Beijing Solarbio Science
& Technology Co., Ltd.) for 60 min at room temperature, and all
the results were detected with DAB chromogenic solution at room
temperature for 4–5 min. After rinsing three times with PBS (pH
7.2-7.6) for 5 min, the slides were stained at room temperature
with hematoxylin for 50 sec, rinsed with running water for 2 min,
differentiated with hydrochloric acid for 3 sec. The percentage of
positively stained area was calculated by two independent
pathologists using ImageJ software and the IHC Profiler plugin
(version 1.8.0; National Institutes of Health).
Statistical analysis
The experiments were performed independently three
times. Quantitative data are presented as the mean ± SD and were
analyzed using one-way ANOVA followed by Bonferroni's multiple
comparisons test. Ordinal data are presented as the median +
interquartile range and were analyzed using Kruskal-Wallis followed
by Dunn's multiple comparisons test. Graphs were generated and data
were analyzed using GraphPad Prism (version 6; GraphPad Software,
Inc.). P<0.05 was considered to indicate a statistically
significant difference.
Results
DEX reduces AHR and inflammatory cell
infiltration in BALF
To assess the effect of DEX on inflammatory cell
infiltration in the airway, the number of inflammatory cells in
BALF was analyzed. Compared with the control group, the number of
total cells and eosinophils in BALF were significantly increased in
the OVA group (Fig. 1A and B).
However, the number of total cells and eosinophils in the OVA + DEX
(30 and 50 µg/kg) groups were reduced compared with the OVA group,
whereas pretreatment with DEX at 20 µg/kg did not decrease the
number of eosinophils (Fig. 1B).
The dose of 30 µg/kg DEX (DEX30) resulted in the largest
decrease.
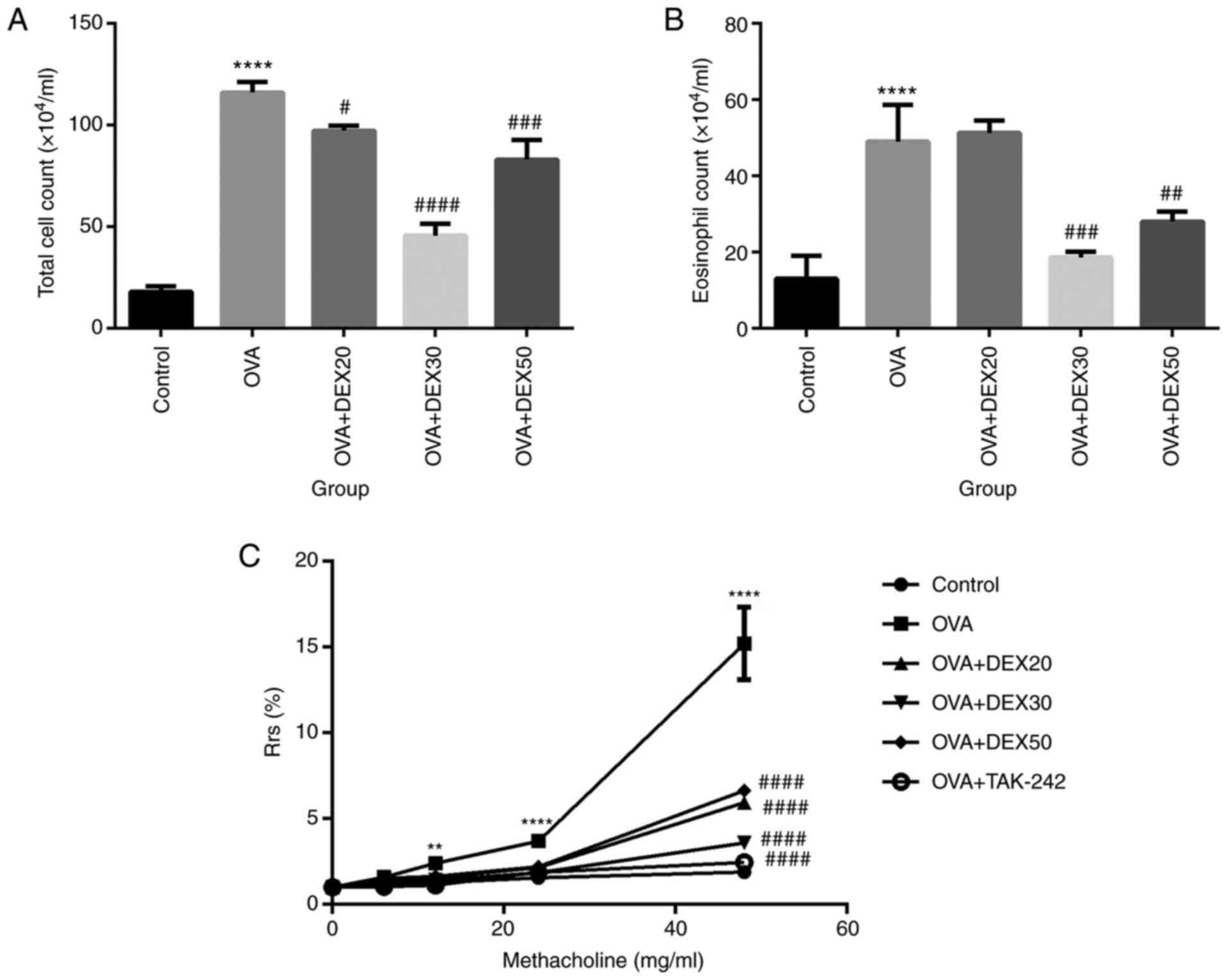 | Figure 1.Effect of DEX on the infiltration of
inflammatory cells in BALF and AHR. Number of (A) total cells and
(B) eosinophils in BALF were measured by Wright-Giemsa staining.
(C) AHR in OVA-induced asthmatic mice treated with DEX and TAK-242.
Mice were nebulized with different concentrations of methacholine
(0, 6, 12, 24 and 48 mg/ml). Results are presented as the
percentage increase in Rrs over the baseline, and the baseline Rrs
of the control group was defined as 100%. Data are presented as the
mean ± SD (n=3). **P<0.01 and ****P<0.0001 vs. control;
#P<0.05, ##P<0.01,
###P<0.001 and ####P<0.0001 vs. OVA.
AHR, airway hyperresponsiveness; BALF, bronchoalveolar lavage
fluid; DEX, dexmedetomidine; OVA, ovalbumin; Rrs, respiratory
resistance. |
Compared with the control group, the mice that
received OVA alone exhibited increased Rrs in response to inhaled
Mch at all concentrations. Compared with the OVA group, the various
DEX treatments significantly reduced Rrs. The OVA + DEX30 group
exhibited the largest reduction in AHR (Fig. 1C).
These results suggested that treatment with DEX30
inhibited the recruitment of inflammatory cells in BALF and reduced
AHR in a murine OVA-induced asthma model.
DEX alleviates airway inflammation and
mucus overproduction
Since inflammatory cell recruitment and mucus
overproduction are the main characteristics of allergic asthma
(9), the subsequent experiments
aimed to determine whether treatment with DEX could attenuate these
symptoms. Inflammatory cell infiltration was assessed using H&E
staining (Fig. 2A). Compared with
the control group, lung tissue from mice in the OVA group exhibited
a significantly higher number of inflammatory cells (Fig. 2B). However, treatment with DEX30
significantly inhibited inflammatory cell infiltration compared
with the OVA group, and DEX at 20 and 50 µg/kg showed inhibitory
effects on inflammatory infiltration, but these effects were not
significant.
Mucus production in goblet cells was assessed using
PAS staining. The percentage of PAS-positive cells in the OVA group
was significantly higher than that of the control group, and
treatment with DEX30 reduced the production of mucus in the airway
compared with the OVA group, while pretreatment with DEX at 20 and
50 µg/kg displayed an inhibitory effect on mucus production that
was not statistically significance (Fig. 2C and D). Thus, 30 µg/kg DEX could
attenuate airway inflammation and mucus overproduction in the
murine OVA-induced asthma model.
DEX inhibits the production of
inflammatory cytokines in the murine OVA-induced asthma model
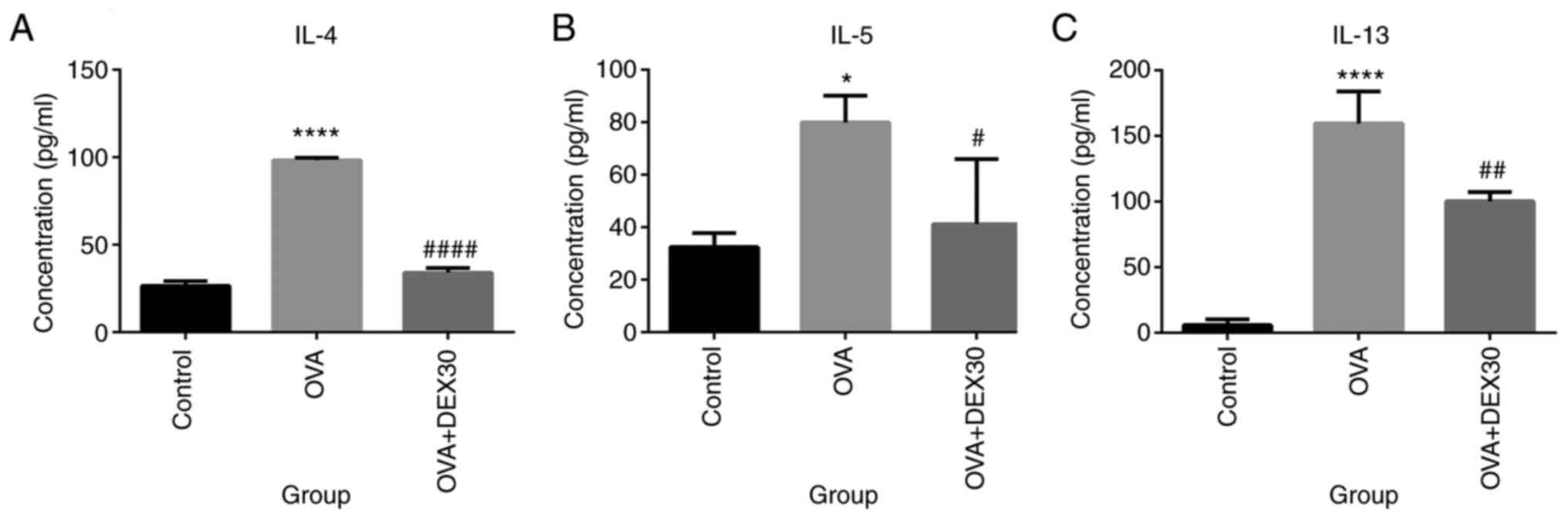
The levels of pro-inflammatory cytokines were
detected in BALF from mice in the Control, OVA and OVA + DEX30
groups. As presented in Fig. 3,
IL-4, IL-5 and IL-13 levels in the OVA group were significantly
higher compared with those in the control group. However, treatment
with DEX30 significantly reduced the levels of these cytokines
compared with the OVA group.
Furthermore, the mRNA expression levels of IL-4,
IL-5 and IL-13 in the lung tissue were also examined (Fig. 4). IL-4, IL-5 and IL-13 mRNA
expression levels in the lung tissue of the OVA group were
significantly higher compared with those of the control group.
Treatment with DEX30 significantly decreased the levels of IL-4,
IL-5 and IL-13 mRNA compared with the OVA group.
TAK-242 attenuates airway inflammation
and AHR in asthma model mice
It was examined whether AHR and airway inflammation
in the OVA-induced asthma model could be ameliorated by TAK-242.
The i.p. injection of TAK-242 or DEX before OVA challenge reduced
airway resistance in response to inhaled Mch (Fig. 1C), and reduced the total number of
cells and eosinophils in the BALF (Fig. 5A). To examine the effect of
TAK-242 on airway inflammation, H&E and PAS staining were
performed to detect inflammatory cell infiltration and the
production of mucus in the lung, respectively. Administration of
TAK-242 or DEX attenuated OVA-induced airway inflammation by
reducing the number of infiltrating inflammatory cells in the
airway (Fig. 5D and F) and the
production of mucus (Fig. 5E and
G). In addition, ELISA and RT-qPCR were used to assess
pro-inflammatory cytokine levels in BALF and mRNA levels in the
lung tissue, respectively. Both TAK-242 and DEX30 treated groups
exhibited decreased levels of inflammatory cytokines in BALF
(Fig. 5B) and lower expression
levels of IL-4, IL-5 and IL-13 mRNA (Fig. 5C) in the lung tissue. These
results indicated that the inhibitor of TLR4 (TAK-242) and DEX
could alleviate the asthmatic symptoms in the murine OVA-induced
asthma model to a similar extent.
 | Figure 5.Effect of TAK-242 and DEX on the
infiltration of inflammatory cells in BALF, and inflammatory
cytokine expression and histological analysis of lung tissues. (A)
Number of total cells and eosinophils in BALF were measured by
Wright-Giemsa staining. (B) Concentrations of IL-4, IL-5 and IL-13
in BALF were determined by ELISA. (C) Gene expression levels of
IL-4, IL-5 and IL-13 in the lung tissues were evaluated by reverse
transcription-quantitative PCR. (D) Lung sections were stained with
H&E to evaluate inflammatory cells. (E) PAS staining was used
to evaluate mucus in the lung tissues. (F) Histological scoring of
inflammatory cell infiltration was based on the morphological
structure. (G) Histological scoring of PAS-positive rate was based
on the morphological structure. Magnification, ×200; scale bar, 100
µm. Quantitative data are presented as the mean ± SD (n=3), and
ordinal data are presented as the median + interquartile range
(n=5). **P<0.01, ***P<0.001 and ****P<0.0001 vs. control;
#P<0.05, ##P<0.01,
###P<0.001 and ####P<0.0001 vs. OVA.
BALF, bronchoalveolar lavage fluid; DEX, dexmedetomidine; OVA,
ovalbumin; PAS, periodic acid-Schiff. |
DEX inhibits the TLR4/NF-κB
pathway
TLR4 and NF-κB serve an important role in
inflammatory responses by promoting the transcription of various
pro-inflammatory cytokines (26).
Immunohistochemistry was performed to detect the expression of
TLR4, NF-κB and p-NF-κB in the lung tissue. As shown in Fig. 6A and B, the expression of TLR4,
NF-κB and p-NF-κB was increased in the OVA group compared with the
control group, whereas DEX at 30 µg/kg significantly downregulated
the expression level of TLR4, NF-κB and p-NF-κB in the lung tissue
of the asthmatic mice. The expression levels of TLR4 and NF-κB mRNA
were also determined by RT-qPCR (Fig.
6C). The mRNA expression of TLR4 and NF-κB in the lung tissue
from the OVA group was elevated, and DEX significantly
downregulated their levels. The aforementioned results suggested
that DEX may inhibit the activation of the TLR4/NF-κB pathway.
 | Figure 6.Effect of DEX on the expression of
TLR4, NF-κB and p-NF-κB. (A) Representative immunohistochemistry
images and (B) quantification of TLR4, NF-κB and p-NF-κB
expression; results are presented as positive area percentage.
Magnification, ×200; scale bar, 100 µm. (C) mRNA expression levels
of TLR4 and NF-κB were examined by reverse
transcription-quantitative PCR. Data are presented as the mean ± SD
(n=3). **P<0.01, ***P<0.001 and ****P<0.0001 vs. control;
##P<0.01 and ###P<0.001 vs. OVA. DEX,
dexmedetomidine; IHC, immunohistochemistry; OVA, ovalbumin; p,
phosphorylated; TLR4, toll-like receptor 4. |
Discussion
AHR is a characteristic of asthma that has emerged
as a major challenge in the perioperative management of asthmatic
patients (27). AHR can be a
life-threatening symptom for patients with asthma during the
perioperative period, as it can result in hypoventilation,
hypoxemia and cardiac arrhythmia. The perioperative management of
asthmatic patients includes the following: i) Detection of upper
airway infection prior to surgery; ii) appropriate choices of
anesthesia; and iii) β-2 adrenergic agonist treatment in the case
of acute attacks (28). Certain
anesthetic agents can dilate the airway, whereas others can also
induce bronchoconstriction (29).
Therefore, it is essential to select appropriate anesthetic agents
that will benefit the patient.
DEX is an anesthetic adjuvant reported to attenuate
perioperative inflammation and improve the immune function of
patients who have undergone surgery (18). Groeben et al (30) reported that intravenous
administration of DEX attenuated bronchoconstriction in dogs
treated with histamine. It was clinically observed that patients
who had received DEX exhibited reduced airway resistance during
mechanical ventilation (31) in
surgery and hemodynamic stability during extubation (32). In the present study, an
OVA-induced murine asthma model was used to examine the effect of
DEX on allergic asthma. TAK-242, a TLR4 inhibitor, was used to
determine whether DEX could affect TLR4 signaling. To the best of
our knowledge, the present study is the first to demonstrate the
inhibitory effect of DEX on allergic airway inflammation in
mice.
According to the dose choices of previous studies on
the role of DEX in inflammatory diseases, most working doses in
mice were in the 20–50 µg/kg range [5, 10 and 20 µg/kg (33); 25 µg/kg (34); 2 and 50 µg/kg (21)], and the safety of DEX at 200 µg/kg
i.p. in BALB/c mice has been demonstrated (35). To further narrow down the range, a
dose-response experiment with different concentrations of DEX (10,
20, 30, 40, 50 and 60 µg/kg) was performed (data not shown).
According to the cell counts and Rrs results, 20, 30 and 50 µg/kg
were used in the present study.
Increasing infiltration of leukocytes around the
airway, particularly eosinophils, is one of the main factors in the
pathogenesis of asthma (36). The
present study demonstrated that DEX effectively decreased
inflammatory cell infiltration in the airway and inhibited mucus
overproduction in OVA-induced asthmatic mice. This indicated that
DEX may suppress inflammatory responses in the lungs of asthmatic
patients.
The imbalance of Th1/Th2 cells is pivotal in
promoting the development of asthma; indeed, the Th2 phenotype is
predominant, and the activity of Th1 cells is suppressed in
allergic asthma (37). Activated
Th2 cells can release type-2 cytokines, such as IL-4, IL-5 and
IL-13, which promotes the development of asthma, including AHR,
mucus overproduction and eosinophil infiltration (9). The present study revealed that
treatment with DEX reduced the levels of IL-4, IL-5 and IL-13 in
BALF of asthmatic mice. Thus, it was demonstrated that DEX may
attenuate airway inflammation in asthmatic mice by suppressing the
Th2 immune response.
AHR is a typical symptom of asthma that can also be
associated with behavioral changes in mice (38). In the present study, following
intranasal administration of OVA, mice in the OVA group were more
irritated and exhibited an elevated respiratory rate, compared with
the OVA + DEX group. In the present study, cyanosis was also
observed in the OVA group (data not shown). These behavioral
changes may be associated with AHR owing to a limited airflow
(39). The mechanism of AHR
remains unclear, although a previous study demonstrated a close
association between inflammatory cytokines and the development of
AHR (40). The results of the
present study demonstrated that DEX may effectively relieve
inflammation in the lungs and attenuate OVA-induced AHR in response
to inhaled Mch.
It has been suggested that the activation of the
TLR4/NF-κB pathway can promote the infiltration of inflammatory
cells in the airway and trigger airway inflammation (41). Although strong evidence that the
α-adrenergic receptor is associated with TLR4/NF-κB signaling is
still lacking, several studies have indicated that DEX can protect
organ function by inhibiting TLR4 signaling (14,30,31). The TLR4/NF-κB signaling pathway
has also been associated with the anti-inflammatory effects of DEX
(42). Activated NF-κB p65 is
phosphorylated, then translocated into the nucleus to promote the
transcription of target genes encoding inflammatory cytokines,
which contribute to the pathogenesis of asthmatic airway
inflammation (43). The present
results indicated that DEX inhibited the activation of NF-κB by
reducing the expression of NF-κB p65 and TLR4 in OVA-induced
asthmatic mice. Thus, DEX may attenuate airway inflammation in the
OVA-induced asthma model by suppressing the activation of the
TLR4/NF-κB pathway.
How DEX affects the TLR4 pathway has not been fully
determined, although it has been demonstrated that microRNA
(miRNA/miR) and long non-coding (lnc)RNAs may serve a role in this
context. miRNAs serve crucial roles in regulating various signaling
pathways, such as miR-30 regulates the MAPK/KRAS pathway (44), miR-486 regulates the PI3K/AKT
pathway (45) and miR-340-5p
regulates the PI3K/AKT pathway (46). Administration of DEX alters the
level of several miRNA molecules in certain murine disease models,
such as neuroinflammation (47),
myocardial ischemia/reperfusion (48) and postoperative cognitive
dysfunction (49). In addition,
it has been reported that DEX can inhibit the NF-κB pathway by
regulating miR-146a-3p (48) and
the TLR4 pathway via the regulation of miR-129 (49). In addition to miRNAs, lncRNAs are
also involved in several diseases through DEX, such as chronic
obstructive pulmonary disease (50), postoperative cognitive dysfunction
(51) and oxygen-glucose
deprivation/reperfusion injury (52). Thus, it may be hypothesized that
miRNAs or lncRNAs could mediate the effect of DEX on TLR4/NF-κB
signaling.
In the present study, DEX treatment at 30 µg/kg
resulted in an improved therapeutic effect compared with treatment
with a higher concentration (50 µg/kg), suggesting that there may
be a dose limit to the therapeutic effect of DEX in the murine
OVA-induced allergic asthma model. Doses higher than the 30 µg/kg
limit may result in reduced therapeutic effect causing unexpected
results, such as worsening symptoms or other side effects. This
dose limit may also differ between mouse strains, administration
mode and other factors.
DEX may be a safer choice as a sedative and an
anesthetic adjunct in patients with acute asthma. Acute asthma
attack causes anxiety and agitation in patients, which hinders
treatment and adversely affects patients (53). DEX allows patients to rest and
increase their tolerance to treatment without respiratory
depression. It is also used as adjunctive treatment for acute and
severe asthma (53,54). DEX has been used to facilitate the
induction of noninvasive positive pressure ventilation for acute
respiratory failure in patients with severe asthma (53) and in monitoring anesthesia care
for bronchial thermoplasty (54).
In conclusion, the present study demonstrated that
DEX attenuated AHR and airway inflammation by decreasing the
production of type-2 cytokines through the inhibition of TLR4/NF-κB
signaling in a murine OVA-induced asthma model. These findings
suggested that DEX may represent an alternative choice for the
perioperative management of patients with asthma and a potential
anti-inflammatory anesthetic.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Plastic Surgery Hospital,
Chinese Academy of Medical Sciences and Peking Union Medical
College (Beijing, China) (grant no. YS202006). Funding was provided
for various projects in the experiment, including model
establishment, experimental drugs and equipment.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QW analyzed and interpreted the data and designed
the study. SX drafted the manuscript and critically revised it for
important intellectual content. QW, SX, XZ and HG performed the
experiments. JZ and DY made substantial contributions to the study
conception. QW and SX confirmed the authenticity of all the raw
data. DY gave final approval of the version to be published. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Experimental animals were handled under a protocol
approved by the Institutional Animal Care and Use Committee of
Plastic Surgery Hospital, Chinese Academy of Medical Sciences and
Peking Union Medical College (approval no. 2021(201); Beijing,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Papi A, Brightling C, Pedersen SE and
Reddel HK: Asthma. Lancet. 391:783–800. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kong S, So WY and Jang S: The association
between vigorous physical activity and stress in adolescents with
asthma. Int J Environ Res Public Health. 18:34672021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vasileiadou S, Ekerljung L, Bjerg A and
Goksör E: Asthma increased in young adults from 2008–2016 despite
stable allergic rhinitis and reduced smoking. PLoS One.
16:e02533222021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Loftus PA and Wise SK: Epidemiology and
economic burden of asthma. Int Forum Allergy Rhinol. 5 (Suppl
1):S7–S10. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Woods BD and Sladen RN: Perioperative
considerations for the patient with asthma and bronchospasm. Br J
Anaesth. 103 (Suppl 1):i57–i65. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Buendía JA and Patiño DG: Cost-utility of
triple versus dual inhaler therapy in moderate to severe asthma.
BMC Pulm Med. 21:3982021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Facchinetti F, Civelli M, Singh D, Papi A,
Emirova A and Govoni M: Tanimilast, A Novel Inhaled Pde4 inhibitor
for the treatment of asthma and chronic obstructive pulmonary
disease. Front Pharmacol. 12:7408032021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hall S and Agrawal DK: Key mediators in
the immunopathogenesis of allergic asthma. Int Immunopharmacol.
23:316–329. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lambrecht BN, Hammad H and Fahy JV: The
cytokines of asthma. Immunity. 50:975–991. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nagase H, Ueki S and Fujieda S: The roles
of IL-5 and anti-IL-5 treatment in eosinophilic diseases: Asthma,
eosinophilic granulomatosis with polyangiitis, and eosinophilic
chronic rhinosinusitis. Allergol Int. 69:178–186. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bagnasco D, Ferrando M, Varricchi G,
Passalacqua G and Canonica GW: A Critical Evaluation of Anti-IL-13
and Anti-IL-4 strategies in severe asthma. Int Arch Allergy
Immunol. 170:122–131. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim G, Hong M, Kashif A, Hong Y, Park BS,
Mun JY, Choi H, Lee JS, Yang EJ, Woo RS, et al: Der f 38 Is a Novel
TLR4-binding allergen related to allergy pathogenesis from
dermatophagoides farinae. Int J Mol Sci. 22:84402021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lu X, Xu C, Yang R and Zhang G: Ganoderic
acid a alleviates ova-induced asthma in mice. Inflammation.
44:1908–1915. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kuzmich NN, Sivak KV, Chubarev VN, Porozov
YB, Savateeva-Lyubimova TN and Peri F: TLR4 signaling pathway
modulators as potential therapeutics in inflammation and sepsis.
Vaccines (Basel). 5:342017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Helal MG, Megahed NA and Abd Elhameed AG:
Saxagliptin mitigates airway inflammation in a mouse model of acute
asthma via modulation of NF-kB and TLR4. Life Sci. 239:1170172019.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Di Stefano A, Ricciardolo LFM, Caramori G,
Adcock IM, Chung KF, Barnes PJ, Brun P, Leonardi A, Andò F, Vallese
D, et al: Bronchial inflammation and bacterial load in stable COPD
is associated with TLR4 overexpression. Eur Respir J.
49:16020062017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Garantziotis S, Li Z, Potts EN, Lindsey
JY, Stober VP, Polosukhin VV, Blackwell TS, Schwartz DA, Foster WM
and Hollingsworth JW: TLR4 is necessary for hyaluronan-mediated
airway hyperresponsiveness after ozone inhalation. Am J Respir Crit
Care Med. 181:666–675. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang K, Wu M, Xu J, Wu C, Zhang B, Wang G
and Ma D: Effects of dexmedetomidine on perioperative stress,
inflammation, and immune function: Systematic review and
meta-analysis. Br J Anaesth. 123:777–794. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bao N and Tang B: Organ-protective effects
and the underlying mechanism of dexmedetomidine. Mediators Inflamm.
2020:61361052020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Heil LB, Santos CL, Santos RS, Samary CS,
Cavalcanti VC, Araújo MM, Poggio H, Maia Lde A, Trevenzoli IH,
Pelosi P, et al: The effects of short-term propofol and
dexmedetomidine on lung mechanics, histology, and biological
markers in experimental obesity. Anesth Analg. 122:1015–1023. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Meng L, Li L, Lu S, Li K, Su Z, Wang Y,
Fan X, Li X and Zhao G: The protective effect of dexmedetomidine on
LPS-induced acute lung injury through the HMGB1-mediated TLR4/NF-κB
and PI3K/Akt/mTOR pathways. Mol Immunol. 94:7–17. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang S, Jiang Z, Li L, Zhang J, Zhang C
and Shao C: Ameliorative effects of eosinophil deficiency on immune
response, endoplasmic reticulum stress, apoptosis, and autophagy in
fungus-induced allergic lung inflammation. Respir Res. 22:1732021.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Myou S, Leff AR, Myo S, Boetticher E, Tong
J, Meliton AY, Liu J, Munoz NM and Zhu X: Blockade of inflammation
and airway hyperresponsiveness in immune-sensitized mice by
dominant-negative phosphoinositide 3-kinase-TAT. J Exp Med.
198:1573–1582. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ford JG, Rennick D, Donaldson DD, Venkayya
R, McArthur C, Hansell E, Kurup VP, Warnock M and Grünig G: Il-13
and IFN-gamma: Interactions in lung inflammation. J Immunol.
167:1769–1777. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Feng R, Adeniran SO, Huang F, Li Y, Ma M,
Zheng P and Zhang G: The ameliorative effect of melatonin on
LPS-induced Sertoli cells inflammatory and tight junctions damage
via suppression of the TLR4/MyD88/NF-κB signaling pathway in
newborn calf. Theriogenology. 179:103–116. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
O'Byrne PM and Inman MD: Airway
hyperresponsiveness. Chest. 123 (Suppl 3):411S–6S. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yamakage M, Iwasaki S and Namiki A:
Guideline-oriented perioperative management of patients with
bronchial asthma and chronic obstructive pulmonary disease. J
Anesth. 22:412–428. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vaschetto R, Bellotti E, Turucz E,
Gregoretti C, Corte FD and Navalesi P: Inhalational anesthetics in
acute severe asthma. Curr Drug Targets. 10:826–832. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Groeben H, Mitzner W and Brown RH: Effects
of the alpha2-adrenoceptor agonist dexmedetomidine on
bronchoconstriction in dogs. Anesthesiology. 100:359–363. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Türktan M, Güleç E, Hatipoğlu Z, Ilgınel
MT and Özcengiz D: The effect of sevoflurane and dexmedetomidine on
pulmonary mechanics in ICU patients. Turk J Anaesthesiol Reanim.
47:206–212. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ambesh SP and Dubey M: Effect of
intramuscular dexmedetomidine administration before extubation on
post-extubation haemodynamics, postoperative sedation, and
analgesic requirements: A double blind placebo controlled study.
Asian J Anesthesiol. 59:102–110. 2021.PubMed/NCBI
|
|
33
|
Hwang L, Ko IG, Jin JJ, Kim SH, Kim CJ,
Chang B, Rho JH, Moon EJ and Yi JW: Dexmedetomidine ameliorates
memory impairment in sleep-deprived mice. Anim Cells Syst (Seoul).
23:371–379. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Geng Y, Li R, He SX, Yang HH, Deng QT,
Shao XY, Wu YS, Xu WW and Ma Q: Dexmedetomidine attenuates acute
lung injury induced by heatstroke and improve outcome. Shock.
52:532–539. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang C, He L, Wang C, Huang Y, Wang A, Li
X and Ao J: Dexmedetomidine alleviated
lipopolysaccharide/D-galactosamine-induced acute liver injury in
mice. Int Immunopharmacol. 72:367–373. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhi Y, Huang H and Liang L:
MFG-E8/integrin β3 signaling contributes to airway inflammation
response and airway remodeling in an ovalbumin-induced murine model
of asthma. J Cell Biochem. 119:8887–8896. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fahy JV: Type 2 inflammation in
asthma-present in most, absent in many. Nat Rev Immunol. 15:57–65.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ren M, Feng M, Long Z, Ma J, Peng X and He
G: Allergic asthma-induced cognitive impairment is alleviated by
dexamethasone. Front Pharmacol. 12:6808152021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lo D, Kennedy JL, Kurten RC, Panettieri RA
Jr and Koziol-White CJ: Modulation of airway hyperresponsiveness by
rhinovirus exposure. Respir Res. 19:2082018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yang M, Kumar RK and Foster PS:
Interferon-gamma and pulmonary macrophages contribute to the
mechanisms underlying prolonged airway hyperresponsiveness. Clin
Exp Allergy. 40:163–173. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Duan J, Kang J, Qin W, Deng T, Liu H, Li
B, Yu W, Gong S, Yang X and Chen M: Exposure to formaldehyde and
diisononyl phthalate exacerbate neuroinflammation through NF-κB
activation in a mouse asthma model. Ecotoxicol Environ Saf.
163:356–364. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kim E, Kim HC, Lee S, Ryu HG, Park YH, Kim
JH, Lim YJ and Park HP: Dexmedetomidine confers neuroprotection
against transient global cerebral ischemia/reperfusion injury in
rats by inhibiting inflammation through inactivation of the
TLR-4/NF-κB pathway. Neurosci Lett. 649:20–27. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Huang W, Li ML, Xia MY and Shao JY:
Fisetin-treatment alleviates airway inflammation through inhbition
of MyD88/NF-κB signaling pathway. Int J Mol Med. 42:208–218.
2018.PubMed/NCBI
|
|
44
|
Tanic M, Yanowsky K, Rodriguez-Antona C,
Andrés R, Márquez-Rodas I, Osorio A, Benitez J and Martinez-Delgado
B: Deregulated miRNAs in hereditary breast cancer revealed a role
for miR-30c in regulating KRAS oncogene. PLoS One. 7:e388472012.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gao ZJ, Yuan WD, Yuan JQ, Yuan K and Wang
Y: MiR-486-5p functions as an oncogene by targeting PTEN in
non-small cell lung cancer. Pathol Res Pract. 214:700–705. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Fan HP, Wang SY, Shi YY and Sun J:
MicroRNA-340-5p inhibits the malignant phenotypes of osteosarcoma
by directly targeting NRF2 and deactivating the PI3K/AKT pathway.
Eur Rev Med Pharmacol Sci. 25:3661–3669. 2021.PubMed/NCBI
|
|
47
|
Bao Y, Zhu Y, He G, Ni H, Liu C, Ma L,
Zhang L and Shi D: Dexmedetomidine attenuates neuroinflammation in
LPS-Stimulated BV2 microglia cells through upregulation of miR-340.
Drug Des Devel Ther. 13:3465–3475. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
He L, Wang Z, Zhou R, Xiong W, Yang Y,
Song N and Qian J: Dexmedetomidine exerts cardioprotective effect
through miR-146a-3p targeting IRAK1 and TRAF6 via inhibition of the
NF-κB pathway. Biomed Pharmacother. 133:1109932021. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wei W, Sun Z, He S, Zhang W and Chen S:
Protective role of dexmedetomidine against sevoflurane-induced
postoperative cognitive dysfunction via the microRNA-129/TLR4 axis.
J Clin Neurosci. 92:89–97. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Du XH, Li SS, Xiong GS, Yang GM, Shen W,
Sun SB, Ye XL, Li L and Weng ZY: Therapeutic efficacy of
dexmedetomidine on chronic obstructive pulmonary disease via
downregulating lncRNA PACER. Eur Rev Med Pharmacol Sci.
24:12963–12970. 2020.PubMed/NCBI
|
|
51
|
Deng F, Cai L, Zhou B, Zhou Z and Xu G:
Whole transcriptome sequencing reveals dexmedetomidine-improves
postoperative cognitive dysfunction in rats via modulating lncRNA.
3 Biotech. 10:2022020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhou Z, Chen Q, Wan L, Zheng D, Li Z and
Wu Z: Dexmedetomidine protects hepatic cells against oxygen-glucose
deprivation/reperfusion injury via lncRNA CCAT1. Cell Biol Int.
42:1250–1258. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Takasaki Y, Kido T and Semba K:
Dexmedetomidine facilitates induction of noninvasive positive
pressure ventilation for acute respiratory failure in patients with
severe asthma. J Anesth. 23:147–150. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lee JA, Rowen DW and Rose DD: Bronchial
thermoplasty: A novel treatment for severe asthma requiring
monitored anesthesia care. AANA J. 79:480–483. 2011.PubMed/NCBI
|