Introduction
Multiple sclerosis (MS) is the most common
inflammatory demyelinating disease of the central nervous system
(CNS), which most commonly affects young adults. Repulsive guidance
molecule a (RGMa) is a glycosylphosphatidylinositol-anchored
membrane protein that fulfils an important role in axonal outgrowth
(1,2). Recent evidence has suggested that
RGMa has a critical role in the pathogenesis of MS (3). Specifically, inhibition of RGMa was
indicated to attenuate clinical symptoms and reduce the ability of
inflammatory cells to invade the CNS (3), and also to modulate T-cell
activation in myelin oligodendrocyte glycoprotein (MOG)-induced
experimental autoimmune encephalomyelitis (EAE) mice (4), findings which demonstrated that
targeting RGMa may be a novel therapeutic strategy in MS. RGMa
expressed in endothelial cells suppressed endothelial tube
formation, angiogenesis and neovascularization, leading to
alterations in the permeability of the blood-brain barrier (BBB) in
the pathogenesis of MS (5), a
phenomenon that is among the earliest observed cerebrovascular
abnormalities to occur in MS (6).
A previous study published by our group also indicated that RGMa
was able to suppress angiogenesis (7). Therefore, the aim of the present
study was to further explore the downstream signalling pathway
featuring RGMa in endothelial cells and in MS.
A previous study suggested that chemokines and
chemokine receptors participate in the recruitment of macrophages
and T lymphocytes into the CNS and this has been considered as the
most important mechanism involved in the pathogenesis of MS
(8). Chemokine ligand 5 (CCL5)
serves a major role in several inflammatory diseases due to its
ability to control the migration of memory B-lymphocytes,
monocytes, macrophages and eosinophils into the CNS (9,10).
The level of CCL5 was indicated to increase both in EAE mice and in
patients with MS, and this molecule has been demonstrated to be
involved in the pathophysiology of MS (9). Knocking out the CCL5 receptor (CCR5)
was indicated to lead to marked improvements in the clinical
scoring and neurological functions in EAE mice, and also suppressed
the expression of inflammatory mediators, including IL-1β, TNF-α,
IFN-γ and monocyte chemoattractant protein-1 (MCP-1) (11). However, how CCL5 participates in
the underlying mechanism of the pathogenesis of MS and how it is
regulated has yet to be fully elucidated. The present study
explored whether RGMa regulates CCL5 expression in a BMP
ligand-dependent manner.
Materials and methods
Experimental animals
Female C57BL/6 mice (weight, 18–20 g; age, 8–10
weeks; n=112) were obtained from the Experimental Animal Centre of
Chongqing Medical University. All mice were housed in groups (five
mice per cage) in a colony room at 22±2°C and 45±10% humidity under
a reverse 12-h light/dark cycle and had ad libitum access to
food and water. All animal procedures were approved by the Ethics
Committee of the First Affiliated Hospital of Chongqing Medical
University (Chongqing, China) and all procedures were performed in
accordance with the Guide for the Care and Use of Laboratory
Animals (12).
Induction of EAE and clinical
scoring
EAE was induced in female C57 BL/6 mice by
subcutaneously injecting 100 µg MOG 35–55 peptide (Sigma-Aldrich;
Merck KGaA) emulsified in Complete™ Freund's adjuvant including
Mycobacterium tuberculosis (Sigma-Aldrich; Merck KGaA) into
the scapular region following anaesthesia with 2% isoflurane. In
addition, intraperitoneal injections of 200 ng of pertussis toxin
(Sigma-Aldrich; Merck KGaA) were performed on days 0 and 2. The
state of EAE in the mice was monitored daily to assess the clinical
scores of disease leading up to the completion of the experiment
using the following scoring system: 1, Complete tail atony; 2,
hindlimb weakness; 3, hindlimb paralysis; 4, complete hind limb
paralysis and front limb weakness; and 5, moribund (13). If mice with EAE were deemed to be
within two scores, a 0.5 value was added to the lower of the
clinical scores. The bodyweight of the mice was also monitored
daily.
Animal treatment
RGMa-specific recombinant adenovirus rAd5-short
hairpin (sh)RNA-RGMa and the same empty carrier recombinant
adenovirus rAd5-HK were supplied by Wuhan Genesil Biotechnology
Co., Ltd. The virus was amplified in 293 cells and purified using a
Sartorius Vivapure® Adeno PACK™ 20 (Sartorius AG).
Details of the titres, delivery efficacy and toxicity of
recombinant adenovirus were the same as those described in a
previously published study by our group (14). Mice were randomly divided into the
EAE control group, the rAd5-HK control group and the
rAd5-shRNA-RGMa group [RGMa RNA interference (RNAi) group] (n=10
mice in each group). The rAd5-HK and rAd5-shRNA-RGMa groups were
individually treated by intracerebroventricular injection on a
stereotaxic instrument at 12 days post-immunization after
anaesthesia (p.i.) (15,16). The injection rate was 0.3 µl/min
and the total volume was 2 µl for each site. At the end of the
injection, the microinjector was kept immobile for 5 min prior to
withdrawal. 2% isoflurane was used for induction and maintenance of
anesthesia. Any of the following criteria were endpoints for
immediate premature euthanasia according to a previous consensus on
EAE model: i) Paralyzed in all four limbs and not mentally alert;
ii) alert but exhibited paralysis in all four limbs for >24 h;
and iii) Dermatitis or posthitis (penile inflammation) with
ulceration from excessive urinary moisture. No EAE animals met
endpoint criteria for disease severity prior to the end of the
experiment in this study. Mice were sacrificed using cervical
dislocation under anesthesia at the designated timepoint on the
examination day (mice used for the immunofluorescence experiment
were subjected to cardiac perfusion after anesthesia). Absence of a
corneal reflex, failure to detect respiration and absence of a
heartbeat for a period of >10 min were used to confirm
death.
Cell culture
bEnd.3 cells [American Type Culture Collection
(ATCC)® CRL2299™; ATCC] were chosen for use in the
present study and grown in ATCC-formulated Dulbecco's modified
Eagle's medium (Gibco; Thermo Fisher Scientific, Inc.) containing
10% foetal bovine serum (Atlanta Biologicals, Inc.) in a humidified
incubator at 37°C in an atmosphere with 5% CO2. The
medium was changed every 48 h. Cells were plated on culture dishes
at a density of 4×104 cells/ml. At 48 h after seeding,
bEnd.3 cells were treated with RGMa (R&D Systems, Inc.; 2
µg/ml) for 0–4 h and subsequently incubated with noggin (R&D
Systems, Inc.; 500 ng/ml) overnight.
ELISA
CCL5 (cat. no. SEA116Po; Wuhan USCN Business Co.,
Ltd.) was used for measuring the concentration of chemokine ligand
in the serum of mice. Samples were measured in duplicate using
ELISA kits following the manufacturer's recommendations. The
results are expressed as the cytokine concentration in pg/ml.
Western blot analysis
Briefly, tissues were lysed in RIPA (cat. no.
P0013B; Beyotime Institute of Biotechnology) with protease and
phosphatase inhibitor cocktail (cat. no. 78441; Thermo Fisher
Scientific, Inc.) and cleared of debris by centrifugation at 14,000
× g for 15 min at 4°C. The protein concentration was determined
with a BCA Protein Assay kit (cat. no. P0011; Beyotime Institute of
Biotechnology). Equal amounts of sample protein (20 µg) were
separated via 10% SDS-PAGE and transferred to PVDF membranes
(MilliporeSigma). The membranes were probed using antibodies that
specifically recognized RGMa (cat. no. sc-46484; 1:1,000 dilution;
Santa Cruz Biotechnology, Inc.) or β-actin (cat. no. sc-58673;
1:2,000; Santa Cruz Biotechnology, Inc.) at 4°C overnight. After
three washes in PBS containing 0.1% Tween-20, blots were probed
with the HRP-conjugated donkey anti-goat IgG (1:1,000; cat. no.
A0181; Beyotime Institute of Biotechnology) or goat anti-mouse IgG
(cat. no. A0216; 1:1,000; Beyotime Institute of Biotechnology)
secondary antibodies for 2 h at room temperature. Protein bands
were visualized using an ECL (cat. no. 32109, Thermo Fisher
Scientific, Inc.) plus Western blotting detection system (Biorad
ChemiDoc MP). β-actin was used as a loading control. Relative
protein expression levels were reflected by the band density of
target proteins relative to β-actin.
Immunofluorescence
Mice were anesthetized with 2% isoflurane and
perfused with PBS and 4% buffered paraformaldehyde (PFA) for
sacrifice. The brains were then post-fixed in 4% PFA for 24 h and
subsequently embedded in paraffin and coronally cut into 4-µm
sections for analysis. Paraffin-embedded sections were
deparaffinized, rehydrated in a graded series of ethanol and then
incubated in H2O2 (0.3% solution) for 15 min
at room temperature. For antigen retrieval, sections were treated
with 10 mmol/l sodium citrate buffer (pH 6.0) and heated in a
microwave oven for 20 min. Tissues were permeabilized with 0.5%
Triton X-100, and the sections were then incubated in 5% goat serum
(cat. no. C0265; Beyotime Institute of Biotechnology) for 30 min at
room temperature. Subsequently, the sections were incubated with
CCL5 primary antibodies (cat. no. AF478; 1:200 dilution; R&D
Systems, Inc.) at 4°C overnight. Sections were then washed and
incubated with tetramethylrhodamine isothiocyanate-conjugated IgG
(cat. no. ab7686; 1:100 dilution; Abcam) in the dark for 1 h at
37°C, prior to the addition of DAPI for 5 min. The resulting
sections were observed under a fluorescence microscope (TCS SP5;
Leica Microsystems GmbH). Images were processed with ImagePro 6.0
software (Media Cybernetics, Inc.).
Total RNA extraction and reverse
transcription-quantitative (RT-q)PCR
Total RNA was purified from mouse whole spinal cord
or b end.3 cells using TRIzol® reagent (Takara
Biotechnology Co., Ltd.) according to the manufacturer's protocol.
The total RNA sample was stored at −80°C until required. The RNA
concentration was determined by measuring the absorption at 260 nm
on a spectrophotometer and the integrity of the RNA was assessed by
running mini-agarose gel electrophoresis. RT was performed using an
RT kit (cat. no. RR047A; Takara Biotechnology Co., Ltd.) according
to the manufacturer's protocol. Oligo-dT primers were used for
generating first-strand cDNA in a reaction mix of 20 µl. Reactions
were performed using SYBR® Green PCR Master Mix (cat.
no. RR820A; Takara Biotechnology Co., Ltd.) in a real-time PCR
apparatus (iCycler iQ5; Bio-Rad Laboratories, Inc.). The sequences
of the primers were as follows: mouse-CCL5 gene sense primer,
5′-AGCCCTCGCTGTCATCCT-3′ and antisense primer,
5′-CACTTGGCGGTTCTTTCG-3′; for the internal control, GAPDH sense
primer, 5′-CCTACCCCCAATGTATCCGTTGTG-3′ and antisense primer,
5′-GGAGGAATGGGAGTTGCTGTTGAA-3′ (Sangon Biotech Co., Ltd.). The
thermocycling conditions for all reactions commenced with a
denaturation step at 95°C for 10 min, followed by 40 cycles at 95°C
for 15 sec and 60°C for 60 sec. Each sample from one set of cells
was analyzed by qPCR in quadruplicate and three sets of cells were
used for the RT-qPCR assays. Results were quantified using the
comparative quantification cycle (Cq) method, 2−ΔΔCq
(17). All calculated
concentrations of the target gene were divided by the endogenous
reference (GAPDH) to obtain the normalized CCL5 expression values
(18).
Histopathological analysis
For the histological evaluation of the samples,
PFA-fixed, paraffin-embedded sections of the spinal cord were
stained with hematoxylin and eosin to assess the level of
inflammation. For each mouse, 20–30 transverse section samples were
examined from the cervical to thoracic spinal cord. Slices were
evaluated by an experienced pathologist in a blinded manner and the
extent of inflammation (specifically, the inflammatory index) was
determined as follows: 0, no inflammation; 1, cellular infiltration
only in the perivascular areas and meninges; 2, mild cellular
infiltration in the parenchyma; 3, moderate cellular infiltration
in the parenchyma; 4, severe cellular infiltration in the
parenchyma (3).
Statistical analysis
The means of two groups of samples were compared
using the unpaired t-test. Other statistical comparisons between
groups were performed by using one-way multiple-range ANOVA and
Tukey's post-hoc test for multiple comparisons. All results were
analyzed using GraphPad Prism version 8.0 (GraphPad Software,
Inc.). P<0.05 was considered to indicate a statistically
significant difference.
Results
RGMa RNAi significantly inhibits the
expression of RGM and is associated with a significant delay of EAE
and a markedly alleviated disease course
As detailed in the Materials and methods section,
the experimental mice were divided into three groups: The EAE
group, the RGMa RNAi group and the rAd5-HK control group. Immunized
mice that followed a monophasic course were characterized by ataxia
and hind-limb paralysis associated with weight loss and faecal and
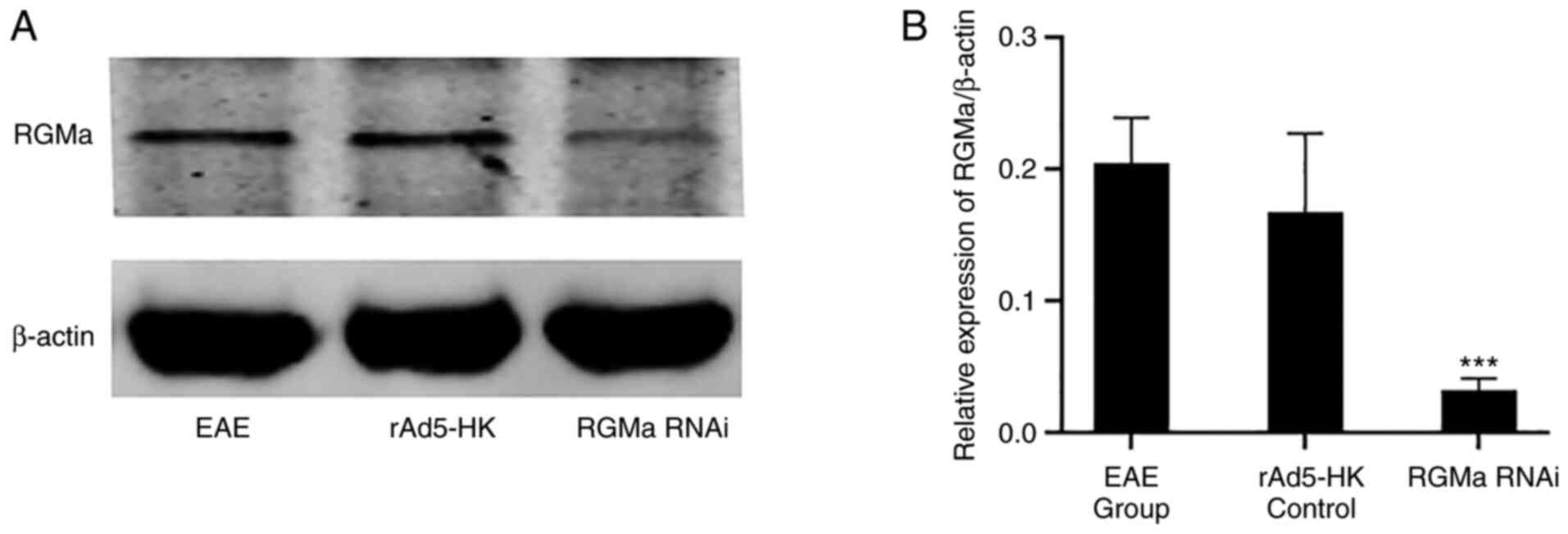
urinary incontinence. The expression of RGMa was indicated to be
significantly lower in the RGMa RNAi group compared with that in
the EAE and rAd5-HK control groups on day 21 p.i. (P<0.01;
Fig. 1). No significant
differences in the incidence of EAE in mice were observed when
comparing between the EAE group (75%, 9/12) and the rAd5-HK control
group (83.3%, 10/12) (χ2=0.253, P=0.615). RGMa RNAi
treatment, however, only led to a slight reduction in the incidence
rate (58.3%, 7/12), while it was not significantly different from
that in the rAd5-HK control group (χ2=1.815, P=0.178;
Table I). RGMa RNAi mice
exhibited a delayed onset of EAE at day 11 p.i. compared with both
the EAE group (day 8) and the rAd5-HK control group (day 9).
Furthermore, RGMa RNAi treatment significantly reduced the daily
mean neurological score for EAE from day 11 p.i. until day 21 p.i.
(Fig. 2A), whereas the maximal
mean score was significantly decreased in the RGMa RNAi group
compared with that in the EAE group (3.4±0.5 vs. 2.3±0.3, P=0.034;
Table I). This result was related
to the finding that RGMa RNAi treatment led to a marked reduction
in the infiltration of cells in the spinal cord at 21 days p.i.
(Fig. 2B and C). These results
indicated that RGMa RNAi ameliorated the clinical severity of EAE
in the model mice and also led to improvements in the neurological
functional in EAE mice.
 | Table I.Incidence and severity of EAE in mice
in each group. |
Table I.
Incidence and severity of EAE in mice
in each group.
| Group | Incidence | P-value | Maximal mean
neurological score | P-value |
|---|
| EAE group | 9/12 (75) | NA | 3.4±0.5 | NA |
| rAd5-HK control | 10/12 (83.3) | 0.615 | 3.2±0.6 | 0.926 |
| RGMa RNAi | 7/12 (58.3) | 0.178 | 2.3±0.3 | 0.034 |
CCL5 expression is upregulated as the
development of EAE takes its course
To evaluate the expression of CCL5 at different
stages during the disease progression of EAE mice, the CCL5 mRNA
and protein levels in the spinal cord of EAE mice were measured by
RT-qPCR and ELISA, respectively, at 0, 7, 14 and 21 days p.i. The
results obtained revealed a significant positive association
between the expression levels of CCL5 mRNA and CCL5 protein over
the total duration of the experimental modelling p.i. (Fig. 3A and B). The expression of CCL5
was indicated to be in parallel with the deterioration of the
clinical course of EAE (Fig.
2A).
 | Figure 3.Expression of CCL5 in different stages
of disease progression in EAE mice, as assessed by RT-qPCR and
ELISA, and the inhibitory effects of RGMa RNAi on the expression of
CCL5 in EAE mice. (A) CCL5 mRNA levels in the spinal cord were
upregulated p.i., as determined by RT-qPCR analysis. (B) CCL5
protein levels in the serum of EAE mice showed a similar trend in
increasing with the expression of CCL5 mRNA as EAE progressed, as
determined by ELISA. (C) CCL5 mRNA in the spinal cord was evaluated
by RT-qPCR in EAE mice at 21 days p.i. CCL5 mRNA levels in the
spinal cord of the RGMa RNAi group were significantly decreased
compared with the rAd5-HK control group. (D) CCL5 protein levels in
the serum of EAE mice were subjected to ELISA at 21 days p.i. CCL5
protein expression levels in the RGMa RNAi group were significantly
decreased compared with the rAd5-HK control group. *P<0.05 and
**P<0.01; ☆P<0.05; #P<0.05;
∆P<0.01. The data were analyzed by one-way ANOVA with
Tukey's post-hoc test and are expressed as the mean ± standard
deviation. p.i., post-immunization; RGMa, repulsive guidance
molecule a; EAE, experimental autoimmune encephalomyelitis; CCL5,
C-C motif chemokine ligand 5; RNAi, RNA interference; RT-qPCR,
reverse transcription-quantitative PCR. |
RGMa RNAi significantly inhibits the
expression of CCL5 in EAE mice
To examine whether RGMa regulates CCL5 expression in
EAE mice, the expression of CCL5 mRNA and protein in EAE mice was
measured by RT-qPCR and ELISA, respectively, after RGMa RNAi
treatment 21 days p.i. The RGMa RNAi group exhibited a significant
reduction in the basal levels of CCL5 mRNA and CCL5 serum protein
expression compared with the EAE group and the rAd5-HK control
group (Fig. 3C and D). These
results suggested that CCL5 is an important downstream cytokine
effector of RGMa in EAE mice.
RGMa regulates the expression of CCL5
mRNA in endothelial cells
The basis of the permeability of the BBB is
determined by the endothelial cell composition. A previous study
demonstrated that CCL5 is involved in this process, as RGMa and
CCL5 were indicated to be expressed in mouse endothelial cells
(19). In the present study, it
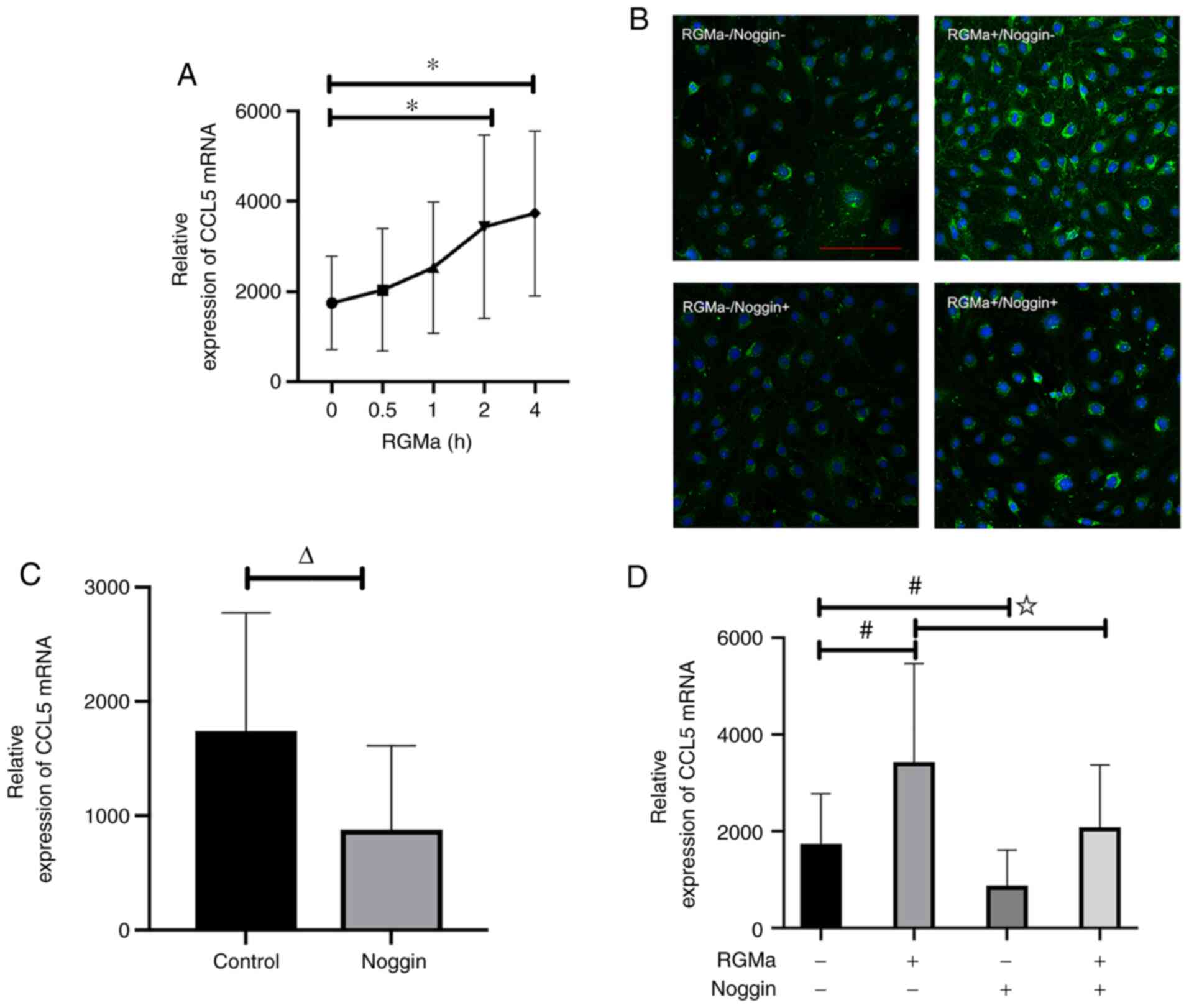
was demonstrated that RGMa led to a marked increase in the
expression level of CCL5 mRNA in bEnd.3 cells, and this effect was
observed as early as 2 h after recombinant RGMa treatment (Fig. 4A). These findings were
corroborated by the similar patterns of CCL5 expression observed in
the immunofluorescence experiments (Fig. 4B).
RGMa regulates the expression of CCL5
through a BMP-receptor-dependent pathway
It has not been previously established whether
RGMa-BMP signalling inhibits CCL5 expression. Incubating the cells
with noggin, an extracellular antagonist of BMP ligand, led to a
marked inhibition of CCL5 mRNA expression (Fig. 4C). These results confirmed that
CCL5 is regulated by RGMa or BMP in endothelial cells. Furthermore,
the addition of exogenous noggin abolished the induction effect of
RGMa on CCL5 mRNA (Fig. 4D).
Taken together, these results suggested that RGMa enhances the
expression of CCL5 through BMP receptor signalling.
Discussion
The present study demonstrated a crucial role for
RGMa in the development of EAE. First, inhibition of RGMa by RNAi
led to a marked improvement in neurological functions of EAE mice.
Furthermore, RGMa was indicated to regulate the expression of CCL5
both in EAE mice and in endothelial cells.
A pathological hallmark of MS is infiltration of
immune cells across the BBB into the CNS, which subsequently causes
myelin destruction and axonal injury (20). Muramatsu et al (3) demonstrated that neutralizing
antibodies against RGMa attenuated the clinical symptoms of mouse
MOG-induced EAE, including a reduced invasion rate of inflammatory
cells into the CNS, although the exact mechanism underlying the
association between RGMa and inflammation remained elusive. In the
present study, it was indicated that specific suppression of RGMa
by RNAi led to a marked inhibition of the expression of RGMa and
this was associated with both a significant delay in the onset of
EAE and an alleviated disease course, findings which are consistent
with those of the previous study. Subsequently, the present study
further focused on exploring the putative role of CCL5 in the
underlying mechanism.
CCL5 induces the migration of T cells across the BBB
(21) and fulfils an important
role in the adhesion of leukocytes in the brain microcirculation in
EAE (22), which are crucial
steps in the pathogenesis of MS (23). The endogenous level of CCL5 is
almost undetectable in the cerebrospinal fluid of healthy
individuals; however, the level of CCL5 increases markedly both at
the onset and during the progression of MS (24) and during the disease progression
of EAE mice (25). Knockout of
CCR5 led to a marked improvement in the clinical scoring and
neurological function of EAE mice, and suppressed the expression of
inflammatory mediators, including IL-1β, TNF-α, IFN-γ and MCP-1
(11). In the present study, the
results revealed that RGMa regulates the expression of both the
mRNA levels in the spinal cord and serum protein levels of CCL5 in
EAE mice, suggesting that the alleviation of the disease severity
in EAE via inhibition of RGMa is at least partially dependent on
the regulation of CCL5. In terms of the planning of the in
vitro experiments in the present study, endothelial cells were
chosen, since these form an essential component in BBB permeability
and migration of activated leukocytes. It was indicated that CCL5
expression is upregulated by RGMa in endothelial cells, which
suggested that RGMa may improve T-cell activation and inflammation
via regulating the expression of CCL5. However, further studies are
required to explore whether RGMa may directly impair BBB function
via CCL5 regulation (such as investigating the role of claudin-5 or
performing a BBB permeability assay) and examining the effects on
inflammatory mediators downstream would also be necessary to
further validate the results. On the other hand, several other
chemokines and cytokines factors besides CCL5 that are also
involved in the pathogenesis of MS and are expected to be modulated
by RGMa. Further studies by our group will focus on the regulatory
interactions of RGMa and those cytokines in oligodendrocytes or
lymphocytes in vitro.
Neogenin has been recognized as a classical receptor
of RGMa (26,27). Furthermore, RGMa is also a BMP
co-receptor, although unlike the well-known neogenin pathway, the
biological role of the RGMa-BMP receptor pathway has not been well
investigated to date. Using in vitro endothelial cell
experiments, it was suggested that RMGa activates CCL5 in a
BMP-receptor signalling-dependent manner. Noggin, a specific BMP
receptor inhibitor, abolished the role mediated by RGMa in
increasing the expression of CCL5 both at the mRNA and protein
levels. Although the exact mechanism of the RGMa-BMP receptor
pathway requires further research for its complete
characterization, including the identification of the specific
subtypes of BMP receptors that are involved, the present study was,
to the best of our knowledge, the first attempt to clarify the
downstream inflammatory molecules in the RGMa-BMP pathway.
In the present study, it was demonstrated that RGMa
leads to an increase in CCL5 expression in a BMP ligand-dependent
manner both in vivo (in EAE mice) and in vitro (in
endothelial cells). This study thereby defined a previously unknown
role of RGMa in modulating a chemokine, i.e. CCL5, via the BMP
signalling pathway. In consideration of the role of CCL5 in
lymphocyte migration, the results of the present study also
potentially underline the mechanism of BBB permeability mediated by
RGMa. The novel role of RGMa that was uncovered has revealed that
RMGa may be a promising therapeutic target for the treatment of
MS.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Science and Technology
Project of Chongqing Education Committee (grant no. KJ1600210), the
National Natural Science Foundation of China (grant no. 81701191)
and the National Key Clinical Specialties Construction Program of
China.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
All authors performed the experiments. ST drafted
the manuscript. BS analyzed and interpreted the data. TT performed
RNAi and cell culture. WY performed EAE, qPCR and
immunofluorescence. RZ performed western blot analysis. XQ and JF
designed the study and revised the manuscript. All authors read and
approved the final manuscript. ST and XQ checked and confirmed the
authenticity of the raw data.
Ethics approval and consent to
participate
All animal procedures were approved by the Ethics
Committee of the First Affiliated Hospital of Chongqing Medical
University (Chongqing, China) and all procedures were performed in
accordance with the Guide for the Care and Use of Laboratory
Animals (12).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siebold C, Yamashita T, Monnier PP,
Mueller BK and Pasterkamp RJ: RGMs: Structural insights, molecular
regulation, and downstream signaling. Trends Cell Biol. 27:365–378.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Monnier PP, Sierra A, Macchi P,
Deitinghoff L, Andersen JS, Mann M, Flad M, Hornberger MR, Stahl B,
Bonhoeffer F and Mueller BK: RGM is a repulsive guidance molecule
for retinal axons. Nature. 419:392–395. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Muramatsu R, Kubo T, Mori M, Nakamura Y,
Fujita Y, Akutsu T, Okuno T, Taniguchi J, Kumanogoh A, Yoshida M,
et al: RGMa modulates T cell responses and is involved in
autoimmune encephalomyelitis. Nat Med. 17:488–494. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fujita Y and Yamashita T: The roles of
RGMa-neogenin signaling in inflammation and angiogenesis. Inflamm
Regen. 37:62017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Harada K, Fujita Y and Yamashita T:
Repulsive guidance molecule A suppresses angiogenesis. Biochem
Biophys Res Commun. 469:993–999. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Minagar A and Alexander JS: Blood-brain
barrier disruption in multiple sclerosis. Mult Scler. 9:540–549.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang Y, Zhang R, Xing X, Guo J, Xie F,
Zhang G and Qin X: Repulsive guidance molecule a suppresses
angiogenesis after ischemia/reperfusion injury of middle cerebral
artery occlusion in rats. Neurosci Lett. 662:318–323. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cheng W and Chen G: Chemokines and
chemokine receptors in multiple sclerosis. Mediators Inflamm.
2014:6592062014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pittaluga A: CCL5-glutamate cross-talk in
astrocyte-neuron communication in multiple sclerosis. Front
Immunol. 8:10792017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tang S, Xiang T, Huang S, Zhou J, Wang Z,
Xie R, Long H and Zhu B: Ovarian cancer stem-like cells
differentiate into endothelial cells and participate in tumor
angiogenesis through autocrine CCL5 signaling. Cancer Lett.
376:137–147. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gu SM, Park MH, Yun HM, Han SB, Oh KW, Son
DJ, Yun JS and Hong JT: CCR5 knockout suppresses experimental
autoimmune encephalomyelitis in C57BL/6 mice. Oncotarget.
7:15382–15393. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
The National Academies Collection, .
Reports funded by National Institutes of Health. Guide for the Care
and Use of Laboratory Animals. 8th edition. Washington (DC):
National Academies Press (US); 2011
|
|
13
|
Feng J, Tao T, Yan W, Chen CS and Qin X:
Curcumin inhibits mitochondrial injury and apoptosis from the early
stage in EAE mice. Oxid Med Cell Longev. 2014:7287512014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Feng J, Wang T, Li Q, Wu X and Qin X: RNA
interference against repulsive guidance molecule A improves axon
sprout and neural function recovery of rats after MCAO/reperfusion.
Exp Neurol. 238:235–242. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang CJ, Qu CQ, Zhang J, Fu PC, Guo SG and
Tang RH: Lingo-1 inhibited by RNA interference promotes functional
recovery of experimental autoimmune encephalomyelitis. Anat Rec
(Hoboken). 297:2356–2363. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Croxford JL, Feldmann M, Chernajovsky Y
and Baker D: Different therapeutic outcomes in experimental
allergic encephalomyelitis dependent upon the mode of delivery of
IL-10: A comparison of the effects of protein, adenoviral or
retroviral IL-10 delivery into the central nervous system. J
Immunol. 166:4124–4130. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang G, Wang R, Cheng K, Li Q, Wang Y,
Zhang R and Qin X: Repulsive guidance molecule a inhibits
angiogenesis by downregulating VEGF and phosphorylated focal
adhesion kinase in vitro. Front Neurol. 8:5042017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ouyang S, Hsuchou H, Kastin AJ, Mishra PK,
Wang Y and Pan W: Leukocyte infiltration into spinal cord of EAE
mice is attenuated by removal of endothelial leptin signaling.
Brain Behav Immun. 40:61–73. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bradl M and Hohlfeld R: Molecular
pathogenesis of neuroinflammation. J Neurol Neurosurg Psychiatry.
74:1364–1370. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ubogu EE, Callahan MK, Tucky BH and
Ransohoff RM: CCR5 expression on monocytes and T cells: Modulation
by transmigration across the blood-brain barrier in vitro. Cell
Immunol. 243:19–29. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dos Santos AC, Roffê E, Arantes RM,
Juliano L, Pesquero JL, Pesquero JB, Bader M, Teixeira MM and
Carvalho-Tavares J: Kinin B2 receptor regulates chemokines CCL2 and
CCL5 expression and modulates leukocyte recruitment and pathology
in experimental autoimmune encephalomyelitis (EAE) in mice. J
Neuroinflammation. 5:492008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
dos Santos AC, Barsante MM, Arantes RM,
Bernard CC, Teixeira MM and Carvalho-Tavares J: CCL2 and CCL5
mediate leukocyte adhesion in experimental autoimmune
encephalomyelitis-an intravital microscopy study. J Neuroimmunol.
162:122–129. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sorensen TL, Tani M, Jensen J, Pierce V,
Lucchinetti C, Folcik VA, Qin S, Rottman J, Sellebjerg F, Strieter
RM, et al: Expression of specific chemokines and chemokine
receptors in the central nervous system of multiple sclerosis
patients. J Clin Invest. 103:807–815. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mecha M, Feliú A, Iñigo PM, Mestre L,
Carrillo-Salinas FJ and Guaza C: Cannabidiol provides long-lasting
protection against the deleterious effects of inflammation in a
viral model of multiple sclerosis: A role for A2A receptors.
Neurobiol Dis. 59:141–150. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rajagopalan S, Deitinghoff L, Davis D,
Conrad S, Skutella T, Chedotal A, Mueller BK and Strittmatter SM:
Neogenin mediates the action of repulsive guidance molecule. Nat
Cell Biol. 6:756–762. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Matsunaga E, Tauszig-Delamasure S, Monnier
PP, Mueller BK, Strittmatter SM, Mehlen P and Chédotal A: RGM and
its receptor neogenin regulate neuronal survival. Nat Cell Biol.
6:749–755. 2004. View
Article : Google Scholar : PubMed/NCBI
|