Introduction
Parkinson's disease (PD) is the second most common
chronic progressive neurodegenerative disorder in the elderly
population (1). The typical motor
symptoms of PD include tremors, bradykinesia, muscle stiffness and
postural instability (2). The
typical pathological characteristics of PD include degeneration and
loss of dopaminergic (DA) neurons in the substantia nigra (SN) pars
compacta (SNpc) and the formation of Lewy bodies in residual
neurons (3). Previous studies
have indicated that aging, mitochondrial defects, oxidative stress,
protein misfolding and aggregation and neuroinflammation are all
involved in PD pathogenesis (4,5).
Defects in mitochondrial complex I activity and reduced ATP
synthesis are routinely observed in PD (6).
In general, the etiology of PD is not clear. Genetic
susceptibility and environmental exposures increase the risk of
developing PD (7,8). As the majority of PD cases are
sporadic, environmental factors may be also important (9). At present, there is no definitive
cure, and the clinical diagnosis of PD is still based on the
characteristic symptoms of motor dysfunction (10). Therefore, the etiology, pathology
and therapeutic approaches for PD require detailed research for
which the selection of appropriate animal models is essential.
Rotenone and
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) are common
neurotoxins used to create animal PD models (11–15). Rotenone is a natural toxic
substance extracted from plants in the Leguminosae family and is
widely used in crop pest control and to eliminate fish from fish
ponds (16). Epidemiological
studies have demonstrated that the incidence of PD in individuals
chronically exposed to rotenone is higher compared with that in the
general population (17).
Rotenone is liposoluble, allowing it to enter neurons directly
without additional metabolic conversion or transporter-mediated
movement, and inhibits mitochondrial complex I inducing neuronal
degeneration (18,19).
MPTP is non-toxic to neurons and freely enters the
brain, where it is taken up by astrocytes, and is rapidly converted
to the toxic metabolite 1-methyl-4-phenylpyridinium-iodide
(MPP+) by monoamine oxidase. Once MPP+ enters
DA neurons via the dopamine transporter (DAT), a part of the
MPP+ is sequestered into synaptic vesicles by the
vesicular monoamine transporter (20). In DA neurons, MPP+
accumulates in the mitochondria, synaptic vesicles and cytoplasm; a
part of the accumulated MPP+ inhibits mitochondrial
complex I (21). Although their
mechanisms of action are not entirely understood, MPTP and rotenone
both effectively induce PD-like symptoms (22).
Both rotenone and MPTP inhibit the activity of
mitochondrial complex I, but they exhibit differences in the manner
in which they enter neurons and their specific intracellular
destinations (23). In the
present study, the rotenone and MPTP-induced mouse PD models were
produced using chronic exposure protocols, then compared based on
aspects of neurobehavior, neuropathology and mitochondrial
function. The aim of the present study was to provide a foundation
to construct and select the most suitable PD animal model to
explore the pathogenesis and mechanism of PD and develop more
effective treatments.
Materials and methods
Experimental animals and groups
The animal research was approved by the Animal Care
and Use Committee of Wenzhou Medical University (approval no.
wydw2020-0840; Wenzhou, China). All animal experiments were
performed in accordance with the Guide for the Care and Use of
Laboratory Animals (NRC 2011) (http://oacu.od.nih.gov/regs/guide/guide_2011.pdf)
and Guidelines for the ethical review of laboratory animal welfare
People's Republic of China National Standard GB/T 35892-2018
(24). All animal experiments
were performed by adequately trained researchers. Appropriate
anesthetics (isoflurane, 3% for induction; 1-1.5% for maintenance)
were administrated to ease pain and suffering.
A total of 80 C57BL/6 male mice (age, 10–12 weeks;
weight, 20–25 g) were purchased from Shanghai Shrek Experimental
Animal Co., Ltd. All of the animal rooms were maintained at 20–25°C
and humidity of 40–60%, with a 12-h light/dark cycle and free
access to food and water. The mice were randomly divided into three
groups. The MPTP group was injected intraperitoneally (IP), first
with 250 mg/kg probenecid (MilliporeSigma), then 1 h later with 20
mg/kg MPTP (MilliporeSigma) dissolved in sterile physiological
saline. The IP injections of probenecid and MPTP occurred twice a
week for a total of 12 doses over 6 consecutive weeks (25). The rotenone group was administered
30 mg/kg rotenone suspended in 0.5% sodium carboxymethyl cellulose
(CMC; MilliporeSigma) via daily administration by gavage for 6
consecutive weeks (26). The
control group was administered the same volume (200–250 µl) of 0.5%
CMC by gavage. After 6 weeks of administration, the survival rates
were 100% (25/25) for the control group, 86.7% (26/30) for the
rotenone group and 80% (20/25) for the MPTP group. In our study,
the control group that received 0.5% CMC by gavage was compared
with the control group that received 250 mg/kg probenecid via IP
injection. The experiment was repeated three times and the results
revealed no significant differences between these two control
groups. Therefore, based on the previously published control data,
the probenecid control group in the present study was not
included.
Pole test
A total of five mice in each group were assessed for
the degree of motor impairment using the pole test. Briefly, each
mouse was placed at the top end of a 50 cm pole that had a radius
of 4 mm. The time each mouse needed to completely turn 180° and
climb down from the pole to the floor was recorded and averaged
over three trials (27). Before
the timed experiments, the mice in each group received 3 days of
training with three trials per day.
Rotarod test
A total of five mice in each group were evaluated
for motor coordination using the rotarod test. Briefly, mice were
trained once a day for 3 days using a rotation speed of 10
revolutions per minute (rpm). The experiment was conducted on the
fourth day using a rotation speed of 40 rpm. The duration each
mouse remained on the rod with a maximum of 300 sec was recorded
(28).
Open field test
A total of five mice in each group were assessed
using the open field test to evaluate their exploratory behavior.
Each mouse was placed in an open field chamber (40×40 cm) for 15
min. The mouse movements were tracked, recorded and analyzed using
the EthoVisionXT 11 software (Shanghai Jiliang Software Technology
Co., Ltd.). The center zone was defined as a 20×20 cm area in the
center of the open field. The following data were analyzed: i)
Total distance traveled; and ii) time in the center zone expressed
as a percentage of the total distance traveled (29,30).
Immunohistochemistry
Following the behavioral tests, five mice in each
group were anesthetized with isoflurane (3% induction; 1-1.5%
maintenance) and perfused transcardially with 4% paraformaldehyde
and post-fixed in PFA at 4°C overnight. The brain was removed and
embedded in paraffin using routine protocols. The
immunohistochemical methods have been previously described
(31). Tissue sections (4-µm)
mounted on glass microscope slides were deparaffinized in xylene
and rehydrated in graded alcohols. Then the tissue sections were
boiled for 15 min in 10 mM citric acid buffer (pH 6.0) for antigen
retrieval. After the sections were cooled to room temperature (RT),
they were incubated with 3% H2O2 for 15 min
at 37°C to block endogenous peroxidase activity. Then the sections
were blocked with 5% goat serum (Scientific Phygene) for 45 min at
room temperature, and incubated with primary antibodies, including
mouse anti-mouse tyrosine hydroxylase (TH; 1:1,000; cat. no. 22941;
Immunostar, Inc.), rabbit anti-mouse phosphorylated (p)S129
α-synuclein (1:8,000; cat. no. ab51253; Abcam), rabbit anti-mouse
glial fibrillary acidic protein (GFAP; 1:1,000; cat. no. ab68428;
Abcam), rabbit anti-mouse ionized calcium-binding adapter molecule
1 (Iba-1; 1:1,000; cat. no. ab178847; Abcam) or mouse anti-mouse
neuronal nuclear antigen (NeuN; 1:500; cat. no. MAB377;
MilliporeSigma), overnight at 4°C. Subsequently, the sections were
incubated with secondary antibodies, including HRP-conjugated goat
anti-mouse IgG antibody (1:1,000; cat. no. ab6823; Abcam) or goat
anti-rabbit IgG antibody (1:1,000; cat. no. ab6112; Abcam), for 90
min at RT. Finally, the substrate color was developed using a
diaminobenzidine substrate kit (Abcam), and the sections were
counterstained with hematoxylin (Beijing Solarbio Science &
Technology Co., Ltd.) for 1 min at room temperature. Image
acquisition was performed using a light microscope (Nikon
Corporation). Immunohistochemistry results were quantified using
ImageJ software (version 1.8.0; National Institutes of Health). One
field was randomly imaged from each of five different sections in
each group to count the positive cells, and the average value was
calculated.
Immunofluorescence
Three mice in each group were anesthetized with
isoflurane as aforementioned and perfused transcardially with 4%
paraformaldehyde to obtain brain tissue sections and post-fixed in
PFA at 4°C overnight. The tissue sections were permeabilized with
0.5% Triton X-100 for 20 min at room temperature and incubated with
5% goat serum for 45 min at room temperature. After washing in PBS,
the sections were incubated overnight in rabbit anti-mouse GFAP
antibody (1:1,000; cat. no. ab68428; Abcam), rabbit anti-mouse
Iba-1 antibody (1:1,000; cat. no. ab178847; Abcam) or mouse
anti-mouse NeuN antibody (1:500; cat. no. MAB377; MilliporeSigma)
at 4°C. Then the sections were incubated with secondary antibodies
at 37°C, Alexa Fluor 555-labeled donkey anti-rabbit IgG antibody
(1:500; cat. no. A0453; Beyotime Institute of Biotechnology) or
Alexa Fluor 488-labeled goat anti-mouse IgG antibody (1:500; cat.
no. A0428; Beyotime Institute of Biotechnology), for 3 h and
stained with DAPI (Beyotime Institute of Biotechnology) for 5 min
at room temperature. Image acquisition was performed using a laser
confocal microscope (Nikon Corporation). Immunofluorescent cells
were quantified using ImageJ software. One field was randomly
imaged from five different sections in each group to count the
positive cells, and the average value was calculated.
Western blotting
A total of six mice in each group were anesthetized
with isoflurane as aforementioned and perfused transcardially with
PBS, the brain was removed and the SN tissue was isolated from both
sides of the brain. The SN tissue was placed in RIPA lysis buffer
(Beyotime Institute of Biotechnology), maintained for 30 min on ice
and then centrifuged at 12,000 × g for 2 min at 4°C. The
supernatant was collected from the homogenized tissue after
centrifugation. The protein concentration was determined using a
BCA reagent (Beyotime Institute of Biotechnology) with bovine serum
albumin as the protein standard. Proteins (20 µg/lane) were
separated on 10% SDS-PAGE and transferred to polyvinylidene
fluoride membranes for immunoblotting. After that, the membrane was
blocked using 5% skim milk powder at room temperature for 90 min,
The membranes were incubated overnight with primary antibodies,
including rabbit anti-mouse TH (1:1,000; cat. no. AF2185; Beyotime
Institute of Biotechnology) and mouse anti-mouse GAPDH (1:5,000;
cat. no. 60004-lg; Wuhan Sanying Biotechnology), at 4°C.
Subsequently, the membranes were incubated at RT with secondary
antibodies, including HRP-labeled goat anti-mouse IgG antibody
(1:1,000; cat. no. A0216; Beyotime Institute of Biotechnology) or
HRP-labeled goat anti-rabbit IgG antibody (1:1,000; cat. no. A0208;
Beyotime Institute of Biotechnology), for 90 min. The blots were
developed utilizing electrochemiluminescence reagent (Beyotime
Institute of Biotechnology), and detection was accomplished using
the ChemiDoc MP Imaging System (Bio-Rad Laboratories, Inc.). The
gray values of the bands were scanned and analyzed using ImageJ
software.
Oxygen consumption measurement
A total of three mice in each group were
anesthetized with isoflurane as aforementioned and perfused
transcardially with PBS, and the SN tissue on both sides of the
dissected brain was removed. Briefly, the SN tissue was homogenized
in ice-cold buffer A {pH 7.4, 5 mM KCl, 5.8 mM NaCl, 2 mM
MgCl2, 0.75 mM CaCl2, 137 mM sucrose and 10
mM 2-[4-(2-hydroxyethyl)piperazin-1-yl)] ethanesulfonic acid
(HEPES)} and centrifuged for 5 min at 1,000 × g at 4°C (32). The supernatant was removed and
further centrifuged for 2 min at 15,000 × g at 4°C. The resulting
pellets were resuspended in Buffer B [20 mM HEPES (pH 7.1), 250 mM
sucrose, 2 mM KH2PO4, 10 mM MgCl2
and 1 mM ADP]. The mitochondrial protein concentration was
determined using a BCA kit (Beyotime Institute of Biotechnology).
Subsequently, 80 µg of the mitochondrial solution was transferred
to an Oxygraph-2k chamber (O2k; Oroboros Instruments GmbH).
Subsequently, 5 mM malic acid, 5 mM glutamic acid and 5 mM
succinate were added to measure the total oxygen consumption of
mitochondrial complexes I and II. Oligomycin (2.5 µg/ml) was added
to record the level of oxygen consumption uncoupled to ATP
synthase. Subsequently, 0.1 µM carbonyl
cyanide-p-trifluoromethoxyphenylhydrazone (FCCP) was added to
determine the maximum respiration rate. All measurements were
performed according to the manufacturer's instructions of the O2k
chamber and the oxygen flux per mass was recorded using Oroboros
DatLab Software 5.2.1.51 (Oroboros Instruments GmbH).
Mitochondrial respiratory chain
complex I enzyme activity
A total of five mice in each group were anesthetized
with isoflurane as aforementioned and perfused transcardially with
PBS, and the SN tissue was removed on both sides of the dissected
brain. Briefly, the tissue was homogenized in ice-cold tissue lysis
buffer (Beijing Solarbio Science & Technology Co., Ltd.) and
centrifuged for 5 min at 1,000 × g at 4°C. The supernatant was
removed and further centrifuged for 10 min at 12,000 × g at 4°C.
The resulting pellets were resuspended in Buffer A as
aforementioned (33). The
mitochondria were frozen and thawed three times using liquid
nitrogen, and the mitochondrial protein concentration was measured
using a BCA kit (Thermo Fisher Scientific, Inc.).
The mitochondrial respiratory chain complex I enzyme
and citrate synthase activity in the SN were detected using a
U-3900 spectrophotometer (Hitachi, Ltd.). The reaction mixture
consisting of 0.5M NaN3, 10 mM nicotinamide adenine
dinucleotide (NADH) and 4 µg mitochondrial protein, was incubated
at 37°C for 2 min. The oxidation rate of NADH was measured by
adding 6 mM ubiquinone and assessed at 340 nm. Subsequently, the
reaction mixture including 1 mM 5,5′-dithiobis (2-nitrobenzoic
acid), 10 mM acetyl coenzyme A and 4 µg mitochondrial protein, was
incubated at 37°C for 2 min. The rate of citric acid generation was
measured by adding oxaloacetic acid and assessed at 412 nm. The
enzyme activity of mitochondrial complex I was normalized with the
activity of citric acid synthase. Enzyme activity measurements were
repeated three times independently.
Statistical analysis
Data are presented as mean ± standard deviation.
P-values were calculated using SPSS 22.0 software (IBM Corp.).
Unpaired Student's t-tests were used to compare data between two
groups. Comparisons among three groups were performed using one-way
ANOVA. First, the homogeneity of variance was tested. If equal
variances were observed, then the P-values were calculated using
Tukey's post hoc test. Otherwise, the P-values were calculated
using Tamhane's T2 test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Results of behavioral tests are
significantly altered in the rotenone and MPTP mouse PD models
compared with the control, but no significant difference is
observed between the models
Behavioral assessments were conducted to investigate
the motor function of the rotenone and MPTP models, including the
pole, rotarod and open field tests, to evaluate changes in
locomotion, motor coordination and exploratory behavior,
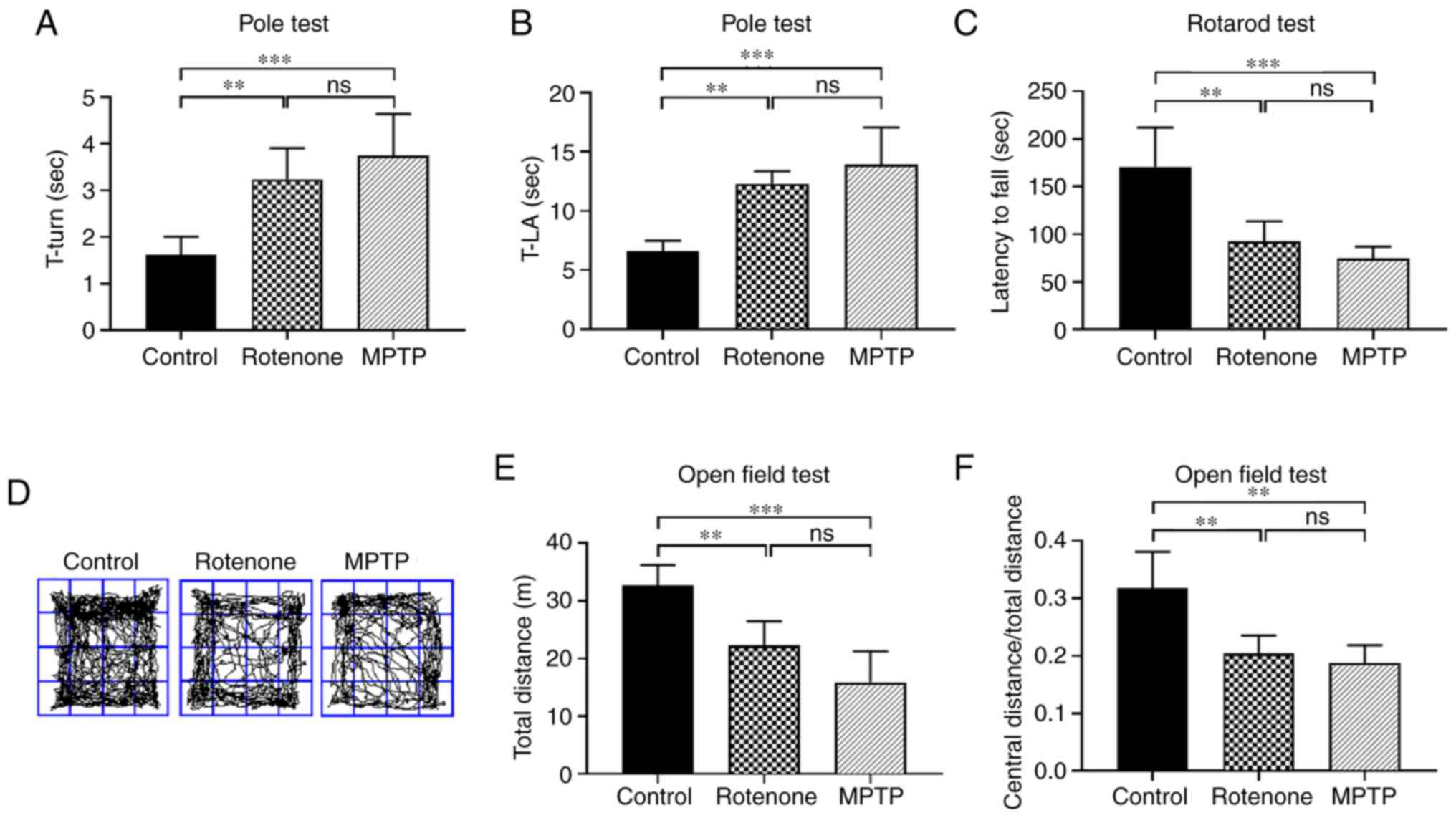
respectively. In the pole test (Fig.
1A and B), rotenone and MPTP treatments significantly extended
the time required to turn 180° and the total time needed for the
mouse to reach the floor (locomotion activity time) compared with
the control group (both P<0.01). There was no significant
difference between the MPTP and rotenone groups. The images of the
pole test are presented in Fig.
S1. In the rotarod test (Fig.
1C), the fall latencies for the rotenone and MPTP groups were
significantly shorter compared with the control group (both
P<0.01). By contrast, there was no significant difference
between the MPTP and rotenone groups. The images of the rotarod
test are presented in Fig.
S2.
In the open field test (Fig. 1D-F), the total distance traveled
was significantly shorter and the ratio of the central distance to
the total distance was smaller in the rotenone and MPTP groups
compared with the control group (all P<0.01). However, there was
no significant difference between the MPTP and rotenone groups.
These results indicated that the rotenone and MPTP-treated mice
exhibited a significant decrease in motor function and exploratory
behavior, but there was no significant difference between the two
experimental groups. After completion of the neurobehavioral tests,
the survival rates for the control, rotenone and MPTP groups were
100, 86.7 and 80%, respectively.
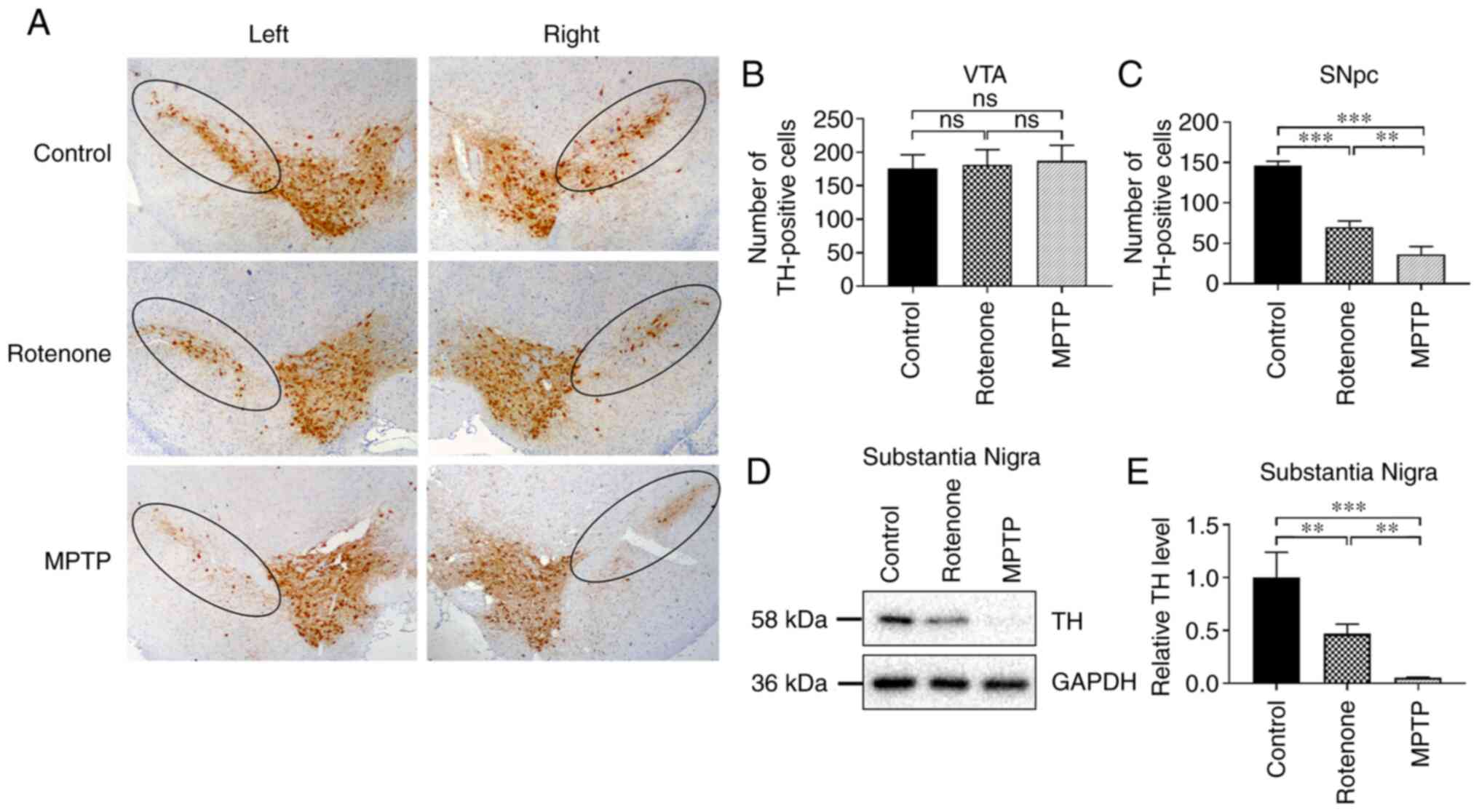
Degeneration and death of DA neurons
in the SNpc of the rotenone and MPTP mouse PD models are
significantly increased compared with the control, especially in
the MPTP model
An important pathological process in the brains of
patients with PD is the degeneration of DA neurons in the SNpc
(34). TH, the rate-limiting
enzyme in dopamine synthesis, is widely recognized as a specific
marker to evaluate the survival status of DA neurons (35). DA neuronal survival was evaluated
by determining the number of TH-positive neurons in the SNpc. The
distribution of neurons in the positively stained region appeared
to be in the shape of an inverted, elongated comma (Fig. 2A). To detect the degree of DA
neuronal damage in the SNpc of the rotenone and MPTP models, the
number of surviving DA neurons in the ventral tegmental area (VTA)
and the SNpc were quantitatively compared in each group. There was
no significant difference in the numbers of DA neurons in the VTA
among all groups (Fig. 2B).
Furthermore, the results demonstrated that exposure to rotenone and
MPTP resulted in no detectable damage in the VTA (Fig. 2B). However, the number of
surviving neurons in the SNpc was significantly lower in the
rotenone and MPTP groups compared with the control group
(P<0.001; Fig. 2C). In
addition, the number of surviving neurons was significantly lower
in the MPTP group compared with the rotenone group (P<0.01;
Fig. 2C). No significant
difference in the number of surviving DA neurons in SNpc and VTA
(Fig. S3A-C) were revealed
between the control group of 0.5% CMC administered via gavage and
the control group of 250 mg/kg probenecid administered via IP
injection.
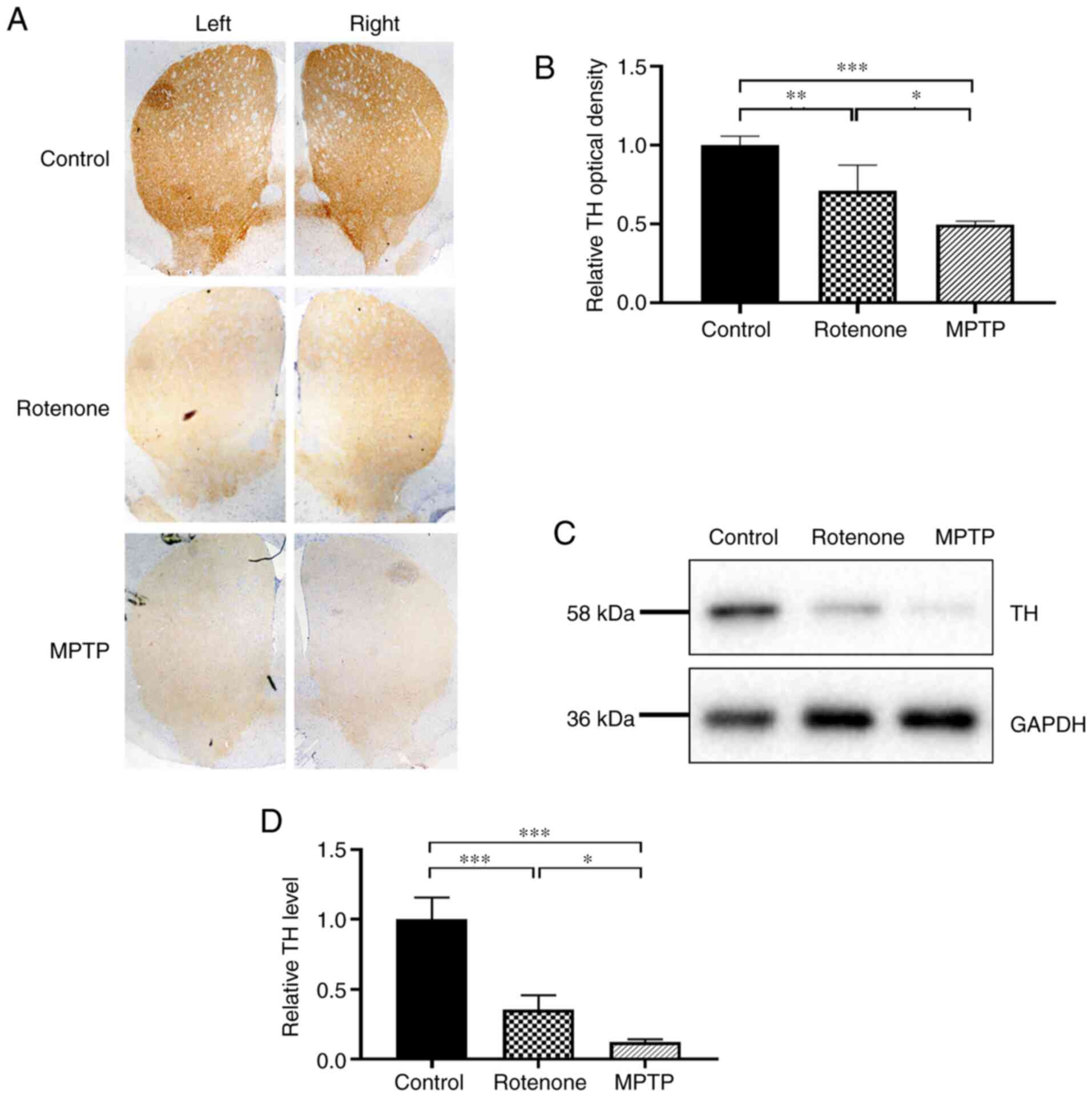
 | Figure 2.Neuronal degeneration and death in
the SNpc in the rotenone and MPTP mouse Parkinson's disease models.
(A) Immunohistochemical staining for TH in the SNpc. TH protein is
expressed in the cytoplasmic regions of dopaminergic neurons; the
inner circle area represents the SNpc; the outer circle area in
brown represents the VTA. Magnification, ×40. Quantitative analysis
of the number of surviving neurons in the (B) VTA and (C) SNpc. (D)
Expression level of TH protein in the SN was detected using western
blotting; GAPDH was used as the internal reference standard. (E)
Quantitative analysis of TH expression in the SN. **P<0.01,
***P<0.001. ns, no significant difference; VTA, ventral
tegmental area; SN, substantia nigra; SNpc, SN pars compacta; MPTP,
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; TH, tyrosine
hydroxylase. |
In PD, degeneration and loss of DA neurons in the SN
results in decreased TH expression (36). To further explore the degree of
damage to DA neurons in the SN in the rotenone and MPTP models, the
expression level of TH in the SN was assessed using western
blotting. TH expression in the SN (Fig. 2D and E) was significantly lower in
the rotenone and MPTP groups compared with the control group (both
P<0.01). Notably, the TH expression was considerably lower in
the MPTP group compared with that of the rotenone group
(P<0.01). These results revealed that rotenone and MPTP caused
the degeneration and death of DA neurons in the SNpc and indicated
that the toxic effects of MPTP were more pronounced.
TH protein content in the striatum of
the rotenone and MPTP mouse PD models is significantly lower
compared with the control, especially in the MPTP model
The TH protein content in the striatum is considered
to be an indirect indication of the striatal dopamine content
(37). The TH protein expression
level in the striatum was detected using immunohistochemistry. The
optical density of TH-positive staining in the striatum (Fig. 3A and B) was significantly lower in
the rotenone and MPTP groups compared with the control group (both
P<0.01), and the staining was significantly lower in the MPTP
group compared with the rotenone group (P<0.05). No significant
difference in the content of dopamine in the striatum (Fig. S3D and E) was revealed between the
control group of 0.5% CMC and that of 250 mg/kg probenecid.
Furthermore, the expression level of TH protein in the striatum was
detected using western blotting. TH expression in the striatum
(Fig. 3C and D) was significantly
lower in the rotenone and MPTP groups compared with the control
group (both P<0.001), and it was significantly lower in the MPTP
group compared in the rotenone group (P<0.05). These results
demonstrated that both rotenone and MPTP treatments significantly
reduced the protein level of TH in the striatum and indicated more
pronounced toxic effects for MPTP.
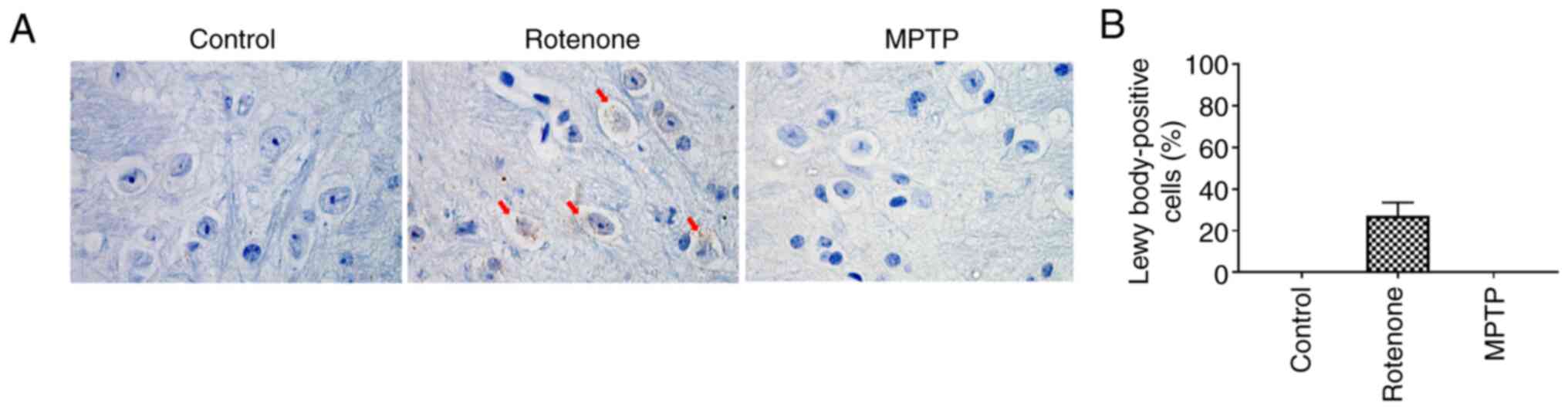
Lewy bodies appear in SNpc neurons in
the rotenone model but not the MPTP model
Another characteristic pathological change observed
in PD is the formation of Lewy bodies in the residual SNpc neurons.
Lewy bodies can activate surrounding microglia, promote the release
of inflammatory mediators, and aggravate neuronal injury (38). A small fraction of α-synuclein is
phosphorylated at the S129 site (<4%) in healthy brains, but it
has been observed to be accumulated (~90%) in the brains of
patients with PD (39).
Therefore, pS129 α-synuclein was used as a marker to evaluate the
pathological changes associated with α-synuclein. Lewy bodies were
observed in the rotenone group using immunohistochemical staining
for pS129 α-synuclein, but not in the control or MPTP groups
(Fig. 4A and B). These results
demonstrated that, compared with MPTP, rotenone could induce the
formation of Lewy bodies in the residual neurons in the SNpc and
provided a more accurate simulation of the pathological processes
that occur in patients with PD.
MPTP and rotenone induce glial
activation and damaged neurons
The importance of neuroinflammation in mediating the
progressive death of DA neurons in PD has been recognized.
Astrocyte and microglial activation in the central nervous system
provides evidence of the existence of inflammation in PD (40,41). GFAP and Iba-1 are molecular
markers of activated astrocytes and microglia, respectively, and
their activation is closely associated with injury or inflammation
in the brain (42). To reveal the
activation of astrocytes and microglia in the SN, GFAP and Iba-1 in
the SN were detected using immunofluorescence and
immunohistochemistry. The number of GFAP-positive cells in the SN
(Fig. 5A, D, G and H) was
significantly increased in the rotenone and MPTP groups compared
with the control group (all P<0.01). Also, the number was
significantly higher in the MPTP group compared with the rotenone
group (both P<0.01). The number of Iba-1-positive cells in the
SN (Fig. 5B, E, G and I) was
significantly increased in the rotenone and MPTP groups compared
with the control group (all P<0.01). The number was
significantly higher in the MPTP group compared with in the
rotenone group (both P<0.01). Thus, these results demonstrated
that treatment with rotenone and MPTP could enhance the activation
of astrocytes and microglia in the SN and revealed that MPTP
exhibited more pronounced toxic effects.
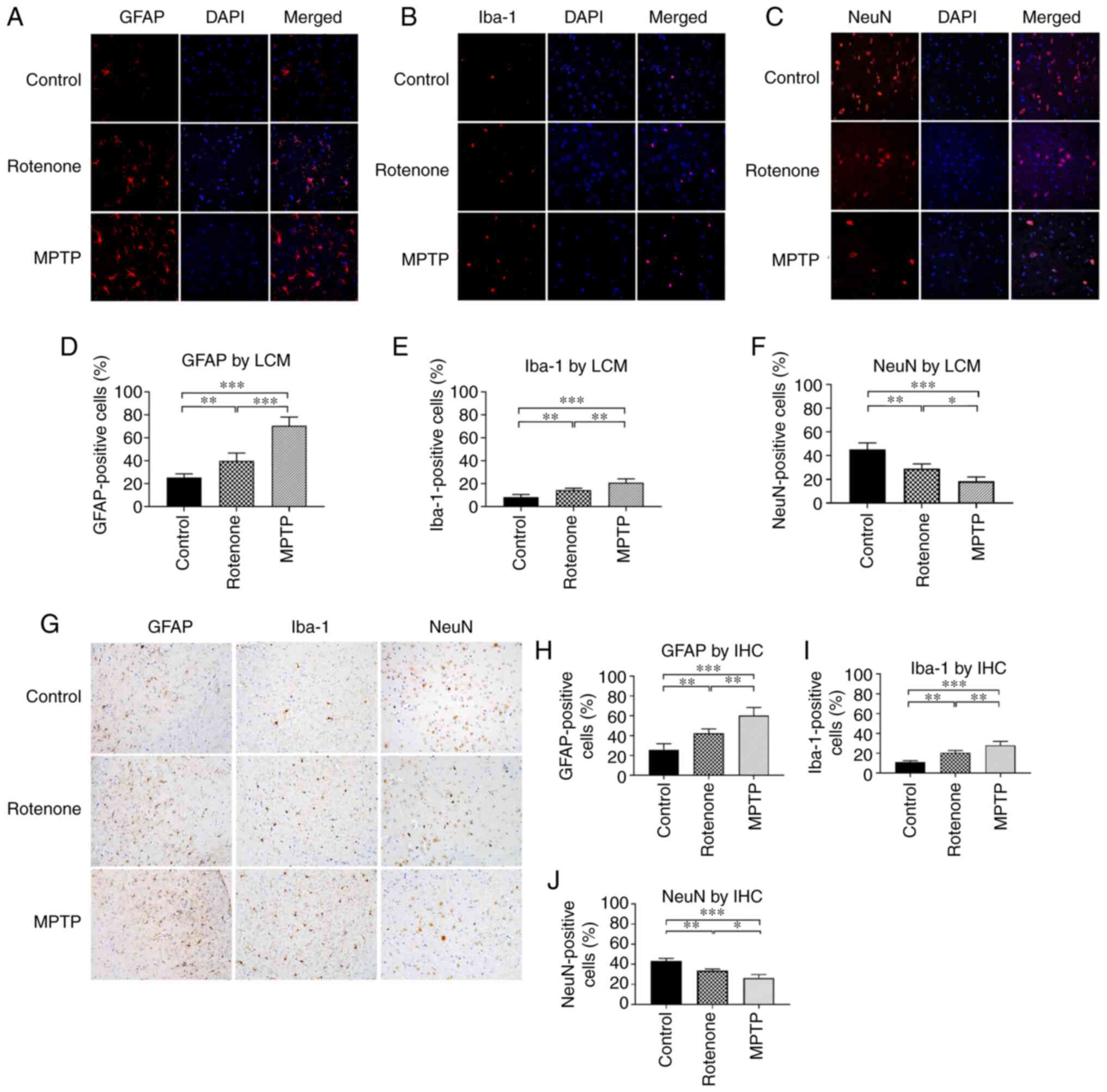 | Figure 5.Number of astrocytes, microglia and
neurons in the substantia nigra of the rotenone and MPTP mouse
Parkinson's disease models. (A) Number of astrocytes
(GFAP-positive) was measured using LCM. GFAP protein (red) is
expressed in the cytoplasmic regions of the astrocytes. DAPI (blue)
indicates the nucleus. Magnification, Annexin V. (B) Number of
microglia (Iba-1-positive) was detected using LCM. Iba-1 protein
(red) is expressed in the cytoplasmic regions of the microglia.
DAPI (blue) indicates the nucleus. Magnification, Annexin V. (C)
Number of NeuN-positive cells was detected using LCM. NeuN protein
was expressed in the nuclei of the majority of neurons. DAPI (blue)
indicates the nucleus. Magnification, 600×. (D) Quantitative
analysis of the proportion of astrocytes (GFAP-positive). (E)
Quantitative analysis of the proportion of microglia
(Iba-1-positive). (F) Quantitative analysis of the proportion of
NeuN-positive cells. (G) Number of astrocytes (GFAP-positive),
microglia (Iba-1-positive) and NeuN-positive cells were detected
using IHC. GFAP, Iba-1 and NeuN proteins are expressed in the
cytoplasmic regions of astrocytes, microglia and the nucleus of the
majority of neurons, respectively. Scale bar, 100 µm. Quantitative
analysis of the proportion of (H) astrocytes, (I) microglia and (J)
NeuN-positive cells. *P<0.05, **P<0.01, ***P<0.001. LCM,
laser confocal microscopy; IHC, immunohistochemistry; NeuN,
neuronal nuclear antigen; GFAP, glial fibrillary acidic protein;
MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; Iba-1 ionized
calcium-binding adapter molecule 1. |
NeuN is expressed in the nucleus of the majority of
neurons in the vertebrate nervous system (43). It is one of the most commonly used
markers for neurons. Thus, the present study examined NeuN
expression to determine whether there was a similar effect as
observed with TH expression. The number of NeuN-positive cells in
the SN (Fig. 5C, F, G and J) was
significantly decreased in the rotenone and MPTP groups compared
with the control group (all P<0.01). Also, the number was
significantly decreased in the MPTP group compared with the
rotenone group (both P<0.05). These observations suggested that
there was neuronal loss in addition to decreased TH expression in
the surviving neurons in the rotenone and MPTP mouse PD models. The
results also suggested that MPTP exhibited more toxic effects
compared with rotenone.
Rotenone and MPTP decreased
mitochondrial-dependent oxygen consumption and complex I enzyme
activity compared with the control, especially in the rotenone
model
Rotenone and MPTP are direct and indirect inhibitors
of mitochondrial complex I, respectively, and the underlying
mechanisms are different (23).
Therefore, the present study determined the oxygen consumption and
complex I activity to assess the effects of rotenone and MPTP on
mitochondrial function.
The mitochondrial oxygen consumption level in the SN
was detected using O2k. In Fig.
6A, complex I and complex II (CI + CII) represented the total
oxygen consumption of mitochondrial complexes I and II, which were
significantly lower in the rotenone and MPTP groups compared with
the control group (both P<0.001). Also, the total oxygen
consumption for the rotenone group was considerably lower compared
with the MPTP group (P<0.05). Oxygen consumption after
oligomycin treatment (Oligo), an inhibitor of complex V (ATP
synthase) of the electron transport chain, was significantly lower
in the rotenone and MPTP groups compared with the control group
(both P<0.05). The oligo was not different between the rotenone
and MPTP groups. FCCP (oxygen consumption after FCCP treatment)
represents the maximum oxygen consumption after uncoupling the
electron transport chain, which was significantly lower in the
rotenone and MPTP groups compared with the control group (both
P<0.001). Also, the FCCP in the rotenone group was considerably
lower compared with the MPTP group (P<0.05). These results
indicated that both rotenone and MPTP significantly altered the
oxygen consumption of mitochondria in the SN and suggested that
rotenone had a more pronounced effect on oxygen consumption.
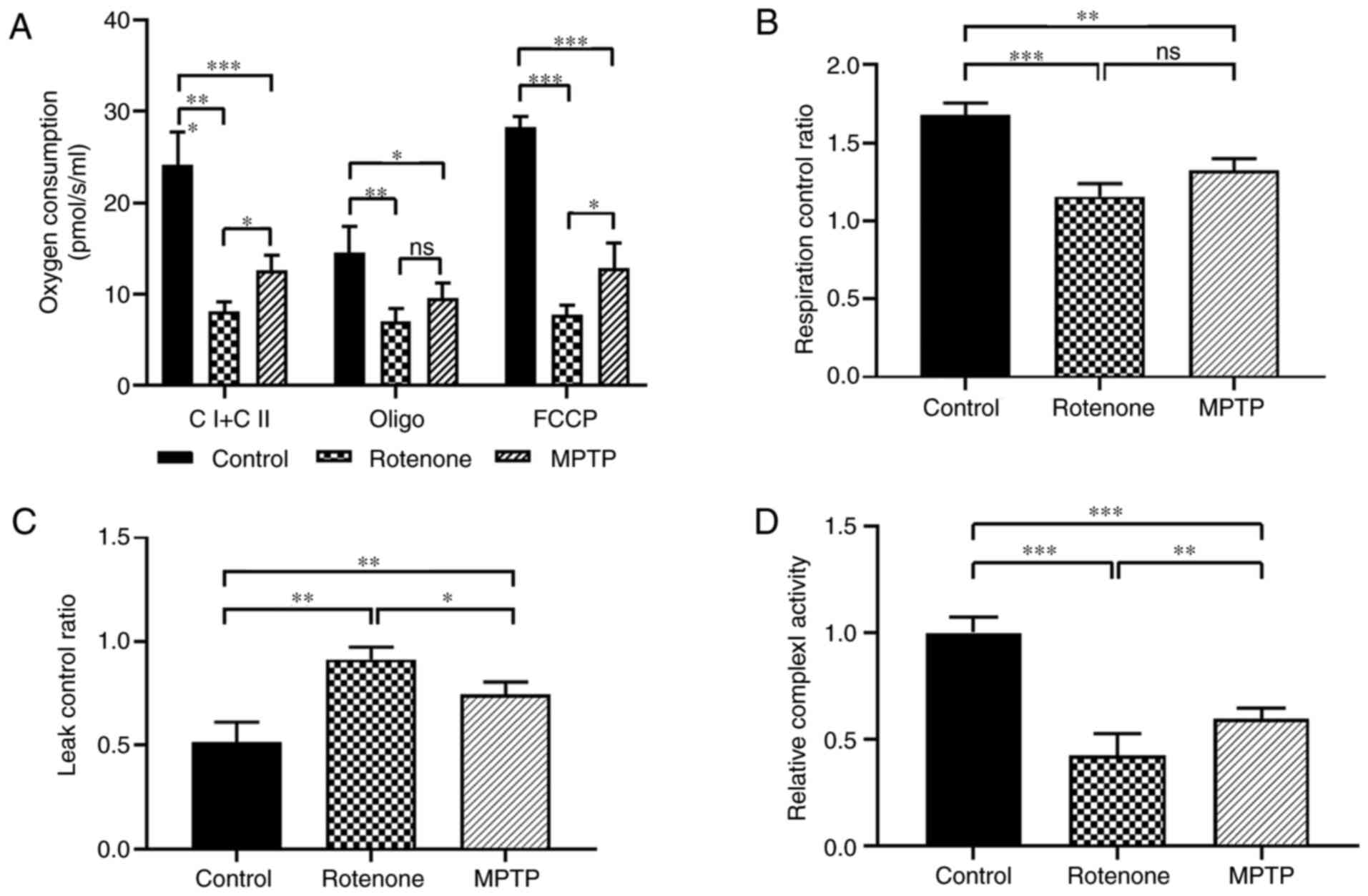 | Figure 6.Oxygen consumption levels and complex
I enzyme activity of mitochondria in the substantia nigra of
rotenone and MPTP Parkinson's disease mouse models. (A)
Mitochondrial oxygen consumption was measured via cell respiration,
including total oxygen consumption of CI + CII, oxygen consumption
after Oligo treatment and oxygen consumption after FCCP treatment.
(B) Respiration control rate was defined as (CI + CII)/oligo. (C)
Leak control rate was defined as oligo/FCCP. (D) Activity of
complex I enzyme in each group was detected using a U-3900
spectrophotometer, with citrate synthase as the internal reference
standard. *P<0.05, **P<0.01, ***P<0.001. ns, no
significant difference; MPTP,
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; Oligo, oligomycin;
FCCP, carbonyl cyanide-p-trifluoromethoxyphenylhydrazone; CI + CII,
mitochondrial complexes I and II. |
To examine whether the coupling of substrate
oxidation to ATP production was impaired, the present study
assessed the mitochondrial coupling efficiency using the
respiratory control ratio (RCR) and leak control ratio (LCR). For
the RCR that was defined as the (CI + CII)/oligo ratio (Fig. 6B), the lower the value, the lower
the efficiency of electron transport chain coupling with oxidative
phosphorylation. The RCR values decreased significantly in the
rotenone and MPTP groups compared with the control group (both
P<0.01). The RCR was not significantly different between the
rotenone and MPTP groups. For the LCR, which was defined as the
oligo/FCCP ratio (Fig. 6C), the
larger the value the lower the coupling efficiency of mitochondria.
The LCR was significantly higher in the rotenone and MPTP groups
compared with in the control group (both P<0.01). Furthermore,
the LCR was considerably higher in the rotenone group compared with
the MPTP group (P<0.05). These results revealed that both
rotenone and MPTP decreased the coupling efficiency of mitochondria
in the SN and suggested that the effects of rotenone were more
pronounced.
Mitochondrial complex I enzyme activity was measured
as the rate of NADH oxidation (Fig.
6D). This assessment was based on the characteristic that
complex I (NADH dehydrogenase) catalyzes NADH dehydrogenation to
NAD+ when measured at 340 nm and was normalized with the
activity of citric acid synthase (44). The activity of mitochondrial
complex I enzyme was significantly lower in the rotenone and MPTP
groups compared with the control group (both P<0.001), and the
activity in the rotenone group was considerably lower compared with
the MPTP group (P<0.01). These results demonstrated that both
rotenone and MPTP could significantly inhibit mitochondrial complex
I enzyme activity and indicated that the effects produced by
rotenone were more important.
Discussion
The incidence of PD may result from genetic and
environmental factors and is divided into two forms, familial and
sporadic. Overall, ~95% of PD cases are sporadic and may be
associated with environmental factors (45). Currently, animal models of PD
primarily include neurotoxin-based animal models and genetic animal
models, each of which have advantages and disadvantages (46). Although the pathogenic mechanisms
underlying the genetic animal models of PD are precise, these
models are complex, time-consuming and expensive to produce.
Compared with the genetic animal models, neurotoxin-based PD animal
models can reproduce the behavioral changes and pathological
characteristics of PD (mainly sporadic PD), and the modeling
protocols are more straightforward (47). The neurotoxin-based PD animal
models reproduce the effects of toxic environmental factors
associated with PD and have been widely used in model construction
(48). For example, rotenone is
widely used as a crop insecticide and fish poison in fish culture
systems (49). Studies have
demonstrated that long-term, low-concentration exposure to
toxicants may be an environmental factor that can lead to PD
(50–52). It also has been reported that MPTP
is associated with PD caused by drug abuse (53). Therefore, the present study is of
considerable practical importance for the neurotoxin-based PD
models.
MPTP administration currently has become the most
commonly used animal model of PD. There are three main models that
use MPTP, including acute, subacute and chronic (54). The acute mouse model of PD has
been created by injecting MPTP four times within 6 h (55,56). The subacute mouse PD model has
been produced by injecting MPTP once a day for several days to
several weeks (57). Finally, the
chronic mouse model has been created by injecting MPTP and
probenecid at 3.5-day intervals for 6 weeks. The acute model causes
neuronal necrosis instead of apoptosis, which is not consistent
with the actual disease (58).
Zhang et al (59) revealed
that it was challenging for the subacute MPTP administration to
accurately simulate the motor dysfunction that occurs in patients
with PD due to the compensatory response of the norepinephrine and
DA systems, although it could produce damage to DA neurons and lead
to the depletion of dopamine in the striatum. The chronic
MPTP-induced mouse models exhibit a low mortality rate, chronic
symptoms and stable pathology, which more accurately simulate the
pathological process of PD (60).
Therefore, MPTP (20 mg/kg) and probenecid were injected
intraperitoneally twice a week for 6 weeks in the present study
(61).
The chronic rotenone-induced mouse model also
simulates the pathological and biochemical characteristics of
patients with PD. The use of gavage administration effectively
avoids severe injury that can result from repeated subcutaneous
injections at the neck and back of the mouse (46). The chronic rotenone-induced mouse
model is useful for studying changes that occur during PD and
developing therapeutic drugs due to the low mortality rate and high
success rate in model establishment (62). Inden et al (63,64) reported that the survival rate of
rotenone-treated mice administered 30 mg/kg via gavage was 70%
within 7 days and remained at 70% until 42 days. Therefore,
rotenone is typically administered by gavage once a day for 6
weeks, using a dose of 30 mg/kg.
In the present study, the results indicated that
this dose selection, route and interval of administration of the
neurotoxins resulted in the development of a stable animal model
and minimized mortality. The survival rates of the rotenone and
MPTP groups were 86.7 and 80%, respectively, which provided
feasible models to study PD. Since the results revealed no
significant differences between the control group of 0.5% CMC
administered via gavage and the control group of 250 mg/kg
probenecid administered via IP injection, the probenecid control
group was not included in the present study.
The results demonstrated that the PD models induced
by rotenone and MPTP produced significant alterations in behavior
with no significant differences between the two groups. This
observation suggested that chronic rotenone and MPTP exposure could
accurately reproduce behavioral changes associated with PD.
Although the differences were not significant, the motor function
in the MPTP group demonstrated a trend to be more impaired compared
with the rotenone group. Also, tremor was observed in the MPTP
group but not in the rotenone group. These differences may be
caused by a more severe damage of the SN-striatum pathway in the
MPTP group, which has a more prominent effect on the regulation of
movement by the basal ganglia (65).
When considering the pathological changes, the
results indicated that the degeneration of DA neurons in the SNpc,
dopamine depletion in the striatum and neuroinflammation in the SN
induced by MPTP were significantly more severe compared with the
rotenone group. It has been previously reported that both rotenone
and MPTP are effective in inhibiting mitochondrial complex I, but
they do so by acting on different sites of the complex (21). Rotenone inhibits complex I by
binding to the coenzyme Q site competitively (66). However, increasing evidence
supports the possibility that rotenone also reduces reactive oxygen
species (ROS) production produced by the electron transport in the
respiratory chain (67). Zawada
et al (68) reported that
the rotenone exposure of MPP+-treated N27 cell cultures
suppressed H2O2 production by 50%. Astrocytes
convert MPTP into the neurotoxic molecule MPP+, which is
taken up into DA neurons via the DAT (21). MPP+ induces an initial
wave of ROS production by binding to complex I and, subsequently,
the second wave of ROS is mediated by extra-mitochondrial NADPH
oxidase, which leads to multiple forms of cell damage (68). Furthermore, both rotenone and MPTP
do not cause damage to DA neurons in the VTA (69). Due to the neuroinflammation in the
SN induced by MPTP and rotenone, it may be necessary to detect the
expression of soluble triggering receptor expressed on myeloid
cells 2 (TREM2) in future studies, since soluble TREM2 in the
cerebrospinal fluid (CSF) has been revealed to be a biomarker of
neurodegeneration and glial activation in Alzheimer's disease
(70). In several recent studies,
soluble TREM2 in CSF has been reported as a potential biomarker of
neuronal injury in Parkinson's disease (71–73).
It has been indicated that ATP-sensitive
K+ (KATP) channels are selectively activated
in the SNpc but not in VTA, suggesting that the death of DA neurons
in the SNpc may be associated with the activation of
KATP channels (74).
KATP channels are hetero-octamers formed with four
regulatory sulphonyl urea receptor (SUR) subunits (SUR1, SUR2A or
SUR2B) and four inwardly rectifying potassium channel subunits
(Kir6.1 or Kir6.2). In a previous study on an MPTP-induced PD mouse
model, SUR1 mRNA expression has been indicated to be two-fold
higher in the SNpc DA neurons compared with the VTA, suggesting
that the selective upregulation of SUR1 leads to DA neuronal damage
(75). In addition to DA neuronal
loss, the presence of Lewy bodies in the remaining neurons
represents a central pathological feature of PD (76). In the present study, chronic,
low-dose rotenone treatment was more likely to lead to the
accumulation of phosphorylated α-synuclein in DA neurons, resulting
in the formation of Lewy bodies, but MPTP treatment did not. This
observation was consistent with several previous reports (21,26).
α-synuclein is a ubiquitously expressed small
protein that is highly enriched in presynaptic nerve terminals,
specifically on the highly curved membrane of synaptic vesicles
(77). It has been demonstrated
that rotenone accelerates the aggregation process of α-synuclein
monomers to form amyloid fibrils (78). Rotenone exposure also leads to the
localization of α-synuclein aggregates in the mitochondria and to
decreased mitochondrial membrane potential, resulting in cell death
(79). Rotenone may lead to
α-synuclein accumulation in the following two ways: i) Increasing
the expression of α-synuclein protein by enhancing the
transcription of the α-synuclein gene; and ii) preventing the
degradation of α-synuclein protein through the
ubiquitin-proteasomal system and increasing phosphorylation of
α-synuclein at S129, which makes it more prone to aggregation
(80). These observations
suggested that the neuropathology of MPTP is more robust compared
with rotenone, but MPTP does not reproduce all the pathological
features of PD.
Mitochondria are indispensable for sustaining the
high energy demand of neurons. It also is well known that
mitochondria regulate programmed cell death, calcium homeostasis
and numerous other important cellular processes (81). Mitochondrial dysfunction has been
strongly implicated in the pathogenesis of PD. Plotegher and Duchen
(82) have reported that a number
of the causative or risk factor genes for PD are associated with
mitochondrial quality-control pathways, ranging from mitochondrial
proteins to proteins that regulate the endo-lysosomal function.
Mitochondrial dysfunction also serves an important role in the
pathogenesis of sporadic PD. Turner and Schapira (83) have reported decreased activity of
respiratory chain complex I in the SN in brain tissue obtained from
patients with PD at autopsy. Betarbet et al (84) observed complex I deficiencies in
skeletal muscle and platelets from patients with PD. When
mitochondrial complex I activity is decreased, a lower
concentration of H2O2 can reduce the membrane
potential of nerve fiber terminals. At the same time, blocking
electronic respiratory chain transmission leads to increased free
radical production and additional damage to complex I. Both
rotenone and MPP+ inhibit the activity of mitochondrial
complex I (85), which impairs
oxidative phosphorylation. Mitochondrial function studies have
revealed a notable decrease in complex I activity and oxygen
consumption in both PD models (86,87). In the present study, rotenone
exhibited a greater effect compared with MPTP. Abnormalities in
mitochondrial function represented by the coupling level of
electron transport and oxidative phosphorylation also were
significantly aggravated in the Rotenone and MPTP groups compared
with the control.
Rotenone exerts a stronger inhibition on
mitochondrial complex I compared with MPTP, which may be attributed
to the fact that rotenone can enter DA neurons directly and
competitively bind to coenzyme Q to inhibit mitochondrial complex I
without the interference of metabolism or the need for
transporters. On the other hand, MPTP must be metabolized into
MPP+ in astrocytes and then enter DA neurons through the
DAT. In addition, MPP+ accumulates in mitochondria,
synaptic vesicles and the cytoplasm in DA neurons. Thus, only a
portion of the MPP+ enters mitochondria and inhibits
complex I (21,88). Moreover, Plotegher and Duchen
(82) have demonstrated that the
inhibition of mitochondrial complex I activity by rotenone impairs
lysosomal function and mitochondrial autophagy, resulting in an
imbalance in the mitochondrial quality control mechanism.
In conclusion, the use of appropriate animal models
will help researchers explore the causes and mechanisms of diseases
and design more effective treatments. The present study indicated
that the effects produced by the rotenone- and MPTP-induced chronic
PD mouse models exhibited several important differences. While
there was no significant difference in neurobehavior between the
two models, the motor function of the animal models decreased
significantly compared with the control group. Based on the current
study, the MPTP model may be more suitable to study the loss of DA
neurons, decreases in dopamine content and neuroinflammation in the
SN. However, the chronic MPTP model had a higher mortality rate and
lacked the most important neuropathological feature of PD, which is
the formation of Lewy bodies. The rotenone model was more suited to
study mitochondrial dysfunction (deficient complex I activity) and
the formation of Lewy bodies in the SNpc. However, the degeneration
of DA neurons in the SNpc, dopamine depletion in the striatum and
neuroinflammation in the SN induced by rotenone were not as severe
as in the MPTP model. Thus, the MPTP and rotenone PD models have
advantages and disadvantages, therefore one or both should be
selected based on the purpose of the study. The present study has
provided a baseline for exploring the mechanism of the pathogenesis
of PD, especially when seeking effective treatment methods for
PD.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the National
Natural Science Foundation of China (grant no. 81971291), the
Natural Science Foundation of Zhejiang Province (grant no.
LY19C070001), the School of Laboratory Medicine and Life Sciences,
Wenzhou Medical University (grant no. 437601607), the Beijing
Friendship Hospital, Capital Medical University (grant no.
YYZZ201920) and the Key Discipline of Zhejiang Province in Medical
Technology (First Class, Category A).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JZ, BS and JY performed the experiments, analyzed
the data and wrote the paper. ZC, ZL and NZ helped to performed the
experiments and analyzed the data. LS and HL designed the research,
analyzed the data and wrote and revised the paper. All authors
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Wenzhou Medical University (approval no.
wydw2020-0840; Wenzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Alamri Y, Pitcher T and Anderson TJ:
Variations in the patterns of prevalence and therapy in
Australasian Parkinson's disease patients of different ethnicities.
BMJ Neurol Open. 2:e0000332020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jankovic J: Parkinson's disease: Clinical
features and diagnosis. J Neurol Neurosurg Psychiatry. 79:368–376.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schneider SA and Obeso JA: Clinical and
pathological features of Parkinson's disease. Curr Top Behav
Neurosci. 22:205–220. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schapira AH and Jenner P: Etiology and
pathogenesis of Parkinson's disease. Mov Disord. 26:1049–1055.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Walter J, Bolognin S, Antony PMA, Nickels
SL, Poovathingal SK, Salamanca L, Magni S, Perfeito R, Hoel F, Qing
X, et al: Neural stem cells of Parkinson's disease patients exhibit
aberrant mitochondrial morphology and functionality. Stem Cell
Reports. 12:878–889. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nakano M, Imamura H, Sasaoka N, Yamamoto
M, Uemura N, Shudo T, Fuchigami T, Takahashi R and Kakizuka A: ATP
maintenance via two types of ATP regulators mitigates pathological
phenotypes in mouse models of Parkinson's disease. EBioMedicine.
22:225–241. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wi S, Lee JW, Kim M, Park CH and Cho SR:
An enriched environment ameliorates oxidative stress and olfactory
dysfunction in Parkinson's disease with α-Synucleinopathy. Cell
Transplant. 27:831–839. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
De Miranda BR and Greenamyre JT:
Trichloroethylene, a ubiquitous environmental contaminant in the
risk for Parkinson's disease. Environ Sci Process Impacts.
22:543–554. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Merhi R, Kalyn M, Zhu-Pawlowsky A and
Ekker M: Loss of Parla function results in inactivity, olfactory
impairment, and dopamine neuron loss in Zebrafish. Biomedicines.
9:2052021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fifel K and Videnovic A: Chronotherapies
for Parkinson's disease. Prog Neurobiol. 174:16–27. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mursaleen L, Noble B, Chan SHY, Somavarapu
S and Zariwala MG: N-Acetylcysteine Nanocarriers protect against
oxidative stress in a cellular model of Parkinson's disease.
Antioxidants (Basel). 9:6002020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang B, Su CJ, Liu TT, Zhou Y, Feng Y,
Huang Y, Liu X, Wang ZH, Chen LH, Luo WF and Liu T: The
Neuroprotection of Low-Dose morphine in cellular and animal models
of Parkinson's disease through ameliorating endoplasmic reticulum
(ER) stress and activating autophagy. Front Mol Neurosci.
11:1202018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou L, Zhang Q, Zhang P, Sun L, Peng C,
Yuan Z and Cheng J: C-Abl-Mediated Drp1 phosphorylation promotes
oxidative stress-induced mitochondrial fragmentation and neuronal
cell death. Cell Death Dis. 8:e31172017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bao XX, Ma HH, Ding H, Li WW and Zhu M:
Preliminary optimization of a Chinese herbal medicine formula based
on the neuroprotective effects in a rat model of rotenone-induced
Parkinson's disease. J Integr Med. 16:290–296. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Abdul-Latif R, Stupans I, Allahham A,
Adhikari B and Thrimawithana T: Natural antioxidants in the
management of Parkinson's disease: Review of evidence from cell
line and animal models. J Integr Med. 19:300–310. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sai Y, Chen J, Ye F, Zhao Y, Zou Z, Cao J
and Dong Z: Dopamine release suppression dependent on an increase
of intracellular Ca(2+) contributed to rotenone-induced
neurotoxicity in PC12 Cells. J Toxicol Pathol. 26:149–157. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
De Lazzari F, Bubacco L, Whitworth AJ and
Bisaglia M: Superoxide radical dismutation as new therapeutic
strategy in Parkinson's disease. Aging Dis. 9:716–728. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qi Z, Miller GW and Voit EO: Rotenone and
paraquat perturb dopamine metabolism: A computational analysis of
pesticide toxicity. Toxicology. 315:92–101. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Benskey MJ, Perez RG and Manfredsson FP:
The Contribution of alpha synuclein to neuronal survival and
function-implications for Parkinson's disease. J Neurochem.
137:331–359. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
San Miguel M, Martin KL, Stone J and
Johnstone DM: Photobiomodulation mitigates cerebrovascular leakage
induced by the Parkinsonian neurotoxin MPTP. Biomolecules.
9:5642019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dauer W and Przedborski S: Parkinson's
disease: Mechanisms and models. Neuron. 39:889–909. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Razali K, Othman N, Mohd Nasir MH,
Doolaanea AA, Kumar J, Ibrahim WN, Mohamed Ibrahim N and Mohamed
WM: The promise of the Zebrafish model for Parkinson's disease:
Today's science and Tomorrow's treatment. Front Genet.
12:6555502021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bhurtel S, Katila N, Srivastav S, Neupane
S and Choi DY: Mechanistic comparison between MPTP and rotenone
neurotoxicity in mice. Neurotoxicology. 71:113–121. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
MacArthur Clark JA and Sun D: Guidelines
for the ethical review of laboratory animal welfare People's
Republic of China national standard GB/T 35892-2018 [Issued 6
February 2018 Effective from 1 September 2018]. Animal Model Exp
Med. 3:103–113. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Han X, Zhu J, Zhang X, Song Q, Ding J, Lu
M, Sun S and Hu G: Plin4-dependent lipid droplets hamper neuronal
mitophagy in the MPTP/p-induced mouse model of Parkinson's disease.
Front Neurosci. 12:3972018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou Q, Chen B, Wang X, Wu L, Yang Y,
Cheng X, Hu Z, Cai X, Yang J, Sun X, et al: Sulforaphane protects
against rotenone-induced neurotoxicity in vivo: Involvement of the
mTOR, Nrf2, and autophagy pathways. Sci Rep. 6:322062016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li T, Zhang W, Kang X, Yang R, Li R, Huang
L, Chen J, Yang Q and Sun X: Salidroside protects dopaminergic
neurons by regulating the mitochondrial MEF2D-ND6 pathway in the
MPTP/MPP+-induced model of Parkinson's disease. J
Neurochem. 153:276–289. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ogrodnik M, Zhu Y, Langhi LGP, Tchkonia T,
Krüger P, Fielder E, Victorelli S, Ruswhandi RA, Giorgadze N,
Pirtskhalava T, et al: Obesity-induced cellular senescence drives
anxiety and impairs neurogenesis. Cell Metab. 29:1061–1077.e8.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu CS, Chen H, Sun H, Zhu J, Jew CP,
Wager-Miller J, Straiker A, Spencer C, Bradshaw H, Mackie K and Lu
HC: GPR55, a G-protein coupled receptor for
lysophosphatidylinositol, plays a role in motor coordination. PLoS
One. 8:e603142013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang W, Zhou M, Lu W, Gong J, Gao F, Li
Y, Xu X, Lin Y, Zhang X, Ding L, et al: CNTNAP4 deficiency in
dopaminergic neurons initiates Parkinsonian phenotypes.
Theranostics. 10:3000–3021. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu J, Liu W, Lu Y, Tian H, Duan C, Lu L,
Gao G, Wu X, Wang X and Yang H: Piperlongumine restores the balance
of autophagy and apoptosis by increasing BCL2 phosphorylation in
rotenone-induced Parkinson disease models. Autophagy. 14:845–861.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhao QY, Ge LH, Zhang K, Chen HF, Zhan XX,
Yang Y, Dang QL, Zheng Y, Zhou HB, Lyu JX and Fang HZ: Assessment
of mitochondrial function in metabolic dysfunction-associated fatty
liver disease using obese mouse models. Zool Res. 41:539–551. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Spinazzi M, Casarin A, Pertegato V,
Salviati L and Angelini C: Assessment of mitochondrial respiratory
chain enzymatic activities on tissues and cultured cells. Nat
Protoc. 7:1235–1246. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu J, Xu F, Nie Z and Shao L: Gut
microbiota Approach-A new strategy to treat Parkinson's disease.
Front Cell Infect Microbiol. 10:5706582020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shi W, Zhang Y, Zhao G, Wang S, Zhang G,
Ma C, Cong B and Li Y: Dysregulation of dopaminergic regulatory
factors TH, Nurr1, and Pitx3 in the ventral tegmental area
associated with neuronal injury induced by chronic morphine
dependence. Int J Mol Sci. 20:2502019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Domanskyi A, Alter H, Vogt MA, Gass P and
Vinnikov IA: Transcription factors foxa1 and foxa2 are required for
adult dopamine neurons maintenance. Front Cell Neurosci. 8:2752014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang XH, Lu G, Hu X, Tsang KS, Kwong WH,
Wu FX, Meng HW, Jiang S, Liu SW, Ng HK, et al: Quantitative
assessment of gait and neurochemical correlation in a classical
murine model of Parkinson's disease. BMC Neurosci. 13:1422012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kelly E, Vyas P and Weber JT: Biochemical
properties and neuroprotective effects of compounds in various
species of berries. Molecules. 23:262017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shin WH and Chung KC: Death-associated
protein kinase 1 phosphorylates α-Synuclein at ser129 and
exacerbates rotenone-induced toxic aggregation of α-Synuclein in
dopaminergic SH-SY5Y Cells. Exp Neurobiol. 29:207–218. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen C, Wei YZ, He XM, Li DD, Wang GQ, Li
JJ and Zhang F: Naringenin produces neuroprotection against
LPS-induced dopamine neurotoxicity via the inhibition of microglial
NLRP3 inflammasome activation. Front Immunol. 10:9362019.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gangapuram M, Mazzio E, Eyunni S, Soliman
KF and Redda KK: Synthesis and biological evaluation of substituted
N-[3-(1H-Pyrrol-1-yl)Methyl]-1,2,5,6-tetrahydropyridin-1-yl]Benzamide/Benzene
sulfonamides as anti-inflammatory agents. Arch Pharm (Weinheim).
347:360–369. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xu SF, Zhang YH, Wang S, Pang ZQ, Fan YG,
Li JY, Wang ZY and Guo C: Lactoferrin ameliorates dopaminergic
neurodegeneration and motor deficits in MPTP-treated mice. Redox
Biol. 21:1010902019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Barbon S, Rajendran S, Bertalot T,
Piccione M, Gasparella M, Parnigotto PP, Di Liddo R and Conconi MT:
Growth and differentiation of circulating stem cells after
extensive ex vivo expansion. Tissue Eng Regen Med. 18:411–427.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Fang Y, Zhao C, Xiang H, Zhao X and Zhong
R: Melatonin inhibits formation of mitochondrial permeability
transition pores and improves oxidative phosphorylation of
frozen-thawed ram sperm. Front Endocrinol (Lausanne). 10:8962019.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gasperi V, Sibilano M, Savini I and Catani
MV: Niacin in the central nervous system: An update of biological
aspects and clinical applications. Int J Mol Sci. 20:9742019.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chia SJ, Tan EK and Chao YX: Historical
perspective: Models of Parkinson's disease. Int J Mol Sci.
21:24642020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Jagmag SA, Tripathi N, Shukla SD, Maiti S
and Khurana S: Evaluation of models of Parkinson's disease. Front
Neurosci. 9:5032015.PubMed/NCBI
|
|
48
|
Goldman SM: Environmental toxins and
Parkinson's disease. Annu Rev Pharmacol Toxicol. 54:141–164. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhang P, Qin D, Chen J and Zhang Z: Plants
in the genus tephrosia: Valuable resources for botanical
insecticides. Insects. 11:7212020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Elbaz A and Moisan F: Update in the
epidemiology of Parkinson's disease. Curr Opin Neurol. 21:454–460.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wirdefeldt K, Adami HO, Cole P,
Trichopoulos D and Mandel J: Epidemiology and etiology of
Parkinson's disease: A review of the evidence. Eur J Epidemiol. 26
(Suppl 1):S1–S58. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Das K, Ghosh M, Nag C, Nandy SP, Banerjee
M, Datta M, Devi G and Chaterjee G: Role of familial, environmental
and occupational factors in the development of Parkinson's disease.
Neurodegener Dis. 8:345–351. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
McKnight S and Hack N: Toxin-Induced
Parkinsonism. Neurol Clin. 38:853–865. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wang LY, Yu X, Li XX, Zhao YN, Wang CY,
Wang ZY and He ZY: Catalpol exerts a neuroprotective effect in the
MPTP mouse model of Parkinson's disease. Front Aging Neurosci.
11:3162019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Tan Y, Xu Y, Cheng C, Zheng C, Zeng W,
Wang J, Zhang X, Yang X, Wang J, Yang X, et al: LY354740 reduces
extracellular glutamate concentration, inhibits phosphorylation of
Fyn/NMDARs, and expression of PLK2/pS129 α-Synuclein in mice
treated with acute or sub-acute MPTP. Front Pharmacol. 11:1832020.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Jackson-Lewis V and Przedborski S:
Protocol for the MPTP mouse model of Parkinson's disease. Nat
Protoc. 2:141–151. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Li G, Luo W, Wang B, Qian C, Ye Y, Li Y
and Zhang S: HMGA1 induction of MiR-103/107 forms a negative
feedback loop to regulate autophagy in MPTP model of Parkinson's
disease. Front Cell Neurosci. 14:6200202020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Meredith GE and Rademacher DJ: MPTP mouse
models of Parkinson's disease: An update. J Parkinsons Dis.
1:19–33. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zhang QS, Heng Y, Mou Z, Huang JY, Yuan YH
and Chen NH: Reassessment of Subacute MPTP-treated mice as animal
model of Parkinson's disease. Acta Pharmacol Sin. 38:1317–1328.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Mustapha M and Mat Taib CN: MPTP-induced
mouse model of Parkinson's disease: A promising direction of
therapeutic strategies. Bosn J Basic Med Sci. 21:422–433.
2021.PubMed/NCBI
|
|
61
|
Sung YH: Effects of treadmill exercise on
hippocampal neurogenesis in an MPTP/Probenecid-induced Parkinson's
disease mouse model. J Phys Ther Sci. 27:3203–3206. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Greenamyre JT, Betarbet R and Sherer TB:
The rotenone model of Parkinson's disease: Genes, environment and
mitochondria. Parkinsonism Relat Disord. 9 (Suppl 2):S59–S64. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Inden M, Kitamura Y, Takeuchi H, Yanagida
T, Takata K, Kobayashi Y, Taniguchi T, Yoshimoto K, Kaneko M, Okuma
Y, et al: Neurodegeneration of mouse nigrostriatal dopaminergic
system induced by repeated oral administration of rotenone is
prevented by 4-Phenylbutyrate, a chemical chaperone. J Neurochem.
101:1491–1504. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Inden M, Kitamura Y, Abe M, Tamaki A,
Takata K and Taniguchi T: Parkinsonian rotenone mouse model:
Reevaluation of long-term administration of rotenone in C57bl/6
mice. Biol Pharm Bull. 34:92–96. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Lieu CA and Subramanian T: The
interhemispheric connections of the striatum: Implications for
Parkinson's disease and drug-induced dyskinesias. Brain Res Bull.
87:1–9. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Pravdic D, Hirata N, Barber L, Sedlic F,
Bosnjak ZJ and Bienengraeber M: Complex I and ATP synthase mediate
membrane depolarization and matrix acidification by isoflurane in
mitochondria. Eur J Pharmacol. 690:149–157. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Murphy MP: How mitochondria produce
reactive oxygen species. Biochem J. 417:1–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Zawada WM, Banninger GP, Thornton J,
Marriott B, Cantu D, Rachubinski AL, Das M, Griffin WS and Jones
SM: Generation of reactive oxygen species in
1-Methyl-4-Phenylpyridinium (MPP+) Treated dopaminergic neurons
occurs as an NADPH oxidase-dependent two-wave cascade. J
Neuroinflammation. 8:1292011. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Brichta L and Greengard P: Molecular
determinants of selective dopaminergic vulnerability in Parkinson's
disease: An update. Front Neuroanat. 8:1522014. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Morenas-Rodríguez E, Alcolea D,
Suárez-Calvet M, Muñoz-Llahuna L, Vilaplana E, Sala I, Subirana A,
Querol-Vilaseca M, Carmona-Iragui M, Illán-Gala I, et al: Different
pattern of CSF glial markers between dementia with Lewy bodies and
Alzheimer's disease. Sci Rep. 9:78032019. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Peng G, Qiu J, Liu H, Zhou M, Huang S, Guo
W, Lin Y, Chen X, Li Z, Li G, et al: Analysis of cerebrospinal
fluid soluble TREM2 and polymorphisms in sporadic Parkinson's
disease in a Chinese population. J Mol Neurosci. 70:294–301. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Wilson EN, Swarovski MS, Linortner P,
Shahid M, Zuckerman AJ, Wang Q, Channappa D, Minhas PS, Mhatre SD,
Plowey ED, et al: Soluble TREM2 is elevated in Parkinson's disease
subgroups with increased CSF Tau. Brain. 143:932–943. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Mo M, Tang Y, Wei L, Qiu J, Peng G, Lin Y,
Zhou M, Dai W, Zhang Z, Chen X, et al: Soluble triggering receptor
expressed on myeloid cells 2 from cerebrospinal fluid in sleep
disorders related to Parkinson's disease. Front Aging Neurosci.
13:7532102021. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Liss B, Haeckel O, Wildmann J, Miki T,
Seino S and Roeper J: K-ATP channels promote the differential
degeneration of dopaminergic midbrain neurons. Nat Neurosci.
8:1742–1751. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
75
|
Han SS, Jiao Q, Bi MX, Du XX and Jiang H:
The expression of KATP channel subunits in
alpha-synuclein-transfected MES23.5 cells. Ann Transl Med.
6:1702018. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Dickson DW: Neuropathology of Parkinson
disease. Parkinsonism Relat Disord. 46 (Suppl 1):S30–S33. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Burré J, Sharma M and Südhof TC: Cell
biology and pathophysiology of α-Synuclein. Cold Spring Harb
Perspect Med. 8:a0240912018. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Silva BA, Einarsdottir O, Fink AL and
Uversky VN: Biophysical characterization of α-Synuclein and
rotenone interaction. Biomolecules. 3:703–732. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Wang Y, Shinoda Y, Cheng A, Kawahata I and
Fukunaga K: Epidermal fatty acid-binding protein 5 (FABP5)
Involvement in alpha-synuclein-induced mitochondrial injury under
oxidative stress. Biomedicines. 9:2021. View Article : Google Scholar
|
|
80
|
Cookson MR and van der Brug M: Cell
systems and the toxic mechanism(s) of alpha-synuclein. Exp Neurol.
209:5–11. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Rocha EM, De Miranda B and Sanders LH:
Alpha-Synuclein: Pathology, mitochondrial dysfunction and
neuroinflammation in Parkinson's disease. Neurobiol Dis.
109:249–257. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Plotegher N and Duchen MR: Crosstalk
between lysosomes and mitochondria in Parkinson's disease. Front
Cell Dev Biol. 5:1102017. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Turner C and Schapira AH: Mitochondrial
dysfunction in neurodegenerative disorders and ageing. Adv Exp Med
Biol. 487:229–251. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Betarbet R, Sherer TB, MacKenzie G,
Garcia-Osuna M, Panov AV and Greenamyre JT: Chronic systemic
pesticide exposure reproduces features of Parkinson's disease. Nat
Neurosci. 3:1301–1306. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
85
|
Udhayabanu T, Manole A, Rajeshwari M,
Varalakshmi P, Houlden H and Ashokkumar B: Riboflavin responsive
mitochondrial dysfunction in neurodegenerative diseases. J Clin
Med. 6:522017. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Ho PW, Ho JW, Liu HF, So DH, Tse ZH, Chan
KH, Ramsden DB and Ho SL: Mitochondrial neuronal uncoupling
proteins: A target for potential disease-modification in
Parkinson's disease. Transl Neurodegener. 1:32012. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Luo Y, Hoffer A, Hoffer B and Qi X:
Mitochondria: A therapeutic target for Parkinson's disease? Int J
Mol Sci. 16:20704–20730. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Patil DA, Patil VA, Bari SB, Surana SJ and
Patil PO: Animal models for Parkinson's disease. CNS Neurol Disord
Drug Targets. 13:1580–1594. 2014. View Article : Google Scholar : PubMed/NCBI
|