Introduction
Ulcerative colitis (UC) is a chronic and idiopathic
inflammatory disease of the colon, which is characterized by
abdominal pain and mucopurulent bloody stools (1). This digestive system disease seriously
affects patients' ability to work and their quality of life
(2). UC can lead to colon cancer
following long-term recurrence (1,2). UC is
a chronic non-specific inflammatory disease of the colon and rectum
with unclear etiology, which is confined to the large intestinal
mucosa and submucosa (3). The
lesions mostly occur in the sigmoid and rectum but can also extend
to the descending colon and even to the entire colon (3).
The majority of patients with UC have a mild disease
that is mainly treated with 5-aminosalicylic acid (5-ASA), whereas
patients with severe disease require hormone-induced remission
(4,5). It can change the transcription of
inflammatory genes, and play anti-inflammatory and
immunosuppressive roles, therefore it is the preferred therapeutic
drug for patients with severe UC (4). However, in some patients, the oral
drugs reach the distal colon, i.e., the second colon, or even part
of the descending colon, at a low concentration, which makes it
difficult to exert the efficacy and this is the site where UC is
most likely to occur (5).
Suppositories can only act on the rectal mucosa, so the
inflammation of the distal colonic mucosa is often prolonged
(1,2). The drugs used often cause different
degrees of bone marrow suppression and immunosuppression, leading
to leukopenia (6). They can also
induce tuberculosis and the formation of tumors (7). As a result, research exploring more
effective treatments for UC is gaining considerable interest.
In recent years, herbal medicine has been
characterized as one of the most common alternative and
complementary therapeutic modalities that has been shown to be
effective in treating UC (8). The
biological activity of herbal medicine has been explored in terms
of basic research and in terms of its clinical application
(6,7). It is of note that the combination of
Chinese and Western medicine has been shown to be more effective
than single conventional treatment for UC (8,9). The
most famous Traditional Chinese medicine (TCM) used is Pulsatilla
decoction (PD) (10). The
successful application of PD suggests that herbal medicine may be a
promising alternative therapy for the treatment of patients with UC
(9).
UC mucosal immune inflammation is characterized by
the accumulation of antigen-presenting cells, such as dendritic
cells or the MHC-I-like molecule CD11d cells, which are present on
the surface of colon epithelial cells (10). A previous study demonstrated that UC
is associated with the presence of non-invasive (type II) natural
killer (NK)T cells, which produce IL-13 and mediate cytotoxicity of
epithelial cells (11). NKT cells
secreting IL-13 is responsible for the primary inflammation caused
in UC, and is a key molecular mediator that can induce colon
epithelial cell apoptosis, inhibit the expression of intercellular
tight junction proteins and prevent the reformation of epithelial
cells, leading to epithelial barrier damage and causing intestinal
bacteria translocation and further activation of macrophages,
neutrophils, eosinophils, NKT and T cells (12). Therefore, the secretion of IL-13 by
NKT cells is a key initiating event in UC.
PD is derived from Zhang Zhongjing's ‘Shang Han Lun’
and consists of four herbs, including radix pulsatillae (11), cortex fraxini, cortex phellodendri
and rhizoma coptidis, which are effective in adjusting homeostasis
and can induce anti-fungal effects in vitro (12). The nuclear transcription factor
(13), NF-κB, is important in
monitoring the development and progression of inflammatory bowel
diseases by regulating the expression levels of a wide range of
pro-inflammatory cytokines (13).
Clinical studies have reported that PD significantly suppresses the
activation of the NF-κB signaling pathway and cytokine expression,
while it modulates the imbalance in the expression levels of the
proinflammatory factor TNF-α and the anti-inflammatory factor IL-10
(14–16). In a previous study, an
oxazolone-induced colitis model was established in BALB/c mice,
which demonstrated typical symptoms of UC, such as diarrhea,
hematochezia and weight loss, apparent hyperemia, edema and
exudation in the diseased colon, pathological changes of chronic
inflammation in the mucosa and a significant increase in the
expression levels of the characteristic UC cytokines IL-13
(14,15). In the present study, the therapeutic
effects of PD were investigated in an oxazolone-induced colitis
model and its underlying molecular mechanism was elucidated.
Materials and methods
Establishment of a C57BL/6 murine
model of oxazolone-induced colitis
A total of 50 male C57BL/6 mice (age, 8 weeks;
weight, 20 g; SPF grade) expressing natural killer (NK)1.1 were
provided by Shanghai SLAC Laboratory Animal Co., Ltd., and housed
in a specific-pathogen-free environment under normal conditions of
humidity (50±5%) and temperature (25±2°C) with a 12-h light/dark
cycle and access to food and water ad libitum. The mucosal
immune response of UC is a Th2 lymphocytic-like response mediated
by NKT cells. Therefore, NK1.1-expressing mice were used as the
research subjects. Subsequently, on day 1, the mice were
anesthetized with 35–40 mg/kg pentobarbital sodium
intraperitoneally and shaved on the back between the shoulders,
subsequently a 2×2 cm area of skin was exposed, which was treated
with 200 µl sensitizing solution (Sigma-Aldrich; Merck KGaA). On
the day 2, the animals developed an allergic reaction. On day 5,
fasting was initiated. On day 6, following pentobarbital sodium
anesthesia, a 3.5 F hose, lubricated with sterile paraffin oil
(Sangon Biotech Co., Ltd.) was slowly inserted into the anus of the
mouse (distance, 4 cm). A total of 150 µl oxazolone gavage solution
(Sigma-Aldrich; Merck KGaA) was slowly injected and the catheter
was removed. The head of the animal was kept down in a vertical
position for 60 sec in order to leave the oxazolone solution in the
intestinal lumen. All protocols were reviewed and approved by the
ethics committee of Shanghai University of Traditional Chinese
Medicine (approval no. 0015; Shanghai, China).
Preparation and intragastric
administration of water-soluble extracts of PD
PD (16) was made up
of 30 g radix pulsatillae, 9 g cortex fraxini, 5 g cortex
phellodendri and 12 g rhizoma coptidis (all purchased from Longhua
Hospital Shanghai University of Traditional Chinese Medicine;
Shanghai, China). To obtain the water-soluble extracts, the samples
were extracted using ethanol, and then concentrated, dried and
powdered (17). The samples
extracted by alcohol were autoclaved and stored at 4°C. The mice
were randomly divided into the following five groups, with 10 mice
in each group: i) PD group; ii) oxazolone-induced colitis group;
iii) IL-13 (1 mg/kg body weight) intervention using anti IL-13
(PeproTech; cat. no. 500-P178) group; iv) 5-ASA (cat. no.
461814-25G; Sigma-Aldrich; Merck KGaA; 20 mg/g body weight)
positive control group; and v) negative control group (equal volume
saline gavage). The PD extracts (20 mg/g body weight) were
administered intragastrically to the oxazolone-induced colitis mice
once a day for 7 days.
Assessment of clinical colitis in
mice
The disease activity index (DAI) was used to
evaluate the grade and extent of intestinal inflammation according
to an established index system (18). During the treatment, the body
weight, stool characteristics and the degree of bloody stool were
recorded daily and were subsequently assessed using a DAI scoring
system. The body weight change in each mouse was calculated using
the following formula: DAI=[(percent weight loss + diarrheal stool
score + bloody stool score)]/3. The scores ranged from 0 to 4,
including the following three parameters: Weight loss, stool
consistency and rectal bleeding.
Reverse transcription-quantitative PCR
(RT-qPCR)
RT-qPCR was used to evaluate mRNA expression in
different colon tissue groups. The extraction of total RNA was
performed using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instruction. Next, purified RNA was subjected to RT using the
PrimeScript RT reagent kit (Takara Bio, Inc.). RT-qPCR was
performed using SYBR Green qPCR Master Mix (Takara Bio, Inc.) as
follows: Initial degeneration at 95°C for 30 sec, followed by 40
cycles of 95°C for 5 sec and 60°C for 30–60 sec. The relative
expression of mRNA (normalized against actin) was calculated by the
2−ΔΔCq method (19).
The following primers were used for each target
gene: Beclin-1 forward, 5′-CCCAGCCAGGATGATGTCTAC-3′ and reverse,
5′-AGGTCTCCAGTGACCTTGAGT-3′; LC3B forward,
5′-CCGTAGTTCGCTGTACGAGG-3′ and reverse, 5′-AACTCACGTCGGATGTCCAG-3′;
PI3K forward, 5′-CTGGAATGTGTGGCTGGAGT-3′ and reverse,
5′-AGGAGGAAGCGGTGGTCTAT-3′; Akt forward,
5′-ATGAACGACGTAGCCATTGTG-3′ and reverse,
5′-TTGTAGCCAATAAAGGTGCCAT-3′; mTORC1 forward,
5′-ATCGTGCTGTTGGGTGAGAG-3′ and reverse, 5′-TGGATCTCCAGCTCTCCGAA-3′;
autophagy-related protein 13 (ATG13) forward,
5′-TGGACCATCAACCGGAAACT-3′ and reverse, 5′-CAAGGGTATGCAGCTGTCCA-3′;
zona occludens protein 1 (ZO-1) forward, 5′-CATGGCCGGGAATGATGAGA-3′
and reverse, 5′-TGAAACTCTTGCCTCGTCCG-3′; claudin-2 forward,
5′-GATCGGCTCCATCGTCAGC-3′ and reverse,
5′-TGTTGGGTAAGAGGTTGTTTTCC-3′; and GAPDH forward,
5′-AGGTCGGTGTGAACGGATTTG-3′ and reverse,
5′-TGTAGACCATGTAGTTGAGGTCA-3′.
ELISA
ELISA kits (cat. no. ab219634; Abcam) were used to
detect the amount of secretory IL-13 in the serum and the
expression of myeloperoxidase (MPO; cat. no. PA5-16672, Gibco;
Thermo Fisher Scientific, Inc.) in the colon tissues of the mice,
according to the manufacturer's instructions. The OD value was
measured at 450 nm using a microplate reader (Thermo Fisher
Scientific, Inc.) and the concentration was calculated using a
standard curve.
Hematoxylin and eosin (H&E)
staining
Colonic mucosal NKT cell infiltration was observed
using H&E staining. The Chiu's scoring system was used to
quantitatively determine the histological scores of the intestine
(20). Following 7 days of growth,
the mice were sacrificed by decapitation and 10 cm colonic mucosa
was removed from four mice in each group, washed with PBS and fixed
in 10% natural buffered formalin at room temperature for 30 min.
The fixed tissues were embedded in paraffin, cut into tissue
sections (5-µm thick) and stained with H&E (25°C; 3 h; Beyotime
Institute of Biotechnology). The slides were examined with a light
microscope (Nikon Corporation) and the data derived were
qualitatively evaluated with QWin software 3.1 (Leica Microsystems,
Inc.) using the following parameter: NKT cell infiltration.
Western blot analysis
Frozen colonic tissues in liquid nitrogen were
mechanically homogenized (20% w/v) in lysis buffer (pH 7.4)
containing 0.1 mM EDTA, 20 mM HEPES, 12.5 mM MgCl2, 150
mM 0.1% Nonidet P40, NaCl, 1 mM dithiothreitol, 0.2 mM
phenylmethylsulfonyl fluoride, and 1 µg/ml leupeptin, aprotinin and
pepstatin. Homogenates were sonicated (120 Hz; 4°C; 20 min) and
subsequently centrifuged for 10 min at 12,000 × g at 4°C. Protein
concentration was assessed using a BCA assay kit (Beyotime
Institute of Biotechnology). Equivalent quantities (20 µg) of
protein from the cell lysates were loaded onto 15% gels (Beyotime
Institute of Biotechnology), separated via SDS-PAGE and then
separated proteins were transferred onto polyvinylidene fluoride
membranes (EMD Millipore). The membranes were blocked with 5%
non-fat milk in Tris-buffered saline (25°C; 30 min) at room
temperature and subsequently incubated with primary antibodies
(4°C; overnight) against Beclin-1 (1:1,000; cat. no. 3738; Cell
Signaling Technology, Inc.), LC3B (1:1,000; cat. no. 43566; Cell
Signaling Technology, Inc.), PI3K (1:1,000; cat. no. 4255; Cell
Signaling Technology, Inc.), Akt (1:1,000; cat. no. 4691; Cell
Signaling Technology, Inc.), mTORC1 (1:1,000; cat. no. 10441-1-AP;
ProteinTech Group, Inc.), phosphorylated (p)-MTORC1 (1:1,000; cat.
no. 5536; Cell Signaling Technology, Inc.), ATG13 (1:1,000; cat.
no. 13273; Cell Signaling Technology, Inc.), Occludin (1:1,000;
cat. no. 91131; Cell Signaling Technology, Inc.), ZO-1 (1:1,000;
cat. no. ab59720; Abcam), claudin-2 (1:1,000; cat. no. 48120; Cell
Signaling Technology, Inc.) and GAPDH (1:1,000; cat. no. 5174; Cell
Signaling Technology, Inc.) overnight at 4°C. The following day,
the membranes were rinsed using TBS with 1% Tween-20 three times
for 8 min each, followed by incubation at room temperature for 1 h
with goat anti-rabbit horseradish peroxidase-conjugated secondary
antibody (1:1,000; Cell Signaling Technology, #7074, Inc.). The
membranes were washed three times and detected using ECL reagent
(1:1,000; Cell Signaling Technology, Inc.). Finally, the
densitometry was analyzed by Image J v1.8.0.112 software (National
Institutes of Health).
Immunofluorescence
LC3B expression levels were analyzed by
immunofluorescence in colonic tissues. The 2-µm paraffin-embedded
sections of the colon tissue sections (4% PFA; 25°C; 24 h) were
dewaxed, rehydrated with gradient alcohol, subjected to antigen
repair (100°C) and subsequently rinsed three times with 0.01M PBS
(25°C) with 0.1% Tween-20 (PBST). The sections were blocked (25°C)
with 5% BSA and 0.3% Triton X-100 for 1 h, placed in a wet box for
an additional 1 h and incubated with a primary antibody against
LC3B (1:1,000; cat. no. 43566; Cell Signaling Technology, Inc.) at
4°C overnight. The following day, the samples were washed three
times with PBS for 5 min each time and then was incubated with
Alexa-488-conjugated donkey secondary antibody against rabbit
antibody (1:3,000; Cell Signaling Technology, Inc.; cat. no. 4412).
The nuclei were counterstained with 10 µg/ml DAPI (25°C; 3 min) at
room temperature. The images were captured using an Olympus
fluorescent microscope (Olympus Corporation).
Statistical analysis
The results are presented as the mean ± standard
deviation. All data presented are representative of ≥3 experiments.
Statistical analysis was conducted using GraphPad Prism 6.2
software (GraphPad Software, Inc.) and significance was determined
using two-way ANOVA and Dunnett's multiple comparisons test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
PD alleviates the symptoms of UC in
mice
In order to investigate the therapeutic effect of PD
on colitis, the present study induced colitis in a mouse model by
skin pre-sensitization and colonic administration of oxazolone. The
results indicated that the mice injected with oxazolone developed
diarrhea, bloody stool and weight loss. Histological changes, such
as monocyte infiltration, intestinal wall thickening, mucosal
hyperplasia and distortion of crypt structures, were observed in
the model group compared with the normal control group (Fig. 1A). Concomitantly, it was found that
PD significantly reduced H&E and DAI scores compared with the
control (Tables I and II). These effects were also noted with
IL-13 intervention and oral administration of 5-ASA (Fig. 1A-C). These findings indicated that
PD mitigated the severity of oxazolone-induced colitis in mice.
 | Table I.Damage to the intestinal mucosa was
graded using Chiu's scoring system. |
Table I.
Damage to the intestinal mucosa was
graded using Chiu's scoring system.
| Feature | Score | Description |
|---|
| Inflammation
severity | 0 | None |
|
| 1 | Mild |
|
| 2 | Moderate |
|
| 3 | Severe |
| Inflammation
extent | 0 | None |
|
| 1 | Mucosa |
|
| 2 | Submucosa |
|
| 3 | Transmural |
| Crypt damage | 0 | None |
|
| 1 | Basal 1/3
damage |
|
| 2 | Basal 2/3
damage |
|
| 3 | Crypt lost; surface
epithelium present |
|
| 4 | Crypt and surface
epithelium lost |
| % of colon
involvement | 0 | 0% |
|
| 1 | 1–25% |
|
| 2 | 26–50% |
|
| 3 | 51–75% |
|
| 4 | 76–100% |
 | Table II.DAI scoring system in
oxazolone-induced colitis mice. |
Table II.
DAI scoring system in
oxazolone-induced colitis mice.
| Score | Weight loss, % | FOB/bloody
stool | Stool
consistency |
|---|
| 0 | None | Normal | Normal |
| 1 | 1–5 |
|
|
| 2 | 5–10 | Loose stools | FOB (+) |
| 3 | 10–20 |
|
|
| 4 | >20 | Diarrhea | Bloody stool |
PD inhibits oxazolone-induced colitis
inflammation
The length of the colon is considered an indirect
indicator of inflammation and is negatively correlated with the
severity of colitis (21). In
addition, the effects of PD were investigated on inflammation of
oxazolone-induced colitis. The colon tissues were significantly
longer and lighter in the normal control, IL-13 intervention, 5-ASA
and PD groups compared with those of the model group; Fig. 2A-C). The accumulation of MPO in the
colon is a marker of neutrophil entry into the tissue and IL-13 is
an inflammatory cytokine characteristic of UC. The ELISA results
indicated that PD caused a significant reduction in the levels of
MPO in the colon tissues and a significant decrease in serum IL-13
levels compared with the model group (Fig. 3A and B). The same results were found
in the IL-13 and 5-ASA groups compared with model group (Fig. 3A and B). These findings illustrated
that PD could inhibit oxazolone-induced colitis inflammation.
PD inhibits the activation of
autophagy by regulating LC3 and Beclin1 expression
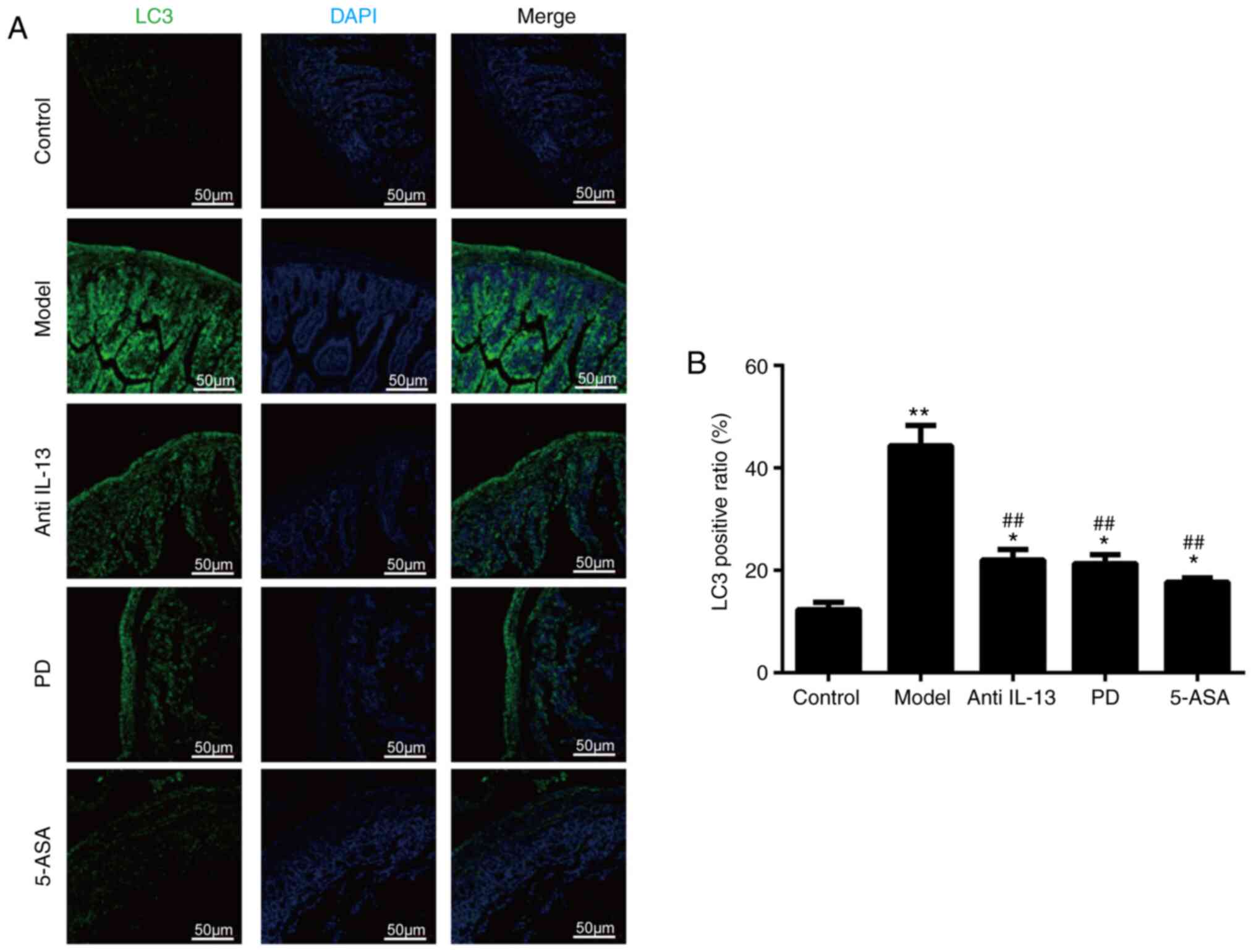
To assess the levels of autophagy in the colon
tissues, western blotting, immunofluorescence and RT-qPCR assays
confirmed that the percentage of cells with low LC3 fluorescence
intensity was significantly higher compared with that of the normal
control group (Fig. 4A and B). The
mRNA and protein expression levels of Beclin1 and LC3 were
upregulated in the model group compared with control (Fig. 5A-E). However, Beclin1 and LC3
expression levels significantly decreased when the mice were
administered PD, 5-ASA or IL-13 (Fig.
5A-E). This indicated that PD significantly inhibited tissue
autophagy in oxazolone-induced colitis. In order to further
investigate the molecular mechanism of action of PD, the expression
levels of the associated molecules in the PI3K-Akt-mTORC1 signaling
pathway were investigated via western blotting and RT-qPCR assays.
The results demonstrated that PI3K, Akt, mTORC1 and ATG13
expression levels were significantly downregulated in the PD, 5-ASA
or IL-13 intervention groups (Fig.
6A-D). In addition, western blot analysis indicated that the of
p-AKT, p-mTORC1 and ATG13 expression levels were increased in the
model group compared with the control group; however, expression
levels were decreased in the PD, 5-ASA and IL-13 groups compared
with the model group. There was no change in the expression levels
of Akt or mTORC1.
(Fig. 6E and F).
Therefore, these results suggested that the protective effects of
PD against oxazolone-induced colitis were exerted via the
regulation of the PI3K-Akt-mTORC1 signaling pathway.
PD protects the colon epithelial
barrier through alteration of tight junction protein
expression
Several studies have shown that the expression
levels of tight junction proteins found between intestinal
epithelial cells, are affected in inflammatory bowel disease,
leading to alterations in tight junction structure and function
(10,22). Therefore, to determine whether PD
maintains the integrity of the intestinal barrier, occludin and
ZO-1 expression levels were detected. The data indicated that they
were decreased, whereas claudin-2 levels were increased compared
with model (Fig. 7A-C). The
relative mRNA expression levels of these markers including occludin
and ZO-1were altered compared with model (Fig. 7A) following PD treatment. These
results suggested that PD protected the intestinal barrier by
altering the expression levels of the colonic tissue tight junction
proteins, which were induced by inflammatory stimuli.
Discussion
Numerous clinical studies have shown that TCM has
unique advantages in the treatment of UC and that the majority of
the herbal preparations exhibit optimal clinical therapeutic
significance (8,23). The treatment process of UC involves
immune regulation and modulation of cytokine levels, signaling
pathways and associated gene expression levels (18). These molecular events are
interconnected through a variety of mechanisms in UC, so as to
relieve intestinal inflammation, restore the normal structure and
function of the colonic mucosa and achieve optimal treatment
efficacy (24–27). The use of PD was first recorded
~1,800 years ago by Zhang Zhongjing in his book ‘Treatise on
Febrile Diseases’, and was a famous prescription for treating
eczema (16,28). Therefore, in the present study, the
efficacy of PD was briefly assessed in oxazolone-induced colitis
and its molecular mechanisms were examined with regard to the
modulation of the PI3K-Akt-mTORC1 and autophagy-related signaling
pathways.
In the present study, the serum levels of IL-13 and
the colon tissue levels of MPO were measured in mice with UC in
order to investigate the possible immunomodulatory effects of PD on
UC. The results indicated that IL-13 and MPO levels in model mice
were significantly higher than those in the control group,
suggesting that increased IL-13 and MPO levels were consistent with
intestinal mucosal injury and inflammation and that they could
serve as an indicator of UC activity, which was consistent with the
results reported in previous studies (29–32).
These data also verified that proinflammatory cytokines caused the
intestinal mucosa to produce an excessive inflammatory response,
thereby triggering intestinal injury. The expression levels of
IL-13 and MPO were inhibited and the histological and DAI scores
were reduced in the treatment group. The weight and length of the
colon tissue were significantly decreased in the treatment group,
indicating that this effect was consistent with previous findings
(16). Overall, the results
highlighted that PD could effectively alleviate the inflammation
and severity of colitis in mice.
Numerous studies that examined the efficacy of
herbal products were not successful in evaluating their mechanisms
of action (14), which hindered
their widespread application. Based on the results of the present
study showing reduced inflammation following PD treatment, it was
speculated that the therapeutic effect of PD may be associated with
the modulation of the intracellular signaling transduction pathway.
It has been shown that the PI3K-Akt-mTORC1 signaling pathway is
also involved in the differentiation and maturation of iNKT cells
(33–36). To assess whether the PI3K-Akt-mTORC1
signaling pathway is involved in regulating inflammation, the mRNA
and protein expression levels of LC3, Beclin1 and several of the
PI3K-Akt-mTORC1 signaling pathway members were assessed via
RT-qPCR, western blotting and immunofluorescence analyses.
Following PD or IL-13 administration, both mRNA and protein
phosphorylation levels of the PI3K-Akt-mTORC1 signaling pathway
were significantly decreased, and total protein expression was not
influenced. The results revealed that the expression levels of
these target genes were significantly increased in the model group
compared with those noted in the control group, indicating that the
PI3K-Akt-mTORC1 signaling pathway was activated in the UC model
group.
Previous studies have reported that impaired
expression of intestinal epithelial tight junction proteins in
inflammatory bowel disease leads to alterations in tight junction
structure and function, resulting in damage to the intestinal
epithelial barrier (37–39). Subsequently, it has been
demonstrated that PD can repair the damaged intestinal epithelial
barrier by regulating the expression levels of junction proteins
(20). In the present study, these
findings were verified by PD or IL-13 neutralization, which
downregulated occludin and ZO-1 protein expression, and upregulated
the expression levels of claudin-2, as confirmed via RT-qPCR and
western blotting.
In summary, the present study elucidated the in
vivo therapeutic effect of PD on UC model mice by a potential
mechanism that may be associated with the activation of the
PI3K-Akt-mTORC1 signaling pathway. The data lay the experimental
foundation for PD as a promising treatment option for UC, however
whether the mechanism of action of PD is related to autophagy,
requires further exploration in the future. The research and
development of UC herbal preparations must follow the developmental
trends of TCM and should aim to accelerate technological innovation
and transformation to develop modern Chinese medicine preparations
that meet market needs and possess successful therapeutic
effects.
Acknowledgements
Not applicable.
Funding
The present study was supported by Putuo District of Shanghai
Science and Technology Commission Research Project (grant no.
ptkwws201806) and Project of Shanghai Municipal Health Commission
of China (grant no. 202040159).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QC and XW participated in the study design,
manuscript writing and confirm the authenticity of all the raw
data. LX performed experiments, analyzed data and participated in
manuscript writing. TW and JX participated in statistical analysis
and manuscript writing. FF, YZ and JW performed experiments and
analyzed data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Ethical approval was given by the Ethics Committee
of Shanghai University of Traditional Chinese Medicine (approval
no. 0015; Shanghai, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Feuerstein JD, Moss AC and Farraye FA:
Ulcerative Colitis. Mayo Clin Proc. 94:1357–1373. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rubin DT, Ananthakrishnan AN, Siegel CA,
Sauer BG and Long MD: ACG Clinical Guideline: Ulcerative Colitis in
Adults. Am J Gastroenterol. 114:384–413. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nakov R: New markers in ulcerative
colitis. Clin Chim Acta. 497:141–146. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ko CW, Singh S, Feuerstein JD, Falck-Ytter
C, Falck-Ytter Y and Cross RK; American Gastroenterological
Association Institute Clinical Guidelines Committee, : AGA Clinical
practice guidelines on the management of Mild-to-Moderate
ulcerative colitis. Gastroenterology. 156:748–764. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cross R, Ko CW and Singh S:
Mild-to-Moderate ulcerative colitis guideline. Gastroenterology.
156:7682019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Teschke R, Wolff A, Frenzel C, Eickhoff A
and Schulze J: Herbal traditional Chinese medicine and its evidence
base in gastrointestinal disorders. World J Gastroenterol.
21:4466–4490. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fan Y, Yi W, Huang H, Mei Z and Feng Z:
Efficacy of herbal medicine (Gegen Qinlian Decoction) on ulcerative
colitis: A systematic review of randomized controlled trials.
Medicine (Baltimore). 98:e185122019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shen Z, Zhou Q, Ni Y, He W, Shen H and Zhu
L: Traditional Chinese medicine for mild-to-moderate ulcerative
colitis: Protocol for a network meta-analysis of randomized
controlled trials. Medicine. 98:e168812019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin JC, Wu JQ, Wang F, Tang FY, Sun J, Xu
B, Jiang M, Chu Y, Chen D, Li X, et al: QingBai decoction regulates
intestinal permeability of dextran sulphate sodium-induced colitis
through the modulation of notch and NF-κB signalling. Cell Prolif.
52:e125472019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang XM, Tian G, Duan QJ, Wu DQ, Shao J,
Wang TM and Wang CZ: Therapeutic potential of n-butanol extract of
Pulsatilla decoction in a murine model of ulcerative colitis
induced by DSS combined with Candida albicans colonization.
Zhongguo Zhong Yao Za Zhi. 43:2979–2984. 2018.(In Chinese).
PubMed/NCBI
|
|
11
|
Wang WH, Zhan ZL, Peng HS, Yang J and Qian
JP: Textual research on the origin and producing area of Baitouweng
(Radix Pulsatillae). Zhonghua Yi Shi Za Zhi. 47:14–18. 2017.(In
Chinese). PubMed/NCBI
|
|
12
|
Yu Z, Liu HJ, Dun HH, Dong Q and Liang C:
Effect of Pulsatilla decoction on the expression of proinflammatory
cytokines in inflammatory bowel disease. Zhongguo Ying Yong Sheng
Li Xue Za Zhi. 27:416–419. 2011.(In Chinese). PubMed/NCBI
|
|
13
|
Pontoglio M: Hepatocyte nuclear factor 1,
a transcription factor at the crossroads of glucose homeostasis. J
Am Soc Nephrol. 11 (Suppl 16):S140–S143. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang X, Fan F and Cao Q: Modified
Pulsatilla decoction attenuates oxazolone-induced colitis in mice
through suppression of inflammation and epithelial barrier
disruption. Mol Med Rep. 14:1173–1179. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu SW, Liu HJ, Zhao W, Li L, Dun HH and
Liang C: Molecular mechanisms involved in the treatment of
inflammatory bowel disease by Pulsatilla decoction. Zhongguo Ying
Yong Sheng Li Xue Za Zhi. 27:106–109. 2011.(In Chinese). PubMed/NCBI
|
|
16
|
Hua YL, Ma Q, Zhang XS, Jia YQ, Peng XT,
Yao WL, Ji P, Hu JJ and Wei YM: Pulsatilla decoction can treat the
dampness-heat diarrhea rat model by regulating glycerinphospholipid
metabolism based lipidomics approach. Front Pharmacol. 11:1972020.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu X, He S, Li Q, Mu X, Hu G and Dong H:
Comparison of the Gut microbiota between pulsatilla decoction and
levofloxacin hydrochloride therapy on Escherichia coli
Infection. Front Cell Infect Microbiol. 10:3192020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang X, Yang J, Cao Q and Tang J:
Therapeutic efficacy and mechanism of water-soluble extracts of
Banxiaxiexin decoction on BALB/c mice with oxazolone-induced
colitis. Exp Ther Med. 8:1201–1204. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ji AL, Li T, Zu G, Feng DC, Li Y, Wang GZ,
Yao JH and Tian XF: Ubiquitin-specific protease 22 enhances
intestinal cell proliferation and tissue regeneration after
intestinal ischemia reperfusion injury. World J Gastroenterol.
25:824–836. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gordon IO, Agrawal N, Willis E, Goldblum
JR, Lopez R, Allende D, Liu X, Patil DY, Yerian L, El-Khider F, et
al: Fibrosis in ulcerative colitis is directly linked to severity
and chronicity of mucosal inflammation. Aliment Pharmacol Ther.
47:922–939. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qian XX, Cai CW, Li HY, Lai LJ, Song DJ,
Qiao YQ, Shen J and Ran ZH: Transcribed ultraconserved region
(T-UCR) uc.261 expression is closely correlated with disease
activity and intestinal permeability in Crohn's disease. Ther Adv
Gastroenter. 12:17562848198807332019.PubMed/NCBI
|
|
23
|
Guo BJ, Bian ZX, Qiu HC, Wang YT and Wang
Y: Biological and clinical implications of herbal medicine and
natural products for the treatment of inflammatory bowel disease.
Ann N Y Acad Sci. 1401:37–48. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Suh SY and An WG: Systems pharmacological
approach of pulsatillae radix on treating Crohn's disease. Evid
Based Complement Alternat Med. 2017:41980352017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Q, Su CP, Zhang HM, Ren YL, Wang W
and Guo SZ: Anti-inflammatory mechanism of heat-clearing and
detoxifying Chinese herbs. Zhongguo Zhong Yao Za Zhi. 43:3787–3794.
2018.(In Chinese). PubMed/NCBI
|
|
26
|
Li S, Li X, Yang R, Wang B, Li J, Cao L,
Xiao S and Huang W: Effects of anemoside B4 on pharmacokinetics of
florfenicol and mRNA expression of CXR, MDR1, CYP3A37 and UGT1E in
broilers. J Vet Med Sci. 81:1804–1809. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shi Y, Zhao M, Yao H, Yang P, Xin T, Li B,
Sun W and Chen S: Rapidly discriminate commercial medicinal
Pulsatilla chinensis (Bge.) Regel from its adulterants using ITS2
barcoding and specific PCR-RFLP assay. Sci Rep. 7:400002017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Frisardi V, Panza F, Seripa D, Farooqui T
and Farooqui AA: Glycerophospholipids and
glycerophospholipid-derived lipid mediators: A complex meshwork in
Alzheimer's disease pathology. Prog Lipid Res. 50:313–330. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tatiya-Aphiradee N, Chatuphonprasert W and
Jarukamjorn K: Immune response and inflammatory pathway of
ulcerative colitis. J Basic Clin Physiol Pharmacol. 30:1–10. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Salem HA and Wadie W: Effect of Niacin on
Inflammation and Angiogenesis in a Murine Model of Ulcerative
Colitis. Sci Rep. 7:71392017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Leung VSY, Benoit-Biancamano MO and Pang
DSJ: Performance of behavioral assays: The Rat Grimace Scale,
burrowing activity and a composite behavior score to identify
visceral pain in an acute and chronic colitis model. Pain Rep.
4:e7182019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hanauer SB: Oral or topical 5-ASA in
ulcerative colitis. Dig Dis. 34:122–124. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lai LJ, Shen J and Ran ZH: Natural killer
T cells and ulcerative colitis. Cell Immunol. 335:1–5. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chang HK and Hou WS: Retinoic acid
modulates interferon-γ production by hepatic natural killer T cells
via phosphatase 2A and the extracellular signal-regulated kinase
pathway. J Interferon Cytokine Res. 35:200–212. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wu S, Yang C, Xu N, Wang L, Liu Y, Wang J
and Shen X: The protective effects of Helix B surface peptide on
experimental acute liver injury induced by carbon tetrachloride.
Dig Dis Sci. 62:1537–1549. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang L, Tschumi BO, Corgnac S, Rüegg MA,
Hall MN, Mach JP, Romero P and Donda A: Mammalian target of
rapamycin complex 1 orchestrates invariant NKT cell differentiation
and effector function. J Immunol. 193:1759–1765. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Landy J, Ronde E, English N, Clark SK,
Hart AL, Knight SC, Ciclitira PJ and Al-Hassi HO: Tight junctions
in inflammatory bowel diseases and inflammatory bowel disease
associated colorectal cancer. World J Gastroenterol. 22:3117–3126.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen T, Xue H, Lin R and Huang Z: MiR-34c
and PlncRNA1 mediated the function of intestinal epithelial barrier
by regulating tight junction proteins in inflammatory bowel
disease. Biochem Biophys Res Commun. 486:6–13. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
He C, Deng J, Hu X, Zhou S, Wu J, Xiao D,
Darko KO, Huang Y, Tao T, Peng M, et al: Vitamin A inhibits the
action of LPS on the intestinal epithelial barrier function and
tight junction proteins. Food Funct. 10:1235–1242. 2019. View Article : Google Scholar : PubMed/NCBI
|