Introduction
Deep vein thrombosis (DVT) is a pathological
condition characterized by the obstruction of veins of the deep
venous circle caused by a thrombotic plug that blocks the normal
blood flow (1). The main causes of
DVT are alterations in the blood flow, damage of the vessel wall
and hypercoagulability of the blood (2). DVT and pulmonary embolism (PE), a
major DVT-induced complication, are often considered two sides of
the same coin leading to a pathological condition called venous
thromboembolism (VTE), which represents a common cause of morbidity
and mortality (3). Various risk
factors are associated with the development of VTE, including
obesity, abnormal platelet activation, alteration of blood oxygen
concentration, genetic factors, trauma and surgery (4–6), as
well as infections (7). Recent
reports demonstrated that a fraction of patients with COVID-19
present with coagulation and fibrinolysis alterations leading to
fatal complications (8–10). Besides these risk factors, chronic
inflammation has been frequently associated with DVT. Some
proinflammatory markers and cytokines, including tumor necrosis
factor (TNF)α, C-reactive protein (CRP), interleukin (IL)6 and IL8,
can promote a procoagulant state inducing the expression of the
tissue factor (11) that actives
the coagulation cascade resulting in fibrin deposition (12). Several studies have demonstrated
that IL6 expression is increased in patients with DVT (13–15),
and its activity may be affected by alterations of the canonical
transduction signaling (cis-pathway) resulting in activation of the
well-known IL6 trans-signaling pathway (16). The soluble form of the IL6 receptor
(sIL6R) has a central role in activating intracellular signaling in
cells that are physiologically IL6-unresponsive but express the
interleukin-6 cytokine family signal transducer (IL6ST, also known
as gp130) (17–19). The plasma levels of sIL6R mainly
depend on the proteolytic activity of a disintegrin and
metalloproteinase (ADAM) family proteins on the IL6R transmembrane
(TM) isoform. IL6R cleavage is enhanced by the IL6R Asp358Ala
mutation (SNP rs2228145) (16). In
addition, alternative splicing of the IL6R gene is responsible for
the expression of sIL6R isoforms characterized by the deletion of
the TM domain (19). Similarly, the
soluble form of gp130 (sgp130) is produced by ADAM cleavage and
alternative splicing of its gene, IL6ST. Unlike the activating role
of sIL6R, the sgp130 inhibits the IL6 trans-signaling by
sequestering the IL6/sIL6R complex (20).
The dysregulation of both cis and trans IL6
signaling may affect the response to IL6 of immune cells involved
in inflammation and its related diseases, including DVT. For
instance, a study reported an accumulation of white blood cells,
mainly leukocytes and neutrophils, inside the thrombus or
surrounding vein wall during DVT, suggesting a possible correlation
between thrombosis and inflammation (21).
In the present study, the expression levels of IL6R
and IL6ST were analyzed in peripheral blood mononuclear cells
(PBMCs) to investigate the involvement of IL6 signaling in
DVT-associated immune responses in a cohort of 19 patients with DVT
and 22 healthy individuals. The expression levels of the
transcriptional isoforms of IL6R and IL6ST associated with their
protein membrane retention were also evaluated. In addition, the
SNP rs2228145 of IL6R was detected to examine its effect on the
balance between soluble and membranous IL6R isoforms in DVT.
Materials and methods
Patients and samples
A consecutive series of 19 patients with DVT of the
lower limbs (age range, 46–71 years) was included in the present
study from January 2019 to March 2020. The patients were admitted
to the Internal Medicine Department of G. Rodolico University
Hospital (Catania, Italy). The DVT diagnosis was performed with
data from ultrasound (US) examination of the venous circulation of
the lower limbs, the non-compression of deep veins induced by the
US probe (CUS test) was considered to diagnose lower limb DVT.
Patients with pregnancy, malignancy, liver disorder and
inflammatory bowel disease were excluded from the study. The
control group consisted of 22 healthy subjects (age range, 41.7–62
years) with no history of any chronic disease.
Patients and controls had similar ethnic background
and originated from the same geographic area. The Institutional
Review Board of University Hospital ‘G. Rodolico’ of Catania
approved all procedures. All participants gave written consent for
blood collection. Venous blood obtained from all subjects was
placed in tubes with or without heparin. Samples were centrifuged
at 2,000 × g for 10 min at room temperature to recover plasma,
serum and buffy coat fractions, then stored at −80°C until
subsequent analysis. The demographic and clinical characteristics
of both patients and controls are reported in Table I.
 | Table I.Demographic and clinical
characteristics of the subjects involved in the present study. |
Table I.
Demographic and clinical
characteristics of the subjects involved in the present study.
| Clinical
characteristics | Control | DVT | P-value |
|---|
| Age in years,
median (range) | 48 (41.7-62) | 56 (46–71) | 0.2392b |
| Sex, number
(%) |
|
|
|
|
Male | 12 (54.5) | 12 (63.2) | 0.7600a |
|
Female | 10 (45.5) | 7 (36.8) |
|
| Median C-reactive
protein level (range), mg/l | 2.06
(0.79-6.96) | 6 (3–11) | 0.0400b |
| Thrombophilic
patients, number (%) | Not applicable | 12 (63) |
|
| Cardiopathic
patients, number (%) | Not applicable | 5 (27.7) |
|
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from buffy coat samples from
patients with DVT and healthy control using the TRIzol®
LS Reagent (cat. no. 10296028; Thermo Fisher Scientific, Inc.),
according to the manufacturer's instructions. For each sample, 750
ng of tota RNA, quantified using a spectrophotometer (NanoDrop
1000; Thermo Fisher Scientific, Inc.), were treated with RNase-free
DNase I (cat. no. EN0525; Thermo Fisher Scientific, Inc.) to remove
possible DNA contamination. A mass of 400 ng treated RNA (final
concentration 20 ng/µl) was then converted into cDNA using the
SuperScript IV Reverse Transcriptase kit (cat. no. 18090050; Thermo
Fisher Scientific, Inc.), following the manufacturer's protocol.
The cDNA obtained from each sample was subsequently analyzed by
qPCR using the Luminaris Color HiGreen qPCR Master Mix, high ROX
(cat. no. K0362; Thermo Fisher Scientific, Inc.). The total and TM
isoforms of both IL6R and IL6ST were amplified with a 7300
Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific,
Inc.) using the primer pairs and thermocycling conditions reported
in Table II. The expression levels
of targets were normalized with those obtained for the GAPDH
housekeeping gene. Relative fold changes in gene expression were
calculated using the 2−ΔΔCq method (22). Furthermore, the IL6R TM/IL6R and
IL6ST TM/IL6R mRNA ratios were calculated by dividing the Cq value
of TM isoform by the Cq value of the total mRNA of each gene.
 | Table II.Primers and PCR conditions. |
Table II.
Primers and PCR conditions.
| A, Expression
profiling |
|---|
|
|---|
| Oligo name | Sequence (5–3) | Thermocycling
conditions |
|---|
| IL6R total F |
GTCCCAGAAGTTCTCCTGCC | Uracil-DNA
glycosylase pre-treatment at 50°C for 2 min, followed by an initial
denaturation step at 95°C for 10 min, then 40 cycles at 95°C for 15
sec, 60°C for 30 sec and 72°C for 30 sec. |
| IL6R total R |
GGCTGCAAGATTCCACAACC |
|
| IL6R TM F |
CACGCCTTGGACAGAATCCA |
|
| IL6R TM R |
CAATGGCAATGCAGAGGAGC |
|
| IL6ST total F |
GCCTCAACTTGGAGCCAGA |
|
| IL6ST total R |
TCCCACTTGCTTCTTCACTCC |
|
| IL6ST TM F |
TGAAACTGCTGTGAATGTGGA |
|
| IL6ST TM R |
GCTAAGCAAACAGGCACGAC |
|
| GAPDH F |
AGAAGGCTGGGGCTCATTTG |
|
| GAPDH F |
AGGGGCCATCCACAGTCTTC |
|
|
| B, IL6R exon 9
sequencing |
|
| Oligo
name | Sequence
(5–3) | Amplification
conditions |
|
| IL6R exon 9 F |
TGTTGGTTGGCAGAGCTGTT | 95°C for 3 min,
followed by 35 cycles of 95°C for 30 sec, |
| IL6R exon 9 R |
CACCTAAAACACGGCTTGGC | 60°C for 3 sec,
72°C for 1 min and final 72°C for 5 min. |
IL6R exon 9 sequencing
Genomic DNA from buffy coat samples from both DVT
patients and healthy controls was extracted using a standard
phenol-chloroform method and quantified using a spectrophotometer
assay (NanoDrop 1000). Exon 9 from the IL6R gene was amplified
using DreamTaq DNA Polymerase (cat. no. EP0702; Thermo Fisher
Scientific, Inc.) with primers and thermocycling conditions
reported in Table II. After PCR
amplification, the DNA amplicons were purified with GeneJET PCR
Purification kit (cat. no. K0702; Thermo Fisher Scientific, Inc.),
according to manufacturer's indications. The purified samples were
sequenced using the Mix2Seq kit (Eurofins Genomics Italy),
according to the manufacturer's instructions. Chromas Lite software
version 2.6.6 (technelysium.com.au/wp/) was used to retrieve and
analyze the DNA sequences.
Statistical analysis
Unpaired Student's t-test was used to compare
normally distributed data. Whereas, the Mann-Whitney test was
performed when data were not normally distributed(Shapiro-Wilk
normality test). The contingency analysis was performed using
Fisher's exact test. To assess the diagnostic performance of
putative biomarkers, the receiver operating characteristic (ROC)
was performed considering specificity, sensitivity and likelihood
ratio (LR), and the cutoff value was reported for each biomarker.
All statistical analyses were performed using GraphPad Prism
software Version 8.0.2 (GraphPad Software, Inc.). P<0.05 was
considered to indicate a statistically significant difference.
Results
The analysis of total IL6R mRNA expression levels
revealed no significant difference between the DVT and control
groups (Fig. 1A). By contrast, the
ratio of IL6R TM to IL6R total mRNA expression was increased in
patients with DVT by 1.66-fold compared with healthy controls
(P<0.01; Fig. 1B). Moreover, a
significant reduction of total IL6ST mRNA was observed in patients
with DVT compared with the healthy controls (P<0.01; Fig. 2A). However, the ratio of IL6ST TM
isoform to IL6ST total mRNA expression was 1.5-fold higher
(P<0.01) in patients with DVT compared with controls (Fig. 2B). Mutation analysis of IL6R
rs2228145 SNP revealed a high frequency of the mutated (AC and CC)
genotypes in both DVT and control groups (DVT, 68.42% and CTRL,
66.67%; Table III and Fig. S1). When the samples were stratified
according to the IL6R sr2228145 SNP, no significant association
between the mutated genotypes and the relative expression of IL6R
TM was observed in any of the groups (Fig. 3).
 | Table III.Association analysis of IL6R and IL6R
rs2228145 expression and the occurrence of DVT. |
Table III.
Association analysis of IL6R and IL6R
rs2228145 expression and the occurrence of DVT.
| Parameter | Patients with DVT,
number (%) | Healthy controls,
number (%) | OR, 95%
CIa |
P-valuea |
|---|
| IL6R TM/IL6R mRNA
ratio |
|
|
|
|
|
≥0.315 | 16 (84.21) | 5 (23.80) | 17.07,
3.48-83.75 | <0.001 |
|
<0.315 | 3 (15.79) | 16 (76.20) |
|
|
| IL6R rs2228145
genotype |
|
|
|
|
| AC and
CC genotypes | 13 (68.42) | 14 (66.67) | 1.08,
0.29-4.08 | 1 |
|
Wild-type | 6 (31.58) | 7 (33.33) |
|
|
| IL6R TM/IL6R mRNA
ratio& rs2228145 genotype |
|
|
|
|
| ≥0.315
& AC and CC | 12 (63.15) | 2 (10.00) | 15.45,
2.73-87.32 | <0.001 |
| ≥0.315 WT; <0.35
WT, AC and CC | 7 (36.84) | 18 (90.00) |
|
|
| IL6ST TM/IL6ST mRNA
ratio |
|
|
|
|
|
≥0.2725 | 14 (77.78) | 5 (25.00) | 10.50,
2.33-47.22 | <0.003 |
|
<0.2725 | 4 (22.22) | 15 (75.00) |
|
|
In order to evaluate the association between the
relative expression of IL6R TM and the occurrence of DVT, all
subjects were stratified according to their IL6R TM/IL6R mRNA ratio
(cut-off value, 0.315), the presence of the IL6R rs2228145(C)
alleles, or both. The results from Fisher's exact tests revealed a
strong association between IL6R TM expression and the occurrence of
DVT [odds ratio (OR), 17.07; confidence interval (CI), 3.478-83.75;
P<0.001; Table III). A similar
association was observed when stratifying the subjects by both the
IL6R TM/IL6R ratio and IL6R rs2228145(C) allele (OR, 15.45; CI,
2.726-87.32; P<0.001; Table
III). By contrast, no significant association was observed when
patients were stratified according to the presence of the IL6R
rs2228145(C) allele (Table
III).
Concerning the IL6ST TM/IL6ST mRNA ratio, the
stratification of samples above the 75th percentile of normal
values (>0.2725) highlighted a strong association between IL6ST
TM/IL6ST mRNA ratio and the occurrence of DVT (OR, 10.50; CI,
2.335-47.22; P<0.003; Table
III).
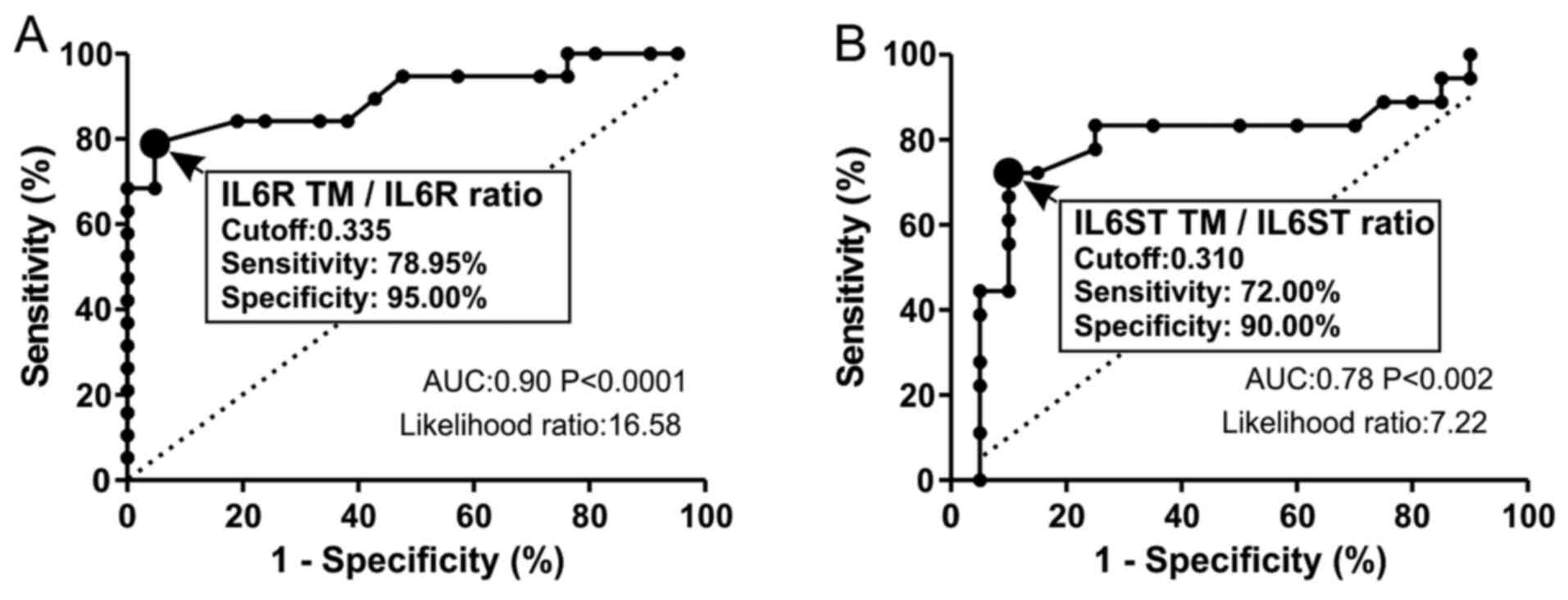
Finally, a ROC curve analysis was performed to
evaluate the diagnostic performance of the IL6R TM/IL6R ratio in
DVT. The cutoff of IL6R TM/IL6R ratio was 0.335 (sensitivity,
78.95%; specificity, 95.00%) with a LR of 16.58, indicating a
significant increase in the probability of DVT given a positive
test (Fig. 4A). Similarly, the
IL6ST TM/IL6ST ratio (cutoff, 0.31) exhibited good diagnostic
performance parameters (sensitivity, 72.00%; specificity, 90.00%)
with a significant association with DVT (LR, 7.22) (Fig. 4B).
Discussion
IL6 is a pleiotropic cytokine involved in several
physiological processes, including the modulation of immune
responses and several vascular disorders such as peripheral
arterial disease, atherosclerosis and VTE (23–27).
The effects of IL6 are mediated by both the IL6R and the gp130 TM
receptors expressed by responsive cells (such as hepatocytes and
leucocytes), activating the IL6 canonical signaling, also known as
IL6 cis-signaling (16). The amount
of these receptors on the membrane surface depends on the
proteolytic cleavage of the IL6 binding domains by specific ADAM
proteinases, as well as on the alternative splicing of the IL6R and
IL6ST mRNA that results in the deletion of their transmembrane
domains (16). The release of the
soluble forms of IL6R and gp130 allows the activation of IL6
trans-signaling in cells lacking IL6R that are normally
unresponsive to IL6 stimulation, which may be activated by IL6 if
the gp130 receptor is present on their membrane surfaces (17–19).
Several autoimmune (such as rheumatoid arthritis and systemic lupus
erythematosus)and inflammatory diseases (such as inflammatory bowel
disease and psoriasis) are sustained by the alterations at
different levels of both cis- and trans-signaling of IL6 (28). Consequently, therapeutic targeting
of IL6 signaling with available drug inhibitors is not always
effective (28,29). Previous evidence has demonstrated
the key role of inflammation in VTE/DVT pathogenesis mediated by
different cytokines, including IL6, which is responsible for
aberrant inflammatory responses (30). Several studies have highlighted how
genetic polymorphisms in IL6 may be predictive of peripheral
arterial disease and DVT in patients with cancer (31,14).
In addition, the activation of leukocytes, as well as endothelial
cells and platelets, can trigger the coagulation cascade by the
formation of microparticles within intact veins (11). The role of leukocytes and their
activation in both thrombus generation and vein remodeling have
been debated (21).
The aim of the present study was to investigate the
involvement of IL6R and IL6ST isoforms in the activation of the IL6
cis-signaling in DVT, focusing on the role of the IL6R Asp358Ala
mutation (SNP rs2228145) in the alternative splicing of IL6R exon
9. In particular, the expression profiling of the different
splicing isoforms of the IL6R and IL6ST receptor was analyzed in
leukocytes obtained from patients with DVT and healthy donors.
The expression analysis revealed that in leucocytes
from DVT patients the expression of TM transcript isoforms of both
IL6R and IL6ST receptors were higher compared with those coding for
the soluble isoforms. These results were supported by the strong
association between higher TM transcript levels of both IL6R and
IL6STand the occurrence of DVT, suggesting a crucial role of IL6
cis-signaling in DVT. Increased expression of IL6R on the membrane
of leukocytes may provide a higher responsiveness to IL6, and this
may be further reinforced by IL6ST being simultaneously
overexpressed on the cellular membrane, thereby also activating the
IL6 trans-signaling. The measurement of these molecular biomarkers
may be useful to identify a subset of high-risk DVT patients that
could develop a hyperinflammatory immune response associated with
severe clinical outcomes (13).
The evaluation of IL6 signaling and the current
results may have a fundamental impact in the prediction of DVT, as
well as in the monitoring of vascular disorders due to other
pathologies, and in particular during the COVID-19 pandemic.
COVID-19 infection induces endothelial damage and cardiovascular
disorders (10,32). Indeed, in some patients with severe
COVID-19 symptomatology, abnormal expression of IL6 was observed,
leading to a cytokine imbalance defined as ‘cytokine storm’
responsible for severe respiratory syndrome (10,33).
Notably, the COVID-19 ‘cytokine storm’ is not observed in all
patients with clinical symptoms, suggesting that genetic factors
affecting key cytokines or immune cells or other comorbidities, in
particular diabetes and cancer, may be related to this complication
(34–37). It remains unknown if IL6
polymorphisms are also associated with COVID-19 severity or with
its vascular complications.
To investigate the molecular mechanisms capable of
driving the expression of IL6R TM, the mutational status of IL6R
was assessed, as it has been demonstrated that the rs2228145 SNP
impairs IL6R transcript splicing (19). However, the present results were not
consistent with this previous report (19), as no significant association between
the IL6R rs2228145 variant and the IL6R TM isoform was observed,
probably due to the small number of cases analyzed. Therefore, it
can be hypothesized that other molecular mechanisms could affect
the relative expression of either the TM or soluble IL6R isoforms.
For instance, it has been demonstrated that DNA methylation can
regulate the splicing mechanism, thus influencing the binding of
spliceosome proteins to nascent pre-mRNA (38,39).
Altogether, the present results support the
hypothesis that IL6 activates leukocytes, which in turn may be
responsible for the inflammatory status in DVT through the
overexpression of both IL6R and IL6ST receptors. The assessment of
the IL6 receptor complex could be a hallmark of the early
inflammatory conditions associated with DVT development and may be
useful for the management of patients at risk of thromboembolic
events. Further studies will be required to confirm the activation
of IL6 cis-signaling in DVT at the protein level and in a larger
patient cohort.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request
Authors' contributions
SSS and SC designed the project and confirm the
authenticity of the raw data. BMT, RS and SC wrote the manuscript
and performed the experiments. GG and RS performed acid nucleic
extraction, PCR and RT-qPCR. SC and BMT performed the statistical
analysis. SSS revised the manuscript. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The study was conducted according to the guidelines
of The Declaration of Helsinki, and ap-proved by the Institutional
Review Board of The University Polyclinic of Catania. Informed
consent was obtained from all subjects involved in the study for
participation and data publication.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Phillippe HM: Overview of venous
thromboembolism. Am J Manag Care. 23 (Suppl 20):S376–S382.
2017.PubMed/NCBI
|
|
2
|
Kyrle PA and Eichinger S: Deep vein
thrombosis. Lancet. 365:1163–1174. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Streiff MB, Agnelli G, Connors JM,
Crowther M, Eichinger S, Lopes R, McBane RD, Moll S and Ansell J:
Guidance for the treatment of deep vein thrombosis and pulmonary
embolism. J Thromb Thrombolysis. 41:32–67. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Martinelli I, Bucciarelli P and Mannucci
PM: Thrombotic risk factors: Basic pathophysiology. Crit Care Med.
38 (Suppl 2):S3–S9. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Reitsma PH, Versteeg HH and Middeldorp S:
Mechanistic view of risk factors for venous thromboembolism.
Arterioscler Thromb Vasc Biol. 32:563–568. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shaheen K, Alraies MC, Alraiyes AH and
Christie R: Factor V Leiden: How great is the risk of venous
thromboembolism? Cleve Clin J Med. 79:265–272. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kaplan D, Casper TC, Elliott CG, Men S,
Pendleton RC, Kraiss LW, Weyrich AS, Grissom CK, Zimmerman GA and
Rondina MT: VTE incidence and risk factors in patients with severe
sepsis and septic shock. Chest. 148:1224–1230. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhai Z, Li C, Chen Y, Gerotziafas G, Zhang
Z, Wan J, Liu P, Elalamy I and Wang C; Prevention Treatment of VTE
Associated with COVID-19 Infection Consensus Statement Group, :
Prevention and Treatment of Venous Thromboembolism Associated with
Coronavirus Disease 2019 Infection: A Consensus Statement before
Guidelines. Thromb Haemost. 120:937–948. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kartsios C, Lokare A, Osman H, Perrin D,
Razaq S, Ayub N, Daddar B and Fair S: Diagnosis, management, and
outcomes of venous thromboembolism in COVID-19 positive patients: A
role for direct anticoagulants? J Thromb Thrombolysis. Sep
10–2020.(Epub ahead of print). doi:
10.1007/s11239-020-02257-7.PubMed/NCBI
|
|
10
|
Tsatsakis A, Calina D, Falzone L, Petrakis
D, Mitrut R, Siokas V, Pennisi M, Lanza G, Libra M, Doukas SG, et
al: SARS-CoV-2 pathophysiology and its clinical implications: An
integrative overview of the pharmacotherapeutic management of
COVID-19. Food Chem Toxicol. 146:1117692020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Branchford BR and Carpenter SL: The role
of inflammation in venous thromboembolism. Front Pediatr.
6:1422018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bovill EG and van der Vliet A: Venous
valvular stasis-associated hypoxia and thrombosis: What is the
link? Annu Rev Physiol. 73:527–545. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang Y, Zhang Z, Wei R, Miao X, Sun S,
Liang G, Chu C, Zhao L, Zhu X, Guo Q, et al: IL (Interleukin)-6
contributes to deep vein thrombosis and is negatively regulated by
miR-338-5p. Arterioscler Thromb Vasc Biol. 40:323–334. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Malaponte G, Polesel J, Candido S,
Sambataro D, Bevelacqua V, Anzaldi M, Vella N, Fiore V, Militello
L, Mazzarino MC, et al: IL-6-174 G>C and MMP-9-1562 C>T
polymorphisms are associated with increased risk of deep vein
thrombosis in cancer patients. Cytokine. 62:64–69. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sharma A, Singh K, Biswas A, Ranjan R,
Kishor K, Pandey H, Kumar R, Mahapatra M, Oldenburg J and Saxena R:
Impact of interleukin 6 promoter polymorphisms (−174 G>C, −572
G>C and −597 G>A) on plasma IL-6 levels and their influence
on the development of DVT: A study from India. Hematology.
23:833–838. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
van Dongen J, Jansen R, Smit D, Hottenga
JJ, Mbarek H, Willemsen G, Kluft C, Penninx BW, Ferreira MA,
Boomsma DI, et al AAGC Collaborators, : The contribution of the
functional IL6R polymorphism rs2228145, eQTLs and other genome-wide
SNPs to the heritability of plasma sIL-6R levels. Behav Genet.
44:368–382. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Müller-Newen G, Küster A, Hemmann U, Keul
R, Horsten U, Martens A, Graeve L, Wijdenes J and Heinrich PC:
Soluble IL-6 receptor potentiates the antagonistic activity of
soluble gp130 on IL-6 responses. J Immunol. 161:6347–6355.
1998.PubMed/NCBI
|
|
18
|
Scheller J and Rose-John S: The
interleukin 6 pathway and atherosclerosis. Lancet. 380:3382012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ferreira RC, Freitag DF, Cutler AJ, Howson
JM, Rainbow DB, Smyth DJ, Kaptoge S, Clarke P, Boreham C, Coulson
RM, et al: Functional IL6R 358Ala allele impairs classical IL-6
receptor signaling and influences risk of diverse inflammatory
diseases. PLoS Genet. 9:e10034442013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Morieri ML, Passaro A and Zuliani G:
Interleukin-6 ‘Trans-signaling’ and ischemic vascular disease: The
important role of soluble gp130. Mediators Inflamm.
2017:13963982017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Saha P, Humphries J, Modarai B, Mattock K,
Waltham M, Evans CE, Ahmad A, Patel AS, Premaratne S, Lyons OT, et
al: Leukocytes and the natural history of deep vein thrombosis:
Current concepts and future directions. Arterioscler Thromb Vasc
Biol. 31:506–512. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bevelacqua V, Libra M, Mazzarino MC,
Gangemi P, Nicotra G, Curatolo S, Massimino D, Plumari A, Merito P,
Valente G, et al: Long pentraxin 3: A marker of inflammation in
untreated psoriatic patients. Int J Mol Med. 18:415–423.
2006.PubMed/NCBI
|
|
24
|
Malaponte G, Libra M, Bevelacqua Y, Merito
P, Fatuzzo P, Rapisarda F, Cristina M, Naselli G, Stivala F,
Mazzarino MC, et al: Inflammatory status in patients with chronic
renal failure: The role of PTX3 and pro-inflammatory cytokines. Int
J Mol Med. 20:471–481. 2007.PubMed/NCBI
|
|
25
|
Signorelli SS, Anzaldi M, Fiore V, Simili
M, Puccia G, Libra M, Malaponte G and Neri S: Patients with
unrecognized peripheral arterial disease (PAD) assessed by
ankle-brachial index (ABI) present a defined profile of
proinflammatory markers compared to healthy subjects. Cytokine.
59:294–298. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Signorelli SS, Anzaldi M, Libra M,
Navolanic PM, Malaponte G, Mangano K, Quattrocchi C, Di Marco R,
Fiore V and Neri S: Plasma levels of inflammatory biomarkers in
peripheral arterial disease: Results of a Cohort Study. Angiology.
67:870–874. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Signorelli SS, Candido S, Salemi R, Fiore
V, Mangiafico M and Libra M: Low levels of inflammation and the
absence of subclinical atherosclerosis in rheumatoid arthritis. Mol
Med Rep. 13:3521–3524. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hunter CA and Jones SA: IL-6 as a keystone
cytokine in health and disease. Nat Immunol. 16:448–457. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kang S, Tanaka T, Narazaki M and Kishimoto
T: Targeting interleukin-6 signaling in clinic. Immunity.
50:1007–1023. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Saghazadeh A and Rezaei N: Inflammation as
a cause of venous thromboembolism. Crit Rev Oncol Hematol.
99:272–285. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Libra M, Signorelli SS, Bevelacqua Y,
Navolanic PM, Bevelacqua V, Polesel J, Talamini R, Stivala F,
Mazzarino MC and Malaponte G: Analysis of G(−174)C IL-6
polymorphism and plasma concentrations of inflammatory markers in
patients with type 2 diabetes and peripheral arterial disease. J
Clin Pathol. 59:211–215. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pennisi M, Lanza G, Falzone L, Fisicaro F,
Ferri R and Bella R: SARS-CoV-2 and the nervous system: From
clinical features to molecular mechanisms. Int J Mol Sci.
21:54752020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Papa A, Di Dato MT, Buonavolonta P,
Saracco E, Salzano AM and Casale B: Clinical management of Il-6
driven cytokine storm related to COVID-19 in a patient with recent
spinal cord stimulator implants: A Case Report. Anesth Pain Med.
10:e1041512020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vivarelli S, Falzone L, Grillo CM,
Scandurra G, Torino F and Libra M: Cancer management during
COVID-19 pandemic: Is immune checkpoint inhibitors-based
immunotherapy harmful or beneficial? Cancers (Basel). 12:22372020.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Vivarelli S, Falzone L, Torino F,
Scandurra G, Russo G, Bordonaro R, Pappalardo F, Spandidos DA,
Raciti G and Libra M: Immune-checkpoint inhibitors from cancer to
COVID 19: A promising avenue for the treatment of patients with
COVID 19 (Review). Int J Oncol. 58:145–157. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zheng M, Wang X, Guo H, Fan Y, Song Z, Lu
Z, Wang J, Zheng C, Dong L, Ma Y, et al: The Cytokine profiles and
immune response are increased in COVID-19 patients with type 2
diabetes mellitus. J Diabetes Res. 2021:95267012021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kaur S, Bansal R, Kollimuttathuillam S,
Gowda AM, Singh B, Mehta D and Maroules M: The looming storm: Blood
and cytokines in COVID-19. Blood Rev. 46:1007432021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shayevitch R, Askayo D, Keydar I and Ast
G: The importance of DNA methylation of exons on alternative
splicing. RNA. 24:1351–1362. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lev Maor G, Yearim A and Ast G: The
alternative role of DNA methylation in splicing regulation. Trends
Genet. 31:274–280. 2015. View Article : Google Scholar : PubMed/NCBI
|