Introduction
Non-alcoholic fatty liver disease (NAFLD) refers to
a large category of liver diseases, the incidence of which is
increasing yearly; studies have reported prevalence rates of ~31%
in South America (1), 25–30% in
Japan (2), 28% in Italy (3) and ~20% in China (4). In general, 15–20% of patients with
NAFLD tend to progress to non-alcoholic steatohepatitis, which, if
not addressed, can progress to liver failure or hepatocellular
carcinoma (5). A number of
pathogenetic factors, such as fat deposition, oxidative stress and
endoplasmic reticulum stress, can induce apoptosis, which serves an
important role in the development of NAFLD (6). The search for effective NAFLD
therapeutic targets and the development of related drugs have
recently attracted attention.
Ginsenoside Rg1 (Rg1) is an active monomer component
isolated from ginseng and Panax notoginseng. Due to its
extensive pharmacological effects and the minor risk of side
effects, it has garnered attention with a number of researchers
(7–12). Monomeric Rg1 has been reported to
exert numerous biological effects, including anti-inflammation and
anti-oxidation (7,8), inhibition of cardiac hypertrophy
(9), anti-aging (10), neuroprotection and memory
improvement (11,12). In addition, Rg1 may reduce the
content of intracellular triglycerides by activating the
AMP-activated protein kinase/NF-κB signaling pathway (13), and could alleviate the fatty
degeneration of HepG2 cells induced by palmitic acid. Peng et
al (14) reported that,
compared with ursodeoxycholic acid, Rg1 better regulated fat
metabolism to reduce liver damage in NAFLD rats. Xiao et al
(15) revealed that the
therapeutic effect of Rg1 on NAFLD rats was mediated through
upregulation of Bcl-2 and pro-caspase-3 expression, and
downregulation of Bax expression, which has an anti-apoptotic
effect on liver cells. However, the specific molecular mechanism
underlying the anti-apoptotic effects of Rg1 in NAFLD needs to be
explored further.
In the present study, the effects of Rg1 on the
apoptosis of steatotic HHL-5 liver cells and the underlying
mechanism were investigated. Furthermore, the role of a key lyase,
sphingosine-1-phosphate lyase 1 (SGPL1), in the sphingosine kinase
signaling pathway and its involvement in the anti-apoptotic effects
of Rg1 on steatotic HHL-5 cells was examined.
Materials and methods
Cell culture
The HHL-5 human hepatocyte cell line was purchased
from Procell Life Science & Technology Co., Ltd. HHL-5 cells
were cultured in RPMI-1640 medium (Gibco; Thermo Fisher Scientific)
containing 10% FBS (Gibco; Thermo Fisher Scientific) at 37°C in a
humidified incubator supplied with 5% CO2. The 293T cell
line was obtained from Kunming Cell Bank of Chinese Academy
Sciences and cultured in DMEM supplemented with 10%
heat-inactivated FBS at 37°C in a humidified incubator supplied
with 5% CO2.
Cell viability assay
HHL-5 cell viability was measured using an MTS assay
(CellTiter 96 AQueous MTS Reagent; Promega Corporation) according
to the manufacturer's protocol. HHL-5 cells were seeded into
96-well plates at a density of 5×104 cells/ml (100
µl/well) and cultured for 24 h. The medium was then discarded and
replaced with 1% medium- and long-chain fat emulsion (MCE; Baxter
Qiaoguang Healthcare) for 24 h at 37°C. Subsequently, 0.2, 0.4 and
0.6 mM Rg1 (Chengdu Push Bio-technology Co., Ltd.) was added to the
HHL-5 cells, followed by incubation for a further 20 h at 37°C
(treatment groups). The model (Mod) group were treated with MCE
only; in the recovery (Rec) group, following 1% MCE treatment for
24 h, MCE was removed without Rg1 treatment. The structure of Rg1
is shown in Fig. 1A. For cell
viability analysis, 20 µl MTS/phenazine methosulfate (20:1 in
volume) was added to the medium, followed by incubation for 4 h at
37°C. Cell viability was finally determined using a microplate
reader (Nanjing DeTie Laboratory Equipment Co., Ltd.) at 490
nm.
 | Figure 1.Effects of Rg1 on steatosis,
proliferation and apoptosis of HHL-5 hepatocytes. (A) Structure of
Rg1. HHL-5 cells were exposed to MCE (1%) for 24 h and treated with
Rg1 (0.2, 0.4 or 0.6 mmol/l) for 24 h. (B) Oil red O staining.
Magnification, ×200; scale bar, 20 µM. (C) Adipose accumulation
(AOD) was semi-quantified using ImageJ software. (D) HHL-5 cell
viability. (E) HHL-5 cells were stained with PI and Annexin V-FITC
and apoptosis was assessed by flow cytometry. (F) Apoptotic rate
(Annexin V+) of HHL-5 cells was estimated. *P<0.05,
**P<0.01 and ***P<0.001. MCE, medium- and long-chain fat
emulsion; Mod, model; Rec, recovery; Rg1, ginsenoside Rg1; AOD,
average optical density. |
Oil-red O staining
HHL-5 cells were seeded at a density of
2×104 cells/ml into 24-well plates, treated with 1% MCE
(Mod group), and then cultured with 0.2, 0.4 and 0.6 mM Rg1 for a
further 24 h (treatment groups). For Rec group, following 1% MCE
treatment for 24 h, MCE was removed without Rg1 treatment. The
cells were washed once with PBS and fixed with 95% ethanol for 20
min at room temperature. Subsequently, the cells were stained with
freshly prepared Oil Red O solution (Wuhan Servicebio Technology
Co., Ltd.; Oil Red:deionized water, 3:2) for 10 min at room
temperature. After staining, the cells were washed with
double-distilled water to remove the unbound staining solution and
observed under an inverted light phase-contrast microscope
(magnification, ×200; PH-XDS5; PhenixOptics). The adipose
accumulation was semi-quantified using ImageJ v2.1.4.7 (National
Institutes of Health), and the result was presented as average
optical density (AOD).
Assessment of cell apoptosis
HHL-5 cells (2×105 cells/ml) in 6-well
plates were treated with various concentrations of Rg1 (0.2, 0.4
and 0.6 mM, treatment groups) at 37°C for 24 h after 24-h 1% MCE
treatment. Similarly, 12- or 24-h 1% MCE-treated HHL-5-SGPL1 cells
were treated with 0.6 mM Rg1 for 24 h. The Mod group were treated
with MCE; in the Rec group, MCE was removed without Rg1 treatment.
After collection by precipitation, cells were washed with PBS and
stained with Annexin V-FITC/PI at room temperature for 15 min in
the dark (Annexin V-FITC Apoptosis Detection kit; MilliporeSigma)
according to the manufacturer's instructions. The numbers of
viable, necrotic and apoptotic cells were assessed using a flow
cytometer (CyFlow Space; Sysmex Partec) and FloMax Software Version
2 (Sysmex Partec GmbH).
Reverse transcription-quantitative PCR
(RT-qPCR)
Following treatment with Rg1 (0.2, 0.4 and 0.6 mM
for 24 h after 24-h 1% MCE treatment, treatment groups), total
cellular RNA was extracted from HHL-5 cells using
TRIzol® reagent (Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. Similarly, total
cellular RNA from HHL-5 cells transfected with empty lentiviral
vector pLVX–IRES-Neo for 48 h was extracted using
TRIzol® reagent (Thermo Fisher Scientific, Inc.). RNA
was reverse transcribed into cDNA using MMLV reverse transcriptase
(cat. no. M1701; Promega Corporation) according to the
manufacturer's instructions. Subsequently, mRNA expression levels
were measured using GoTaq qPCR Master Mix (cat. no. A6001; Promega
Corporation) and an ABI7000 cycler (Applied Biosystems; Thermo
Fisher Scientific, Inc.). The primer sequences used were as
follows: Sphingosine kinase 1 (SPHK1) forward,
5′-CAGTGGATGGGGAATTGATGG-3′ and reverse, 5′-AAGGGCTCTTCTGGCGGTG-3′;
SGPL1 forward, 5′-AGTATGGCTATGCCCCAAAAGG-3′ and reverse,
5′-CCACCCTGCCAATCTGTATCG-3′; Bax forward,
5′-GTCACTGAAGCGACTGATGTCCC-3′ and reverse,
5′-CAAAGATGGTCACGGTCTGCC-3′; Bcl-2 forward,
5′-GTGGATGACTGAGTACCTGAACCG-3′ and reverse,
5′-GACAGCCAGGAGAAATCAAACAGAG-3′; and GAPDH forward,
5′-GGTGAAGCAGGCGTCGGAG-3′ and reverse, 5′-GGAGTGGGTGTCGCTGTTGAA-3′.
The thermocycling conditions were as follows: Initial denaturation
at 94°C for 5 min, followed by 40 cycles at 94°C for 15 sec and
60°C for 20 sec. Relative changes in mRNA expression levels were
calculated and analyzed using the relative quantification
2−ΔΔCq method (16).
GAPDH was used as an internal control to normalize the variability
in expression levels.
Western blotting
After HHL-5 cells were treated with 1% MCE for 24 h
at 37°C, the cells were then treated with different concentrations
(0.2, 0.4 and 0.6 mM) of Rg1 for 24 h (treatment group) at 37°C;
for HHL-5-SGPL1 cells, 1% MCE was first treated for 12 h at 37°C,
and then treated with 0.6 mM Rg1 (treatment group) at 37°C. The
model (Mod) group were treated with MCE only at 37°C; in the
recovery (Rec) group, following 1% MCE treatment for 12 h or 24 h
at 37°C, MCE was removed without Rg1 treatment. The treated cells
were then washed with PBS and lysed with radio immunoprecipitation
assay lysis buffer (MilliporeSigma) on ice for 30 min.
Subsequently, the cells were sonicated on ice with 10 1-sec bursts
with a 3 sec interval by an ultrasonic crusher at medium power. The
cell lysates were centrifuged at 14,000 × g for 15 min at 4°C, and
the supernatants were then collected. The protein concentration was
determined using a BCA assay (BCA Protein Assay Kit; Beyotime
Institute of Biotechnology). Equal amounts of the lysed proteins
(20 µg/lane) were separated by 12% SDS-PAGE. The proteins were then
transferred onto PVDF membranes. The membranes were blocked with 5%
non-fat milk in PBS with 0.05% Tween-20 (PBST) for 1 h at room
temperature. Subsequently, the membranes were incubated with the
primary antibodies at 4°C overnight. After washing with PBST three
times, the secondary antibodies (1:5,000; cat. nos. 31430 and
31460; Thermo Fisher Scientific, Inc.) were added and the membranes
were incubated at room temperature for 1 h, followed by washing
twice with PBST and once with PBS. Enhanced chemiluminescence
reagent (Immobilon Western Chemiluminescent HRP Substrate; cat. no.
WBKLS0500; MilliporeSigma) was then added and the X-ray film was
exposed to the blots. Primary antibodies (dilution 1:500 for
p-Erk1/2 and p-Akt antibodies or 1:1,000 for the rest of
antibodies) against phosphorylated (p)-Erk1/2 (cat. no. 4370),
Erk1/2 (cat. no. 4695), p-Akt (cat. no. 4060), Akt (cat. no. 4691),
Bcl-2 (cat. no. 3498S) and Bax (cat. no. 2772S) were obtained from
Cell Signaling Technology, Inc. A polyclonal antibody against SGPL1
(cat. no. abx103371) was purchased from Abbexa Ltd. The antibody
against SPHK1 (AF5536) was obtained from R&D Systems. β-actin
antibody (1:8,000; cat. no. A1978; MilliporeSigma) was used as a
loading control. Protein bands were scanned and semi-quantified
using ImageJ software (v2.1.4.7; National Institutes of
Health).
Lentiviral packaging and cell
transduction
The full-length coding sequence of SGPL1
(NM_003901.4) was synthesized by Sangon Biotech Co., Ltd.
Subsequently, the SGPL1 fragment was inserted into linearized
pLVX–IRES-Neo plasmid (Clontech Laboratories, Inc.), and the
recombinant plasmid was named pLVX–IRES-Neo-SGPL1. Lentiviruses
carrying SGPL1 were packaged by transfection of three plasmids,
namely pLVX–IRES-Neo-SGPL1, pCMV–VSV-G and pCMV-Δ8.9 (1.5:0.5:1.1
µg in 35-mm dish), into 293T cells after the cells reached 50%
confluence using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) at 37°C. Plasmid extraction and
transfection were performed according to the manufacturer's
instructions. After transfection for 72 h, viruses were collected
by filtering supernatant using a 45-µm filter and added to
pre-seeded HHL-5 cells. Specifically, 200 µl packaged virus and 300
µl medium were added to pre-seeded HHL-5 cells in 24-well plates
(4×104 cells/ml, 500 µl). After lentiviral infection for
24 h, the G418 (0.8 mg/ml) was added to screen for 6 days, during
the screening the dead cells were removed and new culture media
with G418 was added. Viruses were collected by filtering
supernatant using a 45-µm filter, and multiplicity of infection was
1.34. The positive SGPL1-overexpressing HHL-5 cells (HHL-5-SGPL1
cells) were then selected and the virus titer was calculated at 72
h post-infection. The calculated titer of the virus was
1.34×105 IFU/ml. With the virus titer, the number of
HHL-5 cells infected with the viruses can be estimated, and the
multiplicity of infection was 1.34. The HHL-5-SGPL1 cells were then
subjected to gene expression detection and apoptosis analysis after
1% MCE (12 h) and/or Rg1 treatment (0.6 mM for 24 h). HHL-5-SGPL1
cells were maintained at a concentration of 0.8 mg/ml G418.
Statistical analysis
The experiments were performed at least three times
and all data analysis was performed using GraphPad Prism (version
7.0; GraphPad Software, Inc.). All data are presented as the mean ±
SD. Paired Student's t-test was used for comparisons between two
groups, and one-way ANOVA with post-hoc intergroup comparisons
using Tukey test was used for comparisons among multiple groups.
For comparisons between two independent variables, two-way ANOVA
with Bonferroni post hoc test was applied. P<0.05 was considered
to indicate a statistically significant difference.
Results
Rg1 reduces adipose accumulation of
HHL-5 liver cells
During the occurrence of NAFLD, one of the most
significant pathological changes is adipose accumulation. In order
to clarify the anti-steatotic effect of Rg1 on liver cells, HHL-5
hepatocytes were treated with 1% MCE to induce cell steatosis (Mod
group) and were then treated with Rg1. Subsequently, it was
evaluated whether Rg1 reduced the accumulation of fat in HHL-5
cells by Oil Red O staining. Compared with in the Mod group, the
Rec group exhibited a reduction in the number of fat particles, but
still more than in the Rg1 treatment groups (Fig. 1B and C). Furthermore, the cell
state of the Rg1 treatment group gradually returned to normal with
the increase in Rg1 treatment concentration (0.2, 0.4 and 0.6 mM)
(Fig. 1B and C). Additionally, a
cell viability assay was used to detect the effect of Rg1 on the
viability of steatotic HHL-5 cells. The data demonstrated that Rg1
slightly promoted the viability of steatotic HHL-5 cells; however,
the results were not statistically significant (P>0.05; Fig. 1D).
Rg1 reduces the apoptosis of steatotic
HHL-5 cells
In the present study, the anti-apoptotic effect of
Rg1 on steatotic HHL-1 cells was examined using flow cytometry. The
results showed that, compared with that of the control group, the
apoptotic rate (percentage of Annexin V+ cells) in the
Mod and Rec groups was markedly increased, whereas in the treatment
groups, the apoptotic rate gradually decreased with increasing Rg1
concentrations (0.4 and 0.6 mM) compared with that of the Rec group
(Fig. 1E and F).
Effect of Rg1 on the expression levels
of Bcl-2 family proteins
The effect of Rg1 on the expression levels of Bax
and Bcl-2 in steatotic HHL-5 hepatocytes was examined by western
blotting. The data demonstrated that the expression levels of Bax
were significantly increased in the Mod group compared with in the
control group, whereas the expression levels of Bax were decreased
in the treatment group in a Rg1 concentration-dependent manner
(0.2, 0.4 and 0.6 mM) compared with in the Rec group (Fig. 2A and B). Conversely, the protein
expression levels of Bcl-2 were not significantly altered in the
Mod group, but Bcl-2 was significantly upregulated in the treatment
groups compared with in the Rec group (Fig. 2A and B). Additionally, the mRNA
expression levels of Bax and Bcl-2 were detected using RT-qPCR. The
results revealed that the transcription levels of Bax were
upregulated in the Mod and Rec groups compared with in the control
group, whereas these were gradually downregulated in the Rg1
treatment group in a concentration-dependent manner (Fig. 2C). By contrast, the transcription
levels of Bcl-2 were downregulated in the Mod and Rec groups
compared with in the control group, whereas they were gradually
increased in the Rg1 treatment group (Fig. 2C).
Rg1 blocks SGPL1 expression
In order to elucidate whether the relevant molecules
in the sphingosine lipid signaling pathway mediated the
anti-apoptotic effect of Rg1 in the process of hepatocyte
steatosis, the present study first detected the transcription
levels of SPHK1 and SGPL1 in steatotic HHL-5 cells following Rg1
treatment. SPHK1 and SGPL1 are the two key enzymes of
sphingosine-1-phosphate (S1P) metabolism (17). The results revealed that, compared
with in the Rec group, the transcription levels of SPHK1 were
significantly increased in the high-concentration Rg1 treatment
group (0.6 mM), whereas the transcription levels of SGPL1 were
significantly decreased in steatotic HHL-5 cells after Rg1
treatment (0.6 mM; Fig. 3B). The
present study further examined the protein expression levels of
SPHK1 and SGPL1 (Fig. 3A and C).
Notably, the protein expression levels of SPHK1 were not
significantly altered in steatotic HHL-5 hepatocytes following Rg1
treatment. By contrast, the protein expression levels of SGPL1 were
increased in the Mod and Rec groups compared with in the control
group, but were significantly decreased following Rg1 treatment
(0.4 and 0.6 mM). Additionally, the present study further
determined the activities of the anti-apoptotic signaling molecules
Akt and Erk1/2 downstream of the sphingosine signaling pathway
(17,18). The results demonstrated that the
protein expression levels of p-Akt and p-Erk1/2 were downregulated
in the Mod and Rec groups compared with in the control group;
however, the expression levels of p-Akt and p-Erk1/2 were increased
following Rg1 treatment in a concentration-dependent manner
(Fig. 3A and C).
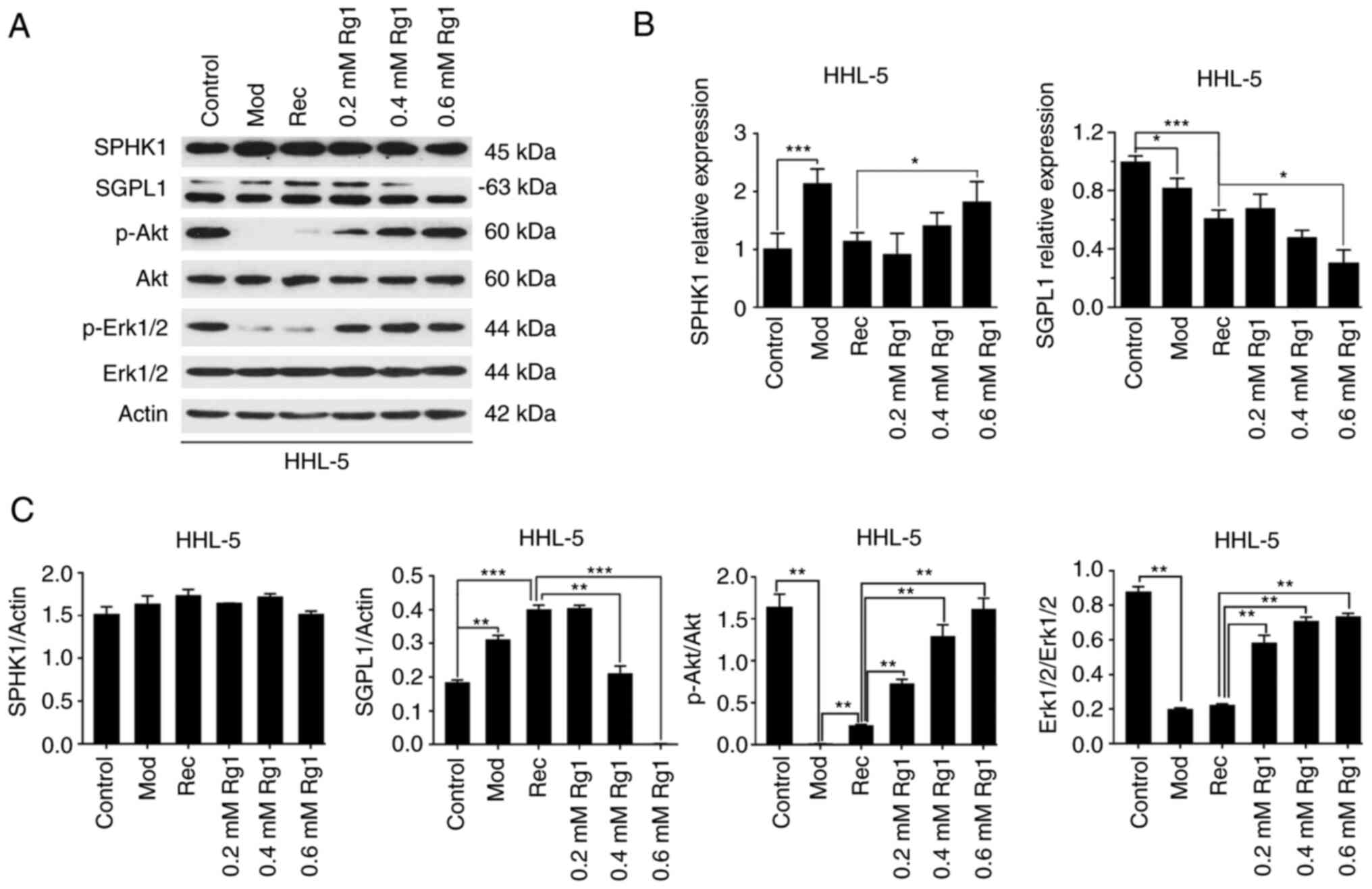 | Figure 3.Effect of Rg1 on sphingolipid pathway
proteins. (A) SPHK1, SGPL1, p-Akt, p-Erk1/2, Akt and Erk1/2
expression levels were detected in HHL-5 cells treated with Rg1
(0.2, 0.4 and 0.6 mM) after exposure to MCE (1%) by western
blotting. (B) SPHK1 and SGPL1 mRNA expression levels were detected
in HHL-5 cells using reverse transcription-quantitative PCR. (C)
SPHK1 and SGPL1 protein expression levels were normalized to actin
expression, and p-Akt and p-Erk1/2 protein expression levels were
normalized to Akt or Erk1/2 using ImageJ software. *P<0.05,
**P<0.01 and ***P<0.001; MCE, medium- and long-chain fat
emulsion; Mod, model; p-, phosphorylated; Rec, recovery; SGPL1,
sphingosine-1-phosphate lyase 1; SPHK1, sphingosine kinase 1; Rg1,
ginsenoside Rg1. |
Overexpression of SGPL1 abrogates the
anti-apoptotic effects of Rg1
In order to further clarify if the anti-apoptotic
effect of Rg1 on steatotic hepatocytes was mediated by
downregulation of SGPL1, SGPL1 was overexpressed in HHL-5
hepatocytes; a hepatocyte line stably overexpressing SGPL1 was
established and named HHL-5-SGPL1. During the establishment of
HHL-5-SGPL1 cells, the lentivirus carrying the overexpressed SGPL1
gene was packaged. The results revealed that the expression levels
of SGPL1 in HHL-5-SGPL1 cells were increased 18.93-fold compared
with SGPL1 expression in normal HHL-5 cells (Fig. 4A), indicating that SGPL1 was
stably expressed in HHL-5-SGPL1 cells. Furthermore, flow cytometry
was used to detect the anti-apoptotic effect of Rg1 on steatotic
HHL-5-SGPL1 cells and wild-type HHL-5 cells. The results revealed
that there was no significant difference in the apoptotic rates
between the Rg1 high-concentration (0.6 mM) treatment group and the
Rec group in steatotic HHL-5-SGPL1 cells (Fig. 4B and C). However, the apoptotic
rate in the Rg1 high-concentration (0.6 mM) treatment group was
decreased compared with the Rec group in wild-type HHL-5 cells
(Fig. 4B and C). Additionally,
western blot analysis demonstrated that high-concentration (0.6 mM)
Rg1 treatment only slightly downregulated SGPL1 expression in
steatotic HHL-5-SGPL1 cells compared with in wild-type HHL-5 cells,
in which SGPL1 expression was substantially decreased (Fig. 4D and E). Furthermore, the
pro-apoptotic protein Bax (control group) was markedly upregulated
in HHL-5-SGPL1 cells compared with in normal HHL-5 cells, and it
was not significantly downregulated following Rg1 treatment in
HHL-5-SGPL1 cells compared with cells in the Rec group (Fig. 4D and E). By contrast, compared
with those in normal HHL-5 cells, the expression levels of Bcl-2
(control group) in HHL-5-SGPL1 cells were significantly
downregulated, and Bcl-2 expression was not significantly increased
after Rg1 treatment in HHL-5-SGPL1 cells compared with in the Rec
group (Fig. 4D and E).
Additionally, the expression levels of p-AKT and p-Erk1/2 (control
group) in HHL-5-SGPL1 cells were significantly decreased compared
with those in normal HHL-5 cells, although Rg1 treatment increased
their expression levels in HHL-5-SGPL1 cells compared with cells in
the Rec group (Fig. 4D and E),
indicating Rg1 may have exerted a pro-proliferative role in
HHL-5-SGPL1 cells. As a control, there was no significant
difference in the expression levels of SGPL1, and apoptosis-related
genes Bax and Bcl-2, between HHL-5 cells and HHL-5 cells infected
with the empty lentiviral vector pLVX–IRES-Neo (Fig. 4F).
 | Figure 4.Overexpression of SGPL1 inhibits the
anti-apoptotic effect of Rg1. (A) SGPL1 expression was analyzed by
western blotting in normal HHL-5 and HHL-5-SGPL1 cells established
by infecting HHL-5 cells with a lentivirus packaged with SGPL1
overexpression plasmids. (B) HHL-5-SGPL1 and HHL-5 cells were
treated with 1% MCE for 12 h (Rec) and with Rg1 (0.6 mM) for 24 h,
and stained with PI and Annexin V-FITC. Cell apoptosis was assessed
by flow cytometry, and (C) analyzed statistically. (D) SGPL1, Bax,
Bcl-2, p-Akt, Akt, p-Erk1/2 and Erk1/2 expression levels in
HHL-5-SGPL1 and HHL-5 cells were examined by western blotting. (E)
SGPL1, Bax and Bcl-2 protein expression levels were normalized to
actin expression, and p-Erk1/2 and p-Akt protein levels were
normalized to Akt or Erk1/2 using ImageJ software. (F) SGPL1, Bax
and Bcl-2 gene relative expression was detected in HHL-5 cells and
HHL-5 cells transfected with the empty lentiviral vector
pLVX–IRES-Neo. ns, no significance. ***P<0.001; HHL-5-SGPL1
cells, SGPL1-overexpressing HHL-5 cells; p-, phosphorylated; Rec,
recovery; SGPL1, sphingosine-1-phosphate lyase 1; Rg1, ginsenoside
Rg1. |
Discussion
NAFLD is the most prevalent liver disease in humans
with the number of patients approaching two billion worldwide
(19). In ~25% of patients, NAFLD
subsequently leads to steatohepatitis, liver cirrhosis and
hepatocellular carcinoma (20).
The search for effective therapeutic targets for NAFLD and the
development of related drugs have attracted increasing attention.
Rg1 is an active monomer isolated from Panax ginseng and
Panax notoginseng, which has been widely studied due to its
extensive pharmacological action and few side effects (7–12).
In the present study, HHL-5 hepatocytes were used to
establish a NAFLD cell model and it was revealed that Rg1 had the
ability to decrease adipose granule aggregation in HHL-5 cells.
Furthermore, Rg1 inhibited the apoptosis of steatotic HHL-5 cells
by regulating the expression levels of Bcl-2 family proteins, Bax
and Bcl-2. Bcl-2 family molecules include two major categories:
Pro-apoptotic protein molecules, such as Bax, and anti-apoptotic
protein molecules, such as Bcl-2 (21). The balance between pro-apoptotic
and anti-apoptotic Bcl-2 family proteins regulates the apoptotic
fate of cells by regulating the stability and integrity of the
mitochondrial membrane (22). In
the present study, Rg1 downregulated the expression levels of Bax
and upregulated the expression levels of Bcl-2, which may enhance
the stability of mitochondria, thereby making HHL-5 hepatocytes
resistant to the pro-apoptotic effects induced by fat accumulation.
The results revealed the regulation effects of Rg1 on Bax and Bcl-2
expression, which was consistent with the anti-apoptotic effects of
Rg1 on hepatocyte apoptosis, as detected by flow cytometry.
Notably, it is well known that the mechanism underlying the
protective effects of Rg1 against NAFLD is multifaceted (14,15,23,24). For example, it has been reported
to involve regulation of the expression of lipid metabolism enzyme
genes, such as peroxisome proliferator-activated receptor α, and
reductions in blood lipids, blood sugar and inflammatory factor
levels, thereby reducing liver fat accumulation and exerting
hepatoprotective effects (25).
The damage caused by hepatocyte steatosis and
apoptosis serves an important role in the pathogenesis of NAFLD
(7). The mechanism underlying
apoptosis in NAFLD has been suggested to be multifaceted (26), and may involve inflammatory
factors (27), lipid peroxidation
damage (28) and endoplasmic
reticulum stress (29). The
sphingosine signaling pathway serves an important role in the
physiological processes of lipid metabolism, cell survival and
apoptosis (30). The present
study revealed that Rg1 downregulated SGPL1 expression in steatotic
HHL-5 hepatocytes. SPHK1 and SGPL1 are two key enzymes in S1P
metabolism. SPHK1 catalyzes sphingosine to produce S1P, whereas
SGPL1 is responsible for the irreversible cleavage of S1P into
hexadecenal and ethanolamine phosphate (17). S1P is transported out of the cell
by the ATP-binding cassette transporter (31), binds to its receptor (including
sphingosine-1-phosphate receptor 1–5), and activates downstream
signaling pathways (17,18), such as the Akt and Erk1/2
signaling pathways. In the present study, although Rg1 treatment
could upregulate the transcription levels of SPHK1 in HHL-5 cells,
Rg1 had no effect on SPHK1 protein expression. The possible reason
is that the regulation of SPHK1 expression by Rg1 is multifaceted,
and that it is not only limited to effects on mRNA transcription
levels, but also involves protein translation and protein
stability. Since Rg1 downregulated SGPL1 expression in HHL-5
steatotic liver cells, it was hypothesized that Rg1 may have the
effect of upregulating S1P levels, thus exerting anti-apoptotic
effects through its downstream signaling pathways. It can be seen
from the present results that the overexpression of SGPL1 abolished
the anti-apoptotic effect of Rg1 on HHL-5-SGPL1 cells, and markedly
downregulated the expression levels of pro-survival molecules, such
as Bcl-2, p-Akt and p-Erk1/2. Conversely, following overexpression
of SGPL1, the expression levels of the pro-apoptotic molecule Bax
in HHL-5-SGPL1 cells were markedly increased. It may be concluded
that the anti-apoptotic effect of Rg1 in steatotic HHL-5
hepatocytes was blocked by overexpression of SGPL1.
Notably, when HHL-5 cells overexpressing SGPL1 were
treated with 1% MCE and flow cytometry was performed, it was
observed that the treatment time of MCE needed to be reduced from
24 to 12 h, since when used for 24 h, it markedly increased the
apoptotic rate (data not shown). Due to too much apoptosis, not
enough cells could be collected for flow cytometry analysis. This
indicated that cells were too sensitive to adipogenesis when SGPL1
was highly expressed, so the treatment time of MCE was shortened.
Too high an apoptotic rate makes the experiment impossible,
indicating that the overexpression of SGPL1 in HHL-5 cells enhanced
the sensitivity of cells to steatosis. The aforementioned findings
revealed that overexpression of SGPL1 promoted apoptosis during
hepatocyte steatosis, whereas Rg1 served an anti-apoptotic role and
protected liver cells by downregulating the expression levels of
SGPL1.
Several studies (32–34) have reported that SGPL1 serves a
role in the inhibition of cell survival and tumor chemotherapeutic
drug sensitivity. The inactivation of SGPL1 has been reported to
induce resistance of tumor cells to chemotherapy drugs and promote
cell survival (32). Matula et
al (32) reported that
resistance to the chemotherapy drugs oxaliplatin, cisplatin and
docetaxel was associated with decreased expression levels of SGPL1
in gastroesophageal cancer. Degagné et al (33) also reported that the loss of SGPL1
promoted colon carcinogenesis, and similar findings were also
reported in prostate cancer (34). Therefore, the downregulation of
SGPL1 and the inhibition of apoptosis in HHL-5 cells mediated by
Rg1 treatment in the present study were similar to the lack of
SGPL1 activity in tumor cells leading to drug resistance and
promoting cell survival, since abolition of SGPL1 promoted cell
survival. Overexpression of SGPL1 promoted HHL-5 hepatocyte
apoptosis, which revealed that Rg1 served an anti-apoptotic effect
by downregulating SGPL1. Furthermore, Rg1 is one of the main
components of the current prescription drug Xuesaitong in China
(35). Xuesaitong is mainly used
to treat cardiovascular and cerebrovascular diseases, such as
cerebral infarction (36,37) and coronary heart disease (38). In the present study, Rg1 reduced
liver cell steatosis and inhibited cell apoptosis. Therefore, it
may be hypothesized that Xuesaitong or Rg1 could have potential
clinical applications in patients with NAFLD.
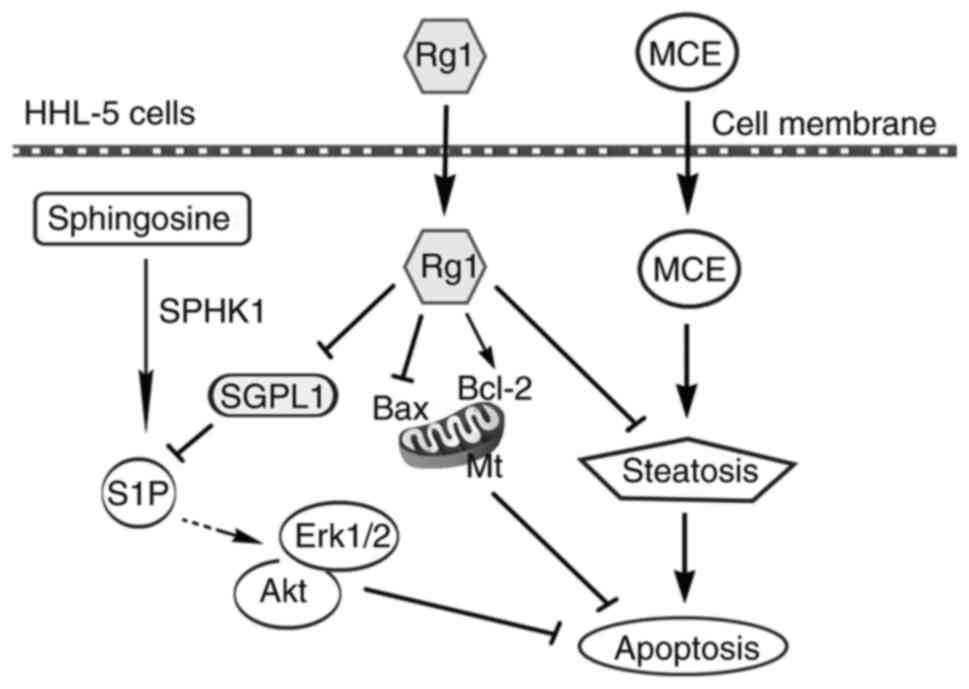
In conclusion, although the mechanism by which Rg1
inhibits cell apoptosis has been widely reported (39–41), to the best of the authors'
knowledge, the present study was the first to report that Rg1
exerted an anti-steatotic effect on hepatocyte apoptosis by
downregulating a key enzyme, SGPL1, in the sphingosine signaling
pathway (Fig. 5). On the one
hand, Rg1 inhibited MCE-induced fat aggregation in HHL-5 cells; on
the other hand, Rg1 upregulated Bcl-2 and downregulated Bax
expression levels to enhance mitochondrial stability. In addition,
Rg1 downregulated SGPL1, and subsequently upregulated pro-survival
p-Erk1/2 and p-Akt proteins via the sphingosine phosphate signaling
pathway. These aforementioned findings may indicate the mechanism
underlying the anti-apoptotic effects of Rg1 on steatotic HHL-5
hepatocytes. However, the present study is limited to the
anti-apoptotic effects of Rg1 on a steatotic hepatocyte model in
vitro, and whether Rg1 can also serve an anti-apoptotic effect
on steatotic hepatocytes by downregulating SGPL1 and sphingosine
kinase pathways in vivo requires further investigation. In
addition, the specific molecular mechanism by which Rg1
downregulates SGPL1 needs to be further elucidated.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural Science
Foundation of China (grant no. 81760109), the National Key Research
and Development Program of China (grant no. 2018YFC2002103), the
Clinical Medicine Development Program of Yunnan Province (grant no.
2019LCZXKF-NM08), the Yunnan Province Dong Birong Academician
Workstation Project (grant no. 202105AF150032) and the Project of
Yunnan Clinical Research Center for Geriatric Diseases (grant no.
202102AA310069).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GL and HX performed all the experiments. GL, YL and
LC conceived and designed the study. GL and HX wrote the main
manuscript. GL, HX, XC and CM analyzed the data. XC and CM confirm
the authenticity of all the raw data. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chalasani N, Younossi Z, Lavine JE,
Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM and Sanyal AJ:
The diagnosis and management of nonalcoholic fatty liver disease:
Practice guidance from the american association for the study of
liver diseases. Hepatology. 67:328–357. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nishioji K, Sumida Y, Kamaguchi M,
Mochizuki N, Kobayashi M, Nishimura T, Yamaguchi K and Itoh Y:
Prevalence of and risk factors for non-alcoholic fatty liver
disease in a non-obese Japanese population, 2011–2012. J
Gastroenterol. 50:95–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Estes C, Anstee QM, Arias-Loste MT, Bantel
H, Bellentani S, Caballeria J, Colombo M, Craxi A, Crespo J, Day
CP, et al: Modeling nafld disease burden in China, France, Germany,
Italy, Japan, Spain, United Kingdom, And United States for the
period 2016–2030. J Hepatol. 69:896–904. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li Z, Xue J, Chen P, Chen L, Yan S and Liu
L: Prevalence of nonalcoholic fatty liver disease in mainland of
China: A meta-analysis of published studies. J Gastroenterol
Hepatol. 29:42–51. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gore E, Bigaeva E, Oldenburger A, Jansen
YJM, Schuppan D, Boersema M, Rippmann JF, Broermann A and Olinga P:
Investigating fibrosis and inflammation in an ex vivo NASH murine
model. Am J Physiol Gastrointest Liver Physiol. 318:G336–G351.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Buzzetti E, Pinzani M and Tsochatzis EA:
The multiple-hit pathogenesis of non-alcoholic fatty liver disease
(NAFLD). Metabolism. 65:1038–1048. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li J, Yang C, Zhang S, Liu S, Zhao L, Luo
H, Chen Y and Huang W: Ginsenoside Rg1 inhibits inflammatory
responses via modulation of the nuclear factor-κB pathway and
inhibition of inflammasome activation in alcoholic hepatitis. Int J
Mol Med. 41:899–907. 2018.PubMed/NCBI
|
|
8
|
Wang ZL, Chen LB, Qiu Z, Chen XB, Liu Y,
Li J, Wang L and Wang YP: Ginsenoside Rg1 ameliorates testicular
senescence changes in D-gal-induced aging mice via
anti-inflammatory and antioxidative mechanisms. Mol Med Rep.
17:6269–6276. 2018.PubMed/NCBI
|
|
9
|
Tang F, Lu M, Yu L, Wang Q, Mei M, Xu C,
Han R, Hu J, Wang H and Zhang Y: Inhibition of TNF-α-mediated NF-κB
activation by ginsenoside Rg1 contributes the attenuation of
cardiac hypertrophy induced by abdominal aorta coarctation. J
Cardiovasc Pharmacol. 68:257–264. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tang YL, Zhou Y, Wang YP, He YH, Ding JC,
Li Y and Wang CL: Ginsenoside Rg1 protects against
sca-1+ HSC/HPC cell aging by regulating the SIRT1-FOXO3
and SIRT3-SOD2 signaling pathways in a γ-ray irradiation-induced
aging mice model. Exp Ther Med. 20:1245–1252. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang SL, He XJ, Li ZF, Lin L and Cheng B:
Neuroprotective effects of ginsenoside Rg1 on oxygen-glucose
deprivation reperfusion in PC12 cells. Pharmazie. 69:208–211.
2014.PubMed/NCBI
|
|
12
|
Wang J, Hou J, Lei H, Fu J, Pan Y and Liu
J: Synergistic neuroprotective effect of microglial-conditioned
media treated with geniposide and ginsenoside Rg1 on hypoxia
injured neurons. Mol Med Rep. 12:5328–5334. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xiao Q, Zhang S, Yang C, Du R, Zhao J, Li
J, Xu Y, Qin Y, Gao Y and Huang W: Ginsenoside Rg1 ameliorates
palmitic acid-induced hepatic steatosis and inflammation in HepG2
cells via the AMPK/NF-κB pathway. Int J Endocrinol.
2019:75148022019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Peng X, Huang D, Yan M and Peng S:
Ginsenoside Rg1 improves liver function by regulating fat
metabolism in rats with non-alcoholic fatty liver disease. Chin J
Pathophysiol. 31:864–870. 2015.
|
|
15
|
Xiao Y, Hou YH, Yin X, Kang F, Li SD, Yang
SK and Tao JP: Ginsenoside Rg1 protects against hepatocyte
apoptosis in a rat model of non-alcoholic fatty liver disease. Chin
J Tissue Eng Res. 23:384–390. 2019.
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kwong E, Li Y, Hylemon PB and Zhou H: Bile
acids and sphingosine-1-phosphate receptor 2 in hepatic lipid
metabolism. Acta Pharm Sin B. 5:151–157. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Strub GM, Maceyka M, Hait NC, Milstien S
and Spiegel S: Extracellular and intracellular actions of
sphingosine-1-phosphate. Adv Exp Med Biol. 688:141–155. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Younossi ZM, Koenig AB, Abdelatif D, Fazel
Y, Henry L and Wymer M: Global epidemiology of nonalcoholic fatty
liver disease-meta-analytic assessment of prevalence, incidence,
and outcomes. Hepatology. 64:73–84. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Singh S, Allen AM, Wang Z, Prokop LJ,
Murad MH and Loomba R: Fibrosis progression in nonalcoholic fatty
liver vs nonalcoholic steatohepatitis: A systematic review and
meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol.
13:643–654. e1–9; quiz e39-40. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Warren CFA, Wong-Brown MW and Bowden NA:
BCL-2 family isoforms in apoptosis and cancer. Cell Death Dis.
10:1772019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Edlich F: BCL-2 proteins and apoptosis:
Recent insights and unknowns. Biochem Biophys Res Commun.
500:26–34. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gao Y, Chu S, Zhang Z and Chen N:
Hepataprotective effects of ginsenoside Rg1-a review. J
Ethnopharmacol. 206:178–183. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu Y, Yang C, Zhang S, Li J, Xiao Q and
Huang W: Ginsenoside Rg1 protects against Non-alcoholic fatty liver
disease by ameliorating lipid peroxidation, endoplasmic reticulum
stress, and inflammasome activation. Biol Pharm Bull. 41:1638–1644.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hou Y, Gu D, Peng J, Jiang K, Li Z, Shi J,
Yang S, Li S and Fan X: Ginsenoside Rg1 regulates liver lipid
factor metabolism in NAFLD model rats. ACS Omega. 5:10878–10890.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cobbina E and Akhlaghi F: Non-alcoholic
fatty liver disease (NAFLD)-pathogenesis, classification, and
effect on drug metabolizing enzymes and transporters. Drug Metab
Rev. 49:197–211. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Arrese M, Cabrera D, Kalergis AM and
Feldstein AE: Innate immunity and inflammation in NAFLD/NASH. Dig
Dis Sci. 61:1294–1303. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Qi J, Kim JW, Zhou Z, Lim CW and Kim B:
Ferroptosis affects the progression of nonalcoholic steatohepatitis
via the modulation of lipid peroxidation-mediated cell death in
mice. Am J Pathol. 190:68–81. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lebeaupin C, Vallée D, Hazari Y, Hetz C,
Chevet E and Bailly-Maitre B: Endoplasmic reticulum stress
signalling and the pathogenesis of non-alcoholic fatty liver
disease. J Hepatol. 69:927–947. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Maceyka M, Harikumar KB, Milstien S and
Spiegel S: Sphingosine-1-phosphate signaling and its role in
disease. Trends Cell Biol. 22:50–60. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yamada A, Nagahashi M, Aoyagi T, Huang WC,
Lima S, Hait NC, Maiti A, Kida K, Terracina KP, Miyazaki H, et al:
ABCC1-Exported Sphingosine-1-phosphate, produced by sphingosine
kinase 1, shortens survival of mice and patients with breast
cancer. Mol Cancer Res. 16:1059–1070. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Matula K, Collie-Duguid E, Murray G,
Parikh K, Grabsch H, Tan P, Lalwani S, Garau R, Ong Y, Bain G, et
al: Regulation of cellular sphingosine-1-phosphate by sphingosine
kinase 1 and sphingosine-1-phopshate lyase determines chemotherapy
resistance in gastroesophageal cancer. BMC Cancer. 15:7622015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Degagné E, Pandurangan A, Bandhuvula P,
Kumar A, Eltanawy A, Zhang M, Yoshinaga Y, Nefedov M, de Jong PJ,
Fong LG, et al: Sphingosine-1-phosphate lyase downregulation
promotes colon carcinogenesis through STAT3-activated microRNAs. J
Clin Invest. 124:5368–5384. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Brizuela L, Ader I, Mazerolles C, Bocquet
M, Malavaud B and Cuvillier O: First evidence of sphingosine
1-phosphate lyase protein expression and activity downregulation in
human neoplasm: Implication for resistance to therapeutics in
prostate cancer. Mol Cancer Ther. 11:1841–1851. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dai G, Jiang Z, Bai Y, Zhang Q, Zhu L, Bai
X, Ju W and Pan R: Pharmacokinetic herb-drug interaction of
Xuesaitong dispersible tablet and aspirin after oral administration
in blood stasis model rats. Phytomedicine. 26:62–68. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li F, Zhao H, Han Z, Wang R, Tao Z, Fan Z,
Zhang S, Li G, Chen Z and Luo Y: Xuesaitong may protect against
ischemic stroke by modulating microglial phenotypes and inhibiting
neuronal cell apoptosis via the STAT3 signaling pathway. CNS Neurol
Disord Drug Targets. 18:115–123. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen H, Cao H, Guo X, Zhao M, Xia Q, Chen
B, Zhao T and Gao W: Naoxuekang, Xinnaoshutong and Xuesaitong
capsules for treating stroke: A protocol for a randomised
controlled trial. BMJ Open. 7:e0159832017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang X, Xiong X, Wang H, Yang G and Wang
J: Xuesaitong soft capsule (Chinese patent medicine) for the
treatment of unstable angina pectoris: A meta-analysis and
systematic review. Evid Based Complement Alternat Med.
2013:9483192013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xu TZ, Shen XY, Sun LL, Chen YL, Zhang BQ,
Huang DK and Li WZ: Ginsenoside Rg1 protects against H2O2-induced
neuronal damage due to inhibition of the NLRP1 inflammasome
signalling pathway in hippocampal neurons in vitro. Int J Mol Med.
43:717–726. 2019.PubMed/NCBI
|
|
40
|
Li H, Xu J, Wang X and Yuan G: Protective
effect of ginsenoside Rg1 on lidocaine-induced apoptosis. Mol Med
Rep. 9:395–400. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang Y, Ding S, Chen Y, Sun Z, Zhang J,
Han Y, Dong X, Fang Z and Li W: Ginsenoside Rg1 alleviates
lipopolysaccharide-induced neuronal damage by inhibiting NLRP1
inflammasomes in HT22 cells. Exp Ther Med. 22:7822021. View Article : Google Scholar : PubMed/NCBI
|